ABSTRACT
Dermanyssus gallinae, also known as the poultry red mite (PRM), is a blood-feeding ectoparasite of poultry and sylvatic birds. This mite is endemic in many parts of the globe and poses a threat to the egg industry, while compromising the health and welfare of hens, both directly and as a vector of diseases. In addition, people attacked by D. gallinae may develop gamasoidosis. Despite the high prevalence in several European countries, epidemiological information on D. gallinae in Portugal is scarce. This study aimed to assess the prevalence and infestation levels in laying farms in Portugal and evaluate the perception and attitudes of producers regarding D. gallinae. A survey was performed between August 2016 – November 2017, which included 24 farms in the NUTS2 regions Centro and Norte. Mites were sampled with corrugated cardboard traps and the perception and attitudes of farmers regarding the PRM were evaluated with the European COREMI questionnaire prepared by WG 1 of the COST action FA1404. D. gallinae was detected in 95.8% of farms (95% CI: 79.8–99.3%). The average number of trapped mites among farms was 5200 ± 16,522, with a median of 359 mites (interquartile range = 46–3135). Results from the questionnaire show that insufficient monitoring, under-detection and late and suboptimal treatment may contribute to the maintenance of significant infestation levels. The present data highlight the need for adequate monitoring of D. gallinae, timely action and effective treatment in order to improve poultry productivity and ensure human and animal health and welfare.
RESEARCH HIGHLIGHTS
A survey on the prevalence of D. gallinae in Portuguese layer farms is presented
The perceived importance of D. gallinae was assessed with a questionnaire
D. gallinae was detected in 95.8% of farms
The results emphasize the need for adequate monitoring and treatment optimization.
Introduction
Dermanyssus gallinae De Geer, 1778 (Mesostigmata: Dermanyssidae) also known as the poultry red mite (PRM) is an obligate blood-feeding ectoparasite of domestic and wild birds. This mite is endemic in many parts of the world, posing a worldwide economic problem. In Europe, D. gallinae is considered the most serious pest in layer farms, with associated prevalences up to 100% (Sparagano et al., Citation2014). D. gallinae has a five-stage life-cycle, which is usually completed within approximately two weeks or even less, allowing large populations of mites to build up in a short time (Wood, Citation1917; Tucci et al., Citation2008). The mites only parasitize birds for 30–60 min to acquire a blood meal, typically during the dark period, and then return to cracks and crevices within the poultry facilities to digest their blood meal and reproduce (Maurer et al., Citation1988). D. gallinae may survive up to 9 months without a blood-meal, allowing the maintenance of populations of mites in poultry houses during the empty period (Nordenfors et al., Citation1999). Several critical hazard categories and critical control points have been identified for the introduction and spread of D. gallinae in poultry facilities, including carrier animals (birds, rats), egg containers, pallets and trays, visitors and farm personnel, cadavers, purchase of growing hens, contaminated material/equipment, and ventilation (Mul & Koenraadt, Citation2009).
Severe infestations cause somatic stress, immunosuppression and anaemia, which may lead to lower body weight, a decrease in egg production and higher mortality in layer hens (Chauve, Citation1998; Wójcik et al., Citation2000; Cosoroaba, Citation2001; Kilpinen et al., Citation2005; Kowalski & Sokół, Citation2009). The negative impact on the welfare of poultry is potentiated by psychogenic stress (Kowalski & Sokół, Citation2009) and stress behaviours like social feather pecking and increased grooming (Kilpinen et al., Citation2005). The economic impact is linked to the high treatment and control costs and to production losses caused by a drop in egg laying rates, a higher susceptibility to poultry diseases, and increased mortality (Sigognault Flochlay et al., Citation2017). Further financial losses arise from the decline in egg quality due to blood spots caused by the crushing of blood-engorged mites in egg collection belts and increased thinning of shells (George et al., Citation2015). Anaemic birds tend to drink more water and increase the feed intake in order to compensate blood losses, leading to extra costs for the farmers (Sparagano et al., Citation2014). Though the global financial impact directly attributable D. gallinae is difficult to determine, production losses and treatment and prevention costs for the approximate population of 600 million laying hens in the EU are estimated at about 250 million Euros per year (Van Riel et al., Citation2016). In the case of high infestation levels or absence of a bird host, D. gallinae may bite mammals, including humans, causing papular pruritic dermatitis (Bellanger et al., Citation2008; Akdemir et al., Citation2009; Dogramaci et al., Citation2010; Abdigoudarzi et al., Citation2014; Gavrilović et al., Citation2015; Şengül et al., Citation2017). The medical significance is aggravated by the potential of D. gallinae to carry and transmit zoonotic bacterial and viral diseases (George et al., Citation2015). Infestation levels may be so high, that workers in some countries demand a significantly higher salary to work in D. gallinae-infested premises (Sahibi et al., Citation2008). Treatment and control of mite infestations have traditionally relied on chemical acaricide spraying of infested premises. However resistance to several acaricidal drugs, including amitraz, carbaryl and permethrin (Beugnet et al., Citation1997; Nordenfors et al., Citation2001; Thind & Ford, Citation2007; Marangi et al., Citation2009) highlight the need for alternative and sustainable control strategies.
