ABSTRACT
The crus haemorrhage is one of the main causes of carcass defects in Pekin duck processing houses. However, its pathologic features are currently unclear. In order to examine the injury to the hind limb veins and illustrate the pathologic characteristics of crus haemorrhage in Pekin ducks, a total of 68 Pekin ducks with crus haemorrhage (test group) and 10 unaffected ducks (control group) were collected in this study. Five ducks randomly selected from each group were examined by computed tomographic venography with 2.0 mm thickness, 120 kVp, and 90 mA. Pathological changes were observed macroscopically, and under a microscope and electron microscope. The computed tomographic venography results showed no differences in the main hind limb veins between Pekin ducks with crus haemorrhage and the control. Macroscopic results demonstrated that the haemorrhage only occurred in crural muscles, most frequently in musculus gastrocnemius and musculus tibialis cranialis. In severe cases, muscular rupture and multiple intermuscular blood clots could be observed. Histological analysis showed rupture of myofibers and massive red blood cells between muscle bundles. Besides, infiltration of connective tissues and inflammatory lesions could be seen. However, no differences were observed in other organs between these two groups. The main ultrastructural characteristics were myofibrillar rupture and split, accompanied by mitochondrial membrane disintegration and vacuolization. All these results indicate that the haemorrhage in crus is a focal myopathy with the characteristics of bleeding, rupture, and inflammatory lesions.
CTV was a feasible method to evaluate the hind limb veins in Pekin ducks.
The focal myopathy presented here only affected crural muscles.
The focal myopathy was characterized by bleeding, rupture and inflammatory lesions.
Research highlights
Introduction
Haemorrhages in muscles are considered as one of the main quality defects in poultry processing houses (Fletcher, Citation2002; Salines et al., Citation2017). Haemorrhages can occur at the stage of pre-slaughter, slaughter and post-slaughter, resulting from various factors (Gregory & Wilkins, Citation1989; Gregory, Citation1994, Citation2005; Kranen et al., Citation1996). The most common cause of muscular haemorrhage in broilers is water bath stunning, which leads to haemorrhage in thigh muscles and pectoralis (Kranen et al., Citation1996). Accordingly, muscular clots or rupture are reported to occur in humans and other animals, such as the ruptured gastrocnemius in humans (tennis leg) (Harwin & Richardson, Citation2017), the ruptured gastrocnemius tendons in broilers which is caused by reovirus and the high load of tendons (Jones et al., Citation1975; Crespo & Shivaprasad, Citation2011), and bilateral gastrocnemius rupture in cattle (Mendes et al., Citation2016). Muscular haemorrhages happen in Pekin ducks as well, and cause a considerable economic loss at Pekin duck processing houses in China.
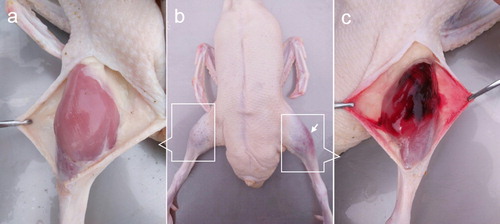
Interestingly, most muscular haemorrhages in Pekin ducks were observed as aubergine regions only in crus after defeathering (). Our previous studies have shown that this kind of haemorrhage typically developed in the latter fattening period, especially in force-fed ducks raised in hot summers. Among ducks that were simultaneously affected by the combined stress of transportation, heat, and force-feeding, the incidence of muscular haemorrhages could reach 7–10%. However, the incidence decreased to 0.1% or less when ducks were affected by only transportation, or heat-stress or force-feeding. It was difficult to make an ante mortem diagnosis because it had little effect on motor function, and only advanced cases involving severe bleeding could be presumptively diagnosed by visual inspection. The typical blood clots in the crural muscles suggest that this kind of haemorrhage is associated with vascular diseases, but no studies have been performed to determine whether or not it is accompanied by injury to the hind limb veins or not. Furthermore, although the histological characterization of muscular haemorrhage in broilers has been reported (Kranen et al., Citation2000), there is no pathological report related to the muscular haemorrhage in Pekin ducks. Hence, the objective of this study was to use indirect computed tomographic venography (CTV) to examine hind limb veins and present a detailed description of the morphological features of the crus haemorrhage in Pekin ducks.
