ABSTRACT
Extinct from nature, captive young Alagoas curassows (Pauxi mitu) were found agonizing or dead with respiratory disease. Intranuclear inclusion bodies were found in the epithelia of the trachea, associated with marked necrotic tracheitis. An Aviadenovirus was isolated in chicken eggs and characterized genetically with 99% identity to the fowl Aviadenovirus A, as based on the hexon protein gene. This is the first report of respiratory disease caused by Aviadenovirus in any cracid species in Brazil, recommending for stricter biosecurity in the conservation premises.
RESEARCH HIGHLIGHTS
Fatal tracheitis in curassows extinct from nature was associated with Aviadenovirus A.
Seven-month-old Alagoas curassows (Aves: Cracidae) died with haemorrhagic tracheitis.
Aviadenovirus A with 99% identity to fowl adenovirus 1 was detected in dead curassows.
Fatal tracheitis by Aviadenovirus was described in Pauxi mitu (Aves: Cracidae).
Introduction
The genus Aviadenovirus presently contains five species (Marek et al., Citation2014) and includes strains causing disease such as inclusion body hepatitis (IBH) in chicken (Schachner et al., Citation2018), cockatiel (Nymphicus hollandicus) (Scott et al., Citation1986) and African grey parrot (Psittacus erithacus) (Capua et al., Citation1995; Droual et al., Citation1995). In addition, severe respiratory disease associated with Aviadenovirus, with tracheal epithelium intranuclear inclusion bodies, was also documented in quails (Coturnix japonica) (Adair and Fitzgerald, Citation2008), and turkeys (Crespo et al., Citation1998). Four natural cases (2008–2012) of quail bronchitis were studied in bobwhite quail chicks (5–8 weeks of age) in Minnesota (Singh et al., Citation2016), with respiratory distress and high mortality, tracheal mucus, lungs with congestion, airsacculitis and pericarditis with caseous exudates, multifocal liver necrosis, splenomegaly and respiratory epithelium karyomegaly with basophilic nuclear inclusions. The experimental infection of one-to-nine-week-old bobwhite quail (Colinus virginianus) with the Aviadenovirus Indiana C strain, obtained from leghorn chickens showing poor eggshell quality, resulted in necrotizing tracheitis, bronchitis, pneumonia, hepatitis and splenitis (Jack & Reed, Citation1994). Natural adenovirus infection in turkeys has been associated with respiratory disease, poult enteritis complex, poult IBH and lower hatchability (Shivaprasad et al., Citation2001; Hess, Citation2013; Moura-Alvarez et al., Citation2013). In chickens, respiratory disease has also been associated with adenovirus infection (Lim et al., Citation1973; Dhillon & Kibenge, Citation1987), with pathogenesis complicated with Escherichia coli and Staphylococcus aureus, and the disease reproduced experimentally (Dhillon et al., Citation1982). Adenovirus tracheitis has also been documented in goslings (Riddell et al., Citation1992), and in ducklings with respiratory disease caused by a strain with genetic identity to duck adenovirus 1 (DAdV1) (Brash et al., Citation2009; Cha et al., Citation2013), an Atadenovirus also described causing the egg drop syndrome in chickens accidentally infected by contaminated Marek’s disease vaccines (McCracken & McFerran, Citation1978).
The Alagoas curassow (Aves: Galliformes: Cracidae) (Pauxi mitu) is a gallinaceous bird only found in captivity. It is extinct in its small natural habitat in the Atlantic Forest in northeastern Brazil, Alagoas and Pernambuco states (BirdLife International, Citation2018). It has been extinct in the wild since the 1970s. Only a few individuals were rescued for breeding in captivity (R. Azeredo, pers. comm.). The captive P. mitu population, of more than 200 birds, includes approximately 40 pure breed individuals. Juvenile birds are kept together in a common enclosure and adult birds are kept separately in pairs for reproduction. The aim of this report was to describe an outbreak of necrotic tracheitis in captive young Alagoas curassows in the world’s largest cracid conservation facility, located Minas Gerais state, Brazil.
Materials and methods
Four captive seven-month-old Alagoas curassows (P. mitu) Aves: Galliformes: Cracidae showed clinical signs and died in July 2013 (). They lived inside a common enclosure at the cracid conservation facility (Crax Brazil; Contagem, Minas Gerais), which maintains a total captive number of approximately 200 young and adult individuals. The total different avian species population at the conservation facility reaches over 1000 individuals.