Despite the considerable economic impact and the high prevalence rates reported from other countries (George et al., Citation2015), epidemiological information on D. gallinae in laying hen farms in Portugal is scarce. In this context, the aim of the present survey was to obtain data on the prevalence, mite burdens and perceived importance of D. gallinae in industrial egg production systems in Portugal.
Materials and methods
Survey area and sampling design
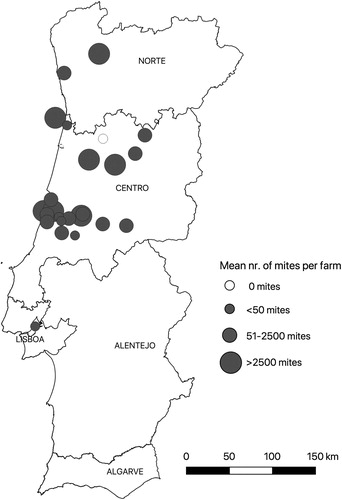
The present survey was performed between August 2016 and November 2017 and included 24 randomly selected laying hen units in the NUTS2 regions Centro and Norte in Portugal (). The study area has a temperate warm Csb climate, which is characterized by mild and dry summers and a threshold temperature value of +10°C for at least four months during the cold months (Köppen climate classification) (Kottek et al., Citation2006). During the study period, there were 133 commercial layer farms in continental Portugal, with a total capacity for 8,315,451 laying hens. Since the majority of these intensive egg production systems are concentrated in the regions Centro and Norte, the sampling frame of the present survey comprised laying hen units owned by commercial producers operating in these areas. The number of layer farms to be sampled (n = 24) was calculated with EpiTools epidemiological calculators (Sergeant, Citation2019), using the following inputs: (1) an assumed prevalence of 92% infected farms, based on the 75th percentile of prevalence records in other countries in Europe (George et al., Citation2015); (2) a population size of 116 commercial producers in the regions Centro and Norte; (3) a confidence level of 95%; and (4) a precision in the estimate of 10%.
Questionnaire survey
A questionnaire survey was conducted to assess the perception and attitudes of poultry farmers regarding the presence of the PRM in layer production systems. The questionnaire was developed within the scope of the actions carried out by the COREMI European Questionnaire Working Group 1 of the COST action FA1404. Questionnaire items were designed to identify (a) the prevalence of D. gallinae in laying hen farms in EU countries, (b) the infestation burden in the laying hen farms, (c) possible risk factors for infestation, and to evaluate the awareness of poultry farmers regarding the presence and impact of the PRM and assess current pest management practices in layer farms in Europe. The forms were explained and handed out to all producers/veterinary practitioners participating in the study. The following farm data were covered: type of production system, mode of operation, housing system, manure disposal system, hen perch structure, ventilation system, current age of flock, and number of hens in the flock. To assess the awareness and attitudes regarding the presence and severity of mite infestation, producers were asked about the following PRM indicators: present and past presence of mites, if mites were seen on the housing, in cracks and crevices, if there were clustered spots of mites on the furniture, bloodspots on eggs, if mites were associated with a reduction in egg production and if the animal care staff complained about red mites and itching skin. Further questions related to the existence of a red mite monitoring system, treatment and hygiene methods employed and treatment timing based on farmers’ perception of mite infestation levels, i.e. before mites were seen, when mites were first seen (low numbers), when mites were clearly visible (medium numbers), when mites were seen as a threat to production (high numbers), routinely (regardless of red mite presence), when animal caretakers started complaining or when mites were felt on the skin. To evaluate the economic impact, producers were asked about the amount of money spent so far on red mite control products and the number of labour hours spent on red mite control in the last month for this particular flock.
Sampling, identification and quantitative assessment of mites
Traps used for collection of mites were made of corrugated cardboard and measured 14 cm × 10 cm × 0.3 cm (Nordenfors & Chirico, Citation2001). Traps were placed on the outside of the housing structures, near to, but out of reach of chickens, at a ratio of 1:1000 birds for aviaries with up to 20,000 hens. For aviaries with more than 20,000 chickens, an extra trap was placed for each additional 5000 birds. Traps were collected after 3–5 days, sealed in zip-lock bags and frozen at −20°C for 24 h or more, in order to kill the mites. The content of each trap was transferred to Petri dishes and weighed on a precision balance with a readability of 0.001 g. A subsample weighing 0.025 g ± 0.001 g was withdrawn from all samples >0.05 g and transferred to a second Petri dish with counting grid (1 mm × 1 mm grid). Mites (adults and nymphs) were identified using the identification keys provided by Moss (Citation1968) and Di Palma et al. (Citation2012) and counted under a stereomicroscope. The total number of mites per trap was determined either by counting all mites in samples weighing up to 0.025 g or by cross-multiplication for subsamples.