Figure 1. The manifestations of crus haemorrhage in Pekin ducks. (a) Shows a normal crus (control group), no haemorrhagic muscle could be seen. (b) Shows an aubergine haemorrhagic region in the left crus (arrow), the right crus was normal. (c) Shows haemorrhagic muscles after incising the skin covering the haemorrhagic region.
Materials and methods
Birds
A total of 1500 commercial fattening Pekin ducks were examined; 68 ducks with suspected crus haemorrhage (test group) and 10 ducks without crus haemorrhage (control group) were used in this study. Among them, five suspected affected ducks and five unaffected ducks were selected randomly to be examined by CTV. The ducks’ crura were wiped with 75% ethanol during the examination. If the aubergine regions occurred in crura, no matter how large or small, the ducks were selected as “suspected” of crus haemorrhage. The “suspected” ducks were reconfirmed after necropsy.
Ducks were 42–45 days old and raised on the floor with dry sandy soil as bedding in summer. The ducks were free to water and were force-fed four times per day. All experimental procedures were approved by the Laboratory Animal Welfare and Animal Experimental Ethical Committee of China Agricultural University.
CTV examination
Ducks for CTV examination were fasted for 4 h but had free access to water. A 24-gauge catheter was placed in the left or right venae metatarsale dorsales, and anaesthesia was induced by injection of propofol via the catheter at a dose of 10 mg/kg body weight (Longley, Citation2008). The ducks were then intubated with a 4.0-mm endotracheal tube without inflation, and anaesthesia was maintained with 2.0–2.5% isoflurane at an oxygen flow rate of 2 l/min. A breathing machine (Kimuramed Co., Ltd., Tokyo, Japan) was used to maintain a positive airway pressure of 6–14 hPa. Respiratory rate and end-tidal carbon dioxide were monitored during the CT scan.
Another 24-gauge catheter was placed in the right or left venae ulnares and connected to a CT power injector (Sino Medical-Device Technology Co., Ltd., Shenzhen, China) containing the non-ionic contrast medium iopamidol (300 mg I/ml). All catheters were secured by adhesive dressing. A sputum aspirator was used if the endotracheal tube was obstructed by mucus.
Each duck was positioned in standard dorsal recumbency with the web entering the gantry first. The CT scan was performed using a 16-detector-row CT scanner (Philips Healthcare, Amsterdam, Netherlands). The region of interest extended from the middle of the ilium shaft to the distal hind limb. The scout scan was performed in the orthogonal plane, and the survey CT scan was then performed with a built-in abdominal scan protocol using the following parameters: standard algorithm in helical scan mode, 2.0-mm thickness, 120 kVp, and 90 mA.
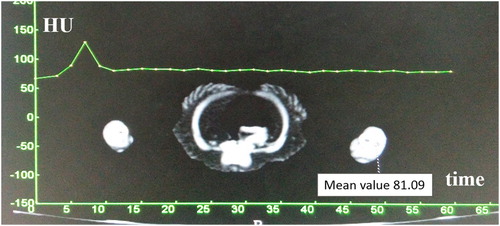
After the survey CT scan, a test bolus of 1 ml/kg of iopamidol was administered over 3 s, and a time–density curve was then fitted by a computer. When the pre-contrast survey scan had been completed, CTV was performed with the same parameters but at a different contrast medium dosage (4 ml per duck over 3 s). An isodose of normal saline was then injected at the same speed. The time–density curve provided the interval of time between injection of the contrast medium and the start of CTV. Approximately 90 s after contrast injection, the delayed-phase image was obtained using the same parameters as in the survey examination.
Anaesthesia was stopped when the scan was finished, but the positive airway pressure and oxygen supply were continued for 10 s. The ducks recovered in a warm, ventilated environment and were continuously monitored until they could move freely.