Figure 1. Seven-month-old Alagoas curassows (Pauxi mitu) which died of natural infection with Aviadenovirus A. Three birds are shown of four birds which died at the outbreak.

Necropsies were performed and the tracheas were collected and stored frozen (−20°C) for PCR and virus isolation. Trachea, lungs, heart, liver, spleen, kidneys and intestine were fixed for histopathology (10% buffered neutral formalin). For virus isolation, tracheal mucosa samples were treated with an antibiotic-antimycotic mixture (Gibco, USA), macerated, added to an equal volume of PBS, and the supernatant inoculated onto the chorioallantoic membrane (CAM) of 10-day-old SPF chicken embryos (Senne, Citation1998) and the CAM was collected at seven days post-infection and fixed in neutral formalin for processing.
DNA extractions from the trachea or CAM were performed with a sodium iodide/silicon dioxide protocol (Boom et al., Citation1990). In addition to the affected birds, all apparently healthy Alagoas curassows and red-billed curassows (Crax blumenbachii) kept in the premises were sampled (cloacal swabs) for PCR testing (n = 289).
Partial Aviadenovirus DNA amplification was performed using a previously described protocol for a 897-bp product of the gene encoding the Aviadenovirus hexon protein (F:5′-CAAGTTCAGGCAGACGGT; 3′ R:5′-TAGTGATGCCGCGACATCAT-3′) (Meulemans et al., Citation2001). PCR products were sequenced using Big Dye Terminator Mix (Applied Biosystems®, EUA Foster, CA, USA) in a capillary sequencer (ABI 310, Applied Biosystems®). Phelps FAdV-1 DNA positive control was kindly provided by Dr. Jane K. A. Cook (Houghton Trust, St Ives, Cambridgeshire, PE27 5BB, UK).
Considering an ongoing outbreak of infectious laryngotracheitis (ILT) in chickens in the same state (Minas Gerais), the DNA extracted from the trachea was also analysed using a PCR to amplify DNA from Gallid herpesvirus 1 (Preis et al., Citation2013).
Results
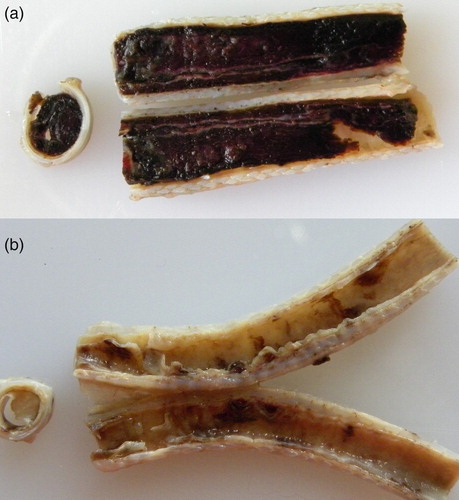
The affected birds () had fibrino-haemorrhagic and necrotic tracheitis with large cylindrical blood clots in the lumen ((a)) or the mucosa was covered by a circumferential diphtheritic whitish membrane ((b)). Lungs were hyperaemic and with moderate oedema. Pock-like lesions were found on the CAM within seven days of infection in laboratory infected chicken embryos.
Figure 2. (a) Formalin-fixed trachea from an Alagoas curassow filled with blood and fibrin. (b) Formalin-fixed trachea from an Alagoas curassow with a diphtheritic membrane.
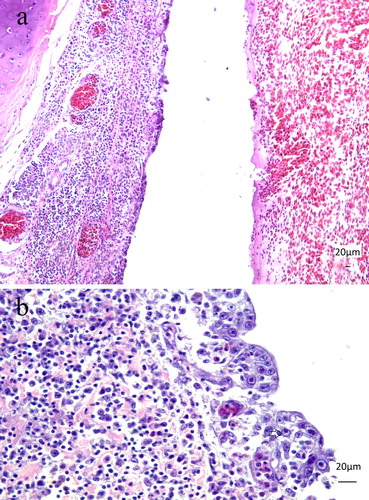
Histopathology revealed diffuse and marked infiltration of lymphocytes and macrophages expanding the lamina propria of the tracheas ((a)). Epithelial cells were necrotic with detachment in multifocal areas, and goblet cells were absent. Multifocally, the normal epithelium was replaced by regenerative non-ciliated cells ((a)). Basophilic intranuclear inclusion bodies (IB), some with a halo ((b)) were present in the respiratory epithelial cells. Cellular debris, some heterophils, fibrin and numerous erythrocytes formed a diphtheritic membrane aligned at the mucosa. Mild lymphocytic airsacculitis with intranuclear IB was found in one bird. In the spleen, there was moderate depletion of the lymphoid follicles and multifocal lymphocytolysis. The inoculated CAM had mild multifocal plasma cell infiltration, extensive necrosis with loss of epithelial chorionic layer and syncytial formation. In some cells, a basophilic or amphophilic material, compatible with intranuclear IB, was found. These nuclei were filled with homogenous basophilic material, and the chromatin was displaced to the periphery. No significant histological lesions were found in the other tissues.