Data analysis
Statistical procedures were performed with the software package R 2.15.0 (R Development Core Team, Citation2008). The 95% confidence intervals for the proportion of positive farms were calculated using Wilson’s score. Normality of data was assessed by visual inspection of histograms and the Shapiro–Wilk test of Normality. Since average mite counts in each farm () were not normally distributed, association between variables was assessed with non-parametric tests: the two-sample Wilcoxon test was used to compare mite counts with answers of farmers regarding PRM indicators, and the Kruskal–Wallis rank sum test was used to compare mite counts with treatment timing based on farmer’s judgement on infestation levels. The relationship between flock size, flock age, money and labour hours spent on the control of the PRM and the average number of trapped mites per farm was analyzed by the Spearman rank correlation method. P values <0.05 were considered statistically significant. The threshold for farmers' awareness of PRM infestation and perceived impact on egg production was determined using a ROC analysis approach, by plotting the average number of trapped mites per farm against the perception of the presence and impact of mites. Geographical distribution maps were constructed using Quantum Geographic Information System (QGIS) software 3.0.3.
Table 1. Average number of mites per trap, standard deviation (SD), minimum and maximum number of mites, flock size and age of birds in each farm.
Results
Farm data
Samples were obtained from a total of 24 laying farms, representing a total of 507,800 laying hens. The surveyed farms were located in 21 civil parishes in 15 municipalities in the regions Norte and Centro (). Average flock size was 29,200 hens and ranged between 9000 and 150,000. Hens were aged between 8 and 70 weeks. Most farmers (91.7%) used a non-free range production system and operated non-organically. Regarding the type of housing, 83.3% of farms used enriched cages, 4.2% single-tier systems, 4.2% multi-tier systems and 8.3% litter systems. Manure disposal was performed with a manure belt in 87.5% of poultry houses and with deep litter in 12.5%. Most poultry houses (95.8%) had a closed ventilation system, while two (4.2%) used open ventilation.
Prevalence and density of mites
D. gallinae was present in 95.8% of the laying farms sampled (95% CI: 79.8–99.3%) (). The mean numbers of mites per trap in each laying farm are presented in . Considering all farms, the mean and median number of trapped mites per farm was 5200 ± 16,522 and 359 mites (interquartile range = 46–3135 mites), respectively. No statistically significant correlation was found between the average number of trapped mites in each farm and the variables of: age of hens (Spearman r = 0.39; P = 0.06), flock size (Spearman r = 0.28; P = 0.18) and money (Spearman r = −0.32; P = 0.2) and labour hours (Spearman r = −0.23; P = 0.45) spent on the control of the PRM.
Perception and attitudes regarding the presence of D. gallinae
Considering indicators of poultry red mite infestation, 62.5% of the responders reported that the flock was currently infested with PRM, while 91.7% had seen red mites in the past. Seventy-five percent noticed PRM in cracks and crevices, 66.7% admitted that animal care staff complained about PRM and itching skin, 45.8% saw mites on the housing, 41.7% spotted clustered spots of mites on the furniture, 25% related the presence of mites to a decrease in egg production and 16.7% reported bloodspots on eggs. Discrepancies between answers were observed in five questionnaires, i.e. farmers answered positively to indicators of PRM presence, where the current presence of mites was excluded but previous infestation was admitted, possibly due to farmers not answering questions as the present situation. Nonetheless, infestation levels were always higher when farmers responded affirmatively to questions on PRM indicators, but this association was only statistically significant for the present awareness (P = 0.004) of mites and when mites were seen in cracks and crevices (P = 0.002). The threshold for farmers' awareness about the presence of PRM in the flock started from 75 mites per trap (sensitivity = 86.7%; specificity = 77.8%) while the perceived impact on egg production started from 1411.2 mites per trap (sensitivity = 66.7%; specificity = 77.8%), as determined by ROC analysis.
