ABSTRACT
Avian pathogenic E. coli (APEC) cause severe respiratory and systemic disease. To address the genetic and immunological basis of resistance, inbred chicken lines were used to establish a model of differential resistance to APEC, using strain O1 of serotype O1:K1:H7. Inbred lines 72, 15I and C.B12 and the outbred line Novogen Brown were inoculated via the airsac with a high dose (107 colony-forming units, CFU) or low dose (105 CFU) of APEC O1. Clinical signs, colibacillosis lesion score and bacterial colonization of tissues after high dose challenge were significantly higher in line 15I and C.B12 birds. The majority of the 15I and C.B12 birds succumbed to the infection by 14 h post-infection, whilst none of the line 72 and the Novogen Brown birds developed clinical signs. No difference was observed after low dose challenge. In a repeat study, inbred lines 72 and 15I were inoculated with low, intermediate or high doses of APEC O1 ranging from 105 to 107 CFU. The colonization of lung was highest in line 15I after high dose challenge and birds developed clinical signs; however, colonization of blood and spleen, clinical signs and lesion score were not different between lines. No difference was observed after intermediate or low dose challenge. Ex vivo, the phagocytic and bactericidal activity of lung leukocytes from line 72 and 15I birds did not differ. Our data suggest that although differential resistance of inbred lines 72, 15I and C.B12 to APEC O1 challenge is apparent, it is dependent on the infectious dose.
Lines 15I and C.B12 are more susceptible than line 72 to a high dose of APEC O1.
Differential resistance is dose-dependent in lines 15I and 72.
Phagocytic and bactericidal activity is similar and dose independent.
Research Highlights
Introduction
Avian pathogenic E. coli (APEC) cause severe respiratory and systemic disease in chickens, commonly termed colibacillosis. APEC exert substantial economic and welfare costs on poultry producers worldwide, with losses due to early mortality, condemnation of carcasses and reduced productivity. APEC infect broilers and layers, particularly at the onset of sexual maturity and during intense laying (reviewed in Guabiraba & Schouler, Citation2015). APEC can act as both a primary and secondary pathogen, with secondary infections following predisposing factors such as poor climatological housing conditions, respiratory viral and Mycoplasma spp. infections or vaccinations (Matthijs et al., Citation2003; Ariaans et al., Citation2008). APEC constitute a large group of diverse serotypes with the O1, O2, O5, O8, O18 and O78 serogroups accounting for 56% of APEC isolates in a recent study in Europe (Schouler et al., Citation2012). Analysis of the genome sequences of prevalent APEC serogroups revealed striking similarity with E. coli causing human extra-intestinal infections, including neonatal meningitis, sepsis and urinary tract infections (Johnson et al., Citation2007). Such APEC serotypes were also readily detected in retail chicken and eggs, raising concern regarding their zoonotic potential (Mitchell et al., Citation2015). The large diversity of APEC also hinders the control of colibacillosis due to poor cross reactivity of responses induced by autologous bacterins across serotypes (reviewed in Dziva & Stevens, Citation2008, and Ghunaim et al., Citation2014).
Inbred chicken lines have proven to be valuable tools to study the genetic and immunological basis of differential resistance to pathogens (reviewed in Lamont et al., Citation2014). Resistance associated immune responses have been described after challenge with various avian pathogens and provide potential novel markers for improved breeding strategies (Bumstead et al., Citation1989; Cavero et al., Citation2009; Sandford et al., Citation2012; Sun et al., Citation2015). Moreover, genome-wide association studies using the progeny of the crosses of inbred lines that differ in resistance has enabled the mapping of resistance-associated quantitative trait loci to inform selective breeding (Fife et al., Citation2009, Citation2011; Tran et al., Citation2012; Psifidi et al., Citation2016). Differential resistance of inbred lines to Salmonella or Campylobacter challenge has been associated with innate immunity, in particular with heterophils and macrophages (reviewed in Kaiser et al., Citation2009). Bumstead et al. (Citation1989) studied the response of multiple inbred chicken lines to co-infections using infectious bronchitis virus (IBV) and a cocktail of APEC representing prevalent serotypes (Bumstead et al., Citation1989). While differences in susceptibility were detected, the extent to which this could be explained by responses to the virus or bacteria was not dissected in depth. A single study involving intramuscular injection of the inbred lines using a pool of four APEC serotypes (O2, O78 and two undetermined serotypes) involving assessment of mortality (LD50) showed that inbred line 72 was most resistant, whereas inbred lines 15I and C were amongst the most susceptible lines studied (Bumstead et al., Citation1989).
Our understanding of the chicken innate immune response to APEC infection is limited and hindered by the large diversity of APEC serotypes. APEC cause localized inflammation in the avian respiratory tract, and birds often develop subacute fibrinopurulent airsacculitis, pericarditis and perihepatitis (Goren, Citation1978; DeRosa et al., Citation1992). It was shown that APEC colonise the lung to a significantly greater extent than non-pathogenic E. coli (Horn et al., Citation2012). Several studies have shown that heterophils and macrophages are recruited to the sites of inflammation in the respiratory tract where they may contribute to bacterial clearance (Pourbakhsh et al., Citation1997; Mellata et al., Citation2003; Ariaans et al., Citation2008; Horn et al., Citation2012). APEC may be able to resist the bactericidal effects of these phagocytes to a greater extent than non-pathogenic strains (Mellata et al., Citation2003). The extent to which lung-resident phagocytic cells may contribute to the differential resistance of inbred lines is unclear.
In the present study we aimed to establish a model of differential resistance to APEC, using a single strain representative of a dominant sequence-type (ST95) and serotype (O1:K1:H7) administered via the respiratory route, to unravel the underlying genetic and immunological basis of resistance to aid breeding strategies and the future design of vaccines.
Materials and methods
Chicken lines
Chickens were provided by the National Avian Research Facility (NARF) at the University of Edinburgh, UK. Studies were performed with inbred lines 72 (B2), 15I (B15) and C.B12 (B12) and with Novogen Brown layers. All inbred chickens were hatched and reared under specified pathogen-free (SPF) conditions, whereas Novogen Brown chicks were hatched in a conventional unit but directly post-hatch were transferred and reared under SPF conditions with ad libitum access to feed and water.
Birds were housed in premises licensed under UK Home Office Establishment Licenses (PEL X212DDDBD and XA40CEF03) in full compliance with the requirements of the Animals (Scientific Procedures) Act 1986. Procedures were conducted under project licence PPL 60/4420 with the consent of Ethical Review Committee of The Moredun Research Institute.
