ABSTRACT
Surveys were conducted with cage and alternative layer production systems to assess the prevalence of fatty liver haemorrhagic syndrome (FLHS). Commercial caged laying hens of different ages from three farms in Queensland were monitored for three months. The mortality rate of flocks ranged from 0.8% (the youngest flock) to 11.6% (the oldest flock). Six hundred and fifty-one birds were necropsied, and approximately 40% of hens died due to FLHS. Hens kept in cages in a controlled environment shed, were at a similar risk of developing FLHS to hens kept in naturally controlled sheds, however, the heavier birds in a flock were more likely to have the condition than lighter birds. In another study, layer flocks kept in cage, barn and free-range housing systems at the University of Queensland facility, were monitored for 50 weeks. Data from necropsies and performance records showed no significant differences in mortality rates between the housing systems (6.1%, 6.4% and 5.8%, for cages, barns and free-range, respectively), but the cause of mortality was different. In cages, 74% of necropsied hens died due to FLHS. In the other systems, only 0–5% of dead hens were diagnosed with the condition. These results are in agreement with previous Australian and overseas findings which have shown that FLHS is one of the main causes of hen death in caged flocks. Factors associated with husbandry practices in different production systems, such as restricted movement, increased production and temperature variations, influence hepatic lipid metabolism and predispose hens to FLHS.
Introduction
Husbandry practices of the egg industry have changed in response to consumers’ concerns about hen welfare. As a result, alternative egg production systems have been introduced during the last 2–3 decades. However, cage production systems still continue to be the predominant system for housing laying hens in many countries (Mench et al., Citation2011). In 2011, around 85% of laying hens were housed in conventional cage systems, worldwide (Windhorst, Citation2011). In 2018, it was reported that, globally, cage systems (and presumably mainly conventional cages) are the predominant egg production method in all major egg producing countries/regions except the EU-15 (Mench & Rodenburg, Citation2018). More than 90% of egg production in three of the largest egg producing countries (China, Japan and United States) comes from caged hens. This figure is 100% for the other four largest egg producing countries (Turkey, India, Russia and Mexico) (Mench & Rodenburg, Citation2018). In Australia, approximately 50% of eggs are produced in cage layer farms, with the balance coming from free-range (40%) and barns (8.5%) (AECL, Citation2016). Previous studies have identified several disease problems being associated with the type of layer housing system (Rodenburg et al., Citation2005; Weitzenburger et al., Citation2005; Fossum et al., Citation2009; Shimmura et al., Citation2010). Some metabolic disorders, including fatty liver haemorrhagic syndrome (FLHS), are associated with the cage system (Scheele, Citation1997; Julian, Citation2005; Leeson, Citation2007).
Some 40 years ago, Neill et al. (Citation1975) described a number of FLHS outbreaks (from 1970 to 1975) in laying hens in south-east Queensland. The presence of severe fat infiltration in a structurally weakened liver was thought to have been the reason for massive hepatic haemorrhage and death of hens (Neill et al., Citation1975). At the same time, Grimes (Citation1975) surveyed two commercial caged flocks in Queensland, and found a high incidence of FLHS in both flocks (approximately 4% of the flocks). Both investigators pointed out that Queensland probably presents “ideal” climatic conditions for outbreaks of this disorder (Grimes, Citation1975; Neill, et al., Citation1975). From these surveys, it was suggested that the sporadic nature of FLHS, and the lack of established diagnostic tests, makes it difficult to detect and predict in laying flocks. Necropsy was required for a definitive diagnosis.
This study was based on the hypothesis that FLHS continues to be the main cause of mortality in commercial hens kept in cages. In view of findings of previous studies, we predicted that hens in the cage system would demonstrate a higher incidence of FLHS compared to hens kept in alternative housing systems.
This paper reports the incidence of FLHS in three commercial caged layer flocks in south-east Queensland. The survey was considered timely as, over the last 30 years, housing and feeding practices have improved, and the genetics of egg laying hens have changed. Moreover, as the modern egg industry is in transition and represented by both conventional and alternative housing systems, the opportunity was taken to examine the mortality rate and occurrence of FLHS in laying hens in relation to current housing systems used for layers. The latter investigation was undertaken in the Poultry Science Unit of the University of Queensland. The Unit maintained layer flocks in three different housing systems; cages, barn and free-range.
Materials and methods
The surveys conducted in this study were approved by the University of Queensland Animal Ethics Committee and complied with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Birds and bird husbandry
A commercial cage farm survey was undertaken of layer flocks on three farms (designated Farms 1, 2 and 3) that were representative of the cage egg industry in Queensland. Details of the farms appear in . As indicated in , there were three flocks of different ages on Farms 1 and 3 but only one flock on Farm 2. A combined total of 21,903 laying hens were monitored during the survey. Farms were visited on three occasions over a 3-month period, and data on mortality and mortality causes, egg production and body weight (BW) were recorded over the monitoring period. Cases of FLHS were confirmed at necropsy on-farm.