None of the producers used a red mite monitoring system. Treatment of premises was performed routinely by 12.5% of the producers, irrespective of PRM presence and none of the others implemented any control methods before mites were first seen. Regarding the timepoint of treatment based on farmers’ assessment of mite infestation, 66.7% considered starting treatment when mites were clearly visible, 45.8% when animal caretakers complained, 41.7% when the number of mites was perceived as a threat for production, 8.3% when mites were first seen by animal care takers and 4.2% when mites were felt on the skin by the farmer. There was no statistical relationship between mite counts and decision to treat based on farmers’ perception of infestation levels. Phoxim was the most used compound (47%) among farmers who indicated treatment and control methods (n = 17), followed by cypermethrin (24%) and spinosad (6%). A further 17.7% mentioned treatment with insecticide/acaricides without specifying the active substance, and 11.8% relied on cleaning measures when the shed was empty. Only 11.76% used a second product for treatment and 17.7% used compressed air every two weeks after first treatment (11.8%) or cleaning (5.9%). The mean age of hens when a treatment was first used in the stocked shed was 35.8 weeks and ranged between 26 and 51 weeks. There was no relationship between the mean number of mites per farm and age of first treatment. All producers considered that treatment was effective in reducing the number of mites. The amount of money spent so far on red mite control products for each 1000 hens in the flock was on average €30.24, with a range of €9.68 to €84.27. Extrapolating for a full year (assuming a pre-lay period of 18–20 weeks before transfer to layer houses), this would represent a mean annual cost of €48.5. Producers spent monthly on average 0.4 labour hours on red mite prevention and control for each 1000 hens, with a range of 0.14–1.78 h.
Discussion
Over the past decade, several surveys have shown that D. gallinae is endemic in many parts of the world, with median prevalence rates above 80% and reaching values higher than 90% in several countries including Spain, Germany and Belgium (George et al., Citation2015). Globally, the studies demonstrate that the impact of D. gallinae is increased in Europe and is expected to further increase due to changes in layer cage systems imposed by the EU directive 1999/74/EC, climate warming, withdrawal of several acaricides from the market and the lack of effective control methods (Sparagano et al., Citation2009). Despite this trend, evidence of D. gallinae in Portugal was limited to a few studies stating its presence in poultry houses (Pereira, Citation2011) and in exotic bird species (Waap et al., Citation2017). Therefore, the aim of the present study was to assess, for the first time, the prevalence of D. gallinae among laying hen farms in Portugal. Similar to previous studies in Europe, results from the present survey point to a very high percentage (95.8%) of farms infested with D. gallinae. Additionally, we intended to evaluate infestation burdens at farm level. Unfortunately, comparison of infestation levels is hampered by the paucity of published information on mite densities in laying farms, the different methods and units used to assess mite burdens and the discrepant grading of infestation levels between authors. In a study that involved 29 farms, Guy et al. (Citation2004) determined a mean mite density of 967 mites per trap in cage systems, whereas Kilpinen et al. (Citation2005) estimated that mite densities may reach 50,000 mites per bird in caged systems, and even escalate to 500,000 mites per bird in severe cases. Gunnarsson (Citation2017), using a similar approach as in our study (1 trap per 1000 layers), graded the degree of infestation as low when the mean number of mites was between 1 and 1000 mites per trap, moderate between 1001 and 2500 and high if >2500. Though our results show an overall high mean number of mites when considering all farms (5200 ± 16,522 mites per trap), the distribution of mite densities among farms was found to be right skewed. In this case, median estimates are a better measure of central tendency, because they are less prone to outliers and therefore may provide a more accurate view of infestation levels. Indeed, 50% of farmers had average counts equal to or lower than 359 mites per trap, with an interquartile range of 46–3135 mites per trap, showing that mite infestation levels were low to moderate in most farms and that high infestation rates were limited to a smaller proportion (29%) of farms (). These data are also supported by the fact that, although a large proportion of farmers spotted mites in cracks and crevices and admitted complaints by the animal care staff, only 16.7% of farmers noticed bloodspots on eggs, which is usually indicative of high infestation rates. In the present survey, mite densities were not found to be associated with age of birds, or flock size. The lack of relationship of mite densities with age of birds and flock size was also evident in Gunnarsson’s survey (Citation2017) involving 54 layer flocks in Sweden. The lack of correlation of mite density with hen age and flock size might indicate that several other factors contribute to variation in mite population dynamics between flocks, which may include farm location, husbandry practices, pre-existing infestation levels, sources of infestation, biosecurity, mite control methods, as well as host immunity.