Bacteria
The genome-sequenced APEC O1 strain (serotype O1:K1:H7) was kindly provided by Prof Lisa Nolan, Iowa State University, USA (Johnson et al., Citation2007). APEC O1 was cultured for 20 h at 37°C in antibiotic-free Luria Bertani (LB) broth in a shaking incubator set to 180 rpm, to reach stationary phase. The inocula were prepared by collection of bacteria from fresh cultures by centrifugation and resuspension in sterile phosphate-buffered saline (PBS) based on previously determined bacterial titres of stationary phase cultures (in colony-forming units (CFU)/ml). The bacterial titres of inocula administered to birds were confirmed by retrospective plating of serial dilutions on selective media. A derivative of APEC expressing an enhanced green fluorescent protein (APEC O1-GFP) was used for the in vitro phagocytosis and killing assays and obtained by transformation of APEC O1 with plasmid pFVP25.1 (Valdivia & Falkow, Citation1996). It was confirmed that this did not alter the plasmid repertoire of APEC O1 or its growth rate in LB medium (not shown).
Experimental design
Two-week-old chickens of all lines were inoculated with 2.0 × 105 CFU (low dose) or 1.9 × 107 CFU (high dose) APEC O1 in 100 µl PBS, administered into the right caudal thoracic air sac (n = 10 per line per dose). At 2 and 7 days post-infection (dpi) five birds of each line were culled by cervical dislocation. Clinical signs, colibacillosis lesions and the bacterial loads in lung, blood and liver were determined.
In the repeat study two-week-old chickens of the 72 and 15I lines (indicated to be relatively resistant and susceptible, respectively, in the previous study) were inoculated with 7.3 × 104 CFU (low dose), 1.1 × 106 CFU (intermediate dose) or 8.8 × 106 CFU (high dose) APEC O1 in 100 µl PBS or with 100 µl PBS as control administered into the right thoracic air sac. Birds were culled at 14 hours post-infection (hpi), 3 dpi or 7 dpi (n = 6 APEC O1 inoculated birds per time interval, n = 3 PBS controls). Clinical signs, colibacillosis lesions and the bacterial loads in lung, blood and spleen were determined.
Clinical signs and post mortem examination
Clinical signs of APEC infection were monitored twice daily after inoculation. A bird was recorded as showing mild clinical signs if its posture was hunched, or as showing moderate clinical signs if its posture was hunched and it was lethargic or reluctant to move and/or dyspnoeic (erratic breathing). Birds which died suddenly without showing any previous clinical signs were recorded as unexpected deaths. Colibacillosis lesions were scored macroscopically during post mortem examination as described previously (Goren, Citation1978; Vandemaele et al., Citation2006) in the left and right cranial and caudal thoracic air sacs, left and right lungs, liver and pericardium (). A maximum lesion score of 16 per bird could be reached.
Table 1. Colibacillosis lesion scoring.
Bacteriological analysis
Right lung (transversal section between the second and third costal grooves), liver (distal part of the right lobe) and spleen tissues were collected in 500 µl PBS and the weight of the tissue recorded. Tissues were homogenized using a TissueLyser II instrument (twice 20 Hz pulses for 2 min, Qiagen, UK) before serial ten-fold dilutions in PBS were prepared and 100 µl of the dilutions plated onto antibiotic-free MacConkey agar plates (MCA, Oxoid, UK). Heparinized blood samples were collected and 200 µl mixed with 200 µl 1% saponin (Sigma Aldrich, UK) in double-distilled H2O (ddH2O) for 5 min at room temperature (RT). Serial ten-fold dilutions in PBS were plated onto MCA plates. All MCA plates were incubated at 37 °C overnight before colonies were counted and the bacterial loads determined as CFU/g of tissues or CFU/ml of blood.
Phagocytosis and killing assays
In vitro phagocytosis and killing assays were performed with gradient-purified lung cells of naïve inbred line 72 and 15I chickens. The right and left lungs were collected in sterile PBS and enzymatically digested for 30 min at 37 °C with 1 mg/ml DNAse I (Sigma Aldrich, UK) and 3 mg/ml collagenase A (Sigma Aldrich, UK) after which the tissue was homogenized with a GentleMACSTM tissue dissociator (Miltenyi Biotec, UK), to form a single cell suspension that was then filtered through a 70 µm cell strainer. The cells were gradient purified using Histopaque® 1.077 (Sigma Aldrich, UK) as previously described (Jansen et al., Citation2013). Lung leukocytes from the interface and above were collected, washed twice with PBS (5 min, 350 × g, RT), and their cell number and viability determined by trypan blue exclusion.
Lung leukocytes were inoculated for 30 min at 41 °C with APEC O1-GFP at a multiplicity of infection (MOI) of 100 or 10 in 3 ml antibiotic-free RPMI 1640 medium supplemented with 10% heat-inactivated foetal calf serum (FCS) and 1% L-Glutamine in FACS tubes. After 30 min, ceftazidime hydrate (Sigma Aldrich, UK) was added (1000 µg/ml with MOI = 100 or 500 µg/ml with MOI = 10) for 30 min to kill extracellular bacteria before the cells were washed with PBS three times by centrifugation (5 min, 350 × g, RT). The cells were incubated for 15 min with TrypLETM Express (ThermoFisher, UK) at RT to detach adherent cells, centrifuged and re-suspended in PBS. At this point, inoculated cells were equally distributed between FACS tubes (1.5 × 106 cells per tube) and either lysed directly with 0.5% saponin (T = 0 h) in ddH2O for 15 min at RT to release and enumerate phagocytosed bacteria, or cultured for 2, 4 and 6 h at 41 °C and 5% CO2 in RPMI 1640 supplemented with 10% heat inactivated FCS, 1% L-Glutamine and 20 µg/ml ceftazidime hydrate, to determine their bactericidal activity over time. At each sample time point the cells were washed three times with PBS, incubated with TrypLETM Express and lysed with 0.5% saponin before viable ceftazidime hydrate-protected intracellular bacteria were enumerated. Serial ten-fold dilutions in PBS were plated onto antibiotic-free MacConkey agar plates in duplicate to enumerate viable bacteria. Phagocytosis and killing assays with lung leukocytes from each inbred line were performed with an MOI of 100 (n = 6 birds, two independent experiments) and MOI of 10 (n = 3 birds, one experiment).