Pathological sample collection and observation
Ducks were narcotized by pentobarbital sodium and exsanguinated by cutting the jugular vein unilaterally. Necropsy was performed immediately after exsanguination. After evaluation of the skin, head, wings, liver, kidney, genitals, lungs, heart and vessels, the crural muscles were exposed by bluntly dissecting the legs. Both crura were visually evaluated for colour change, bleeding severity and bleeding site. After necropsy, the 54 affected muscle samples from the test group and 10 muscle samples from left crus of the control group were collected. Other samples including kidney, liver, aortic arch and arteria ischiadica were taken randomly from 10 ducks in both the test group and the control group. All samples were fixed in phosphate-buffered solutions containing 4% paraformaldehyde for 48 h and stained with haematoxylin and eosin (H&E) for histological observation. Another three muscle samples from both the test group and the control group, were sectioned and fixed in 2.5% glutaraldehyde at 4°C for 2 h, then transferred to 1% solution of cold osmium tetroxide (OsO4) for 2 h. After that, the tissues were rapidly dehydrated through ascending grades of ethanol and transferred to a 1:1 mixture of propylene oxide and epoxy araldite. Semi-thin sections (1 μm) were cut, and stained with toludine blue. Then, for the electron microscope examination, these sections were viewed with a light microscope to select the suitable areas. The ultrathin sections (60–100 nm) were cut by a glass knife with LKB microtome, and stained with uranyl acetate followed by lead citrate. These sections were examined under the transmission electron microscope (JEM-1230, JEOL Ltd., Tokyo, Japan) operating at 80 kV.
Image post-processing and data analysis
All CTV images were reconstructed with a standard algorithm of a 2-mm thickness and 1-mm interval. The CT images were reconstructed by multi-planar reconstruction (MPR) in various planes (coronal, sagittal, and curved). Three-dimensional reconstruction was also used to acquire images of the hind limb veins in the Pekin ducks. A soft tissue window (width, 350 HU; level, 40 HU) was used for pre-contrast and post-contrast observation. The following parameters of the hind limb veins were assessed and compared: position, filling degree, continuity, and smoothness.
The numbers of male/female and bilateral/unilateral affected ducks were analysed to evaluate the predisposition of the lesion, in which an individual bird was considered as an experimental unit. Individual crus or specific muscle was used as an experimental unit to evaluate the number of haemorrhagic locations in each crus or the susceptible muscle. Data were compared using the chi-square test in SPSS Statistics software 20.0 and P < 0.05 was considered as significant.
Results
CTV examination
The five suspected ducks for CTV examination were finally diagnosed with crus haemorrhage based on the characteristic blood clots in the gastrocnemius. They all had unilateral crus haemorrhage (left crus in four ducks and right crus in one duck). The five healthy ducks had no lesions in the crural muscles. All ducks were smoothly anaesthetized, their respiratory rate and end-tidal carbon dioxide remained within the reference range during the CT scan. The CTV procedure under anaesthesia was successful in all ducks, and high-quality venous images were obtained. On the time–density curve created by the test bolus, the enhancement peak in the popliteal vein appeared 7 s after contrast medium administration ().
Three-dimensional reconstruction of the main hind limb veins was feasible with CTV (). In addition, MPR in various planes was also used to evaluate the main hind limb veins in both the healthy ducks and ducks with crus haemorrhage (). The shape of the veins in the healthy and affected ducks had no obvious abnormalities. The venous walls were smooth and continuous and had no significant stenosis or dilatation. Three of the five affected ducks showed increased branching of the vena poplitea, whereas this phenomenon did not appear in the healthy ducks. No adverse effects were observed in the ducks during or after contrast administration.
Figure 3. Three-dimensional reconstruction imaging of the main hind limb veins in the Pekin ducks. (a) Shows the image of a healthy duck (control group), and (b) shows the image of a duck with haemorrhage in the left crus. The MPR images in the mid-femur section (a and d), knee joint section (b and e), and mid-tibia section (c and f) are shown in . The image of the vena ischiadica was also visible (triangle). The bladder was filled with contrast medium because of the test bolus in figure (b) (double arrows). R = right.
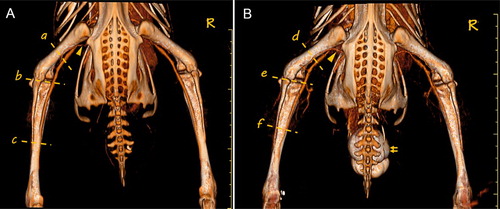
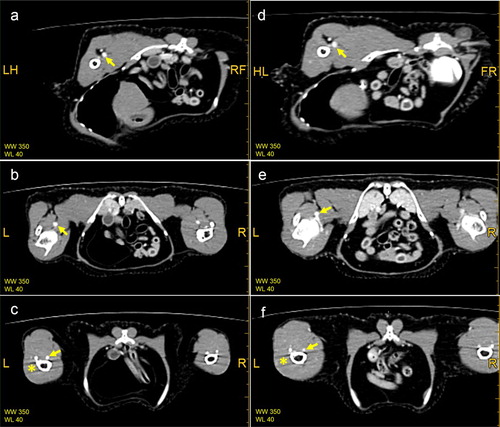
Figure 4 . The MPR images in various sections. (a), (b) and (c) are images of a healthy duck (control group), whereas (d), (e) and (f) are images of a duck with haemorrhage in the left crus. The main veins were clearly observed (arrow). Vascular branching was observed in (e). The vena ischiadica, which was near the vena femoralis, was also visible in (a) and 4(d). Artifacts appeared in some sections (* on (c) and (f)). L = left, R = right.