Figure 3. (a) Seven-month-old Alagoas curassow (Pauxi mitu) with tracheitis due to Aviadenovirus. The respiratory epithelium is replaced by a single layer of epithelium, with loss of goblet cells. The lamina propria is expanded by the lymphoplasmacytic infiltration. H&E. Obj. 20. Bar = 20 µm. (b) Seven-month-old Alagoas curassow (P. mitu) with tracheitis by Aviadenovirus. Lymphoplasmacytic tracheitis with epithelial necrosis, loss of goblet cells and basophilic intranuclear inclusion bodies (arrow) were detected. H&E. Obj. 40. Bar = 20 µm.
The partial sequences of the hexon protein-encoding gene detected in the P. mitu virus strains, in the trachea and in the inoculated CAM (Accession numbers KJ666643.1, KJ666644.1, KJ666645.1 and KJ666646.1), were phylogenetically grouped with Aviadenovirus A. These sequences were found to have high (99%) nucleotide identity to fowl adenovirus 1 (FAdV-1) (AF339914.1) and fowl Aviadenovirus A of chickens in Canada (EF685382.1; EF685393.1; EF685377; EF685412.1), quail adenovirus in the USA (KT380842.1) and chicken adenovirus in Hungary (JQ647514.1). The cracid isolates differed from previously published Brazilian Aviadenovirus hexon gene sequences, of fowl Aviadenovirus D in São Paulo [FAdV-11 (FJ360747.1 and FJ360748.1)] and Minas Gerais (Pereira et al., Citation2014) and Aviadenovirus E in São Paulo (JF288952.1 and JF288937.1), and grouped separately to other known serotypes with published hexon gene sequences: Fowl Adenovirus 2 ATCC VR-827 P7-A (AF339915.1), Fowl Adenovirus 3 ATCC VR-828 IBH-2A (AF339916.1), Fowl adenovirus 4 (India; AY514902.1), Fowl Adenovirus 4 ATCC VR-829 J2-A (AF339917.1), Fowl adenovirus 5 ATCC VR-830 T8-A (AF339919.1), Fowl adenovirus 6 ATCC VR-831 75-1A-1 (AF339921.1), Fowl adenovirus 7 ATCC VR-832 B-3A (AF339922.1), Fowl adenovirus 8 ATCC VR-833 A-2A (AF339918.1), Fowl adenovirus 9 ATCC VR-834 C-2B (AF339923.1), Fowl adenovirus 10 ATCC VR-835X-11A (AF339924.1), Fowl adenovirus 11X11 (AF339920.1), Fowl Aviadenovirus B 8844/2010 (JF304111.1), Fowl Aviadenovirus D 508-14A (KR781101), Fowl Aviadenovirus D 508-14A (KR781101.1) and Fowl Aviadenovirus E SA84-08 (HQ117911.1).
No relevant bacterium was isolated from the tracheal lesions and no significant lesions were found in other organs. The total DNA from the trachea analysed for ILT virus was found to be negative for Gallid herpesvirus 1. In addition, there were no histology lesions indicative of ILT.
Discussion
Similar necrotic and haemorrhagic tracheitis associated with Aviadenovirus A was previously described in other species, such as quails and turkeys (Hess, Citation2013), and characterized as a severe fatal tracheal disease, as described here. A recent study in Pakistan described Aviadenovirus (FAdV) as an emerging cause of respiratory disease (Gowthaman et al., Citation2012), characterized by eosinophilic nuclear inclusions in the respiratory epithelium. In contrast, our findings were of basophilic nuclear inclusions, in agreement with Singh et al. (Citation2016), although no lesions were found in the other tissues (trachea, lungs, heart, liver, kidneys and intestine), except for splenic lymphocytic depletion, and were confined to the trachea.
