ABSTRACT
Dermanyssus gallinae, the poultry red mite, is a global threat to the commercial egg-laying industry. Control of D. gallinae is difficult, with only a limited number of effective pesticides and non-chemical treatments available. Here, we characterize the candidate vaccine antigen D. gallinae cathepsin D-1 (Dg-CatD-1) and demonstrate that purified refolded recombinant Dg-Cat-D1 (rDg-CatD-1) is an active aspartyl proteinase which digests haemoglobin with a pH optimum of pH 4. Soluble protein extracts from D. gallinae also have haemoglobinase activity, with a pH optimum comparable to the recombinant protein, and both proteinase activities were inhibited by the aspartyl proteinase inhibitor Pepstatin A. Enzyme activity and the ubiquitous localization of Dg-CatD-1 protein in sections of adult female mites is consistent with Dg-CatD-1 being a lysosomal proteinase. Using Dg-CatD-1 as a model vaccine antigen, we compared vaccine delivery methods in laying hens via vaccination with: (i) purified rDg-CatD-1 with Montanide™ ISA 71 VG adjuvant; (ii) recombinant DNA vaccines for expression of rDg-CatD-1 and (iii) transgenic coccidial parasite Eimeria tenella expressing rDg-CatD-1. In two independent trials, only birds vaccinated with rDg-CatD-1 with Montanide™ ISA 71 VG produced a strong and long-lasting serum anti-rDg-Cat-D1 IgY response, which was significantly higher than that in control birds vaccinated with adjuvant only. Furthermore, we showed that egg-laying rates of D. gallinae mites fed on birds vaccinated with rDg-CatD-1 in Montanide™ ISA 71 VG was reduced significantly compared with mites fed on unvaccinated birds.
RESEARCH HIGHLIGHTS
Dermanyssus gallinae cathepsin D-1 (Dg-CatD-1) digests haemoglobin
Vaccination of hens with rDg-CatD-1 in Montanide™ ISA 71 VG results in long-lasting IgY levels
Serum anti-rDg-CatD-1 antibodies reduce egg laying in D. gallinae after a single blood meal
Introduction
The poultry red mite (PRM), Dermanyssus gallinae (De Geer) is a blood-feeding ectoparasite that infests many avian species. Globally, from both economic and welfare perspectives, PRM is the most important parasite to the egg-laying industry. Across Europe, PRM prevalence is high, with up to 83% of commercial egg-laying facilities infested (George et al., Citation2015). In Europe alone, heavy infestations of poultry houses with PRM cost the industry an estimated 231 million Euro per annum (Sigognault Flochlay et al., Citation2017; Van Emous, Citation2017). PRM are difficult to control as they live off-host in inaccessible areas of the poultry house, and emerge during darkness to feed from birds for up to 1 h every 2–4 days (Sparagano et al., Citation2014; George et al., Citation2015). Heavy infestations of birds can reach up to 500,000 mites per bird and cause serious welfare issues, including anaemia, irritation and restlessness as well as a significant reduction in egg production (Kilpinen et al., Citation2005; Sigognault Flochlay et al., Citation2017). Currently, PRM populations are controlled during periods when hen-houses are empty using synthetic acaricides, or during production cycles using an isoxazoline-based drug (Sparagano et al., Citation2014; Miglianico et al., Citation2018). However, long-term repeated use of the older classes of acaricides has resulted in emergence of resistant PRM populations (Beugnet et al., Citation1997; Abbas et al., Citation2014). In addition, due to concerns about safety and environmental contamination, many synthetic acaricides have been withdrawn from use, exacerbating the problem of PRM control (Sparagano et al., Citation2014).
Vaccination can provide high levels of protection against blood-feeding ectoparasites, thereby reducing reliance on chemical control methods (de la Fuente & Kocan, Citation2003; Willadsen, Citation2004). For example, the commercial cattle vaccines, TickGARD™ and Gavac™, both based on administration of recombinant Bm86 protein, have been used to protect vaccinated cattle against the tick, Rhipicephalus microplus (Rand et al., Citation1989; Rodriguez et al., Citation1995). Moreover, studies show that vaccination has the potential to control PRM infestations of chickens. Experimental vaccination with protein extracts from D. gallinae mites (Harrington et al., Citation2009; Wright et al., Citation2009; Bartley et al., Citation2015; Makert et al., Citation2016), or with recombinant-expressed D. gallinae antigens (Bartley et al., Citation2009, Citation2012, Citation2015; Harrington et al., Citation2009; Wright et al., Citation2016), induces antigen-specific serum IgY that reduces mite survival when fed to mites in in vitro feeding devices. Furthermore, we recently showed that vaccination of laying hens, housed in a commercial-style poultry facility, with a native PRM soluble protein extract results in protective immunity, reducing average mite counts in cages of vaccinated birds by 78% compared to cages of control, unvaccinated birds (Bartley et al., Citation2017).
Vaccine delivery methodology is particularly salient in the poultry layer sector. Vaccination programmes operate at large scales in hatchlings, or immature hens, and need to induce and sustain effective immunity through the full laying cycle, which commonly ranges between 60 and 80 weeks. This need for prolonged protection is one of the most challenging aspects of developing vaccines for laying flocks, particularly for ectoparasites where immunoprotective vaccine antigens (including Bm86) may be “hidden” because they are not naturally exposed to the host immune system during parasite feeding. This means there is no opportunity for natural immunological recall responses (boosting) against this type of antigen when hosts are exposed to the parasite.
In the current study, we used a prototype vaccine antigen D. gallinae cathepsin D1 (Dg-CatD-1) (Bartley et al., Citation2012), and compared antigen delivery to laying hens by: (i) immunization with purified recombinant Dg-CatD-1 in adjuvant; (ii) recombinant DNA vaccination for expression of Dg-CatD-1 and (iii) oral challenge with populations of the live transgenic coccidial parasite Eimeria tenella expressing Dg-CatD-1. These delivery platforms were chosen to compare antigen-specific antibody titres in laying hens using tried and tested protein-in-adjuvant vaccination (Bartley et al., Citation2017), with new vaccine technologies which include DNA vaccination (Gupta et al., Citation2016; Meunier et al., Citation2016) and a novel experimental E. tenella antigen delivery system (Clark et al., Citation2012; Marugan-Hernandez et al., Citation2016; Pastor-Fernandez et al., Citation2018), in order to identify the optimal system. We assessed each delivery platform for the ability to induce specific anti-Dg-CatD-1 IgY in laying hens and monitor longevity of the response over a commercially relevant period. With the delivery method that gave the highest antibody responses and the longest duration of response, we went on to test vaccine efficacy of the prototype vaccine antigen using a recently-developed novel in vivo challenge system (Nunn et al., Citation2019).
Materials and methods
Purification and refolding of recombinant Dg-CatD-1
Expression of recombinant Dg-CatD-1 (rDg-CatD-1) was carried out in BL21-CodonPlus®(DE3)-RIPL E. coli cells as previously described (Bartley et al., Citation2012). Following expression, cells were lysed by sonication and the insoluble inclusion bodies were isolated by centrifugation at 13,000×g for 20 min at 4°C. Inclusion bodies were solubilized in binding buffer (8M urea, 20 mM Tris-HCl, 0.5M NaCl, 45 mM imidazole, pH8.0) and rDg-CatD-1 was purified by nickel-affinity chromatography on HisTrap™ HP columns using an ÄKTA™ chromatography system (GE Healthcare Life Sciences, Marlborough, MA, USA). rDg-CatD-1 was eluted in binding buffer with a linear imidazole gradient (up to 500 mM imidazole), within five column volumes.
Fractions containing purified rDg-CatD-1 were pooled and refolded at 4°C by step-wise dialysis into refolding buffer (100 mM Tris-HCl, 400 mM L-arginine, 2 mM EDTA, 0.5 mM oxidized glutathione, 5 mM reduced glutathione, pH8.0) with decreasing concentrations of urea. Briefly, rDg-CatD-1 was contained within cellulose membrane dialysis tubing (Sigma-Aldrich Co Ltd, Dorset, UK) and dialyzed for 3 h into refolding buffer with 6 M urea; 3 h into refolding buffer with 4 M urea; overnight into refolding buffer with 2 M urea; 3 h into refolding buffer with 1 M urea; and finally overnight into 10 mM Tris-HCl (pH 7.4), 0.5 M NaCl. After refolding, rDg-CatD-1 was concentrated using a Centriprep® centrifugal filter with Ultracel-10 membrane (Merck, Kenilworth, NJ, USA) followed by filtration through a 0.2 µm sterile filter (Sartorious, Goettingen, Germany). rDg-CatD-1 protein concentration was determined by BCA assay (Thermo Fisher Scientific, Waltham, MA, USA) with BSA standards, and purity was confirmed by SDS-PAGE analysis. Purified, refolded rDg-CatD-1 was stored at 4°C prior to use.