Flow cytometric analysis
Flow cytometric analysis was performed on uninfected lung leukocytes to determine the frequencies of antigen-presenting cells in the inbred lines. All incubations and washes were performed using FACS buffer (PBS with 0.5% bovine serum albumin, 0.05% sodium azide and 0.05% horse serum). The cells were incubated for 20 min at 4 °C with the primary antibodies, washed three times, and incubated with the secondary antibodies for 20 min at 4 °C and washed three times before being analysed on an LSRFortessaTM flow cytometer (BD Bioscience, UK). The following antibodies and isotype controls were used: mouse anti-chicken CD45 (clone UM16-6, 1:4000, Bio-Rad, UK), mouse anti-chicken MRC1L-B (KUL01, 1:100, Bio-Rad, UK), mouse anti-chicken CD11 (clone 8F2, 1:1000, kind gift from Dr. S. Härtle, LMU, Germany), goat anti-mouse IgG2a:RPE (1:2500, Southern Biotech, UK), goat anti-mouse IgG1:AF647 (1:5000, ThermoFisher, UK), negative control for mouse IgG1 (1:40, ThermoFisher, UK) and negative control for mouse IgG2a (1:800, ThermoFisher, UK). Data were compensated on the LSRFortessaTM, gated according to fluorescence minus one (FMO) controls, antibody specificity checked with the relevant isotype controls, and SYTOXTM Blue (1:2000, ThermoFisher, UK) included as viability dye. The cell-associated fluorescence of single, live CD45+ leukocytes was analysed and data expressed as percentages thereof by using FlowJo® 10.4 (FlowJo, US). Flow cytometric analysis with both lines was performed twice independently.
Statistical analysis
As data were not normally distributed, all data comparing two chicken lines were analysed by Mann Whitney tests, and all data comparing four chicken lines by Kruskal–Wallis tests with Dunn's multiple comparisons tests using GraphPad Prism 7.00 (GraphPad, US). Correlations between bacterial load in different tissues were analysed using Spearman rank correlation tests and Minitab 17 (Minitab, UK). The probability level for significance was taken as P < 0.05.
Results
Differential resistance of inbred lines 72, 15I, and C.B12 to a high dose but not to a low dose of APEC O1
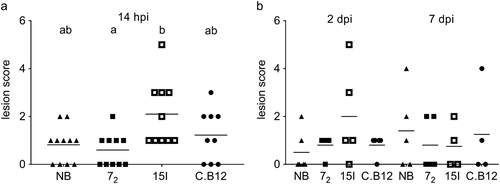
The response of inbred lines 72, 15I, and C.B12 to APEC O1 challenge was investigated. After inoculation with APEC O1, the birds were examined for clinical signs and macroscopic lesions. Several of the line 15I and C.B12 birds unexpectedly succumbed to the high dose within 14 hpi and, as a consequence, all birds inoculated with this dose were culled at 14 hpi. In sharp contrast to these lines, none of the line 72 and the Novogen Brown birds developed clinical signs after a high dose challenge (). After challenge with a low dose of APEC O1, one bird of inbred line 15I developed moderate clinical signs at 14 hpi and was culled together with the high dose birds. None of the remaining birds of any line inoculated with the low dose developed clinical signs.
Table 2. Clinical signs at 14 hpi after intra-airsac inoculation of a high dose of APEC O1.
Colibacillosis lesion scores were significantly different between line 72 and line 15I, but not between lines 72 and C.B12 after high dose challenge ((a)). At this infection dose, the Novogen Brown birds developed colibacillosis lesions similar to the line 72 birds. After a low dose APEC O1 inoculation, lesion scores were not significantly different between the lines at either 2 or 7 dpi ((b)).
Figure 1. Colibacillosis lesion scores. Inbred lines 72, 15I and C.B12 and the outbred Novogen Brown chickens were inoculated with (a) the high or (b) the low dose of APEC O1. Colibacillosis lesions were recorded at different time points. Each dot represents the total lesion score of an individual bird with a maximum score of 16. Four to six birds of each line were sampled at each time point after low dose challenge. Nine to eleven birds of each line were sampled after high dose challenge as, unexpectedly, several of the relatively susceptible birds succumbed to the infection and as a consequence all high dose birds were culled at 14 hpi (not at 2 and 7 dpi as planned). Bird numbers vary due to the availability of birds for this study. NB = Novogen Brown, inbred lines as indicated. The mean is shown. Groups with different letters are significantly different (P < 0.05).
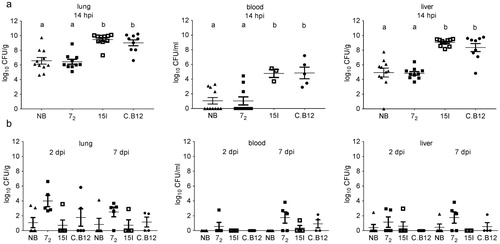
The bacterial loads in lung, blood and liver after high dose challenge were significantly lower in line 72 (which appeared relatively resistant to clinical signs and macroscopic lesions) compared to lines 15I and C.B12, which were relatively susceptible to disease ((a)). The Novogen Brown birds had bacterial loads similar to the line 72 birds after high dose inoculation. In contrast, no significant differences in bacterial colonization between the lines were observed at 2 and 7 dpi after inoculation with a low dose ((b)). The bacterial loads in lung and blood and in lung and liver were positively correlated across all birds of all lines (lung and blood: P < 0.001, R = 0.568; lung and liver: P < 0.001, R = 0.918). The correlation was independent of both the inoculation dose and the time point post inoculation, suggesting a rapid systemic dissemination of bacteria.
Figure 2. Bacterial colonization of tissues. Inbred lines 72, 15I and C.B12 and the outbred Novogen Brown chickens were inoculated with (a) the high or (b) the low dose of APEC O1. Bacterial loads in lung, blood and liver were determined at different time points. Four to six birds of each line were sampled at each time point after low dose challenge. One bird of the low dose group developed moderate clinical signs and was culled at 14 hpi and thus is not included in the presented data (colibacillosis lesion score 7, bacterial load in lung, blood and liver 7.08, 3.08 and 5.21 log10 CFU/g or ml respectively). Nine to eleven birds of each line were sampled after high dose challenge as, unexpectedly, several of the susceptible birds succumbed to the infection and as a consequence all high dose birds were culled at 14 hpi (not at 2 and 7 dpi as planned). Blood samples could only be obtained from surviving birds. Bird numbers vary due to the availability of birds for this study. NB = Novogen Brown, inbred lines as indicated. The mean with SEM is shown. Groups with different letters are significantly different (P < 0.05).
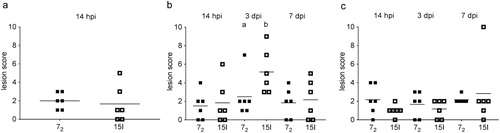
Since a dose-dependent effect was noted, a range of different APEC O1 doses (low (7.3 × 104 CFU), intermediate (1.1 × 106 CFU) and high (8.8 × 106 CFU)) were examined in the lines that exhibited the greatest difference in resistance (72 and 15I). After inoculation with the high dose of APEC O1, no differences in clinical signs were observed at 14 hpi as all birds developed moderate clinical signs (). All birds that received the intermediate dose developed mild clinical signs within 14 hpi and the signs resolved in all birds of both lines by 3 dpi. Birds inoculated with the low dose of APEC O1 did not develop clinical signs.
Table 3. Clinical signs at 14 hpi after intra-airsac inoculation of a high or intermediate dose of APEC O1 in resistant line 72 and susceptible line 15I.