Table 1. Description of commercial cage farms that were surveyed in Queensland.
The housing systems survey was carried out at the Poultry Science Unit (Layer Facility) on the Gatton campus of the University of Queensland. This facility provided a unique resource as Hy-Line Brown layers of the same hatch were housed under three different housing systems; conventional cages, barn (a cage-free egg production system, where hens are housed loose in a naturally ventilated shed, with litter, perches and nest boxes), and free-range. Birds had been reared on the same farm and under the same conditions as they were placed during the laying cycle, fed the same diet, and subjected to the same lighting (a standard lighting programme was used for all systems, 16L:8D) and vaccination programmes. Pullets were placed in the respective layer facilities at 17 weeks of age and fed a commercial layer diet with the following specifications: crude protein (CP) 17.5%; metabolizable energy (ME) 11.5 MJ/kg; calcium (Ca) 4.1%; available phosphorus (P) 0.40%; sodium (Na) 0.18%; lysine 0.85%, methionine + cystine 0.77%. Water and feed were provided ad libitum. All the caged laying hens and barn system hens were beak-trimmed at day-old but, in accordance with the Royal Society for the Prevention of Cruelty to Animals recommendations, free-range hens were not beak-trimmed. The free-range system comprised three separate units or enclosures located adjacent to each other which allowed a density of five hens/m2 of floor space or 600 hens per shed, five hens per nest box, 176 mm of perch per hen, cupped nipple drinkers (1/20 birds) and automatic feed chain feeders (giving a space of 4.8 cm/hen); hens had access to a grassed outdoor free-range area suitable for 1500 hens/hectare from 10:00 to 18:00 daily. The barn system (Big Dutchman flat chain system) was represented by three replicate units, each housing 600 hens at approximately seven hens/m2. Within each unit, 1/3 of the floor area was covered by litter and the remaining 2/3 by a slatted floor which also contained perches, automated nest boxes, cupped nipple drinkers (1/20 birds) and an automatic chain feeder (4.9 cm space/hen). Cages (Big Dutchman, Euro-vent, six hens/cage) were located in an environmentally controlled shed (23 ± 0.5°C) with tunnel ventilation and a pad system for summer cooling and fan assisted heaters for winter. Cages were in three tiers (three replicates), with 48 units or cages per tier, and in two rows back to back. There were 576 hens per replicate, providing 550 cm2/hen, and two nipple drinkers/cage and 10 cm of feeding space/hen.
Bird performance
For the commercial cage farm survey, production and mortality data were provided by farmers and used to calculate percentage hen-day egg production (HDP%), and mortality rates (%) expressed as cumulative mortality from the start of lay. To assess body weight (BW), measurements were taken during the time when each farm was visited to conduct necropsies (once a month). The same birds, taken at three different locations in the shed, were weighed on three separate occasions. For an accurate BW monitoring in layers, most breeder companies recommend individually weighing 3–5% of the flock at each weighing (Hy-Line, Citation2008). Others suggest that 2% of the flock is sufficient, provided that a minimum of 50 birds are weighed irrespective of flock size (Leeson & Summers, Citation2000). For Farm 1, with approximately 5000 birds per flock, individual birds in 40 cage units (240 birds or 4.8% of birds per flock) were weighed; for Farm 2, with approximately 3240 birds per flock, birds in 48 cage units (144 birds or 4.4% of birds) were weighed; for Farm 3, with approximately 1500 birds per flock, birds in 24 cage units (72 birds or 4.8% of birds per flock) were weighed. Individual birds from each cage unit were weighed and the results are expressed as an average BW (g) per bird.
For the housing systems survey, egg production and body weight data were collected from 19 to 70 weeks of age. Eggs were collected daily between 09:30 h and 12:30 h. The number of eggs laid in a flock was calculated as HDP% and BW was measured monthly. Fifty birds from each enclosure in the free-range and barn systems, and all birds in a cage (six hens) from nine cages (54 birds per replicate) were weighed at each time point. Data on feed consumption were recorded inconsistently for all systems, and therefore not used in this study.