Incidence
The inspected ducks showed no signs of drowsiness, emaciation or dyspnoea, but limping was observed in two ducks with crus haemorrhage. All of the 68 suspected ducks showed aubergine or dark green regions only in crus, no other muscles were affected. It seemed that this condition occurred more in females (43/68, 63.2%) than males (25/68, 36.8%) (P < 0.05). Twenty-six ducks were affected bilaterally and 42 ducks unilaterally, including 23 ducks in left crus and 19 ducks in right crus. In the total of 95 affected crura, 75 crura had one lesion (79.9%), which showed that this kind of haemorrhage mostly resulted in one lesion per crus (P < 0.01). Furthermore, musculus gastrocnemius medialis (60.5%) was the most affected muscle in the total of 114 lesions (P < 0.01). Haemorrhage also occurred in musculus gastrocnemius lateralis (24.5%), musculus tibialis cranialis (8.8%), musculus peroneus longus (4.4%) and musculus flexor perforans et perforates digitis II (1.8%) ( and ).
Figure 5. Haemorrhagic muscles in crura of Pekin ducks. (a) Musculus gastrocnemius medialis (medial surface) and (b) musculus gastrocnemius lateralis (medial surface). (c) Musculus tibialis cranialis, and (d) musculus peroneus longus.
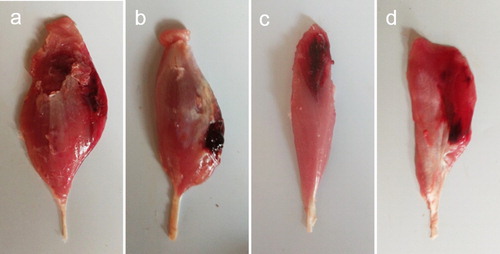
Table 1. The predisposition of crus haemorrhage in Pekin ducks.
Macroscopic findings
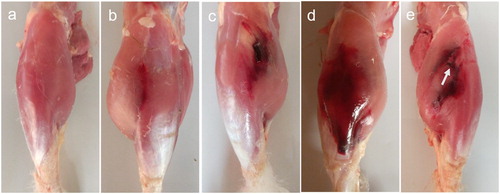
The lesions were grouped into four degrees depending on the macroscopic characteristics (). Mild lesions only showed slight bleeding, and most were located on the junction of musculus gastrocnemius medialis and musculus peroneus longus, occasionally on the surface of musculus gastrocnemius. Moderate lesions had small muscular blood clots, and severe lesions were characterized by large blood clots. In this case, blood cells infiltrated into subcutaneous tissues, which resulted in aubergine or dark green regions on the skin. When haemorrhage in crural muscles was very severe, the multiple blood clots accumulated and macroscopic muscular rupture could be seen, it was hard to distinguish haemorrhagic muscles. Very severe lesions were seen in two ducks with limping. Additionally, these two ducks showed intramuscular cysts, with bean-size, yellowish-white-colour and clear serous fluid inside.
Figure 6. Different degrees of crus haemorrhage in Pekin ducks. (a) Normal crus with no haemorrhage. (b) Mild haemorrhagic profile of thin slight bleeding. In moderate degree, few intermuscular blood clots could be seen. (c) When severe haemorrhage occured, large blood clots could be seen between crural muscles, and blood cells infiltrated into subcutaneous tissue. (d) Multiple large blood clots could be seen between crural muscles in very severe cases. (e) In addition, macroscopic muscular rupture could be seen (arrow). Subcutaneous fascia with blood had been removed.
Some ducks showed a greasy lustre on the liver section; only one duck in the test group showed liver hepatomegaly. Other organs such as kidney, spleen, heart, aortic arch and arteria ischiadica showed no macroscopic differences between ducks in the test group and control group.