Colibacillosis lesion scores were not significantly different between the two lines after inoculation with the high dose. After inoculation with the intermediate dose colibacillosis lesions were significantly greater in line 15I birds at 3 dpi. Similar to the previous study, inoculation with a low dose did not induce significant differences in lesion scores between the lines at either 3 or 7 dpi ().
Figure 3. Colibacillosis lesion scores. Inbred lines 72 and 15I chickens were inoculated with (a) the high, (b) the intermediate or (c) the low dose of APEC O1. Colibacillosis lesions were recorded at different time points. Each dot represents the total lesion score of an individual bird with a maximum score of 16. Six birds of each line were sampled at each time point after each challenge dose. The mean is shown. Groups with different letters are significantly different (P < 0.05).
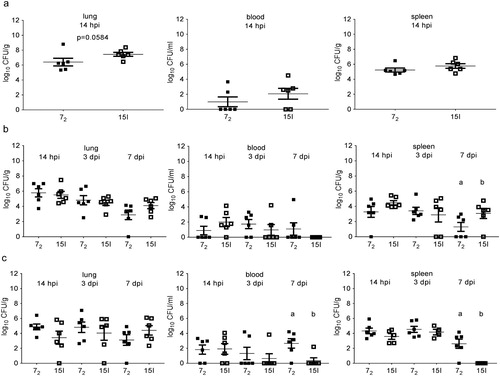
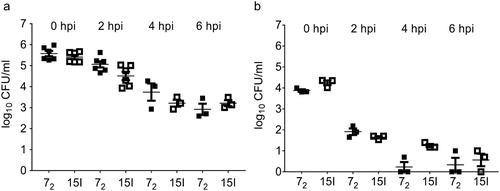
Bacterial colonization of the lung was highest in line 15I after inoculation with the high dose of APEC O1 ((a)). However, in blood and spleen samples no significant differences in bacterial numbers were observed in the different chicken lines at the high dose. No overall differences were observed after intermediate or low dose APEC O1 challenge, with the exception of bacterial loads in spleen at 7 days post intermediate dose challenge and in blood and spleen at 7 days post low dose challenge, where bacterial loads differed between the lines ((b) and (c)).
Figure 4. Bacterial colonization of tissues. Inbred lines 72 and 15I chickens were inoculated with (a) the high, (b) the intermediate or (c) the low dose of APEC O1. Bacterial loads in lung, blood and spleen were determined at different time points. Six birds of each line were sampled at each time point after each challenge dose. The mean with SEM is shown. Groups with different letters are significantly different (P < 0.05).
Phagocytic and bactericidal activity of lung leukocytes toward APEC O1-GFP is similar between inbred lines
Since the in vivo studies suggested differences in the colonization of E. coli or differences in the innate immune response between inbred lines 72 and 15I, resulting in differential resistance to a high dose of APEC O1, the phagocytic and bactericidal activity of lung leukocytes and the frequencies of phagocytic cells in the lungs of these lines were studied.
Phagocytosis and killing assays were performed using ex vivo leukocytes with an MOI = 100 and MOI = 10, of APEC O1. The number of intracellular bacteria, representing the balance of bacterial replication and death, was determined over time using a ceftazidime hydrate-protection assay. The phagocytic and bactericidal activity was similar between the inbred lines after both an MOI = 100 and MOI = 10, indicating that both inbred lines control APEC ().
Figure 5. Phagocytic and bactericidal activity of lung leukocytes. Gradient purified lung leukocytes of inbred lines 72 and 15I were inoculated with (a) an MOI = 100 or (b) an MOI = 10 in vitro, and the phagocytic (0 hpi) and bactericidal (2, 4, 6 hpi) activity determined by enumerating viable bacteria. (a) n = 3–6 (n = 3 at 4 and 6 hpi due to limitations with bird numbers), (b) n = 3. The mean with SEM is shown.
In addition, the frequencies of phagocytic cells, based on expression of macrophage markers including the mannose receptor MRC1L-B and CD11 expressed on both chicken macrophages and heterophils, were determined by flow cytometry and expressed as percentages of single live CD45+ leukocytes in uninfected birds. The frequencies of CD11 were similar between lines, whilst the frequency of MRC1L-B was lower in 15I birds (Supplementary Figure 1).
Discussion
Two-week-old inbred chicken lines were used to investigate if reliable differential resistance exists to APEC challenge, using a strain of APEC O1:K1:H7 administered intra-air sac. The inbred lines and age at challenge were chosen based on previous studies by Bumstead et al. (Citation1989) in which they determined the median lethal dose (LD50) of a mixture of APEC serotypes inoculated via the intramuscular route, which is arguably of questionable relevance to the natural routes of exposure. Whilst all ages and breeds of chickens are susceptible to APEC, younger chickens are more frequently affected and the severity of disease is greater in young chickens, including developing embryos (reviewed in Barnes et al., Citation2008). Older chickens can be more resistant to APEC infection (reviewed in Barnes et al., Citation2008), and higher resistance to disease in older chickens was also observed after Salmonella challenge (Gast & Beard, Citation1989; Beal et al., Citation2005). The higher susceptibility of young chickens may be due to the still developing immune system (Sutton et al., Citation2018). In this study we used two-week-old inbred line chickens to enable comparisons with previous studies in which susceptibility to APEC and Salmonella was investigated (Bumstead et al., Citation1989; Fife et al., Citation2009). The APEC O1 strain of serotype O1:K1:H7 was chosen because it is a well-characterized and sequenced strain of APEC (Johnson et al., Citation2006a, Citation2006b, Citation2007) and represents a dominant serotype and sequence-type lineage responsible for colibacillosis worldwide. Owing to the genetic diversity of APEC (reviewed in Collingwood et al., Citation2014 and Guabiraba & Schouler, Citation2015), we cannot preclude the possibility that other APEC would behave in the same way across lines; however, our study represents an attempt to disentangle the differential resistance of lines reported by Bumstead et al. following a complex co-infection of IBV and cocktail of APEC. We also cannot exclude the possibility that other respiratory routes of infection would provide differing results, especially with the same dose of inoculation. One could speculate that intranasal or intratracheal inoculation may result in a less pronounced differential resistance as physical barriers including mucus and ciliary activity and local immunity at the site of infection may start to clear bacteria before systemic dissemination.