Mortality rate and cause
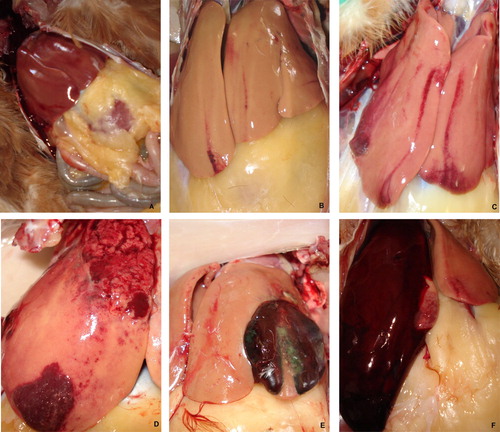
All dead birds from three commercial cage farms were collected and data recorded. Due to insufficient freezer storing space, post mortem examination was only carried out on birds that died and were stored in the freezer during the first 10 days of each month for three months. A complete necropsy was carried out on each bird. Particular attention was paid to the presence of an excess amount of fat and blood or blood clots in the coelomic cavity. The liver was carefully removed, examined for haemorrhages and haematomas, and weighed. Haemorrhage was assessed on both the dorsal and ventral surfaces of the liver and was graded on a scale from 0 to 5 (), with score 0 indicating no haemorrhages; score 1, up to 10 subcapsular petechial or ecchymotic haemorrhages; score 2, more than 10 subcapsular petechial or ecchymotic haemorrhages; and scores 3–5, large haematomas and massive liver haemorrhage accompanied by rupture of liver capsule. A haemorrhagic score of 3 to 5 was considered highly characteristic of FLHS and was diagnosed as the cause of death. Histological, bacteriological, parasitological or other laboratory examinations were not performed in any of the surveys.
Figure 1. Livers of hens showing various stages of FLHS (petechial haemorrhages, haematomas and massive haemorrhages) scored 0 (A), 1 (B), 2 (C) and 3–5 (D–F).
For the housing systems survey, data on mortality were collected from 19 to 70 weeks of age. Mortality rate was calculated as a percentage of the initial number of hens in a flock. Mortality causes were determined from gross necropsy of birds that died between 30 and 62 weeks of age. Only gross pathological findings were used to determine the cause of death. Birds that died were collected daily, refrigerated and necropsied weekly. The results presented in this paper are only for birds necropsied during the survey period (240 dead birds); not all dead birds recorded during this period were necropsied (in total 263) due to advanced decomposition. Other problems such as parasite infestations and other pathologies that did not lead to the death of hens are not included or discussed in this study.
Statistics
For the commercial cage farm survey, a direct comparison of the data from different farms was not valid statistically, due to variations in management. Hence, results from each farm were summarized into three periods (at each sampling point) and used to evaluate trends in mortality, BW and egg production within each farm. The main independent variable of interest, in this case, was the housing system, while BW, mortality and the frequency of FLHS were the dependent variables. To calculate the minimum sample size (i.e. the number of birds that needed to be necropsied from each flock at each sampling point), the occurrence rate was predicted to be 20–50% of dead birds, as previously found in field investigations (Couch, Citation1956; Butler, Citation1975; Dimitrov et al., Citation1980; Weitzenburger et al., Citation2005; Valkonen et al., Citation2008). Therefore, for an estimated prevalence of disease 20% or more and with a confidence level 95%, a minimum sample size of 14 necropsied birds was found to be sufficient to detect FLHS in a flock with a population of >1000 birds (Cannon & Roe, Citation1982). Data on BW measured on farms were subjected to one-way ANOVA. All analyses were performed using the General Linear Model procedure of the SAS version 8.0 software (SAS Institute Inc., Citation2001). Significant differences between sampling points were determined using the Tukey's HSD (Honestly Significant Difference) test. Statements of significance are based on P < 0.05.
For the housing systems survey, data were subjected to one-way ANOVA for repeated measure tests and SAS programme (SAS Inc., Citation2001) was used to test the effect of housing system (the independent variable) on dependent variables, such as hen performance and mortality. For further interpretation of data, General Linear Model procedures were used. When significant differences were found, comparisons among systems were conducted by Duncan's multiple comparison tests. Statements of significance are based on P < 0.05. Correlations between BW and mortality rate, and BW and HDP% for birds housed in cages were determined using Pearson correlation coefficients. The means (n = 3) were calculated for each system and are presented in Figures and Tables.
Results
Commercial farm survey
Performance parameters
Hen performance data (HDP%, BW and cumulative mortality) for commercial cage farms surveyed are presented in . Data from the layer breeding companies (Management Guides: ISA, Citation2000; Hy-Line, Citation2008) are included in for comparisons. In general, the results obtained from this survey were comparable with the breeder's recommendations, especially given possible differences in climatic conditions and diets fed. Body weight increased with age for birds in Farms 2 and 3 (). At 73 weeks of age, there was a numerical decrease in BW of birds in Farm 1. The average BW of dead birds in Farms 1, 2 and 3 were 2008 ± 107, 1821 ± 78 and 1954 ± 92 g, respectively. In terms of HDP%, the age trend for three flocks from Farm 1 is presented in . From the start of lay until the peak the HDP% increased (P < 0.001) with age. From 26 weeks of age, birds had an HDP% close to 90% and this continued until 42 weeks of age. At 32 weeks of age, HDP% was over 90% in all farms, and comparable with the breeder's recommendations for the respective age ().