Concerning the perception of farmers regarding the PRM, nearly one third of the producers were unaware that their flock was presently infested. Low mite densities are difficult to detect and may go unnoticed, which is corroborated by the results obtained, suggesting a threshold of an average of 75 mites per trap for the recognition of infestation. Early detection of D. gallinae requires an adequate mite monitoring system, which was virtually absent in all the surveyed farms and, therefore, the main reason for under-detection. Furthermore, the questionnaire showed that control methods were usually implemented at a late stage of infestation, i.e. when mites were clearly visible, and/or the animal care staff complained, in contrast to evidence that early intervention results in better mite control (Mul & Koenraadt, Citation2009; Mul et al., Citation2015). D. gallinae also poses a risk of disease transmission within the flock, being implicated as vector for numerous poultry pathogens (Sparagano et al., Citation2014). Therefore, monitoring of mite population growth is essential to take timely pest management actions aimed at reducing the damaging effects of infestation (Mul & Koenraadt, Citation2009; Mul et al., Citation2015). The estimated mean annual costs for the control of D. gallinae in the studied farms (€45.8/1000 hens) were in the same range as the estimates of Lubac et al. (Citation2003) in France, who calculated the annual costs at national levels to be €43.3/1000 hens and €38.3/1000 hens for cage and alternative systems, respectively. In a more recent study in the Netherlands (Van Emous, Citation2017), which took into account current changes in layer husbandry practices (ban on beak trimming, longer production periods, transition to alternative housing systems) the current total cost of red mite infestation was estimated at €0.60 per hen per year, including €0.15 for treatment costs and €0.45 for productivity losses. Though utilized control products were considered effective, the fact that most farmers relied on a single treatment only, and the lack of relationship between mite counts and the money and labour hours spent on mite control, indicates a suboptimal implementation of pest management practices. Besides the impact on production and welfare of hens, D. gallinae poses a hazard to other birds, mammals and humans. The presence of D. gallinae was proposed as an occupational hazard for poultry workers (Cafiero et al., Citation2011). Furthermore, D. gallinae was implicated as a putative vector of several zoonotic pathogens, including bacteria, such as Chlamydia psittaci (Circella et al., Citation2011), Coxiella burnetii (Zemskaya & Pchelkina, Citation1967; Raele et al., Citation2018), Salmonella spp. (Moro et al., Citation2009; Hamidi et al., Citation2011; Sylejmani et al., Citation2016), Erysipelothrix rhusiopathiae (Chirico et al., Citation2003; Eriksson et al., Citation2009, Citation2010), Borrelia burgdorferi (Raele et al., Citation2018) and Bartonella-like bacteria (Hubert et al., Citation2017) and several viruses, including equine encephalitis viruses (Howitt & Dodge, Citation1948; Miles et al., Citation1951; Durden et al., Citation1992; Durden et al., Citation1993), West Nile virus (Miles et al., Citation1951) and avian influenza virus (Sommer et al., Citation2016). The increasing number of reports on dermatitis caused by dermanissoid mite bites (George et al., Citation2015) in humans and pets suggests that D. gallinae may be of increasing medical and veterinary concern.
Conclusion
The present survey shows a high prevalence of D. gallinae among industrial laying farms in Portugal. Quantitative data point to low to moderate infestation levels amongst farms, with massive infestations concentrated in individual holdings. Though D. gallinae is recognized as a threat to egg production by poultry farmers, insufficient monitoring, under-detection and late and suboptimal treatment contribute to the maintenance of significant infestation levels. Considering the impact on production, as well as their veterinary and human medical importance, adequate monitoring and early action, encompassing effective treatment approaches and biosecurity measures, are needed in order to ensure human and animal welfare and improve poultry productivity.
Acknowledgements
We wish to express our gratitude to Paulo Leite, Zoetis Portugal, without whom this work would not have been possible, to Dra Leonor Amaral for her valuable support with the field work and to Lucinda Marques and Maria do Carmo Ramos (INIAV/Parasitology Laboratory) for laboratory assistance. The authors thank Direção Geral de Alimentação e Veterinária (DGAV) for providing statistical data on commercial layer farms in Portugal. We further acknowledge networking support by the COST Action FA1404, “Improving current understanding and research for sustainable control of the poultry red mite D. gallinae (COREMI)” (www.coremi.eu) and all participating members of COREMI working group 1 in developing the questionnaire, with particular thanks to Lisette Graat, Rick Van Emous, Johan Zoons and Robert Finn.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abdigoudarzi, M., Mirafzali, M.S. & Belgheiszadeh, H. (2014). Human infestation with Dermanyssus gallinae (Acari: Dermanyssidae) in a family referred with pruritus and skin lesions. Journal of Arthropod-Borne Diseases, 8, 119–123.
- Akdemir, C., Gülcan, E. & Tanritanir, P. (2009). Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitolojii Dergisi, 33, 242–244.