Haemoglobin digest assay
Hydrolysis of haemoglobin by purified refolded rDg-CatD-1 was determined using a haemoglobin digest assay, according to Houseman & Downe (Citation1982). As a positive control, the haemoglobin digest assay was also performed with a soluble mite extract, with protein extracts prepared as described previously (Wright et al., Citation2009). For each assay, equal volumes of 20 mg/ml bovine haemoglobin (Sigma-Aldrich Co Ltd, Dorset, UK) in 0.15 M NaCl, and a 200 mM buffer of varying pH (pH 2 glycine-HCl buffer; pH 3–6 citrate buffer; pH 7 HEPES buffer) were mixed and incubated at 37°C for 5 min. Digestion was initiated by adding refolded rDg-CatD-1, or soluble mite extract to the haemoglobin/buffer mixture and assays were incubated at 37°C for 20 h. Reactions were terminated by addition of 10% (w/v) trichloroacetic acid (TCA) (final concentration) and precipitated polypeptides were removed by centrifugation at 13,000 × g for 10 min at 4°C. Absorbance values of TCA-soluble peptides in supernatants were measured at 280 nm and used to calculate enzyme activity. For inhibition assays, a 1 mg/ml pepstatin A (Sigma-Aldrich Co Ltd, Dorset, UK) stock was prepared in 10% acetic acid in methanol (9:1 methanol:acetic acid). Inhibition assays were performed as described above, in pH 4 citrate buffer, with the addition of 2 µM pepstatin A (final concentration; control reactions contained no pepstatin A, but an equivalent volume of 10% acetic acid in methanol). For all assays, controls lacking test samples were run in parallel and activity data were corrected for any spontaneous haemoglobin breakdown. One haemoglobin enzyme unit (Hb.E.U.) is defined as an increase of one absorbance unit at 280 nm, per mg protein, per hour.
SDS-PAGE and western blot analysis
Proteins were analysed by SDS-PAGE using the NuPAGE® electrophoresis system (Thermo Fisher Scientific, Waltham, MA, USA). Briefly, protein samples were prepared in NuPAGE LDS sample buffer with reducing agent and heated to 70°C for 10 min prior to loading on NuPAGE 4–12% Bis-Tris gels. Gels were run in MES SDS running buffer and either stained with SimplyBlue™ SafeStain (Thermo Fisher Scientific, Waltham, MA, USA) or prepared for western blots.
For western blot analysis, proteins were transferred to nitrocellulose membranes by electroblotting in 1X NuPAGE transfer buffer, according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, USA). After transfer, to prevent non-specific protein binding, membranes were blocked by incubation at room temperature for 2 h with 5% (w/v) milk powder in TBST (20 mM Tris-HCl, pH7.6, 150 mM NaCl, 0.1% (v/v) TWEEN® 20). Blots were washed three times with TBST, then incubated with antiserum raised in rabbits against rDg-Cat-D1. Antiserum was generated using a custom service provided by ImmunoTools (Friesoythe, Germany) and used at 1:3000 dilution in 5% milk powder in TBST. After washing three times in TBST, membranes were incubated with swine anti-rabbit IgG HRP (Agilent, Santa Clara, CA, USA) at 1:3000 dilution in TBST for 1 h at room temperature. Membranes were washed three times with TBST and specifically-bound antibodies were detected using SIGMAFAST™ 3,3′-Diaminobenzidine (DAB) substrate.
Immunohistochemical localization of Dg-CatD-1 in D. gallinae tissues
Mites were collected and stored at room temperature for 7 days to allow complete digestion of their blood meal, and then fixed in Carnoy’s fixative (60% absolute ethanol, 30% chloroform, 10% glacial acetic acid) for 5 days at 4°C. After fixation, mites were washed in 150 mM NaCl then orientated and pre-embedded in 2% agar:2.5% gelatin according to Jones & Calabresi (Citation2007). Tissues were dehydrated using a graded ethanol series and then infiltrated with paraffin wax using a Tissue-Tek VIP 6 AI automated vacuum infiltration processor (Sakura, Rijn, The Netherlands). After processing, tissues were embedded in paraffin wax blocks and sections of 5 µm thickness were cut and placed on Superfrost® Plus glass slides (Thermo Fisher Scientific, Waltham, MA, USA). Tissue sections were deparaffinized in Histo-Clear (National Diagnostics, Atlanta, GA, USA) and then rehydrated in graded alcohols. Sections were washed in PBS (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4) and endogenous peroxidase was quenched with 3% (v/v) H2O2 at room temperature for 20 min. Tissue sections were blocked by incubation in 25% normal goat serum (v/v) (Vector Laboratories, Burlingame, CA, USA) in PBS with 0.5% (v/v) TWEEN® 80 (PBST80), then incubated overnight at 4°C with primary rabbit anti-Dg-CatD-1 antisera at 1:10,000 (final concentration) in 10% (v/v) normal goat serum in PBST80. Bound antibodies were detected using EnVision+ System-HRP/DAB (Agilent, Santa Clara, CA, USA), according to the manufacturer’s instructions and then counterstained with haematoxylin. Sections incubated with pre-immune rabbit sera were processed in parallel and used as negative controls. Images of tissue sections were captured on an Olympus microscope equipped with a CCD camera.
Formulation of rDg-CatD-1 in Montanide™ ISA 71 VG adjuvant
Purified refolded rDg-Cat-D1 protein was formulated in Montanide™ ISA 71 VG (SEPPIC, Paris, France) according to manufacturer’s guidelines. Briefly, rDg-Cat-D1 in 10 mM Tris-HCl (pH7.4), 0.5 M NaCl was mixed with Montanide™ ISA 71 VG (adjuvant/antigen ratio of 70:30, based on weight) and a stable water-in-oil emulsion was produced using an IKA® T25 Ultra Turrax® high shear mixer with a S25N-8G head at 15,600 rpm for 3 min. For adjuvant-only control injections, 10 mM Tris-HCl (pH 7.4), 0.5 M NaCl was mixed with Montanide™ ISA 71 VG and processed as described above.
Preparation of IL21::Dg-CatD-1::mCherry DNA Vaccine
Three synthetic IL21::Dg-CatD-1::mCherry fusion genes were designed for DNA vaccination and synthesized by GeneArt gene synthesis (Thermo Fisher Scientific, Waltham, MA, USA). Each synthetic gene contained a continuous open reading frame, and encoded sequence variants of the following fusion partners: (i) Gallus gallus IL-21 (NM_001024835); (ii) Dg-CatD-1 (HE565350); and (iii) fluorescent reporter mCherry (AY678264). In each of the synthetic genes, fusion partners were joined by an alanine (ala-ala-ala) linker region. Full details of each synthetic gene are shown in , and sequences of each synthetic gene are shown in Supplementary File 1.
Table 1. Summary of Dg-CatD-1 delivery platforms.
To produce DNA vaccines, each synthetic gene was PCR amplified using CloneAmp HiFi PCR premix (Takara, Shiga, Japan) and directionally cloned into the XhoI site of pVAX1 (Thermo Fisher Scientific, Waltham, MA, USA) using the In-Fusion HD cloning kit (Takara, Shiga, Japan), according to the manufacturer’s instructions. All synthetic IL21::Dg-CatD-1::mCherry fusion genes were sequenced after gene synthesis (Thermo Fisher Scientific, Waltham, MA, USA), and re-sequenced using Sanger sequencing after cloning into pVAX1 to ensure correct orientation of each of the genes. Sequence data were edited and assembled into contiguous sequence using Contig Express module in Vector Nti (version 11) (Thermo Fisher Scientific, Waltham, MA, USA). For both pilot and long-term vaccine trials, endotoxin-free plasmid DNA for pVAX1 DNA vaccines (Constructs 1–3) was isolated using NucleoBond PC 10000 EF DNA purification kit (Macherel-Nagel, Düren, Germany) and quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Prior to DNA vaccination, purified pVAX1 vaccine DNA (Constructs 1–3) and negative control DNA (empty pVAX1 DNA) were validated by transfection of HEK-293 cells, and mCherry fluorescence monitored using a Nikon fluorescence microscope and images captured with a Nikon DXM1200F digital camera.