Figure 2. Average body weight (BW) of hens for Farms 1, 2 and 3. Data are presented as mean ± SD of hens weighed at each sampling point (every 4 weeks) and only for one flock per farm. Number of birds weighed at each sampling point was: Farm 1 (n = 240), Farm 2 (n = 144) and Farm 3 (n = 72).
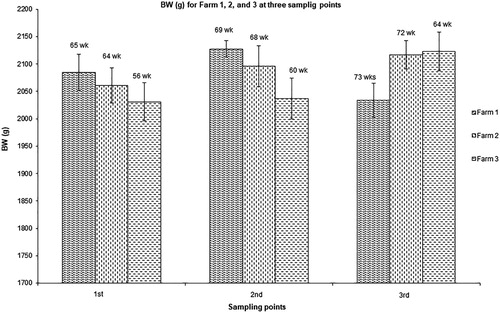
Figure 3. Egg production (HDP%) of hens from Farm 1, flocks of different ages: flock 1 (a), flock 2 (b) and flock 3 (c). Data shown only for the period of study for each flock.
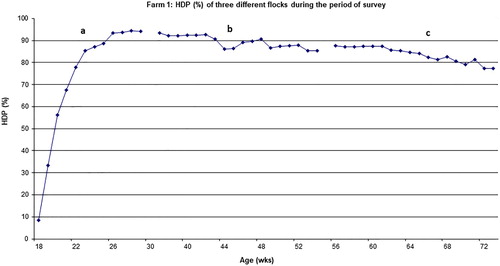
Table 2. Comparative data on performance monitored in three commercial cage farms in Queensland vs. data recommended by breeders in the management guides (Hy-Line Brown for Farms 1 and 2; and ISA Brown for Farm 3).
Mortality rate and necropsy findings
shows cumulative mortality and the number of dead birds and necropsies that were conducted on all three commercial cage farms surveyed. The mortality rate of flocks ranged from 0.8% (the youngest flock) to 11.6% for the oldest one. The mortality rates increased with age (P < 0.001). The age trend in mortality rates for the three flocks in Farm 1 are presented in . Each part of the graph represents one flock, and the entire graph represents three flocks from the same farm jointly. The results indicate that at 29, 54 and 73 weeks of age, the percentage cumulative mortality of flocks (1, 2 and 3) was 2%, 4.8% and 11.6%, respectively. The monthly mortality rate for these flocks during the study ranged from 1% to 1.2% per month. At 72 weeks of age, mortality rate in Farm 2 was 7.4%. At 31, 49 and 64 weeks of age the mortality rate in Farm 3 was 0.8%, 2.5% and 4.8% (for flocks 1, 2 and 3, respectively) (). As indicated in the methodology, only 30–50% of dead birds were necropsied. On Farm 1, 42% of birds necropsied showed large subcapsular hepatic haemorrhage and blood clotting in the abdominal cavity indicative of FLHS, while in Farms 2 and 3, 28% and 34% of dead birds, respectively, exhibited signs of FLHS (). On Farm 1, 63% of birds that died between 42 and 54 weeks of age were found to have clinical signs of FLHS.
Figure 4. Data on hen mortality for Farm 1: flock 1 (a), flock 2 (b) and flock 3 (c). Mortality is presented for flocks of different ages and only for the period of study as a cumulative mortality of birds housed in the shed at start of lay.
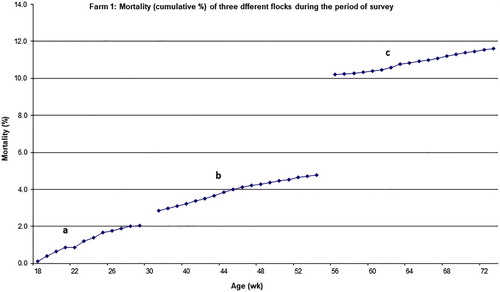
Table 3. Mortality (%), number of post mortem examinations conducted, and frequency of FLHS in laying hens found on three commercial cage farms in Queensland.