White Leghorn inbred lines 72, 15I and C.B12 and the outbred Leghorn Novogen Brown birds were inoculated with a high, intermediate or low dose of APEC O1. Differential resistance to APEC O1 based on mortality, clinical signs, colibacillosis lesions and the bacterial colonization of tissues was only observed after inoculation with a high dose of APEC. This differential resistance was less pronounced between inbred line 72 (relatively resistant) and inbred line 15I (relatively susceptible) challenged with 8.8 × 106 CFU. No significant difference was noted in the bacterial colonization of the lung with this dose of infection in contrast to 1.9 × 107 CFU challenge. One bird of line 72, however, had a high bacterial load in lung, which was in fact the highest bacterial load amongst both inbred lines in response to infection with 8.8 × 106 CFU, which led to the statistically non-significant result ((a), P = 0.0043 omitting this single outlying bird). These findings are in line with observations made by Bumstead et al. (Citation1989) which identified inbred lines 15I and C.B12 as relatively susceptible, and inbred line 72 as relatively resistant to a mixture of APEC serotypes, administered intramuscularly. The outbred Novogen Brown birds were resistant to 107 CFU APEC O1 challenge with similar bacterial loads in tissues to the line 72 birds, indicating that these outbred chickens are relatively resistant to APEC O1 infection as a primary pathogen. The relative resistance of outbred broiler and layer birds to APEC challenge was also observed in other studies using a respiratory route of infection and doses of 108 CFU or higher (Horn et al., Citation2012; Sandford et al., Citation2012).
The relative resistance of the inbred chicken lines was also studied in response to other enterobacteria. Wigley et al. (Citation2002) intravenously inoculated three-week-old line N and line C birds with Salmonella enterica serovar Gallinarum or Typhimurium and showed that, whilst initial colonization of organs is similar between these lines, the more resistant line N birds limit the infection mainly to the liver and spleen where the bacteria are subsequently eliminated, whilst the more susceptible line C birds fail to control the infection and succumb to salmonellosis. Studying the faecal shedding and intestinal colonization of S. enterica serovar Typhimurium or Enteritidis in six-week-old inbred lines, Barrow et al. (Citation2004) identified inbred lines 61 and W as more resistant, and inbred lines N, 72, 15I and C as more susceptible to Salmonella infection. Sadeyen et al. (Citation2004) orally inoculated one-week old line 61 and 15I birds with S. enterica serovar Enteritidis and showed that whilst no difference was observed in the initial colonization of the spleen, bacterial clearance in the spleen was delayed in more susceptible line 15I birds. In the same study, line 15I birds appeared to be more resistant than line 61 birds to caecal colonization. Beal et al. (Citation2005) orally infected either 10- or 40-day-old line N and line 61 birds with S. enterica serovar Typhimurium and showed significantly higher numbers of viable bacteria in the caecal contents of more susceptible line N birds when inoculating 40-day-old birds, but no differential resistance between inbred lines when inoculating 10-day-old birds. These observations are largely in line with previous findings by Bumstead and Barrow (Citation1988) and Bumstead and Barrow (Citation1993) which identified inbred lines 61, W and N as more resistant and inbred lines 72, 15I and C as more susceptible to Salmonella infection using a variety of strains, with some reported differences likely due to differences in bacterial strain, route of inoculation, inoculation dose, age of birds, and the choice of inbred lines for a relative comparison. All these variables likely also impact on differential resistance studies after APEC challenge, for which the current literature is more limited. Interestingly, inbred lines 15I and C were identified as relatively susceptible to APEC and Salmonella in several studies, whilst inbred line 72 seems relatively resistant to APEC but not to Salmonella.
Our data suggest a potential role for innate components and immune responses in the differential resistance to APEC O1 early after challenge. Physical barriers such as the mucociliary clearance and host defence peptides (HDP) may prevent APEC from establishing an infection throughout the respiratory tract (reviewed in de Geus et al., Citation2012; Cuperus et al., Citation2013; Wigley, Citation2013). Differential resistance to bacterial infections such as Escherichia coli, Pasteurella multocida and Salmonella enterica has been previously shown to be correlated to HDP such as Mannan Binding Lectin (MBL) (Norup et al., Citation2009; Schou et al., Citation2010; Ulrich-Lynge et al., Citation2016). Using primary intestinal epithelial cell cultures from inbred lines 6 and 15I infected with S. Enteritidis, Derache et al. (Citation2009) showed that the expression of β-defensins 1 and 2 was unchanged, but the baseline expression levels were higher in the resistant line 6 cells. Such baseline expression differences were also observed in vivo in intestinal tissue of adults and young birds of the same inbred lines (Sadeyen et al., Citation2004). Greater baseline expression of a range of β-defensins in more resistant Fayoumi chickens compared to less resistant commercial broiler Ross chickens have also been described after Eimeria maxima challenge (Su et al., Citation2018). Transcriptomic studies indicated upregulation of β-defensins in peripheral blood leukocytes at 1 dpi after intra-air sac APEC O1 challenge, indicating a role for HDP in the innate immune response against APEC (Sandford et al., Citation2012). Additional transcriptomic studies described the immune response to intra-air sac APEC O1 challenge in bone marrow, thymus, bursa and spleen but not the respiratory tract (Sandford et al., Citation2011, Citation2012; Nie et al., Citation2012; Sun et al., Citation2015, Citation2016a, Citation2016b). They revealed both innate and adaptive immune responses in commercial broiler chickens at 1 and 5 dpi, and at 1 dpi differences in β-defensins and IL-8 expression between challenged and control birds are indicative of an important role of innate responses to APEC infection. In addition to physical barriers, the mucociliary clearance and HDP during the early response to APEC, differences in the early bacterial response may also contribute to the differential resistance observed. Bacterial colonization, growth, invasion and serum survival may be affected by potential differences in the local tissue environment and serum of different inbred lines, including the availability of iron, which is known to be critical for APEC virulence (reviewed in Dziva & Stevens, Citation2008).
Innate cells such as heterophils and macrophages contribute to rapid clearance of E. coli in the respiratory tract. Multiple studies have described local infiltration of heterophils and macrophages after infection with pathogenic and non-pathogenic E. coli, but their detailed role in APEC clearance remains to be studied (Pourbakhsh et al., Citation1997; Mellata et al., Citation2003; Ariaans et al., Citation2008; Dwars et al., Citation2009; Horn et al., Citation2012). Interestingly, both pathogenic and non-pathogenic E. coli caused similar lung pathology whilst bacterial loads in lung after APEC inoculations were significantly higher, for which the mechanisms are yet unknown (Horn et al., Citation2012). The in vitro phagocytic and bactericidal activity of lung phagocytes of inbred lines 72 and 15I birds was investigated using different MOI of APEC O1 (MOI = 100 and MOI = 10). Whilst both inbred lines had similar ratios of CD11+ cells, and 15I birds had a lower ratio of MRC1L-B+ cells, we did not determine the absolute number of phagocytic cells in their lungs. The number of bacteria that were phagocytosed and killed over time were not significantly different, suggesting additional differences between the lines. This would not be unexpected as it was shown that APEC uptake by phagocytes differs significantly between serotypes (Pourbakhsh et al., Citation1997; Mellata et al., Citation2003).