Microscopic findings
Ten muscular sections from the control group and 54 muscular sections from the test group were observed. The muscle samples from the control group showed no significant histological changes. The myofibers configurated regularly on the transection and had striped shape with myocyte nucleus on both sides of the vertical section ((a)). Haemorrhage, which was seen on most of the muscle samples from the test group, was the primary histological feature. Numerous red blood cells could be seen between muscle bundles ((b)). It was often accompanied by a small number of phagocytic cells and inflammatory cells which mainly consisted of heterophile cells. Meanwhile, myofibers ruptured with increased spacing. In some sections, red blood cells dissolved and only myocyte nuclei were visible.
Figure 7. Histopathological characterizations of crus haemorrhage in Pekin ducks. (a) Normal myofibers (H&E, ×200) whereas (b) Rupture of the myofibers with massive red blood cells (H&E, ×200). (c) Massive inflammatory cells gathered between the muscle bundles; hyperplasia of connective tissues could also be seen (H&E, ×100). Besides swelling and vacuolation of the myofibers, inflammatory cells and the hyperplasia of connective tissues could be seen in the muscular interstitium in (d) (H&E, ×200). (e) Represents necrotizing inflammation in myofibers. Lymphoid cells could be seen between the myofibers, which were surrounded by connective tissues (H&E, ×200). (f) Shows the histological morphology of a muscular cyst; the cyst wall was formed by connective tissues, with a few myoblast cells (H&E, ×200).
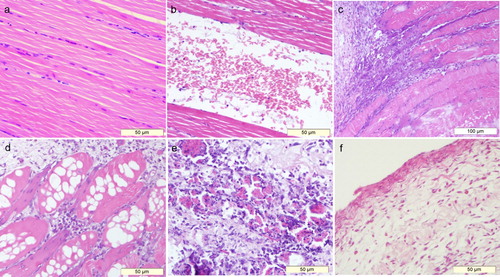
Inflammation in muscular interstitium could be seen on 41/54 sections ((c) and 7(d)). Massive inflammatory cells infiltrated between muscle bundles, and necrosis of myofibers was visible under a microscope, characterized by swelling, vacuolation, rupture and fuzzy border. In this case, the infiltration of connective tissues was common. In addition, inflammatory lesions could also be seen in myofibers ((e)); most of them were necrotized with the infiltration of lymphoid cells and connective tissues. The intramuscular cysts collected from two ducks consisted of loose connective tissues and sporadic myoblast cells ((f)).
Some liver samples showed cellular swelling and vacuolation, which were seen both in the test group and control group. Other organs from ducks with crus haemorrhage showed no histological differences from those of ducks in the control group.
Ultrastructural findings
The ultrastructure of the crural muscle in the control group was normal with clear Z line and M line under a transmission electron microscope ((a)). The affected muscles showed that the myofibrils ruptured and degenerated, with irregular, displaced and degenerated Z lines ((b)). In addition, abnormal myofibrils also showed a “broom”-like change, which was characterized by splitting myofibrils and ruptured Z lines ((c)).
Figure 8. Ultrastructural characterizations of crus haemorrhage in Pekin ducks. (a) Shows normal ultrastructure of Pekin ducks’ skeletal muscles, mitochondria, Z line and M line could be seen in the vertical sections of the skeletal muscles of Pekin ducks. (TEM, ×15,000). (b) shows the rupture of myofibrils; loss of myofibril continuity and rupture of Z line could also be seen (TEM, ×15,000). “Broom”-like myofibers are shown in (c). With increased myofilament spacing and rupture of Z line, myofibrils were just like a “broom” (TEM, ×30,000). (d) Represents the disintegration of mitochondrial cristae (TEM, ×20,000), and (e) shows the vacuolization in mitochondria (TEM, ×15,000). In (f), the mitochondrion in the centre was swollen (arrow), and the other mitochondrion on the left side showed total membranous dissolution (TEM, ×30,000).

The quantity of mitochondria increased and their structures were swollen. Meanwhile, mitochondrial cristae dissolved, which resulted in wide electron light zone ((d)). In addition, different sizes of vacuoles could be seen in mitochondria with disintegrated cristae ((e)). Furthermore, abnormal shapes of mitochondria and dissolution of mitochondrial membranes could be seen ((f)).