Generation of transgenic Eimeria tenella expressing Dg-CatD-1::mCherry
A synthetic Dg-CatD-1::mCherry reporter fusion gene was designed for expression in E. tenella to allow fluorescence detection of Dg-CatD-1::mCherry protein in transformed parasites. The Dg-CatD-1 coding sequence (HE565350) without the N-terminal signal sequence and stop codon (corresponding to 49–1150 bp of the Dg-CatD-1 coding sequence) was fused to an in-frame C-terminal mCherry reporter (AY678264) via an alanine (ala-ala-ala) linker region, and included a C-terminal 6x-His-tag and glycophosphatidyl inositol (GPI) signal from E. tenella surface antigen SAG D09 (AIR91965). The synthetic gene was codon optimized for E. tenella (http://www.kazusa.or.jp/codon/cgi-bin/showcodon.cgi?species=5802&aa=1&style=GCG), and included a 5′ XbaI site and 3′ BglII site to facilitate cloning into Eimeria transformation vectors. The sequence of the synthetic gene is shown in Supplementary File 1.
The synthetic Dg-CatD-1::mCherry reporter fusion gene was used to produce E. tenella transformation vectors for: (i) cytoplasmic expression of Dg-CatD-1::mCherry; (ii) secreted expression of Dg-CatD-1::mCherry and (iii) GPI-anchored expression of Dg-CatD-1::mCherry. To produce transformation vectors for cytoplasmic and secreted expression of Dg-CatD-1::mCherry the synthetic Dg-CatD-1::mCherry reporter fusion gene was PCR amplified using MyTaq DNA polymerase (Bioline, Memphis, TN, USA) with a forward primer with an XbaI site (5′-CATTCAAAATGTCTAGAGACCTG-3′), and a reverse primer with a BglII site and stop codon (5′-CGTAGATCT TTAGTGGTGGTGGTGGTGG-3′); both restriction enzyme sites are underlined and the stop codon shown in bold. The PCR product was cloned using the pGEM-T Easy vector system (Promega, Madison, WI, USA), according to the manufacturer’s instructions, fully sequenced (GATC Biotech, Ebersberg, Germany) and sequence data assembled into a contiguous sequence using CLC Main Workbench (Qiagen, Venlo, The Netherlands). The sequenced coding region was digested with XbaI and BglII and cloned into the respective sites of either: p5M2-mChe (Marugan-Hernandez et al., Citation2017) to produce “Cytoplasmic Dg-CatD-1::mCherry” E. tenella transformation vector; or p5M2-SP2-mChe (Marugan-Hernandez et al., Citation2017) to produce “Secreted Dg-CatD-1::mCherry” E. tenella transformation vector. The GPI-anchored Dg-CatD-1::mCherry variant was produced by digesting the original (unamplified) Eurofins synthetic Dg-CatD-1::mCherry reporter fusion gene with XbaI and BglII and cloned into the respective sites of p5M2-SP2-mChe-GPI to produce “GPI-anchored Dg-CatD-1::mCherry”. All Eimeria p5M2 transformation vectors used here, were generated in a previous study (Marugan-Hernandez et al., Citation2017), and were a kind gift from Virginia Marugan-Hernandez (Royal Veterinary College, UK).
For transformation of E. tenella Wisconsin strain (McDougald & Jeffers, Citation1976), oocysts were propagated in 3–4 week old specific pathogen free (SPF) Light Sussex or Lohmann Selected Leghorn chickens (supplied by Millennium Hatchery, Studley, UK) using established techniques (Long et al., Citation1976). Oocysts were recovered and sporulated, then sporozoites were purified through columns of nylon wool and DE-52, using standard methods (Schmatz et al., Citation1984; Shirley et al., Citation1995). Sporozoites transformed with 10 µg PsiI linearized p5M2-Dg-CatD-1::mCherry transfection plasmids (Plasmids 1–6) by restriction enzyme-mediated integration (REMI) transfection using the Nucleofector 4D system (Lonza, Basel, Switzerland) according to Marugan-Hernandez et al. (Citation2017). Transformation vectors included: p5M2-Dg-CatD-1::mCherry, for cytoplasmic expression of Dg-CatD-1::mCherry; p5M2-SP2-Dg-CatD-1::mCherry, for secreted expression of Dg-CatD-1::mCherry; and p5M2-SP2-Dg-CatD-1::mCherry-GPI, for GPI-anchored expression of Dg-CatD-1::mCherry. For control E. tenella populations, sporozoites were excysted and transformed as described above using p5M2 transformation vectors without the Dg-CatD-1 coding sequence (control vectors included: p5M2-mChe; p5M2-SP2-mChe and p5M2-SP2-mChe-GPI). To obtain stable transgenic populations, transfected sporozoites were used for cloacal administration in birds (12–24 × 103 sporozoites/bird). Oocysts were harvested 7 days after infection, sporulated and used for subsequent in vivo passages after population enrichment for fluorescent parasites by FACS (BD FACS Aria III) (Marugan-Hernandez et al., Citation2016). A full list of transgenic E. tenella strains generated in this study is shown in .
Dg-CatD-1 pilot (P) immunization trial
A pilot vaccination trial was designed to compare circulating serum anti-Dg-CatD-1 IgY antibody levels in Hy-Line Brown hens after Dg-CatD-1 antigen delivery by: (i) vaccination with purified rDg-CatD-1 formulated in Montanide™ ISA 71 VG; (ii) vaccination with DNA for transient expression of Dg-CatD-1::mCherry; or (iii) oral vaccination with transgenic E. tenella expressing Dg-CatD-1::mCherry. The pilot trial was performed under specific pathogen free (SPF) conditions and ran for 8 weeks. Prior to the start of the trial, 72× one-day-old chicks (supplied by Millennium Hatchery, Studley, UK) were co-housed for 8 days in a loose-litter floor pen (diameter 2.4 m) with layer mash and water provided ad libitum. At the beginning of the trial (experimental day 0, eight days of age) six groups of six chicks were randomly selected, assigned to groups P7-P12, and housed in loose-litter floor pens of 1.0 m diameter. The remaining chicks, representing groups P1-P6, were co-housed in the original 2.4 m diameter pen. All chicks were wing-tagged to facilitate identification. All groups received a standard layer mash and water ad libitum throughout the trial. Blood samples were taken from two birds per group prior to each treatment and at intervals throughout the trial. Groups received the following treatments:
Groups P1 & P2. Recombinant protein vaccination
Each bird from the protein vaccination group (Group P1), received three doses of 50 µg rDg-CatD-1 in Montanide™ ISA 71 VG via intramuscular (IM) injection into the thigh at days 2, 12, and 29 of the trial. The adjuvant-only negative control group (Group P2) received equivalent injections with 10 mM Tris-HCl (pH 7.4), 0.5 M NaCl in Montanide™ ISA 71 VG.
Groups P3 – P6. DNA vaccination
Each bird was vaccinated three times with 56 µg DNA vaccine with 1 mg/kg bupivacaine via IM injection into the thigh at days 2, 12, and 29 of the trial. Group P3 birds were vaccinated with DNA Construct 1; Group P4 birds were vaccinated with DNA Construct 2; Group P5 birds were vaccinated with DNA Construct 3. Details of all DNA constructs are shown in . The empty vector negative control group (Group P6) received equivalent injections with 56 µg pVAX1 (empty vector) with 1 mg/kg bupivacaine.
Groups P7 – P12. E. tenella – Dg-CatD-1::mCherry oral challenge
Each bird was orally infected three times with E. tenella Dg-CatD-1::mCherry or the appropriate control E. tenella population, with each bird receiving 1000, 3000 and 5000 oocysts at days 2, 12, and 22 of the trial, respectively. Groups P7, P9 and P11 were infected with the experimental E. tenella Populations 1, 3, or 5, respectively. Group P8, P10 and P12 were infected with the control E. tenella Populations 2, 4, or 6, respectively. Details of all transgenic Eimeria Populations are shown in .
Dg-CatD-1 long-term immunization trial
To investigate longevity of the immune response in immunized laying hens, a longer-term trial was performed under SPF conditions for 28 weeks. At the beginning of the trial (experimental day 0, eight days of age), 80× one-day-old chicks (supplied by Millennium Hatchery, Studley, UK) were randomly assigned to eight groups (Group L1–L8) of 10 and housed in a loose-litter floor pen (diameter 1.6 m) with layer mash and water provided ad libitum. Blood samples were taken from two birds per group prior to each treatment and throughout the trial. Groups received the following treatments:
Group L1 & L2. Recombinant protein vaccination
Each bird from the protein vaccination group (Group L1), received four injections with 50 µg rDg-CatD-1 in Montanide™ ISA 71 VG via IM injection into the thigh at days 3, 13, 30 and 177 of the trial. The adjuvant-only negative control group (Group L2) received equivalent injections with 10 mM Tris-HCl (pH7.4), 0.5 M NaCl in Montanide™ ISA 71 VG.