Chicken lines that differ in heterophil functionality have been described and shown to exhibit differential resistance to bacterial infections. Lines with less active heterophils were more susceptible to challenges with Enterococcus gallinarum (Swaggerty et al., Citation2005), and Campylobacter jejuni (Li et al., Citation2008). Comparison of heterophils from three different chicken lines (Broiler, Leghorn, and Fayoumi) showed significant differences in respect to phagocytosis and extracellular trap production, which are associated with clearance of invasive bacteria (Chuammitri et al., Citation2011), and correlated with their susceptibility to Salmonella. The same genetically distinct chicken lines also differentially expressed cytokines and chemokines in the spleen, including CXCLi2, IL-10, and IL-12α at 2 and 18 h post S. Enteritidis challenge (Cheeseman et al., Citation2007). In macrophages isolated from Salmonella resistant W1 and susceptible line 72 inbred birds, a magnitudinal and temporal differential expression of cytokines and chemokines following Salmonella challenge was described (Wigley et al., Citation2006). A rapid and more pronounced expression of IL-1β, IL-6, CXCLi1 and CCLi2 was detected at 1 hpi in the resistant line W1 macrophages. One could speculate that differences in innate immune chemokine and cytokine expression at baseline level prior to APEC challenge, or in response to APEC challenge, could significantly impact on heterophil and macrophage numbers and activity at the site of infection, and thus the localized immune response to APEC. Redundancies in innate immune mechanisms may explain the high dose of APEC O1 required to detect differential resistance. In conclusion, the differential resistance of inbred lines 72, 15I and C.B12 proved to be dependent on the dose of APEC O1, which makes the model suboptimal for further genetic or immunological studies to unravel the mechanisms of resistance to APEC O1. Further studies will focus on the early innate immune responses in the respiratory tract and mechanisms of dissemination to unravel which cells and innate components, such as HDP, are of importance for clearance of APEC.
Supplemental Material
Download MS Word (215 KB)Acknowledgements
We would like to dedicate this paper to our colleague Prof Pete Kaiser. We express our gratitude to Prof Lisa Nolan for providing the APEC O1 strain and Dr Sonja Härtle and Prof Bernd Kaspers for providing the CD11 antibody. We wish to thank the animal caretakers of the National Avian Research Facility for supply of the inbred lines. We wish to thank our collaborators for fruitful discussions.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Lonneke Vervelde http://orcid.org/0000-0003-2241-1743
Additional information
Funding
References
- Ariaans, M.P., Matthijs, M.G., van Haarlem, D., van de Haar, P., van Eck, J.H., Hensen, E.J. & Vervelde, L. (2008). The role of phagocytic cells in enhanced susceptibility of broilers to colibacillosis after infectious bronchitis virus infection. Veterinary Immunology and Immunopathoogy, 123, 240–250. doi: 10.1016/j.vetimm.2008.02.003
- Barnes, H.J., Nolan, L.K. & Vaillancourt, J.P. (2008). Colibacillosis. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nolan, & D.E. Swayne (Eds.), Diseases of Poultry 12th edn (pp. 691–732). Blackwell Publishing Professional.
- Barrow, P.A., Bumstead, N., Marston, K., Lovell, M.A. & Wigley, P. (2004). Faecal shedding and intestinal colonization of Salmonella enterica in in-bred chickens: the effect of host-genetic background. Epidemiology and Infection, 132, 117–126. doi: 10.1017/S0950268803001274
- Beal, R.K., Powers, C., Wigley, P., Barrow, P.A., Kaiser, P. & Smith, A.L. (2005). A strong antigen-specific T-cell response is associated with age and genetically dependent resistance to avian enteric salmonellosis. Infection and Immunity, 73, 7509–7516. doi: 10.1128/IAI.73.11.7509-7516.2005
- Bumstead, N., Huggins, M.B. & Cook, J.K. (1989). Genetic differences in susceptibility to a mixture of avian infectious bronchitis virus and Escherichia coli. British Poultry Science, 30, 39–48. doi: 10.1080/00071668908417123
- Bumstead, N. & Barrow, P. (1993). Resistance to Salmonella gallinarum, S. pullorum, and S. enteritidis in inbred lines of chickens. Avian Diseases, 37, 189–193. doi: 10.2307/1591473
- Bumstead, N. & Barrow, P.A. (1988). Genetics of resistance to Salmonella typhimurium in newly hatched chicks. British Poultry Science, 29, 521–529. doi: 10.1080/00071668808417078
- Cavero, D., Schmutz, M., Philipp, H.C. & Preisinger, R. (2009). Breeding to reduce susceptibility to Escherichia coli in layers. Poultry Science, 88, 2063–2068. doi: 10.3382/ps.2009-00168
- Cheeseman, J.H., Kaiser, M.G., Ciraci, C., Kaiser, P. & Lamont, S.J. (2007). Breed effect on early cytokine mRNA expression in spleen and cecum of chickens with and without Salmonella enteritidis infection. Developmental and Comparative Immunology, 31, 52–60. doi: 10.1016/j.dci.2006.04.001
- Chuammitri, P., Redmond, S.B., Kimura, K., Andreasen, C.B., Lamont, S.J. & Palic, D. (2011). Heterophil functional responses to dietary immunomodulators vary in genetically distinct chicken lines. Veterinary Immunology and Immunopathology, 142, 219–227. doi: 10.1016/j.vetimm.2011.05.019
- Collingwood, C., Kemmett, K., Williams, N. & Wigley, P. (2014). Is the concept of avian pathogenic Escherichia coli as a single pathotype fundamentally flawed? Frontiers in Veterinary Science, 1, 5–8. doi: 10.3389/fvets.2014.00005
- Cuperus, T., Coorens, M., van Dijk, A. & Haagsman, H.P. (2013). Avian host defense peptides. Developmental and Comparative Immunology, 41, 352–369. doi: 10.1016/j.dci.2013.04.019
- de Geus, E.D., Jansen, C.A. & Vervelde, L. (2012). Uptake of particulate antigens in a nonmammalian lung: phenotypic and functional characterization of avian respiratory phagocytes using bacterial or viral antigens. Journal of Immunology, 188, 4516–4526. doi: 10.4049/jimmunol.1200092