Nevertheless, both Aviadenovirus infection and fatal tracheal disease are described here for the first time in the host species (P. mitu) and avian family (Cracidae). The phylogenetic evaluation of the cracid adenovirus strain was limited to part of the hexon protein-encoding gene and its characterization may benefit from more detailed genomic analysis (Marek et al., Citation2014), considering that turkey adenovirus (TAdV) strains were evaluated at the hexon gene and found to present phylogenetic and G+C content diversity which merit the separation of strains into two species, TAdV C and TAdV D (Schachner et al., Citation2016).
In quails, Aviadenovirus A respiratory infections can be severe and with pathology similar to that described in the cracids of the present study. Quail chicks (5–8 weeks-old) had fibrinonecrotic tracheitis, pneumonia, airsacculitis, pericarditis, hepatitis, etc., and quail bronchitis virus hexon gene sequences had high identity (99%) to the CELO strain, although different from Aviadenovirus A of goose, duck, turkey and pigeon (Singh et al., Citation2016). Aviadenoviruses are more commonly associated with IBH, the hydropericardium syndrome in chickens, turkeys and other species, and gizzard erosion (Hess et al., Citation1998; Shivaprasad et al., Citation2001; Schachner et al., Citation2018). Specific strains or genotypes are involved in such natural diseases (Mazaheri et al., Citation1998; Schachner et al., Citation2016).
However, the experimental demonstration of a primary role for Aviadenovirus has been difficult, possibly due to the diversity of intervening factors, such as field or geographic conditions, the genetic profile of birds, and immune-suppressing pathogens, such as the chicken anaemia virus (restricted to chickens) (Toro et al., Citation2000) or mycotoxins (Singh et al., Citation2016). The cracids of the present outbreak were fed with quality control industrial chicken feed and, although not evaluated, the presence of low levels of determined mycotoxins, such as aflatoxins, fumonisins, fusarin and zearalenone is likely (Oliveira et al., Citation2017), and may have contributed to the pathogenesis.
The potential explosive upsurge of Aviadenovirus as a pathogen was documented in epidemics of IBH in broiler and turkey flocks, with elevated mortality, haemorrhagic and enlarged livers in most continents (McFerran et al., Citation1976; Guy et al., Citation1988; Christensen & Saifuddin, Citation1989; Toro et al., Citation1999). IBH was also documented in aplomado and peregrine falcons, and the molecular characterization has identified the falcon virus as a new member of Aviadenovirus and closely related to the Aviadenovirus A (Oaks et al., Citation2005). Aviadenovirus D and E strains with low identity to falcon strains but high identity to chicken strains were detected in IBH outbreaks in chickens and gyrfalcons (Falco rusticolus) in Saudi Arabia suggesting cross-species transmission (Mohamed et al., Citation2018).
Aviadenovirus strains appear to be reemerging worldwide as relevant respiratory pathogens (Gowthaman et al., Citation2012) although they may be involved in a diversity of pathologies, such as pancreatitis in chickens with diabetes mellitus (Matos et al., Citation2017), and the strain JM1/1 was described in cases of broiler gizzard erosion in Japan, with hexon gene sequence 99% identical to the CELO strain (Thanasut et al., Citation2017), and a novel adenovirus infecting the cloacal bursa of gulls (Bodewes et al., Citation2013) was described. A previous study revealed Aviadenovirus D in chickens of the regional (Minas Gerais, Brazil) poultry industry and in free-range poultry (Pereira et al., Citation2014), suggesting that local chickens could act as a potential source of infection for wild birds.
Considering an ongoing (mild) outbreak of ILT in a large (8 million chickens) industrial layer chicken region in the same state (Minas Gerais), approximately 400 km away, the tracheas were also analysed for Gallid herpesvirus 1 DNA, and shown to be negative.