Group L3 & L4. DNA vaccination
Each bird from the DNA vaccination group (Group L3), received four injections with 120 µg DNA vaccine Construct 3 with 1 mg/kg bupivacaine via IM injection into the thigh at days 3, 13, 30 and 177 of the trial. Details of all DNA constructs are shown in . The empty vector negative control group (Group L4) received equivalent injections with 120 µg pVAX1 (empty vector) with 1 mg/kg bupivacaine.
Group L5 & L6. DNA vaccination prime/recombinant protein vaccination boost
Each bird from the DNA vaccination prime/recombinant protein vaccination boost (Group L5), received two injections of 120 µg DNA vaccine Construct 3 with 1 mg/kg bupivacaine via IM injection into the thigh at days 3 and 13 of the trial and injections of 50 µg rDg-CatD-1 in Montanide™ ISA 71 VG via IM injection into the thigh at days 30 and 177 of the trial. The control group (Group L6) received two injections with 120 µg pVAX1 (empty vector) with 1 mg/kg bupivacaine via IM injection into the thigh at days 3 and 13 of the trial and two injections of 50 µg rDg-CatD-1 in Montanide™ ISA 71 VG via IM injection into the thigh at days 30 and 177 of the trial.
Group L7 & L8. Eimeria tenella – Dg-CatD-1::mCherry oral challenge
Each bird from the Eimeria challenge group (Group L7), received four oral administrations of E. tenella Population 1, with each bird receiving 1000, 3000, 5000 and 10,000 oocysts at days 3, 13, 23 and 72 of the trial. Control birds (Group L8) each received equivalent doses of E. tenella Population 2. For all Eimeria challenged birds, cage litter was replaced at day 59 of the trial.
Evaluation of immune responses to Dg-CatD-1
Levels of serum anti-Dg-CatD-1 IgY were monitored throughout the pilot and long-term trials by ELISA. Microtitre plates were coated overnight at 4°C with 50 µl rDg-CatD-1 (10 μg/ml in 50 mM bicarbonate/carbonate coating buffer, pH 9.6). Plates were washed six times with phosphate-buffered saline with TWEEN20 (PBST; 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 0.1% v/v TWEEN20), and then blocked with 10% soya milk powder in Tris-buffered saline with TWEEN20 (TBST; 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.05% v/v TWEEN20) for 2 h at room temperature. After washing, plates were incubated with 50 μl sera (diluted 1:1600 in TBST) and incubated for 1 h at room temperature. Plates were washed again and incubated with 50 μl rabbit anti-chicken IgY-peroxidase conjugate (Sigma-Aldrich Co Ltd, Dorset, UK) at 1:30,000 in TBST for 1 h at room temperature. Plates were washed, and specifically bound antibodies were detected by the addition of 50 μl SIGMAFAST™ OPD (Sigma-Aldrich Co. Ltd, Dorset, UK) to each well. After 20 min in the dark, the colour reaction was stopped by addition of 25 μl of 2.5 M H2SO4 to each well and the OD values read at 490 nm. Each serum sample from individual birds was assayed in duplicate.
On-bird D. gallinae vaccine trial
The Dg-CatD-1 vaccine trial was performed under SPF conditions and ran for 37 days. Prior to the start of the trial, 10× 19-week-old Hy-Line Brown pullets were randomly assigned to two groups (Group V1 and Group V2) of five. Throughout the trial birds were housed in an enclosed, loose-litter floor pen of 2.1 m × 0.9 m with natural lighting. All groups received layer mash and water ad libitum throughout the trial; perches and nest boxes were provided. At the beginning of the trial (experimental day 0, 17 weeks of age) and throughout the trial, blood samples were taken from birds in each group. Each bird from the Dg-CatD-1 protein vaccination group (Group V1), received two injections of 50 µg rDg-CatD-1 in Montanide™ ISA 71 VG via IM injection into the breast at day 2 and day 16 of the trial. The adjuvant-only negative control group (Group V2) received equivalent injections with 10 mM Tris-HCl (pH7.4), 0.5 M NaCl in Montanide™ ISA 71 VG.
For on-bird D. gallinae feeding trials, mixed-stage and mixed-sex mites were collected from commercial egg-laying facilities and stored in vented 75 cm2 tissue culture flasks (Corning, NY, USA) at room temperature (RT) for seven days, after which they were stored at 4°C for 3 weeks without access to food. On-bird feeding trials were performed, according to methods previously described (Nunn et al., Citation2019). Briefly, sealed mesh feeding devices were constructed from 105 μm aperture width, 63 μm depth polyester mesh, and each mesh feeding device was filled with 100 conditioned adult female D. gallinae mites. Prior to feeding assays, feathers were plucked from the outer thigh of each hen, and a single feeding pouch containing mites was placed on the plucked thigh and held in place using Leucoplast tape and further secured using a cohesive bandage (Central Medical Supplies Ltd., Pontefract, UK), according to methods described by Nunn et al. (Citation2019). Feeding devices were left on the birds for 3 h, after which devices were removed and engorged fed mites were recovered and transferred to individual wells of a 96-well tissue culture plate (Costar, Corning, NY, USA), sealed with AeraSeal tape (Sigma-Aldrich Co. Ltd, Dorset, UK). Plates were incubated at 25°C with 85% relative humidity, and mites were monitored daily over 8 days and scored for survival and cumulative egg production. The experiment was repeated three times at day 28, day 30 and day 32 of the experiment (at peak levels of serum anti-Dg-CatD-1 IgY), with each experiment using fresh batches of conditioned adult female D. gallinae mites and the same Dg-CatD-1 vaccinated birds (Group V1) and adjuvant-only vaccinated birds (Group V2).
Statistical analyses
Analysis of antibody levels induced by different modes of immunization in the pilot and long-term vaccine trials, analysis of anti-Dg-CatD-1 IgY antibody titres in egg yolks, and analysis of mite feeding rates on vaccinated and unvaccinated chickens were analysed using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, La Jolla, CA, USA, www.graphpad.com). Datasets were analysed using either: Student’s t-test, one-way ANOVA with Dunnett’s multiple comparison test, or Mann–Whitney U test (as indicated). P values of <0.05 were considered significant. For the analysis of numbers of eggs per mite in the on-hen vaccine trial study, data were analysed with a Poisson generalized linear mixed model (GLMM) including an offset to account for the number of fed mites.
Results
Expression and validation of recombinant Dg-CatD-1 vaccine antigen
To investigate antigen delivery platforms in laying hens, recombinant vaccine antigen Dg-CatD-1 was expressed in E. coli and purified as an insoluble protein ((A)); encoded as a Dg-CatD-1::mCherry fusion in DNA vaccines ((B)); or expressed as a Dg-CatD-1::mCherry fusion in transgenic E. tenella parasites ((C)).
Figure 1. Expression of rDg-CatD-1 and rDg-CatD-1::mCherry. (A) SDS-PAGE analysis of purified refolded rDg-CatD-1 with Coomassie staining. Arrow indicates purified rDg-CatD-1 at 42 kDa. (B) Validation of Dg-CatD-1::mCherry DNA vaccine constructs in HEK 293 cells transfected with Construct 1 (panel 1), Construct 2 (panel 2) and Construct 3 (panel 3). Negative control cells (panel 4) were transfected with empty pVAX1. In each panel localization of mCherry reporter (red) is shown. Scale bar is 50 µm. (C) Expression of Dg-CatD-1::mCherry in transgenic E. tenella oocysts. Panels 1–6 represent E. tenella Populations 1–6, respectively. In each panel localization of mCherry (red) and mCitrine (green) reporters is shown. Scale bar is 10 µm.
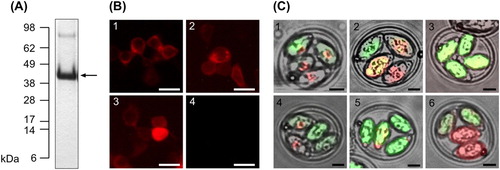
In E. coli, rDg-CatD-1 was expressed as an insoluble protein at approx. 60 mg/litre cell culture. Purified refolded rDg-CatD-1 migrated as a 42 kDa protein when analysed by reducing SDS-PAGE, in agreement with its calculated molecular weight ((A)). To aid detection of the expressed antigen in DNA vaccines and transformed Eimeria parasites, Dg-CatD-1 was fused at the C-terminus to an in-frame mCherry reporter tag. All vaccine constructs are detailed in . Prior to DNA vaccination of hens, expression of the encoded transgenes in each DNA vaccine construct was validated by transfection and expression in HEK 293 cells ((B)). For DNA vaccine Constructs 1–3, production of rDg-CatD-1::mCherry was confirmed by the presence of the fused mCherry reporter by fluorescence microscopy ((B)). E. tenella transformants were selected by FACS enrichment of mCitrine expressing parasites (background transfection control). After five passages, the number of mCitrine positive transformants was increased between 10- and 20-fold. Positive transformants were selected by FACS sorting before each trial, ensuring >95% of inoculated oocysts were expressing the transgene. All transgenic Eimeria parasites generated in this study are detailed in . Expression of Dg-CatD-1 protein was confirmed by detection of the fused mCherry reporter by fluorescence microscopy ((C)).