Discussion
CTV showed no differences in the main hind limb veins between the healthy ducks and those with crus haemorrhage, suggesting that this kind of haemorrhage is not accompanied by injury to the main hind limb veins. However, its ante mortem diagnosis is difficult, and the number of affected ducks was limited to five. Therefore, further studies involving more diseased ducks are necessary.
The macroscopic observation showed that this type of haemorrhage only occurred in crural muscles and was restricted to specific muscles. Meanwhile, mental status and nutritional status were not affected by the haemorrhage, which indicated that this kind of haemorrhage was not a generalized disease. In the total 68 affected ducks, only two ducks were limping, which might be caused by severe haemorrhage, haematoma compression and multiple muscular rupture. In this study, musculus gastrocnemius seemed to be the most susceptible muscle. This muscle is the most superficial muscle of the calf and is composed of two muscle heads, medial head and lateral head. The function of musculus gastrocnemius is to flex the knee and extend the tarsus. Another susceptible muscle was musculus tibialis cranialis, which is used to flex the tarsal articulation and was haemorrhaged in 10 of 68 affected ducks. Excessive and inappropriate contraction of these muscles might occur when birds are being driven, captured and force-fed, which might result in muscular injury (Paterson et al., Citation2007; Crespo & Shivaprasad, Citation2011).
In the present study, no ducks showed macroscopic necrosis in the heart or vessels. One duck showed hepatomegaly. Besides, liver samples both in the test group and the control group showed cellular swelling and vacuolation. This might be caused by force-feeding, which could result in liver steatosis and hepatomegaly (Rodenburg et al., Citation2005; Wen et al., Citation2012).
Haemorrhage and rupture of myofibers were the primary histological changes. Meanwhile, rupture of myofibrils could be seen under the transmission electron microscope. Haemorrhage could be caused by the rupture of muscle, which has been reported in humans and birds. “Tennis leg” results from sports-related activities or daily activities such as running to catch a bus or climbing stairs (Delgado et al., Citation2002). Its pathogenesis is characterized by rupture of the medial head of the musculus gastrocnemius or the rupture of the musculus plantaris (Delgado et al., Citation2002; Harwin & Richardson, Citation2017). The rupture of gastrocnemius tendons in broiler chickens is considered to be due to reovirus infection (Jones et al., Citation1975), but inflammatory changes were not detected in all cases (Dinev, Citation2008). Rupture of the gastrocnemius muscles in cattle could occur due to vigorous contraction (Mendes et al., Citation2016). In this study, we detected inflammatory changes in both muscular interstitium and myofibers, but further study should be designed to detect whether microbe factors participate in the occurrence of crus haemorrhage in Pekin ducks.
Mitochondria, which supply energy for cells, are involved in many cellular events of growth, development, aging, apoptosis, as well as diseases (Chan, Citation2006). The distribution of mitochondria in cells is related to cellular function and status. If mitochondria gather around a specific region, its metabolism tended to be active and needed more ATP. Mitochondria distributed on both sides of myofibrils in the muscles from the control group, whereas mitochondria showed increased quantities and abnormal shapes in the muscles from the test group; these findings indicated that affected muscles needed more energy. Haematomas could compress adjacent tissues, resulting in tissue ischaemia and hypoxia, then causing mitochondrial hyperplasia. Additionally, the disintegration of mitochondrial cristae and mitochondrial membranes were also associated with ischaemic necrosis which was observed in the ultrastructure of deep pectoral myopathy (Siller & Wight, Citation1978; Wight & Siller, Citation1980).
In conclusion, this study found that crus haemorrhage in Pekin ducks was a focal myopathy with the characteristics of bleeding, rupture, and inflammatory lesions. However, the definitive pathogenesis and mechanism of this kind of haemorrhage are currently unclear and further research is needed.