The Alagoas curassow is a gallinaceous bird only found in captivity, extinct from its small natural habitat (www.iucnredlist.org) in the Atlantic Forest in northeastern Brazil (Alagoas and Pernambuco states). The captive stocks should be kept in biosecure premises and have been tested for poultry-relevant a etiologies (Salmonella spp., Mycoplasma gallisepticum and M. synoviae, Newcastle disease and influenza viruses) before the future reintroduction into the wild, for reducing the risk of introducing exotic diseases in the natural environment. The extinction in the wild since the 1970s was mostly due to habitat loss from sugar cane plantations (Silveira et al., Citation2004). Only a few individuals were rescued for breeding in captivity (R. Azeredo, pers. comm.). The captive population (∼200), includes approximately 40 pure breed individuals. Juvenile birds are kept together in a common enclosure until close to adulthood. Adult birds are separated into pairs for reproduction. The occurrence of a severe outbreak by Aviadenovirus in young birds has highlighted the importance of biosecure management policies for conservation facilities, especially regarding proximity to chickens.
This is the first report of a severe respiratory disease caused by an Aviadenovirus in Alagoas curassow or in any cracid bird in Brazil. Considering conservation and breeding of the Alagoas currasow, vertical transmission of Aviadenovirus may cause concern, and breeders should be kept in biosecurity and monitored, due to the risk of reintroducing infected progeny into the wild.
Acknowledgements
We are grateful to Roberto Azeredo, of Crax Brasil, for providing the avian specimens for analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Nelson Rodrigo da Silva Martins http://orcid.org/0000-0001-8925-2228
Additional information
Funding
References
- Adair, B.M. & Fitzgerald, S.D. (2008). Adenovirus infections. In Y.M. Saif (Ed.). Diseases of Poultry 12th edn (pp. 251–296) Ames, Iowa: Iowa State University.
- BirdLife International. (2018). Mitu mitu. The IUCN Red List of Threatened Species 2018: e.T22678486A132315266.
- Bodewes, R., van de Bildt, M.W., Schapendonk, C.M., van Leeuwen, M., van Boheemen, S., de Jong, A.A., Osterhaus, A.D., Smits, S.L. & Kuiken, T. (2013). Identification and characterization of a novel adenovirus in the cloacal bursa of gulls. Virology, 440, 84–88. doi: 10.1016/j.virol.2013.02.011
- Boom, R., Sol, C.J., Salimans, M.M., Jansen, C.L., Wertheim-van Dillen, P.M. & Van der Noordaa, J. (1990). Rapid and simple method for purification of nucleic acids. Journal of Clinical Microbiology, 28, 495–503.
- Brash, M.L., Swinton, J.N., Weisz, A. & Ojkić, D. (2009). Isolation and identification of duck adenovirus 1 in ducklings with proliferative tracheitis in Ontario. Avian Diseases, 53, 317–320. doi: 10.1637/8559-121508-Case.1
- Capua, I., Liberti, L., Gough, R.E., Casaccia, C. & Asdrubali, G. (1995). Isolation and characterization of an adenovirus associated with inclusion body hepatitis in psittacine birds. Avian Pathology, 24, 717–722. doi: 10.1080/03079459508419110
- Cha, S.Y., Kang, M., Moon, O.K., Park, C.K. & Jang, H.K. (2013). Respiratory disease due to current egg drop syndrome virus in Pekin ducks. Veterinary Microbiology, 165, 305–311. doi: 10.1016/j.vetmic.2013.04.004
- Christensen, N.H. & Saifuddin, M. (1989). A primary epidemic of inclusion body hepatitis in broilers. Avian Diseases, 33, 622–630. doi: 10.2307/1591135
- Crespo, R., Shivaprasad, H.L., Droual, R., Chin, R.P., Woolcock, P.R. & Carpenter, T.E. (1998). Inclusion body tracheitis associated with avian adenovirus in turkeys. Avian Diseases, 42, 589–596. doi: 10.2307/1592687
- Dhillon, A.S. & Kibenge, F.S. (1987). Adenovirus infection associated with respiratory disease in commercial chickens. Avian Diseases, 31, 654–657. doi: 10.2307/1590755
- Dhillon, A.S., Winterfield, R.W., Thacker, H.L. & Feldman, D.S. (1982). Lesions induced in the respiratory tract of chickens by serologically different adenoviruses. Avian Diseases, 26, 478–486. doi: 10.2307/1589893
- Droual, R., Woolcock, P.R., Nordhausen, R.W. & Fitzgerald, S.D. (1995). Inclusion body hepatitis and hemorrhagic enteritis in two African grey parrots (Psittacus erithacus) associated with adenovirus. Journal of Veterinary Diagnostic Investigation, 7, 150–154. doi: 10.1177/104063879500700125
- Gowthaman, V., Singh, S.D., Dhama, K., Barathidasan, R., Kumar, M.A., Desingu, P.A., Mahajan, N.K. & Ramakrishnan, M.A. (2012). Fowl adenovirus (FAdV) in India: evidence for emerging role as primary respiratory pathogen in chickens. Pakistan Journal of Biological Sciences, 15, 900–903. doi: 10.3923/pjbs.2012.900.903
- Guy, J.S., Schaeffer, J.L. & Barnes, H.J. (1988). Inclusion-body hepatitis in day-old turkeys. Avian Diseases, 32, 587–590. doi: 10.2307/1590936
- Hess, M. (2013). Aviadenovirus Infections. In David E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez, & V.L. Nair (Eds.). Diseases of Poultry 13th edn (pp. 289–332). Ames, Iowa: Wiley-Blackwell.