Characterization of Dg-CatD-1 haemolytic activity
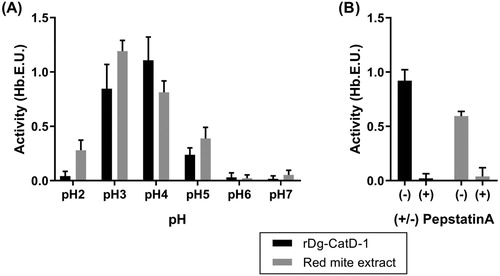
Purified refolded rDg-CatD-1 is an enzymatically active aspartyl proteinase, with detectable haemoglobin digestion activity. High levels of protease activity were detected in acidic conditions (pH 2–5), with a pH optimum at pH 4. In comparison, D. gallinae soluble protein extracts also had high levels of haemoglobin digestion protease activity in acidic conditions, with a comparable pH profile and pH optimum to rDg-CatD-1 ((A)). Protease activity of both rDg-CatD-1 and D. gallinae soluble protein extracts were inhibited by 2 µM Pepstatin A ((B)).
Figure 2. Haemoglobinolytic protease activity analysis of Dg-CatD-1. (A) Activity of refolded rDg-CatD-1 and soluble D. gallinae protein extract at pH 2 – pH 7 (as indicated in the figure). (B) Activity of refolded rDg-CatD-1 and soluble D. gallinae protein extract at pH 4 in the presence (+) or absence (-) of pepstatin A (2 µM final concentration). Each value is the mean ± SEM, n = 4.
Localization of Dg-CatD-1 in D. gallinae whole mite sections
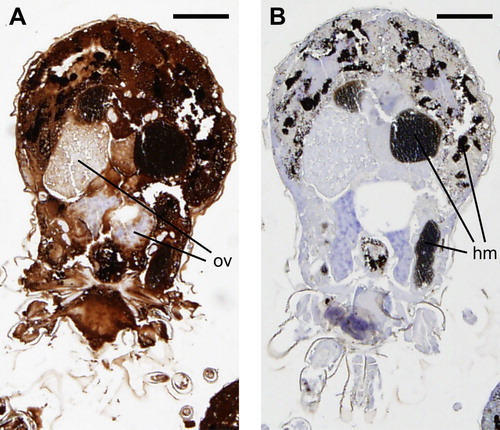
Antiserum raised against rDg-CatD-1 was first validated in western blot experiments. Rabbit anti-rDg-CatD-1 antiserum was found to specifically bind purified rDg-CatD-1 (Supplementary Figure 1, Lanes 1 and 2) as well as native Dg-CatD-1 in soluble protein extracts derived from both fed and starved D. gallinae mites (Supplementary Figure 1, Lanes 3, 4 and 5). Control western blots probed with pre-immune sera from the same rabbit showed no non-specific cross-reactivity (Supplementary Figure 1). For localization experiments, consecutive sections of fixed, wax-embedded mites were probed with either rabbit anti-rDg-CatD-1 sera or rabbit pre-immune sera (). Mite tissue sections probed with anti-rDg-CatD-1 antisera showed ubiquitous staining, with the exception of ovary tissue in female mites ((A)). As a control, a consecutive section of the same mite probed with pre-immune sera showed no non-specific antibody staining ((B)).
Figure 3. Immunohistochemical localization of Dg-CatD-1 in D. gallinae. Sequential D. gallinae tissue sections (5 µm thickness) were probed with either: (A) rabbit polyclonal anti-Dg-CatD-1 sera; or (B) pre-immune rabbit sera. Specifically bound antibodies were detected using EnVision+ System-HRP (DAB) (Dako) and counterstained with haematoxylin. Ov, ovary; hm, haematin granules. Scale bar is 125 µm.
Comparative analysis of anti-Dg-CatD-1 IgY levels – pilot study
Recombinant protein vaccination
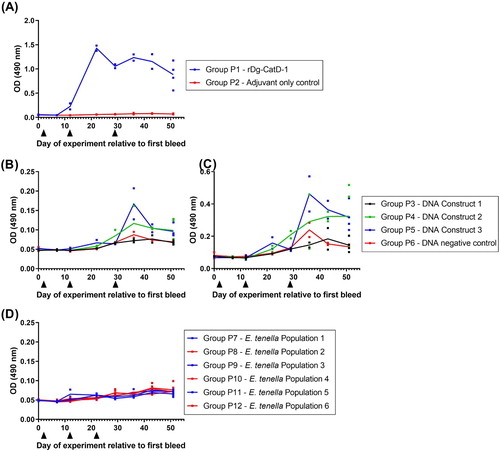
Group P1 birds immunized three times (V1, V2, V3) with 50 µg rDg-CatD-1 in Montanide™ ISA 71 VG generated a specific serum anti-Dg-CatD-1 IgY response, with a peak serum IgY level 10 days after V2 ((A)). Anti-Dg-Cat-1 IgY levels remained above levels observed in adjuvant-only immunized birds (Group P2) for at least 22 days post V3 ((A)). Adjuvant-only control birds (Group P2) did not generate anti-Dg-CatD-1 IgY antibodies ((A)).
Figure 4. Comparative analysis of serum anti-Dg-CatD-1 IgY levels during the pilot vaccine trial. Comparative ELISA showing levels of circulating anti-Dg-CatD-1 IgY antibodies in chicken sera after delivery of Dg-CatD-1 antigen by: (A) recombinant protein vaccination; (B) and (C) DNA vaccination; and (D) transgenic Eimeria. Black triangles indicate time points for either rDg-CatD-1 protein vaccination, Dg-CatD-1 DNA vaccination, or transgenic Dg-CatD-1 Eimeria challenge. Sera in panels (A), (B) and (D) were used at 1/1600; serum in panel (C) was used at 1/200. Individual data points are shown for each replicate sample. At each time point n = 2, except day 51 where n = 4. Connecting line represents mean value at each time point.
DNA vaccination
Group P3, P4 and P5 birds were vaccinated three times with 56 µg DNA Constructs 1, 2 and 3, respectively. All DNA vaccine constructs (Constructs 1–3) are detailed in . Birds vaccinated with DNA vaccine Construct 1 did not generate an anti-Dg-CatD-1 IgY response and were comparable to control birds vaccinated with empty pVAX1 DNA vector. Birds vaccinated with DNA vaccine Construct 2 and DNA Construct 3 had elevated sera anti-Dg-CatD-1 IgY level 7–22 days after V3 ((B,C)). However, anti-Dg-CatD-1 IgY levels at 22 days post V3 were not significantly different to control birds immunized with empty pVAX1 DNA vector (one-way ANOVA with Dunnett’s multiple comparison test, n = 4).
Eimeria tenella – Dg-CatD-1::mCherry oral challenge
Group P7, P9 and P11 birds were immunized orally with Dg-CatD-1 expressing transgenic E. tenella populations 1, 3 or 5, respectively. All transgenic E. tenella parasites (populations 1–6) generated in this study are detailed in . Chickens challenged with transgenic E. tenella expressing three different variants of Dg-CatD-1::mCherry, did not generate a specific anti-Dg-CatD-1 IgY response ((D)), and showed no difference from control birds immunized orally with transgenic E. tenella populations expressing only mCherry ((D)).
Comparative analysis of anti-Dg-CatD-1 IgY levels – long term study
Recombinant protein vaccination
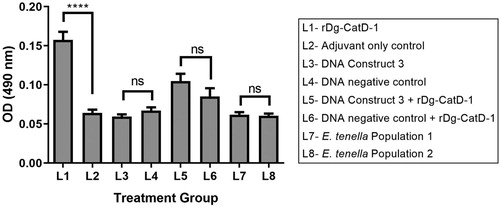
Group L1 birds immunized three times (V1, V2, V3) with 50 µg rDg-CatD-1 in Montanide™ ISA 71 VG generated a specific anti-Dg-CatD-1 IgY response, with a peak serum IgY level 7 days after V2 ((A)). Serum anti-Dg-Cat-1 IgY levels remained above those observed in adjuvant-only control birds (Group L2) for at least 100 days post V3 ((A)). Adjuvant-only control birds (Group L2) did not generate anti-Dg-CatD-1 IgY antibodies ((A)). Finally, a fourth rDg-CatD-1 immunization (V4) at day 177 of the experiment elevated the circulating anti-Dg-CatD-1 IgY level in immunized birds (Group L1) relative to adjuvant-only control birds (Group L2). Specific anti-Dg-CatD-1 antibodies were also detectable in egg yolks from Group L1 birds at day 64 and day 65 (relative to first bleed), and were significantly higher than in control adjuvant-only vaccinated birds (t-test P < 0.0001) ().