- Derache, C., Esnault, E., Bonsergent, C., Le Vern, Y., Quere, P. & Lalmanach, A.C. (2009). Differential modulation of beta-defensin gene expression by Salmonella enteritidis in intestinal epithelial cells from resistant and susceptible chicken inbred lines. Developmental and Comparative Immunology, 33, 959–966. doi: 10.1016/j.dci.2009.03.005
- DeRosa, M., Ficken, M.D. & Barnes, H.J. (1992). Acute airsacculitis in untreated and cyclophosphamide-pretreated broiler chickens inoculated with Escherichia coli or Escherichia coli cell-free culture filtrate. Veterinary Pathology, 29, 68–78. doi: 10.1177/030098589202900109
- Dwars, R.M., Matthijs, M.G., Daemen, A.J., van Eck, J.H., Vervelde, L. & Landman, W.J. (2009). Progression of lesions in the respiratory tract of broilers after single infection with Escherichia coli compared to superinfection with E. coli after infection with infectious bronchitis virus. Veterinary Immunology and Immunopathology, 127, 65–76. doi: 10.1016/j.vetimm.2008.09.019
- Dziva, F. & Stevens, M.P. (2008). Colibacillosis in poultry: unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathology, 37, 355–366. doi: 10.1080/03079450802216652
- Fife, M.S., Howell, J.S., Salmon, N., Hocking, P.M., van Diemen, P.M., Jones, M.A., Stevens, M.P. & Kaiser, P. (2011). Genome-wide SNP analysis identifies major QTL for salmonella colonization in the chicken. Animal Genetics, 42, 134–140. doi: 10.1111/j.1365-2052.2010.02090.x
- Fife, M.S., Salmon, N., Hocking, P.M. & Kaiser, P. (2009). Fine mapping of the chicken salmonellosis resistance locus (SAL1). Animal Genetics, 40, 871–877. doi: 10.1111/j.1365-2052.2009.01930.x
- Gast, R.K. & Beard, C.W. (1989). Age-related changes in the persistence and pathogenicity of Salmonella typhimurium in chicks. Poultry Science, 68, 1454–1460. doi: 10.3382/ps.0681454
- Ghunaim, H., Abu-Madi, M.A. & Kariyawasam, S. (2014). Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: potentials and limitations. Veterinary Microbiology, 172, 13–22. doi: 10.1016/j.vetmic.2014.04.019
- Goren, E. (1978). Observations on experimental infection of chicks with Escherichia coli. Avian Pathology, 7, 213–224. doi: 10.1080/03079457808418274
- Guabiraba, R. & Schouler, C. (2015). Avian colibacillosis: still many black holes. FEMS Microbiological Letters, 362, 118. doi: 10.1093/femsle/fnv118
- Horn, F., Correa, A.M., Barbieri, N.L., Glodde, S., Weyrauch, K.D., Kaspers, B., Driemeier, D., Ewers, C. & Wieler, L.H. (2012). Infections with avian pathogenic and fecal Escherichia coli strains display similar lung histopathology and macrophage apoptosis. PloS One, 7, e41031. doi: 10.1371/journal.pone.0041031
- Jansen, C.A., de Geus, E.D., van Haarlem, D.A., van de Haar, P.M., Londt, B.Z., Graham, S.P., Gobel, T.W., van Eden, W., Brookes, S.M. & Vervelde, L. (2013). Differential lung NK cell responses in avian influenza virus infected chickens correlate with pathogenicity. Scientific Reports, 3, 2478–2487. doi: 10.1038/srep02478
- Johnson, T.J., Johnson, S.J. & Nolan, L.K. (2006a). Complete DNA sequence of a ColBM plasmid from avian pathogenic Escherichia coli suggests that it evolved from closely related ColV virulence plasmids. Journal of Bacteriology, 188, 5975–5983. doi: 10.1128/JB.00204-06
- Johnson, T.J., Kariyawasam, S., Wannemuehler, Y., Mangiamele, P., Johnson, S.J., Doetkott, C., Skyberg, J.A., Lynne, A.M., Johnson, J.R. & Nolan, L.K. (2007). The genome sequence of avian pathogenic Escherichia coli strain O1:K1:H7 shares strong similarities with human extraintestinal pathogenic E. coli genomes. Journal of Bacteriology, 189, 3228–3236. doi: 10.1128/JB.01726-06
- Johnson, T.J., Wannemeuhler, Y.M., Scaccianoce, J.A., Johnson, S.J. & Nolan, L.K. (2006b). Complete DNA sequence, comparative genomics, and prevalence of an IncHI2 plasmid occurring among extraintestinal pathogenic Escherichia coli isolates. Antimicrobial Agents and Chemotheapy, 50, 3929–3933. doi: 10.1128/AAC.00569-06
- Kaiser, P., Howell, M.M., Fife, M., Sadeyen, J.R., Salmon, N., Rothwell, L., Young, J., Poh, T.Y., Stevens, M., Smith, J., Burt, D., Swaggerty, C. & Kogut, M. (2009). Towards the selection of chickens resistant to salmonella and campylobacter infections. Bulletin et Memoires de L'Academie Royale de Medecine de Belgique, 164, 17–25.
- Lamont, S.J., Dekkers, J.C.M. & Zhou, H. (2014). Immunogenetics and the Mapping of Immunological Functions. In K.A. Schat, B. Kaspers, & P. Kaiser (Eds.), Avian Immunology 2nd edn (pp. 205–222). Academic Press, Elsevier.
- Li, X., Swaggerty, C.L., Kogut, M.H., Chiang, H., Wang, Y., Genovese, K.J., He, H., Stern, N.J., Pevzner, I.Y. & Zhou, H. (2008). The paternal effect of Campylobacter jejuni colonization in ceca in broilers. Poultry Science, 87, 1742–1747. doi: 10.3382/ps.2008-00136
- Matthijs, M.G., van Eck, J.H., Landman, W.J. & Stegeman, J.A. (2003). Ability of Massachusetts-type infectious bronchitis virus to increase colibacillosis susceptibility in commercial broilers: a comparison between vaccine and virulent field virus. Avian Pathology, 32, 473–481. doi: 10.1080/0307945031000154062
- Mellata, M., Dho-Moulin, M., Dozois, C.M., Curtiss, R., III, Lehoux, B. & Fairbrother, J.M. (2003). Role of avian pathogenic Escherichia coli virulence factors in bacterial interaction with chicken heterophils and macrophages. Infectious Immunology, 71, 494–503. doi: 10.1128/IAI.71.1.494-503.2003
- Mitchell, N.M., Johnson, J.R., Johnston, B., Curtiss, R., III & Mellata, M. (2015). Zoonotic potential of Escherichia coli isolates from retail chicken meat products and eggs. Applied Environmental Microbiology, 81, 1177–1187. doi: 10.1128/AEM.03524-14
- Nie, Q., Sandford, E.E., Zhang, X., Nolan, L.K. & Lamont, S.J. (2012). Deep sequencing-based transcriptome analysis of chicken spleen in response to avian pathogenic Escherichia coli (APEC) infection. PLoS One, 7, e41645. doi: 10.1371/journal.pone.0041645
- Norup, L.R., Dalgaard, T.S., Friggens, N.C., Sorensen, P. & Juul-Madsen, H.R. (2009). Influence of chicken serum mannose-binding lectin levels on the immune response towards Escherichia coli. Poultry Science, 88, 543–553.