- Bellanger, A.P., Bories, C., Foulet, F., Bretagne, S. & Botterel, F. (2008). Nosocomial dermatitis caused by Dermanyssus gallinae. Infection Control and Hospital Epidemiology, 29, 282–283. doi: 10.1086/528815
- Beugnet, F., Chauve, C., Gauthey, M. & Beert, L. (1997). Resistance of the red poultry mite to pyrethroids in France. The Veterinary Record, 140, 577–579. doi: 10.1136/vr.140.22.577
- Cafiero, M.A., Galante, D., Camarda, A., Giangaspero, A. & Sparagano, O. (2011). Why dermanyssosis should be listed as an occupational hazard. Occupational and Environmental Medicine, 68, 628–628. doi: 10.1136/oemed-2011-100002
- Chauve, C. (1998). The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. Veterinary Parasitology, 79, 239–245. doi: 10.1016/S0304-4017(98)00167-8
- Chirico, J., Eriksson, H., Fossum, O. & Jansson, D. (2003). The poultry red mite, Dermanyssus gallinae, a potential vector of Erysipelothrix rhusiopathiae causing erysipelas in hens. Medical and Veterinary Entomology, 17, 232–234. doi: 10.1046/j.1365-2915.2003.00428.x
- Circella, E., Pugliese, N., Todisco, G., Cafiero, M.A., Sparagano, O.a.E. & Camarda, A. (2011). Chlamydia psittaci infection in canaries heavily infested by Dermanyssus gallinae. Experimental and Applied Acarology, 55, 329–338. doi: 10.1007/s10493-011-9478-9
- Cosoroaba, I. (2001). Observation d'invasions massives par Dermanyssus gallinae (De Geer 1778), chez les poules élevées en batterie en Roumanie. Revue de Médecine Vétérinaire, 152, 89–96.
- Di Palma, A., Giangaspero, A., Cafiero, M. A. & Germinara, G. S. (2012). A gallery of the key characters to ease identification of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) and allow differentiation from Ornithonyssus sylviarum (Acari: Gamasida: Macronyssidae). Parasites & Vectors, 5, 104. doi: 10.1186/1756-3305-5-104
- Dogramaci, A.C., Culha, G. & Ozçelik, S. (2010). Dermanyssus gallinae infestation: an unusual cause of scalp pruritus treated with permethrin shampoo. The Journal of Dermatological Treatment, 21, 319–321. doi: 10.3109/09546630903287437
- Durden, L.A., Linthicum, K.J. & Turell, M.J. (1992). Mechanical transmission of Venezuelan equine encephalomyelitis virus by hematophagous mites (Acari). Journal of Medical Entomology, 29, 118–121. doi: 10.1093/jmedent/29.1.118
- Durden, L.A., Linthicum, K.J. & Monath, T.P. (1993). Laboratory transmission of eastern equine encephalomyelitis virus to chickens by chicken mites (Acari: Dermanyssidae). Journal of Medical Entomology, 30, 281–285. doi: 10.1093/jmedent/30.1.281
- Eriksson, H., Jansson, D.S., Johansson, K.-E., Båverud, V., Chirico, J. & Aspán, A. (2009). Characterization of Erysipelothrix rhusiopathiae isolates from poultry, pigs, emus, the poultry red mite and other animals. Veterinary Microbiology, 137, 98–104. doi: 10.1016/j.vetmic.2008.12.016
- Eriksson, H., Brännström, S., Skarin, H. & Chirico, J. (2010). Characterization of Erysipelothrix rhusiopathiae isolates from laying hens and poultry red mites (Dermanyssus gallinae) from an outbreak of erysipelas. Avian Pathology, 39, 505–509. doi: 10.1080/03079457.2010.518313
- Gavrilović, P., Kecman, V. & Jovanović, M. (2015). Diagnosis of skin lesions caused by Dermanyssus gallinae in five patients. International Journal of Dermatology, 54, 207–210. doi: 10.1111/ijd.12009
- George, D.R., Finn, R.D., Graham, K.M., Mul, M.F., Maurer, V., Moro, C.V. & Sparagano, O.A. (2015). Should the poultry red mite Dermanyssus gallinae be of wider concern for veterinary and medical science? Parasites & Vectors, 8, 178. doi: 10.1186/s13071-015-0768-7
- Gunnarsson, E. (2017). Poultry red mites in Swedish laying hen flocks: occurrence and efficacy to a selection of acaricides (Master thesis).