Figure 5. Comparative analysis of serum anti-Dg-CatD-1 IgY levels during the long-term vaccine trial. Comparative ELISA showing levels of circulating anti-Dg-CatD-1 IgY antibodies in chicken sera after delivery of Dg-CatD-1 antigen by: (A) recombinant protein vaccination; (B) DNA vaccination; (C) DNA vaccination prime followed by a recombinant protein boost; and (D) transgenic Eimeria. Black triangles indicate rDg-CatD-1 protein vaccination; dark grey triangles indicate Dg-CatD-1 DNA vaccination; light grey triangles indicate transgenic Dg-CatD-1 Eimeria inoculation; open triangle indicates litter change for chickens receiving transgenic Eimeria. All sera were tested at 1/1600. Individual data points are shown for each replicate sample. At each time point n = 2, except day 191 where n = 4–8. Connecting line represents mean value at each time point.
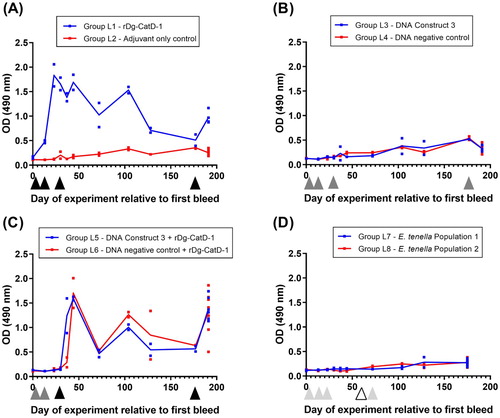
Figure 6. Comparative analysis of egg yolk anti-Dg-CatD-1 IgY antibodies from the long-term vaccine trial. Comparative ELISA showing levels of anti-Dg-CatD-1 IgY antibodies in egg yolk from birds in treatment Groups L1 – L8 (as indicated in the figure). Eggs were collected from each group at days 64 and 65 relative to the first bleed, and diluted yolks from individual eggs were used at 1/1600. Each bar represents mean ± SEM, n = 3–10. Non-significant (ns) and significant differences (****) between treatment groups are shown (t-test, P < 0.0001).
DNA Vaccination
Group L3 birds vaccinated four times (V1, V2, V3 and V4) with 120 µg DNA Construct 3 did not generate an anti-Dg-CatD-1 IgY response and were comparable to negative control birds (Group L4) vaccinated with control DNA Construct 4 ((B)).
DNA vaccination prime/recombinant protein vaccination boost
Group L5 birds vaccinated twice (V1, V2) with 56 µg DNA Construct 3 and then once (V3) with rDg-CatD-1 in Montanide™ ISA 71 VG generated a specific anti-Dg-CatD-1 IgY response after the protein boost immunization ((C)). However, vaccination with DNA Construct 3 did not prime a response, and comparable anti-Dg-CatD-1 IgY levels were observed in negative control birds vaccinated with pVAX1 followed by a rDg-CatD-1 protein boost immunization ((C)).
Eimeria tenella – Dg-CatD-1::mCherry oral challenge
The pilot study demonstrated that birds challenged with E. tenella expressing three different variants of Dg-CatD-1::mCherry did not generate a specific anti-Dg-CadD-1 IgY response. As an extension of this preliminary trial, we performed an additional long-term trial to test whether prolonged exposure to E. tenella expressing Dg-CatD-1::mCherry was sufficient to generate a detectable anti-Dg-CatD-1 IgY response. For the long-term study, Group L7 and L8 birds administered with transgenic E. tenella Population 1 or 2, respectively, generated an anti-Eimeria IgY response, confirming establishment of E. tenella infection (Supplementary Figure 2). However, birds infected with E. tenella test Population 1 did not generate an anti-Dg-CatD-1 IgY response and were comparable to negative control birds infected with control E. tenella Population 2 expressing only mCherry ((D)).
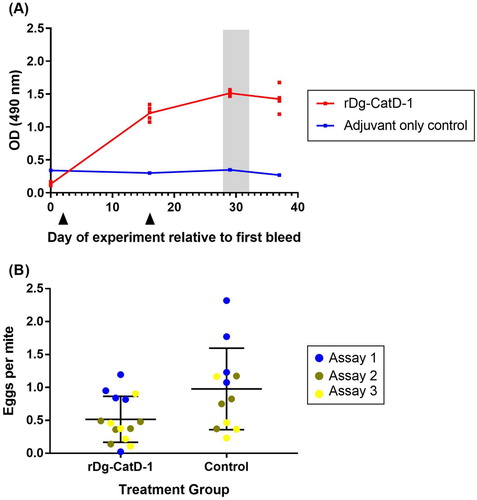
On-bird D. gallinae vaccine trial
Birds immunized twice (V1 and V2) with 50 µg rDg-CatD-1 in Montanide™ ISA 71 VG generated a specific serum anti-Dg-CatD-1 IgY response, with similar dynamics to those in the pilot vaccine trial () and the long-term vaccine trial (). Peak serum anti-Dg-CatD-1 IgY levels were observed 13 days after V2 ((A)), while birds immunized with only adjuvant did not generate anti-Dg-CatD-1 IgY antibodies. On-bird D. gallinae feeding trials, where conditioned adult female mites in mesh pouches were fed on either Dg-Cat-D-1 vaccinated or control birds, were conducted between days 28–32 (relative to the first bleed), at peak levels of serum anti-Dg-CatD-1 IgY antibodies ((B)). Feeding rates of female D. gallinae mites on chickens vaccinated with either rDg-CatD-1 or adjuvant only control were 62% ± 5.4 (n = 12) and 70% ± 7.1 (n = 10), respectively, with no statistically significantly difference between groups (Mann–Whitney U test, P = 0.2276). The total number of fed female mites recovered varied within group and experiment, and the total number of recovered fed female mites for the three repetitions of the experiment were as follows: rDg-CatD-1 n = 186, 337 and 151; Control n = 232, 258 and 146. ((B)). Cumulative mite egg production per fed mite over 7 days was analysed using a Poisson GLMM including an offset to account for the varied number of fed mites. The results showed statistically significant differences between treatment groups, with fed mites from the rDg-CatD-1 vaccinated group (Group V1) producing fewer eggs than mites which had fed on control hens (Group V2) (P = 0.0033, LRT P = 0.01456).
Figure 7. D. gallinae feeding trial on hens vaccinated with rDg-CatD-1. (A) Comparative ELISA showing levels of circulating anti-Dg-CatD-1 IgY antibodies in chicken sera after vaccination with rDg-CatD-1 in Montanide ISA 71 VG, or adjuvant only control. Black triangles indicate vaccination time points. All sera were tested at 1/1600. Individual data points are shown for each replicate sample (n = 5), with exception of the control group (n = 4) as a bird was removed from the trial due to health issues unrelated to the study. Following vaccination, a D. gallinae on-bird feeding trial was conducted on day 28, day 30 and day 32 (shaded grey). (B) Egg production of D. gallinae mites after feeding on birds vaccinated with either rDg-CatD-1 or adjuvant only (control). Eggs per fed mite in assay is shown at 196 h post-feeding time point. The feeding assay was repeated three times (assay 1–3) using the same vaccinated birds. Individual data points are shown for each assay with mean ± SEM (n = 15, rDg-CatD-1 group; n = 12, control treatment group).
Discussion
We have characterized the activity and localization of the aspartyl proteinase Dg-CatD-1 in the poultry red mite, demonstrating that Dg-CatD-1 digests haemoglobin, and has a ubiquitous localization, with the exception of the ovary, in female mite tissues. In blood-feeding mites, haemoglobin digestion takes place in intracellular vesicles through the combined activities of lysosomal endopeptidases Cathepsin D (Dg-CatD-1) and Cathepsin L (Nisbet & Billingsley, Citation2000; Filimonova, Citation2013; Pritchard et al., Citation2015). While Dg-CatD-1 is predicted to function in the gut as a digestive proteinase, based on its localization, it is also likely to function in lysosomes throughout mite tissues. Using Dg-CatD-1 as a model vaccine antigen, we analysed different methods of delivering this protein as a vaccine to chickens in order to induce high and sustained serum antibody levels, and demonstrated its potential for inclusion in a vaccine to control red mite infestations.