- Hess, M., Prusas, C., Vereecken, M. & De Herdt, P. (1998). Isolation of fowl adenoviruses serotype 4 from pigeons with hepatic necrosis. Berliner und Münchener tierärztliche Wochenschrift, 111, 140–142.
- Jack, S.W. & Reed, W.M. (1994). Experimental infection of bobwhite quail with Indiana C adenovirus. Avian Diseases, 38, 325–328. doi: 10.2307/1591957
- Lim, A.C., Mustaffa-Babjee, A., Bains, B.S. & Spradbrow, P.B. (1973). An avian adenovirus associated with respiratory disease. Avian Diseases, 17, 690–696. doi: 10.2307/1589035
- Marek, A., Ballmann, M.Z., Kosiol, C., Harrach, B., Schlötterer, C. & Hess, M. (2014). Whole-genome sequences of two turkey adenovirus types reveal the existence of two unknown lineages that merit the establishment of novel species within the genus Aviadenovirus. Journal of General Virology, 95, 156–170. doi: 10.1099/vir.0.057711-0
- Matos, M., Dublecz, K., Grafl, B., Liebhart, D. & Hess, M. (2018). Pancreatitis is an important feature of broilers suffering from inclusion body hepatitis leading to dysmetabolic conditions with consequences for zootechnical performance. Avian Diseases, 62, 57–64. doi: 10.1637/11755-092717-Reg.1
- Mazaheri, A., Prusas, C., Voss, M. & Hess, M. (1998). Some strains of serotype 4 fowl adenoviruses cause inclusion body hepatitis and hydropericardium syndrome in chickens. Avian Pathology, 27, 269–276. doi: 10.1080/03079459808419335
- McCracken, R.M. & McFerran, J.B. (1978). Experimental reproduction of the egg drop syndrome 1976 with a haemagglutinating adenovirus. Avian Pathology, 7, 483–490. doi: 10.1080/03079457808418304
- McFerran, J.B., McCracken, R.M., Connor, T.J. & Evans, R.T. (1976). Isolation of viruses from clinical outbreaks of inclusion body hepatitis. Avian Pathology, 5, 315–324. doi: 10.1080/03079457608418201
- Meulemans, G., Boschmans, M., Berg, T.P. & Decaesstecker, M. (2001). Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenovirus. Avian Pathology, 30, 655–660. doi: 10.1080/03079450120092143
- Mohamed, M.H., El-Sabagh, I.M., Abdelaziz, A.M., Al-Ali, A.M., Alramadan, M., Lebdah, M.A., Ibrahim, A.M. & Al-Ankari, A.R.S. (2018). Molecular characterization of fowl aviadenoviruses species D and E associated with inclusion body hepatitis in chickens and falcons indicates possible cross-species transmission. Avian Pathology, 47, 1–7. doi: 10.1080/03079457.2018.1457769
- Moura-Alvarez, J., Chacon, J.V., Scanavini, L.S., Nunez, L.F., Astolfi-Ferreira, C.S., Jones, R.C. & Piantino Ferreira, A.J. (2013). Enteric viruses in Brazilian turkey flocks: single and multiple virus infection frequency according to age and clinical signs of intestinal disease. Poultry Science, 92, 945–955. doi: 10.3382/ps.2012-02849
- Oaks, J.L., Schrenzel, M., Rideout, B. & Sandfort, C. (2005). Isolation and epidemiology of falcon adenovirus. Journal of Clinical Microbiology, 43, 3414–3420. doi: 10.1128/JCM.43.7.3414-3420.2005
- Oliveira, M.S., Rocha, A., Sulyok, M., Krska, R. & Mallmann, C.A. (2017). Natural mycotoxin contamination of maize (Zea mays L.) in the South region of Brazil. Food Control, 73, 127–132. doi: 10.1016/j.foodcont.2016.07.033