- Guy, J.H., Khajavi, M., Hlalel, M.M. & Sparagano, O. (2004). Red mite (Dermanyssus gallinae) prevalence in laying units in Northern England. British Poultry Science, 45, S15–S16. doi: 10.1080/00071660410001698001
- Hamidi, A., Sherifi, K., Muji, S., Behluli, B., Latifi, F., Robaj, A., Psotoli, R., Hess, C., Hess, M. & Sparagano, O. (2011). Dermanyssus gallinae in layer farms in Kosovo: a high risk for salmonella prevalence. Parasites & Vectors, 4, 136. doi: 10.1186/1756-3305-4-136
- Howitt, B.F. & Dodge, H.R. (1948). Virus of eastern equine encephalomyelitis isolated from chicken mites, Dermanyssus gallinae and chicken lice, Eomenacanthus stramineus. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.), 68, 622–625. doi: 10.3181/00379727-68-16577
- Hubert, J., Erban, T., Kopecky, J., Sopko, B., Nesvorna, M., Lichovnikova, M., Schicht, S., Strube, C. & Sparagano, O. (2017). Comparison of microbiomes between red poultry mite populations (Dermanyssus gallinae): predominance of bartonella-like bacteria. Microbial Ecology, 74, 947–960. doi: 10.1007/s00248-017-0993-z
- Kilpinen, O., Roepstorff, A., Permin, A., Nørgaard-Nielsen, G., Lawson, L.G. & Simonsen, H.B. (2005). Influence of Dermanyssus gallinae and Ascaridia galli infections on behaviour and health of laying hens (Gallus gallus domesticus). British Poultry Science, 46, 26–34. doi: 10.1080/00071660400023839
- Kottek, M., Grieser, J., Beck, C., Rudolf, B. & Rubel, F. (2006). World Map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. doi: 10.1127/0941-2948/2006/0130
- Kowalski, A. & Sokół, R. (2009). Influence of Dermanyssus gallinae (poultry red mite) invasion on the plasma levels of corticosterone, catecholamines and proteins in layer hens. Polish Journal of Veterinary Sciences, 12, 231–235.
- Lubac, S., Dernburg, A., Bon, G., Chauve, C. & Zenner, L. (2003). Problématiques et pratiques d’élevage en poules pondeuses dans le sud est de la France contre les nuisibles: poux rouges et mouches. In Cinquièmes journées de la Recherche Avicole. Tours, France.
- Marangi, M., Cafiero, M. A., Capelli, G., Camarda, A., Sparagano, O. A. E. & Giangaspero, A. (2009). Evaluation of the poultry red mite, Dermanyssus gallinae (Acari: Dermanyssidae) susceptibility to some acaricides in field populations from Italy. Experimental and Applied Acarology, 48, 11–18. doi: 10.1007/s10493-008-9224-0
- Maurer, V., Bieri, M. & Folsch, D.W. (1988). Das Suchverhalten von Dermanyssus gallinae in Hühnerställen. Host-finding of Dermanyssus gallinae in poultry-houses. Archiv für Geflükelkunde, 52, 209–215.
- Miles, V.I., Howitt, B.F., Gorrie, R. & Cockburn, T.A. (1951). Encephalitis in Midwest. V. Western equine encephalomyelitis virus recovered from mites Dermanyssus americanus Ewing. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.), 77, 395–396. doi: 10.3181/00379727-77-18789
- Moro, C.V., Luna, C.J.D., Tod, A., Guy, J.H., Sparagano, O.A.E. & Zenner, L. (2009). The poultry red mite Dermanyssus gallinae: a potential vector of pathogenic agents. Experimental and Applied Acarology, 48, 93–104. doi: 10.1007/s10493-009-9248-0
- Moss, W.W. (1968). An illustrated key to the species of the acarine genus Dermanyssus (Mesostigmata: Laelapoidea: Dermanyssidae). Journal of Medical Entomology, 5, 67–84. doi: 10.1093/jmedent/5.1.67
- Mul, M.F. & Koenraadt, C.J.M. (2009). Preventing introduction and spread of Dermanyssus gallinae in poultry facilities using the HACCP method. Experimental and Applied Acarology, 48, 167–181. doi: 10.1007/s10493-009-9250-6
- Mul, M.F., van Riel, J.W., Meerburg, B.G., Dicke, M., George, D.R. & Groot Koerkamp, P.W.G. (2015). Validation of an automated mite counter for Dermanyssus gallinae in experimental laying hen cages. Experimental and Applied Acarology, 66, 589–603. doi: 10.1007/s10493-015-9923-2
- Nordenfors, H. & Chirico, J. (2001). Evaluation of a sampling trap for Dermanyssus gallinae (Acari: Dermanyssidae). Journal of Economic Entomology, 94, 1617–1621. doi: 10.1603/0022-0493-94.6.1617
- Nordenfors, H., Höglund, J. & Uggla, A. (1999). Effects of temperature and humidity on oviposition, molting, and longevity of Dermanyssus gallinae (Acari: Dermanyssidae). Journal of Medical Entomology, 36, 68–72. doi: 10.1093/jmedent/36.1.68
- Nordenfors, H., Höglund, J., Tauson, R. & Chirico, J. (2001). Effect of permethrin impregnated plastic strips on Dermanyssus gallinae in loose-housing systems for laying hens. Veterinary Parasitology, 102, 121–131. doi: 10.1016/S0304-4017(01)00528-3
- Pereira, D.M. da C. (2011). Dermanyssus gallinae em galinhas poedeiras em bateria: carga parasitária, acção vectorial e ensaio de campo de um biopesticida (Master thesis).