Almost every licensed vaccine available on the market for use against infectious agents confers protection by inducing pathogen-/antigen-specific antibody production in the host (reviewed by Rappuoli et al., Citation2016). The efficacy of vaccine-induced antibody responses against eukaryotic haematophagous parasites is established for some ecto- and endo-parasites (e.g. see Nisbet & Huntley, Citation2006; Nisbet et al., Citation2016). However, the requirement for long-lasting (>1 year) immunity in commercial laying hens is a particular challenge, given the scale of these operations and associated difficulties in administering booster vaccinations by injection during the laying cycle. For this reason, we investigated three different methods of vaccine administration to attempt to gain high, sustained levels of antigen-specific circulating antibody.
Montanide™ ISA 71 VG has been used previously to immunize hens with Eimeria recombinant profilin protein because of the enhanced immune repertoire generated by this adjuvant (Jang et al., Citation2013). In the current study, this method of antigen administration proved to be the most superior for inducing antigen-specific serum IgY. A series of three immunizations, administered to hens before 6 weeks of age, led to high antigen-specific serum IgY levels which were sustained for >100 days.
Vaccination of chickens with DNA vaccines encoding Dg-CatD-1 fused with chicken IL-21 and mCherry reporter did not induce a significant level of antigen-specific antibodies, unless boosted by immunization with the rDg-CatD-1 protein, though antigen-specific antibody levels were short-lived following the booster. The Gallus gallus IL-21 coding sequence was included in the construct of the DNA vaccines, as it has been shown to facilitate specific humoral responses in DNA vaccines against Toxoplasma (Chen et al., Citation2014). Although a number of interleukins had shown some promise, IL-21 was one of the few Gallus gallus interleukin genes available in a public database at the time of design of DNA constructs in this project. IL-21 had shown some promise to induce B-cell differentiation into antibody-secreting plasma cells (Ettinger et al., Citation2005) and to increase levels of humoral responses after DNA-vaccination in synergy with other interleukins after being included in DNA constructs (Li et al., Citation2014). Since higher levels of antibody production towards the mite protein Dg-CatD-1 was the purpose of this study, we hoped these additional gene fragments could facilitate humoral responses in our experiments. For IL-21, there is now conflicting evidence suggesting that although, it may facilitate humoral responses in some cases, the opposite may also occur (Leonard and Wan, Citation2016). The failure to provoke an anti-Dg-CatD-1 humoral response in these studies may therefore be explained by this double- edged effect of IL-21.
Oral inoculation of birds with transgenic populations of E. tenella expressing a selected exogenous antigen has been shown to confer partial protection against challenge with E. maxima, with levels of efficacy comparable to those obtained using recombinant protein or DNA vaccines (Pastor-Fernandez et al., Citation2018). However, this method of antigen delivery did not generate a specific humoral immune response against rDg-CatD-1 in the present study, possibly due to low levels of antigen expression or misfolding of the expressed protein, among other recurrent issues when ectopically expressing proteins in evolutionarily unrelated organisms. Serial E. tenella infection induces a robust immune response including elevated serum antibody levels specific for soluble E. tenella oocyst antigen (Gilbert et al., Citation1988). However, while recent studies using transgenic E. tenella to deliver vaccine antigens targeting Campylobacter jejuni, infectious laryngotracheitis virus or infectious bursal disease virus demonstrated capacity to induce specific antibody responses, all were generally low titre and variable between individuals (Clark et al., Citation2012; Marugan-Hernandez et al., Citation2016). Comparison of these results suggest that current transfection protocols for E. tenella promote more effective induction of cell-mediated than humoral transgene-specific immune responses.
Immunization of hens with rDg-CatD-1 in Montanide™ ISA 71 VG resulted in antigen-specific serum IgY which, when consumed by adult female D. gallinae feeding on the immunized hens in in vivo feeding devices, induced an ∼50% reduction in egg production by the mites ((B)). Dg-CatD-1 was selected as a model antigen for our work because its expression is induced by blood feeding (Bartley et al., Citation2012), it is ubiquitously expressed in tissues accessible to ingested IgY ( and Nisbet et al., Citation2006) and antibodies to Dg-CatD-1 increased mortality of D. gallinae in in vitro assays (Bartley et al., Citation2012). In other haematophagous parasites, aspartyl proteinases, including orthologues of Dg-Cat-D1, initiate haemoglobin digestion and are vital for parasite nutrition (Williamson et al., Citation2004). A similar role has been proposed for aspartyl proteinases in D. gallinae marking them out as important potential vaccine candidates (Nisbet & Billingsley, Citation2000; Hamilton et al., Citation2003).
In this study, we have made substantial progress towards identifying a method to administer prototype vaccines for D. gallinae in hens to stimulate long-lasting circulating antibody responses. With the recent publication of the D. gallinae genome (Burgess et al., Citation2018), we expect an increase in activity in development of prototype vaccines for this species. However, for any vaccine which relies on induction of protective antibodies there is a threshold level of antigen-specific antibody below which efficacy is compromised. This threshold needs to be determined empirically, along with detailed analysis of the population dynamics of D. gallinae (see Mul et al., Citation2017), to establish the required levels of both antibody and vaccine efficacy to impact on PRM numbers and to keep parasite numbers below the economic threshold of infestation.
Ethics statement
All experimental procedures were approved by the Moredun Research Institute Experiments and Ethics Committee and conducted under the legislation of UK Home Office Project License (reference P46F495BD), or by the Royal Veterinary College Animal Welfare & Ethical Review Body and conducted under the legislation of UK Home Office Project License (reference 70-7781).
Supplemental Material
Download Zip (266.3 KB)Acknowledgements
We are very grateful to Javier Palarea-Albaladejo (Biomathematics & Statistics Scotland) for assistance in analysis of the data and to the staff of Bioservices Division, Moredun Research Institute for expert care of the experimental animals.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Daniel R. G. Price http://orcid.org/0000-0002-4819-0654
José Francisco Lima Barbero http://orcid.org/0000-0002-2694-0215
Additional information
Funding
References
- Abbas, R.Z., Colwell, D.D., Iqbal, Z. & Khan, A. (2014). Acaricidal drug resistance in poultry red mite (Dermanyssus gallinae) and approaches to its management. World's Poultry Science Journal, 70, 113–124. doi: 10.1017/S0043933914000105
- Bartley, K., Huntley, J.F., Wright, H.W., Nath, M., & Nisbet, A.J. (2012). Assessment of cathepsin D and L-like proteinases of poultry red mite, Dermanyssus gallinae (De Geer), as potential vaccine antigens. Parasitology, 139, 755–765. doi: 10.1017/S0031182011002356
- Bartley, K., Nisbet, A.J., Offer, J.E., Sparks, N.H., Wright, H.W. & Huntley, J.F. (2009). Histamine release factor from Dermanyssus gallinae (De Geer): characterization and in vitro assessment as a protective antigen. International Journal for Parasitology, 39, 447–456. doi: 10.1016/j.ijpara.2008.09.006
- Bartley, K., Turnbull, F., Wright, H.W., Huntley, J.F., Palarea-Albaladejo, J., Nath, M. & Nisbet, A.J. (2017). Field evaluation of poultry red mite (Dermanyssus gallinae) native and recombinant prototype vaccines. Veterinary Parasitology, 244, 25–34. doi: 10.1016/j.vetpar.2017.06.020