- Pereira, C.G., Marin, S.Y., Santos, B.M., Resende, J.S., Resende, M., Gomes, A.M. & Martins, N.R.S. (2014). Occurrence of Aviadenovirus in chickens from the poultry industry of Minas Gerais. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 66, 801–808. doi: 10.1590/1678-41625899
- Preis, I.S., Braga, J.F.V., Couto, R.M., Brasil, B.S.A.F., Martins, N.R.S. & Ecco, R. (2013). Outbreak of infectious laryngotracheitis in large multi-age egg layer chicken flocks in Minas Gerais, Brazil. Pesquisa Veterinária Brasileira, 33, 591–596. doi: 10.1590/S0100-736X2013000500007
- Riddell, C., van den Hurk, J.V., Copeland, S. & Wobeser, G. (1992). Virus tracheitis in goslings in Saskatchewan. Avian Diseases, 36, 158–163. doi: 10.2307/1591732
- Schachner, A., Marek, A., Grafl, B. & Hess, M. (2016). Detailed molecular analyses of the hexon loop-1 and fibers of fowl Aviadenoviruses reveal new insights into the antigenic relationship and confirm that specific genotypes are involved in field outbreaks of inclusion body hepatitis. Veterinary Microbiology, 186, 13–20. doi: 10.1016/j.vetmic.2016.02.008
- Schachner, A., Matos, M., Grafl, B. & Hess, M. (2018). Fowl adenovirus-induced diseases and strategies for their control – a review on the current global situation. Avian Pathology, 47, 111–126. doi: 10.1080/03079457.2017.1385724
- Scott, P.C., Condron, R.J. & Reece, R.L. (1986). Inclusion body hepatitis associated with adenovirus-like particles in a cockatiel (Psittaciformes; Nymphicus hollandicus). Australian Veterinary Journal, 63, 337–338. doi: 10.1111/j.1751-0813.1986.tb02879.x
- Senne, D.A. (1998). Virus Propagation in Embryonating eggs. In D. Swayne, J.R. Glisson, M.W. Jackwood, J.E. Pearson, & W.M. Reed. A Laboratory Manual for the Isolation and Identification of Avian Pathogens 4th edn (pp. 235–247) American Association of Avian Pathologists. Kennett Square, PA: New Bolton Center.
- Shivaprasad, H.L., Woolcock, P.R. & McFarland, M.D. (2001). Group I avian adenovirus and avian adeno-associated virus in turkey poults with inclusion body hepatitis. Avian Pathology, 30, 661–666. doi: 10.1080/03079450120092152
- Silveira, L.F., Olmos, F. & Long, A.J. (2004). Taxonomy, history, and status of Alagoas Curassow Mitu mitu (Linnaeus, 1766), the world’s most threatened cracid. Ararajuba, 12, 43–50.
- Singh, A., Bekele, A.Z., Patnayak, D.P., Jindal, N., Porter, R.E., Mor, S.K. & Goyal, S.M. (2016). Molecular characterization of quail bronchitis virus isolated from bobwhite quail in Minnesota. Poultry Science, 95, 2815–2818. doi: 10.3382/ps/pew217
- Thanasut, K., Fujino, K., Taharaguchi, M., Taharaguchi, S., Shimokawa, F., Murakami, M. & Takase, K. (2017). Genome sequence of Fowl Aviadenovirus A strain JM1/1, which caused gizzard erosions in Japan. Genome Announcements, 5, e00749-17. doi: 10.1128/genomeA.00749-17
- Toro, H., Gonzalez, C., Cerda, L., Hess, M., Reyes, E. & Geisse, C. (2000). Chicken anemia virus and fowl adenoviruses: association to induce the inclusion body hepatitis/hydropericardium syndrome. Avian Diseases, 44, 51–58. doi: 10.2307/1592507
- Toro, H., Prusas, C., Raue, R., Cerda, L., Geisse, C., Gonzalez, C. & Hess, M. (1999). Characterization of fowl adenoviruses from outbreaks of inclusion body hepatitis/hydropericardium syndrome in Chile. Avian Diseases, 43, 262–270. doi: 10.2307/1592616