- Raele, D.A., Galante, D., Pugliese, N., La Salandra, G., Lomuto, M. & Cafiero, M.A. (2018). First report of Coxiella burnetii and Borrelia burgdorferi sensu lato in poultry red mites, Dermanyssus gallinae (Mesostigmata, Acari), related to urban outbreaks of dermatitis in Italy. New Microbes and New Infections, 23, 103–109. doi: 10.1016/j.nmni.2018.01.004
- R Development Core Team. (2008). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
- Sahibi, H., Sparagano, O., & Rhalem, A. (2008). Dermanyssus gallinae: Acari parasite highly aggressive but still ignored in Morocco. In BSP Spring, Trypanosomiasis/Leishmaniasis and Malaria Meetings (p. 173). March 30th, April 2nd, Newcastle Upon Tyne.
- Sergeant, E.S.G. (2019). Epitools epidemiological calculators. Ausvet Pty Ltd.
- Şengül, M., Kaçar, N., Karaca, M., Öner, S.Z. & Ergin, Ç. (2017). A case with scalp pruritus caused by Dermanysus gallinae (order: mesostigmata). Mikrobiyoloji Bulteni, 51, 293–298. doi: 10.5578/mb.57351
- Sigognault Flochlay, A., Thomas, E. & Sparagano, O. (2017). Poultry red mite (Dermanyssus gallinae) infestation: a broad impact parasitological disease that still remains a significant challenge for the egg-laying industry in Europe. Parasites & Vectors, 10, 357. doi: 10.1186/s13071-017-2292-4
- Sommer, D., Heffels-Redmann, U., Köhler, K., Lierz, M. & Kaleta, E.F. (2016). Role of the poultry red mite (Dermanyssus gallinae) in the transmission of avian influenza A virus. Tierarztliche Praxis. Ausgabe G, Grosstiere/Nutztiere, 44, 26–33. doi: 10.15653/TPG-150413
- Sparagano, O.a.E., George, D.R., Harrington, D.W.J. & Giangaspero, A. (2014). Significance and control of the poultry red mite, Dermanyssus gallinae. Annual Review of Entomology, 59, 447–466. doi: 10.1146/annurev-ento-011613-162101
- Sparagano, O., Pavlićević, A., Murano, T., Camarda, A., Sahibi, H., Kilpinen, O., Mul, M., van Emous, R., le Bouquin, S., Hoel, K. & Cafiero, M.A. (2009). Prevalence and key figures for the poultry red mite Dermanyssus gallinae infections in poultry farm systems. Experimental and Applied Acarology, 48, 3–10. doi: 10.1007/s10493-008-9233-z
- Sylejmani, D., Musliu, A., Ramadani, N., Sparagano, O. & Hamidi, A. (2016). Associations between the level of biosecurity and occurrence of Dermanyssus gallinae and Salmonella spp. in layer farms. Avian Diseases, 60, 454–459. doi: 10.1637/11327-111415-Reg
- Thind, B.B. & Ford, H.L. (2007). Assessment of susceptibility of the poultry red mite Dermanyssus gallinae (Acari: Dermanyssidae) to some acaricides using an adapted filter paper based bioassay. Veterinary Parasitology, 144, 344–348. doi: 10.1016/j.vetpar.2006.10.002
- Tucci, E.C., Prado, A.P. & Araújo, R.P. (2008). Development of Dermanyssus gallinae (Acari: Dermanyssidae) at different temperatures. Veterinary Parasitology, 155, 127–132. doi: 10.1016/j.vetpar.2008.04.005
- Van Emous, R. (2017). Verwachtte schade bloedluis 21 miljoen euro. Pluimveeweb.nl.
- Van Riel, J.W., Mul, M.F., Guy, J.H. & George, D.R. (2016). Investigations on economics of operational control of Dermanyssus gallinae. In Abstract book of the 2nd COST Conference and management committee meeting (p. 39). Zagreb, Croatia.
- Waap, H., Paulino, D. & Cardoso, R. (2017). Occurrence of Ornithonyssus sylviarum in pet birds from the district of Setúbal, Portugal. Parasitology Research, 116, 2041–2046. doi: 10.1007/s00436-017-5486-y
- Wójcik, A.R., Grygon-Franckiewicz, B., Zbikowska, E. & Wasielewski, L. (2000). Invasion of Dermanyssus gallinae (De Geer, 1778) in poultry farms in the Toruń region. Wiadomosci Parazytologiczne, 46, 511–515.
- Wood, H.P. (1917). The chicken mite: its life history and habits. Bulletin of the U.S. Department of Agriculture, 553, 1–14.
- Zemskaya, A.A. & Pchelkina, A.A. (1967). Gamasid mites and Q fever. In Markevich (Ed.), Problemy Parazitologii (pp. 258–259). Kiev, Ukraine.