Housing systems survey
Performance parameters
Data on egg production (HDP%) are shown in , and data on BW for all systems are presented in . As shown in , hens in cages started laying eggs earlier than hens in other systems; however, egg production peaked at about 33 weeks of age (with HDP% over 90%) for all systems. At the end of the laying period, there were no significant differences in HDP% (P > 0.05) between systems. Data on BW () show that all birds started laying with a similar BW (ca. 1700 g per bird). From the start to the peak of lay BW increased significantly (P < 0.01) in all systems. Birds were heaviest (P < 0.001) around 49 and 53 weeks of age compared to their BW at peak of lay. Hens in cages maintained their high BW till the end of the study, while hens in barns and free-range lost weight and were significantly lighter (P < 0.05) than caged birds at 69 weeks. There was a positive correlation between BW and mortality rate (r = 0.78, P < 0.001), and BW and HDP% (r = 0.46, P < 0.01) for the cage system. A weak, but not significant, correlation (r = 0.15, P > 0.05) was found between mortality rate and HDP% ().
Figure 5. Monthly body weight (g) average1 of laying hens from 21 to 70 weeks of age in three different housing systems. 1Each value represents the average (n = 3) of each unit (replicate) calculated on the basis of number of hens weighed at each time point.
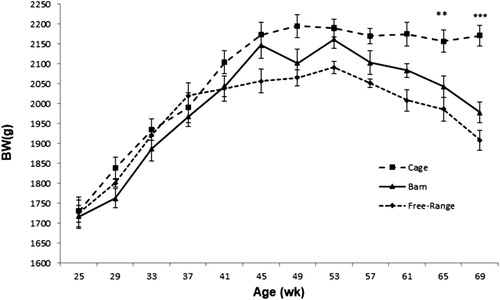
Table 4. Egg production (HDP%) and mortality (%) of laying hens from 19 to 70 weeks of age kept in three different housing systems.
Table 5. Pearson's correlation of body weight (BW), mortality rate and hen-day production (HDP%) for hens kept in the cage system.
Mortality rates and necropsy findings
Cumulative mortality from 19 to 70 weeks of age is shown in , and data on causes of mortality are presented in . At 70 weeks of age, the mean of cumulative mortality rate was similar (P = 0.7) at 6.1%, 6.4%, 5.8%, respectively, for cages, barn and free-range hens. The gross pathology findings showed that causes of mortality for birds in different systems were different. The necropsy results indicated that in the cage system 74% of birds died due to FLHS. Several birds from the cage system (7% of all birds necropsied) showed prolapse (but no signs of cannibalism), while 5% of examined birds were pecked and died due to cannibalism. For six cadavers, the cause of death was unclear. In the barn system, necropsy revealed that 59% of deaths were due to cannibalism. There were 16 birds (or 17% of birds examined) in which the cause of death was unclear. Abdominal haemorrhage was present in 5% of examined birds. Ten examined birds showed inflammation of the ovary, oviduct and cloaca (salpingitis), and one bird showed necrotic alterations of the intestine. In the free-range system, 77% of dead birds demonstrated evidence of cannibalism due to the presence of fresh wounds on the back, tail and vent. There were 11 cachectic birds (or 14% of examined birds) but no signs of cannibalism were found. Examination of the reproductive tract of cachectic birds revealed an atrophied ovary (i.e. lack of large yellow follicles). The cause of death was unclear in seven cadavers from the free-range system.
Table 6. Causes of death determined following necropsy of hens in the housing systems study.
Discussion
A survey was conducted of three commercial cage egg operations in south-east Queensland, to assess the effect of the production system on hen health and performance. This study was followed by another survey of research layer flocks kept in cages, barn or free-range housing systems at the University of Queensland poultry facility. The unique feature of the current study was that the different housing systems were populated by hens of the same genotype and the same hatch reared together and fed the same diets during growth and production, thus removing many of the factors that can confound comparisons of different housing systems. From necropsies conducted in both studies, it was found that FLHS was the main cause of mortality in hens kept in cages. The results are in agreement with previous Australian and overseas findings, which have demonstrated that FLHS was one of the main causes of death in caged commercial flocks irrespective of mortality rate (Grimes, Citation1975; Lee et al., Citation1975; Neill, et al., Citation1975; Peckham, Citation1984; Rodenburg, et al., Citation2005; Weitzenburger, et al., Citation2005; Valkonen, et al., Citation2008).