- Pourbakhsh, S.A., Boulianne, M., Martineau-Doize, B., Dozois, C.M., Desautels, C. & Fairbrother, J.M. (1997). Dynamics of Escherichia coli infection in experimentally inoculated chickens. Avian Diseases, 41, 221–233. doi: 10.2307/1592463
- Psifidi, A., Fife, M., Howell, J., Matika, O., van Diemen, P.M., Kuo, R., Smith, J., Hocking, P.M., Salmon, N., Jones, M.A., Hume, D.A., Banos, G., Stevens, M.P. & Kaiser, P. (2016). The genomic architecture of resistance to Campylobacter jejuni intestinal colonisation in chickens. BMC Genomics, 17, 293–320. doi: 10.1186/s12864-016-2612-7
- Sadeyen, J.R., Trotereau, J., Velge, P., Marly, J., Beaumont, C., Barrow, P.A., Bumstead, N. & Lalmanach, A.C. (2004). Salmonella carrier state in chicken: comparison of expression of immune response genes between susceptible and resistant animals. Microbes and Infection, 6, 1278–1286. doi: 10.1016/j.micinf.2004.07.005
- Sandford, E.E., Orr, M., Balfanz, E., Bowerman, N., Li, X., Zhou, H., Johnson, T.J., Kariyawasam, S., Liu, P., Nolan, L.K. & Lamont, S.J. (2011). Spleen transcriptome response to infection with avian pathogenic Escherichia coli in broiler chickens. BMC Genomics, 12, 469–482. doi: 10.1186/1471-2164-12-469
- Sandford, E.E., Orr, M., Shelby, M., Li, X., Zhou, H., Johnson, T.J., Kariyawasam, S., Liu, P., Nolan, L.K. & Lamont, S.J. (2012). Leukocyte transcriptome from chickens infected with avian pathogenic Escherichia coli identifies pathways associated with resistance. Results in Immunology, 2, 44–53. doi: 10.1016/j.rinim.2012.02.003
- Schou, T.W., Permin, A., Christensen, J.P., Cu, H.P. & Juul-Madsen, H.R. (2010). Mannan-binding lectin (MBL) in two chicken breeds and the correlation with experimental Pasteurella multocida infection. Comparative Immunology, Microbiological & Infectious Disease, 33, 183–195. doi: 10.1016/j.cimid.2008.08.010
- Schouler, C., Schaeffer, B., Bree, A., Mora, A., Dahbi, G., Biet, F., Oswald, E., Mainil, J., Blanco, J. & Moulin-Schouleur, M. (2012). Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. Journal of Clinical Microbiology, 50, 1673–1678. doi: 10.1128/JCM.05057-11
- Su, S., Miska, K.B., Fetterer, R.H., Jenkins, M.C., Lamont, S.J. & Wong, E.A. (2018). Differential expression of intestinal nutrient transporters and host defense peptides in Eimeria maxima-infected Fayoumi and Ross chickens. Poultry Science.
- Sun, H., Bi, R., Liu, P., Nolan, L.K. & Lamont, S.J. (2016a). Combined analysis of primary lymphoid tissues’ transcriptomic response to extra-intestinal Escherichia coli (ExPEC) infection. Developmental and Comparative Immunology, 57, 99–106. doi: 10.1016/j.dci.2015.12.013
- Sun, H., Liu, P., Nolan, L.K. & Lamont, S.J. (2015). Novel pathways revealed in bursa of Fabricius transcriptome in response to extraintestinal pathogenic Escherichia coli (ExPEC) infection. PLoS One, 10, e0142570. doi: 10.1371/journal.pone.0142570
- Sun, H., Liu, P., Nolan, L.K. & Lamont, S.J. (2016b). Thymus transcriptome reveals novel pathways in response to avian pathogenic Escherichia coli infection. Poultry Science, 95, 2803–2814. doi: 10.3382/ps/pew202
- Sutton, K., Costa, T., Alber, A., Bryson, K., Borowska, D., Balic, A., Kaiser, P., Stevens, M. & Vervelde, L. (2018). Visualisation and characterisation of mononuclear phagocytes in the chicken respiratory tract using CSF1R-transgenic chickens. Veterinary Research, 49, 104–117. doi: 10.1186/s13567-018-0598-7
- Swaggerty, C.L., Ferro, P.J., Pevzner, I.Y. & Kogut, M.H. (2005). Heterophils are associated with resistance to systemic Salmonella enteritidis infections in genetically distinct chicken lines. FEMS Immunology and Medical Microbiology, 43, 149–154. doi: 10.1016/j.femsim.2004.07.013
- Tran, T., Beaumont, C., Salmon, N., Fife, M., Kaiser, P., Le Bihan-Duval, E., Vignal, A., Velge, P. & Calenge, F. (2012). A maximum likelihood QTL analysis reveals common genome regions controlling resistance to salmonella colonization and carrier-state. BMC Genomics, 13, 198–207. doi: 10.1186/1471-2164-13-395
- Ulrich-Lynge, S.L., Juul-Madsen, H.R., Kjaerup, R.B., Okimoto, R., Abrahamsen, M.S., Maurischat, S., Sorensen, P. & Dalgaard, T.S. (2016). Broilers with low serum Mannose-binding Lectin show increased fecal shedding of Salmonella enterica serovar Montevideo. Poultry Science, 95, 1779–1786. doi: 10.3382/ps/pew101
- Valdivia, R.H. & Falkow, S. (1996). Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Molecular Microbiology, 22, 367–378. doi: 10.1046/j.1365-2958.1996.00120.x
- Vandemaele, F., Bleyen, N., Abuaboud, O., van der Meer, E., Jacobs, A. & Goddeeris, B.M. (2006). Immunization with the biologically active lectin domain of PapGII induces strong adhesion-inhibiting antibody responses but not protection against avian pathogenic Escherichia coli. Avian Pathology, 35, 238–249. doi: 10.1080/03079450600710997
- Wigley, P. (2013). Immunity to bacterial infection in the chicken. Developmental and Comparative Immunology, 41, 413–417. doi: 10.1016/j.dci.2013.04.008
- Wigley, P., Hulme, S., Rothwell, L., Bumstead, N., Kaiser, P. & Barrow, P. (2006). Macrophages isolated from chickens genetically resistant or susceptible to systemic salmonellosis show magnitudinal and temporal differential expression of cytokines and chemokines following Salmonella enterica challenge. Infectious Immunology, 74, 1425–1430. doi: 10.1128/IAI.74.2.1425-1430.2006
- Wigley, P., Hulme, S.D., Bumstead, N. & Barrow, P.A. (2002). In vivo and in vitro studies of genetic resistance to systemic salmonellosis in the chicken encoded by the SAL1 locus. Microbes and Infection, 4, 1111–1120. doi: 10.1016/S1286-4579(02)01635-0