Acknowledgements
The authors thank Ran Liu and Qi Wei for providing technical assistance with the CT scans. The authors also thank Dr. Zhiqi Song for providing pathological consult.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chan, D.C. (2006). Mitochondria: dynamic organelles in disease, aging, and development. Cell, 125, 1241–1252. doi: 10.1016/j.cell.2006.06.010
- Crespo, R. & Shivaprasad, H. (2011). Rupture of gastrocnemius tendon in broiler breeder hens. Avian Diseases, 55, 495–498. doi: 10.1637/9669-012711-Case.1
- Delgado, G.J., Chung, C.B., Lektrakul, N., Azocar, P., Botte, M.J., Coria, D., Bosch, E. & Resnick, D. (2002). Tennis leg: clinical US study of 141 patients and anatomic investigation of four cadavers with MR imaging and US. Radiology, 224, 112–119. doi: 10.1148/radiol.2241011067
- Dinev, I. (2008). Clinical and morphological studies on spontaneous rupture of the gastrocnemius tendon in broiler breeders. British Poultry Science, 49, 7–11. doi: 10.1080/00071660701751393
- Fletcher, D.L. (2002). Poultry meat quality. World’s Poultry Science Journal, 58, 131–145. doi: 10.1079/WPS20020013
- Gregory, N.G. (1994). Pathology and handling of poultry at the slaughterhouse. World’s Poultry Science Journal, 50, 66–67. doi: 10.1079/WPS19940010
- Gregory, N.G. (2005). Recent concerns about stunning and slaughter. Meat Science, 70, 481–491. doi: 10.1016/j.meatsci.2004.06.026
- Gregory, N.G. & Wilkins, L.J. (1989). Effect of stunning current on carcase quality in chickens. The Veterinary Record, 124, 530–532. doi: 10.1136/vr.124.20.530
- Harwin, J.R. & Richardson, M.L. (2017). “Tennis leg”: gastrocnemius injury is a far more common cause than plantaris rupture. Radiology Case Reports, 12, 120–123. doi: 10.1016/j.radcr.2016.10.012
- Jones, R.C., Jordan, T.W. & Lioupis, S. (1975). Characteristics of reovirus isolated from ruptured gastrocnemius tendons of chickens. The Veterinary Record, 96, 153–154. doi: 10.1136/vr.96.7.153
- Kranen, R.W., Lambooy, E., Veerkamp, C.H., Kuppevelt, T.H.V. & Veerkamp, J.H. (2000). Histological characterization of hemorrhages in muscles of broiler chickens. Poultry Science, 79, 110–116. doi: 10.1093/ps/79.1.110
- Kranen, R.W., Veerkamp, C.H., Lambooy, E., Van Kuppevelt, T.H. & Veerkamp, J.H. (1996). Hemorrhages in muscles of broiler chickens: the relationships among blood variables at various rearing temperature regimens. Poultry Science, 75, 570–576. doi: 10.3382/ps.0750570
- Longley, L. (2008). Anaesthesia of Exotic Pets. Philadelphia: Elsevier Saunders.
- Mendes, R.E., Lucca, N.J., Henker, L.C., Schwertz, C.I., Stedile, F.A., Christ, R., Gheller, L., Marques, E.J., Pereira, W.B., Mori, A.M. & Broll, F. (2016). Bilateral gastrocnemius rupture in Friesian cattle in Santa Catarina State, Brazil. Journal of Comparative Pathology, 154, 93. doi: 10.1016/j.jcpa.2015.10.087
- Paterson, J., West, G., Heard, D. & Caulkett, N. (2007). Zoo animal & wildlife immobilization & anesthesia. Oxford: Blackwell Publishing.
- Rodenburg, T.B., Bracke, M.B.M., Berk, J., Cooper, J., Faure, J.M., Guémené, D., Guy, G., Harlander, A., Jones, T., Knierim, U., Kuhnt, K., Pingel, H., Reiter, K., Serviére, J. & Ruis, M.A.W. (2005). Welfare of ducks in European duck husbandry systems. World’s Poultry Science Journal, 61, 633–646. doi: 10.1079/WPS200575
- Salines, M., Allain, V., Roul, H., Magras, C. & Le, B.S. (2017). Rates of and reasons for condemnation of poultry carcases: harmonised methodology at the slaughterhouse. The Veterinary Record, 180, 516.
- Siller, W.G. & Wight, P.A. (1978). The pathology of deep pectoral myopathy of turkeys. Avian Pathology, 7, 583–617. doi: 10.1080/03079457808418313
- Wen, Z., Hou, S., Xie, M., Huang, W. & Yu, J. (2012). Amounts of force-feeding affect growth performance, serum biochemical parameters and liver histology of Pekin ducks. Chinese Journal of Animal Nutrition, 24, 69–77.
- Wight, P.A.L. & Siller, W.G. (1980). Pathology of deep pectoral myopathy of broilers. Veterinary Pathology, 17, 29–39. doi: 10.1177/030098588001700103