Necropsies of differently aged flocks in three commercial cage farms indicated that, of all birds examined, on average approximately 34% died due to FLHS, demonstrating that FLHS was the most significant cause of death of laying hens in these farms. However, only 600 birds were necropsied, representing approximately 40% of all birds that died from the three farms during the three months study. Thus, the incidence calculated in this study may be an underestimate of actual prevalence FLHS in commercial cage farms. Data from the housing system survey revealed that housing system did not affect mortality rate, but had a major influence on the cause of a hen's death. The most common cause of death in cages was FLHS with 74% of necropsied hens dying from this condition. The majority of hens kept in free-range died from cannibalism (77%) and cachexia (14%), while hens in barns succumbed to cannibalism (59%), reproductive tract infections (10%) and few to cachexia. The difference in the occurrence of FLHS in hens kept in cages noted in this study may be due to a number of factors. Estimates of the prevalence of FLHS, in the study with research layer flocks, were based on necropsies that were conducted systematically throughout the laying cycle (from 19 to 70 weeks of age). In contrast, necropsies on the commercial cage farms were conducted only over a 3-month period, and only from 40% of dead birds examined. The high incidence of FLHS in caged hens, found in the current surveys, is in agreement with previous studies, which have suggested that FLHS is a metabolic condition of hens kept in conventional cages (Couch, Citation1956; Ringer & Sheppard, Citation1963; Neill et al., Citation1975; Butler, Citation1976; Simonsen, Citation1978; Peckham, Citation1984; Weitzenburger et al., Citation2005).
Previous studies have suggested that increased body weight had a major impact on hen mortality and, in many cases, was associated with fatty livers and FLHS (Harms et al., Citation1972; Pearson & Butler, Citation1978b; Walzem et al., Citation1993; Schumann et al., Citation2003). Likewise, these studies have also suggested an increased incidence of FLHS in high-producing flocks (Couch, Citation1956; Harms, et al., Citation1972; Lee, et al., Citation1975; Squires & Leeson, Citation1988; Julian, Citation2005; Leeson, Citation2007). In this study, increased body weight correlated well with the incidence of FLHS. It was shown that there was a positive and significant correlation between BW (g) and mortality rate (%), and BW (g) and egg production (HDP%) in the cage system. In contrast, there was a weak correlation between mortality rate and egg production indicating that the level of production was a lower contributor to the mortality rate, compared to the BW, and subsequently to the occurrence of FLHS. At peak production (ca. 33 weeks of age), the mortality rate was under 2% in all systems. Birds in cages increased BW over time, relative to birds in other systems; presumably, fat deposition was also increased, most likely due to a positive energy balance and lack of physical activity (Richards & Proszkowiec-Weglarz, Citation2007). Providing laying hens with more space for movement and exercise could reduce excess fat deposition in the liver. Greuel & Hartfiel (Citation1968) and Hartfiel et al. (Citation1972) investigated the effect of the housing system on liver fat content, a predisposing factor to FLHS, and concluded that the percentage of hens with a relatively high liver fat content was decreased when hens were kept on the floor.
The results from both surveys indicated that laying hens, in cages in an environmentally controlled shed, are at a similar risk of developing FLHS as hens kept in naturally controlled commercial sheds. This is the first demonstration of a similar effect of a thermo-neutral environmental temperature and a diurnal fluctuating natural environmental temperature on the occurrence of FLHS in caged hens. Lee et al. (Citation1975), had suggested that keeping temperature controlled at a thermo-neutral zone does not decrease the incidence of FLHS. However, other studies that examined the effect of temperature on the occurrence of FLHS were conducted approximately 40 or 50 years ago, when controlled environment sheds were not widely used in the laying hen production system. In these studies, increased mortality due to FLHS was found during hot weather (Couch, Citation1956; Greuel & Hartfiel, Citation1968; Ivy & Nesheim, Citation1973; Schexnailder & Griffith, Citation1973; Pearson & Butler, Citation1978a). Interestingly, Wolford (Citation1971) observed that the liver fat content in birds housed at 17°C for 28 days was significantly lower than in hens housed at 26.7°C for the same period. Lee et al. (Citation1975), however, did not see any effect of changing the environmental temperature from 22.2°C to 30.6°C or from 30.6°C to 22.2°C on total liver fat or liver wet weight. There is some evidence that daily fluctuations in temperature, affected by the season of the year, stimulates hens to over consume feed. Jensen et al. (Citation1976) observed more FLHS in warmer vs. cooler regions of Georgia, despite feed intake being reduced at high temperatures and increased in cold temperatures. The relationship between feed intake and hepatic lipid accumulation in laying hens is not straightforward, and is likely to be modulated by the rate of egg production and feed composition (Leeson, Citation2012).
It is pertinent to remember that death from FLHS occurs only in extreme cases following massive liver haemorrhage, suggesting that a significant number of hens within a flock might suffer from “sub-acute and chronic FLHS”. The chronic form of FLHS may cause a drop in egg production, but little or no change in mortality; such hens may exhibit reproductive dysfunction (Chen et al., Citation2006). This could be the case for hens in Farm 1 (commercial cage farm survey), where there was a drop in HDP% in hens from 46 to 54 weeks of age, while the mortality rate was constant.
In conclusion, this study confirms that FLHS continues to be a disease with high prevalence in caged flocks in Queensland. It is an unresolved malady in laying hens (anecdotally called “the silent killer”) and both the acute and chronic forms of the disease are a significant source of loss for cage egg producers. Factors associated with husbandry practices in different production systems, such as restricted movement, increased production and other system-related stressors, such as temperature and humidity variations, influence hepatic lipid metabolism and predispose the hen to FLHS. It is recommended that the systematic monitoring of flocks for increased BW, signs of decreased egg production, and increased mortality, along with post mortem examinations of dead hens, may assist producers in detecting FLHS and making strategic management decisions for their flocks while maximizing egg production and hen welfare.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AECL. (2016). Australian Egg corporation limited – Annual Report 2016.
- Butler, E. (1975). Lipid metabolism in the fowl under normal and abnormal circumstances. Proceedings of the Nutrition Society, 34, 29–34. doi: 10.1079/PNS19750007
- Butler, E. (1976). Fatty liver diseases in the domestic fowl – a review. Avian Pathology, 5, 1–14. doi: 10.1080/03079457608418164
- Cannon, R.M. & Roe, R.T. (1982). Livestock disease surveys. A field manual for veterinarians. Bureau of Resource Science, Department of Primary Industry.
- Chen, S.E., McMurtry, J.P. & Walzem, R.L. (2006). Overfeeding-induced ovarian dysfunction in broiler breeder hens is associated with lipotoxicity. Poultry Science, 85, 70–81. doi: 10.1093/ps/85.1.70
- Couch, J.R. (1956). Fatty livers in laying hens – a condition which may occur as a result of increased strain. Feedstuffs, 28, 46–54.
- Dimitrov, A., Antonov, S., Stoianov, P., Petrova, L. & Aleksandrova, E. (1980). Fatty liver syndrome in laying hens. Veterinarno-medicinski Nauki, 17, 81–89.
- Fossum, O., Jansson, D.S., Etterlin, P.E. & Vågsholm, I. (2009). Causes of mortality in laying hens in different housing systems in 2001 to 2004. Acta Veterinaria Scandinavica, 51, 3. doi: 10.1186/1751-0147-51-3
- Greuel, E. & Hartfiel, W. (1968). Changes in fat content and fatty acid pattern in chickens with fatty liver syndrome. Deutsche Tierärztliche Wochenschrift, 75, 294–296.
- Grimes, T.M. (1975). Causes of disease in two commercial flocks of laying hens. Australian Veterinary Journal, 51, 337–343. doi: 10.1111/j.1751-0813.1975.tb15942.x
- Harms, R.H., Simpson, C.F. & Damron, B.L. (1972). Some new observations on “fatty liver syndrome” in laying hens. Avian Diseases, 16, 1042–1046. doi: 10.2307/1588827
- Hartfiel, W., Splittgerber, H. & Wein, F.K. (1972). Untersuchungen zum fettlebersyndrom der legehennen. II. mitteilung: vergleich von boden-und kafighaltung. Archiv für Geflugelkunde, 36, 11–15.
- Hy-Line. (2008). Hy-Line Variety Brown Commercial Management Guide 2006-2008.
- ISA Brown. (2000). ISA Brown Management Guide.
- Ivy, C.A. & Nesheim, M.C. (1973). Factors influencing the liver fat content of laying hens. Poultry Science, 52, 281–291. doi: 10.3382/ps.0520281
- Jensen, L.S., Chang, C.H. & Maurice, D.V. (1976). Liver lipid accumulation and performance of hens as affected by cage density and initial body weight. Poultry Science, 55, 1926–1932. doi: 10.3382/ps.0551926
- Julian, R.J. (2005). Production and growth related disorders and other metabolic diseases of poultry – a review. The Veterinary Journal, 169, 350–369. doi: 10.1016/j.tvjl.2004.04.015
- Lee, K., Flegal, C.J. & Wolford, J.H. (1975). Factors affecting liver fat accumulation and liver hemorrhages associated with fatty liver-hemorrhagic syndrome in laying chickens. Poultry Science, 54, 374–380. doi: 10.3382/ps.0540374
- Leeson, S. (2007). Metabolic challenges: past, present, and future. The Journal of Applied Poultry Research, 16, 121–125. doi: 10.1093/japr/16.1.121
- Leeson, S. (2012). Future considerations in poultry nutrition. Poultry Science, 91, 1281–1285. doi: 10.3382/ps.2012-02373
- Leeson, S. & Summers, J.D. (2000). Broiler breeder production. Nottingham: University Press.
- Mench, J.A. & Rodenburg, T.B. (2018). Sustainability of laying hen housing systems. In J.A. Mench (Ed.), Advances in Poultry Welfare (pp. 199–225). Oxford, UK: Woodhead Publishing.
- Mench, J.A., Sumner, D.A. & Rosen-Molina, J.T. (2011). Sustainability of egg production in the United States--the policy and market context. Poultry Science, 90, 229–240. doi: 10.3382/ps.2010-00844
- Neill, A.R., McKenzie, R.A., Schultz, K. & Connor, J.K. (1975). Reticulolysis and fatty liver syndrome in commercial laying fowls. Australian Veterinary Journal, 51, 104–105. doi: 10.1111/j.1751-0813.1975.tb09423.x
- Pearson, A.W. & Butler, E.J. (1978a). Environmental temperature as a factor in the aetiology of fatty liver-haemorrhagic syndrome in the fowl. Research in Veterinary Science, 25, 133–138. doi: 10.1016/S0034-5288(18)32967-9
- Pearson, A.W. & Butler, E.J. (1978b). Pathological and biochemical observations on subclinical cases of fatty liver-haemorrhagic syndrome in the fowl. Research in Veterinary Science, 24, 65–71. doi: 10.1016/S0034-5288(18)33100-X
- Peckham, M.C. (1984). Diseases of caged layers. In M.S. Hofstad, H.J. Barnes, B.W. Calnek, W.M. Reid & H.W. Yoder (Eds.). Diseases of poultry 8th edn (pp. 775–776). Ames: Iowa State Press.
- Richards, M.P. & Proszkowiec-Weglarz, M. (2007). Mechanisms regulating feed intake, energy expenditure, and body weight in poultry. Poultry Science, 86, 1478–1490. doi: 10.1093/ps/86.7.1478
- Ringer, R.K. & Sheppard, C.C. (1963). Report of fatty liver syndrome in a Michigan caged layer operation. Quarterly Bulletin of the Michigan Agricultural Experimental Station, 45, 426–427.
- Rodenburg, T.B., Tuyttens, F.A., Sonck, B., De Reu, K., Herman, L. & Zoons, J. (2005). Welfare, health, and hygiene of laying hens housed in furnished cages and in alternative housing systems. Journal of Applied Animal Welfare Science, 8, 211–226. doi: 10.1207/s15327604jaws0803_5
- SAS Institute Inc. (2001). SAS/STAT guide for personal computers. Cary, NC, USA.
- Scheele, C.W. (1997). Pathological changes in metabolism of poultry related to increasing production levels. Veterinary Quarterly, 19, 127–130. doi: 10.1080/01652176.1997.9694756
- Schexnailder, R. & Griffith, M. (1973). Liver fat and egg production of laying hens as influenced by choline and other nutrients. Poultry Science, 52, 1188–1194. doi: 10.3382/ps.0521188
- Schumann, B.E., Squires, E.J., Leeson, S. & Hunter, B. (2003). Effect of hens fed dietary flaxseed with and without a fatty liver supplement on hepatic, plasma and production characteristics relevant to fatty liver haemorrhagic syndrome in laying hens. British Poultry Science, 44, 234–244. doi: 10.1080/0007166031000087065
- Shimmura, T., Hirahara, S., Azuma, T., Suzuki, T., Eguchi, Y., Uetake, K. & Tanaka, T. (2010). Multi-factorial investigation of various housing systems for laying hens. British Poultry Science, 51, 31–42. doi: 10.1080/00071660903421167
- Simonsen, H.B. (1978). Battery-cages as the cause of environmental and behavioural dependent diseases. Nordisk Veterinaermedicin, 30, 241–252.
- Squires, E.J. & Leeson, S. (1988). Aetiology of fatty liver syndrome in laying hens. British Veterinary Journal, 144, 602–609. doi: 10.1016/0007-1935(88)90031-0
- Valkonen, E., Venalainen, E., Rossow, L. & Valaja, J. (2008). Effects of dietary energy content on the performance of laying hens in furnished and conventional cages. Poultry Science, 87, 844–852. doi: 10.3382/ps.2007-00237
- Walzem, R.L., Simon, C., Morishita, T., Lowenstine, L. & Hansen, R.J. (1993). Fatty liver hemorrhagic syndrome in hens overfed a purified diet. Selected enzyme activities and liver histology in relation to liver hemorrhage and reproductive performance. Poultry Science, 72, 1479–1491. doi: 10.3382/ps.0721479
- Weitzenburger, D., Vits, A., Hamann, H. & Distl, O. (2005). Effect of furnished small group housing systems and furnished cages on mortality and causes of death in two layer strains. British Poultry Science, 46, 553–559. doi: 10.1080/00071660500303206
- Windhorst, H. (2011). The changing global egg industry. International Egg Commission Special Economic Report. London, UK.
- Wolford, J.H. (1971). The effect of temperature and iodinated casein on liver lipids of laying chickens. Poultry Science, 50, 1331–1335. doi: 10.3382/ps.0501331