- Bartley, K., Wright, H.W., Huntley, J.F., Manson, E.D., Inglis, N.F., McLean, K., Nath, M., Bartley, Y. & Nisbet, A.J. (2015). Identification and evaluation of vaccine candidate antigens from the poultry red mite (Dermanyssus gallinae). International Journal for Parasitology, 45, 819–830. doi: 10.1016/j.ijpara.2015.07.004
- Beugnet, F., Chauve, C., Gauthey, M. & Beert, L. (1997). Resistance of the red poultry mite to pyrethroids in France. Veterinary Record, 140, 577–579. doi: 10.1136/vr.140.22.577
- Burgess, S.T.G., Bartley, K., Nunn, F., Wright, H.W., Hughes, M., Gemmell, M., Haldenby, S., Paterson, S., Rombauts, S., Tomley, F.M., Blake, D.P., Pritchard, J., Schicht, S., Strube, C., Øines, Ø, Van Leeuwen, T., Van de Peer, Y. & Nisbet, A.J. (2018). Draft genome assembly of the poultry Red mite, Dermanyssus gallinae. Microbiology Resource Announcements, 7: e01221-18. doi: 10.1128/MRA.01221-18
- Chen, J., Li, Z.Y., Huang, S.Y., Petersen, E., Song, H.Q., Zhou, D.H. & Zhu, X.Q. (2014). Protective efficacy of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) adjuvated with recombinant IL-15 and IL-21 against experimental toxoplasmosis in mice. BMC Infectious Diseases, 14, 487. doi: 10.1186/1471-2334-14-487
- Clark, J.D., Oakes, R.D., Redhead, K., Crouch, C.F., Francis, M.J., Tomley, F.M. & Blake, D.P. (2012). Eimeria species parasites as novel vaccine delivery vectors: anti-Campylobacter jejuni protective immunity induced by Eimeria tenella-delivered CjaA. Vaccine, 30, 2683–2688. doi: 10.1016/j.vaccine.2012.02.002
- de la Fuente, J. & Kocan, K.M. (2003). Advances in the identification and characterization of protective antigens for recombinant vaccines against tick infestations. Expert Review of Vaccines, 2, 583–593. doi: 10.1586/14760584.2.4.583
- Ettinger, R., Sims, G.P., Fairhurst, A.M., Robbins, R., da Silva, Y.S., Spolski, R., Leonard, W.J. & Lipsky, P.E. (2005). IL-21 induces differentiation of human naïve and memory B cells into antibody-secreting plasma cells. Journal of Immunology, 175, 7867–7879. doi: 10.4049/jimmunol.175.12.7867
- Filimonova, S.A. (2013). Morphological aspects of blood digestion in a parasitic mite Bakericheyla chanayi. Arthropod Structure & Development, 42, 265–276. doi: 10.1016/j.asd.2013.02.006
- George, D.R., Finn, R.D., Graham, K.M., Mul, M.F., Maurer, V., Moro, C.V. & Sparagano, O.A. (2015). Should the poultry red mite Dermanyssus gallinae be of wider concern for veterinary and medical science? Parasites & Vectors, 8, 178. doi: 10.1186/s13071-015-0768-7
- Gilbert, J.M., Bhanushali, J.K. & McDougald, L.R. (1988). An enzyme-linked immunosorbent assay for coccidiosis in chickens: correlation of antibody levels with prior exposure to coccidia in the laboratory and in the field. Avian Diseases, 32, 688–694. doi: 10.2307/1590986
- Gupta, S.K., Dey, S. & Chellappa, M.M. (2016). DNA vaccination in chickens. Methods in Molecular Biology, 1404, 165–178. doi: 10.1007/978-1-4939-3389-1_11
- Hamilton, K.A., Nisbet, A.J., Lehane, M.J., Taylor, M.A. & Billingsley, P.F. (2003). A physiological and biochemical model for digestion in the ectoparasitic mite, Psoroptes ovis (Acari: Psoroptidae). International Journal for Parasitology, 33, 773–785. doi: 10.1016/S0020-7519(03)00089-4
- Harrington, D., Canales, M., de la Fuente, J., de Luna, C., Robinson, K., Guy, J. & Sparagano, O. (2009). Immunisation with recombinant proteins subolesin and Bm86 for the control of Dermanyssus gallinae in poultry. Vaccine, 27, 4056–4063. doi: 10.1016/j.vaccine.2009.04.014
- Harrington, D., Din, H.M., Guy, J., Robinson, K. & Sparagano, O. (2009). Characterization of the immune response of domestic fowl following immunization with proteins extracted from Dermanyssus gallinae. Veterinary Parasitology, 160, 285–294. doi: 10.1016/j.vetpar.2008.11.004
- Houseman, J.G. & Downe, A.E.R. (1982). Characterization of an acidic proteinase from the posterior midgut of Rhodnius prolixus Stål (hemiptera: Reduviidae). Insect Biochemistry, 12, 651–655. doi: 10.1016/0020-1790(82)90052-X
- Jang, S.I., Kim, D.K., Lillehoj, H.S., Lee, S.H., Lee, K.W., Bertrand, F., Dupuis, L., Deville, S., Ben Arous, J. & Lillehoj, E.P. (2013). Evaluation of Montanide ISA 71 VG adjuvant during profilin vaccination against experimental coccidiosis. PLoS One, 8, e59786. doi: 10.1371/journal.pone.0059786
- Jones, M.V. & Calabresi, P.A. (2007). Agar-gelatin for embedding tissues prior to paraffin processing. Biotechniques, 42, 569–570. doi: 10.2144/000112456
- Kilpinen, O., Roepstorff, A., Permin, A., Norgaard-Nielsen, G., Lawson, L.G. & Simonsen, H.B. (2005). Influence of Dermanyssus gallinae and Ascaridia galli infections on behaviour and health of laying hens (Gallus gallus domesticus). British Poultry Science, 46, 26–34. doi: 10.1080/00071660400023839
- Leonard, W.J. & Wan, C.K. (2016). IL-21 Signalling in Immunity. F1000Res, 5. doi: 10.12688/f1000research.7634.1
- Li, Z.Y., Chen, J., Petersen, E., Zhou, D.H., Huang, S.Y., Song, H.Q. & Zhu, X.Q. (2014). Synergy of mIL-21 and mIL-15 in enhancing DNA vaccine efficacy against acute and chronic Toxoplasma gondii infection in mice. Vaccine, 32, 3058–3065. doi: 10.1016/j.vaccine.2014.03.042
- Long, P.L., Millard, B.J., Joyner, L.P. & Norton, C.C. (1976). A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Veterinaria Latina, 6, 201–217.
- Makert, G.R., Vorbruggen, S., Krautwald-Junghanns, M.E., Voss, M., Sohn, K., Buschmann, T. & Ulbert, S. (2016). A method to identify protein antigens of Dermanyssus gallinae for the protection of birds from poultry mites. Parasitology Research, 115, 2705–2713. doi: 10.1007/s00436-016-5017-2
- Marugan-Hernandez, V., Cockle, C., Macdonald, S., Pegg, E., Crouch, C., Blake, D.P. & Tomley, F.M. (2016). Viral proteins expressed in the protozoan parasite Eimeria tenella are detected by the chicken immune system. Parasites & Vectors, 9, 463. doi: 10.1186/s13071-016-1756-2
- Marugan-Hernandez, V., Long, E., Blake, D., Crouch, C. & Tomley, F. (2017). Eimeria tenella protein trafficking: differential regulation of secretion versus surface tethering during the life cycle. Scientific Reports, 7, 4557. doi: 10.1038/s41598-017-04049-1
- McDougald, L.R. & Jeffers, T.K. (1976). Comparative in vitro development of precocious and normal strains of Eimeria tenella (Coccidia). Journal of Protozoology, 23, 530–534. doi: 10.1111/j.1550-7408.1976.tb03834.x
- Meunier, M., Chemaly, M. & Dory, D. (2016). DNA vaccination of poultry: the current status in 2015. Vaccine, 34, 202–211. doi: 10.1016/j.vaccine.2015.11.043
- Miglianico, M., Eldering, M., Slater, H., Ferguson, N., Ambrose, P., Lees, R.S., Koolen, K.M.J., Pruzinova, K., Jancarova, M., Volf, P., Koenraadt, C.J.M., Duerr, H.P., Trevitt, G., Yang, B., Chatterjee, A.K., Wisler, J., Sturm, A., Bousema, T., Sauerwein, R.W., Schultz, P.G., Tremblay, M.S. & Dechering, K.J. (2018). Repurposing isoxazoline veterinary drugs for control of vector-borne human diseases. Proceedings of the National Academy of Sciences, 115, E6920–E6926. doi: 10.1073/pnas.1801338115
- Mul, M.F., van Riel, J.W., Roy, L., Zoons, J., Andre, G., George, D.R., Meerburg, B.G., Dicke, M., van Mourik, S. & Groot Koerkamp, P.W.G. (2017). Development of a model forecasting Dermanyssus gallinae's population dynamics for advancing Integrated Pest Management in laying hen facilities. Veterinary Parasitology, 245, 128–140. doi: 10.1016/j.vetpar.2017.07.027
- Nisbet, A.J. & Billingsley, P.F. (2000). A comparative survey of the hydrolytic enzymes of ectoparasitic and free-living mites. International Journal for Parasitology, 30, 19–27. doi: 10.1016/S0020-7519(99)00169-1
- Nisbet, A.J. & Huntley, J.F. (2006). Progress and opportunities in the development of vaccines against mites, fleas and myiasis-causing flies of veterinary importance. Parasite Immunology, 28, 165–172. doi: 10.1111/j.1365-3024.2006.00803.x
- Nisbet, A.J., Huntley, J.F., Mackellar, A., Sparks, N. & McDevitt, R. (2006). A house dust mite allergen homologue from poultry red mite Dermanyssus gallinae (De Geer). Parasite Immunology, 28, 401–405. doi: 10.1111/j.1365-3024.2006.00862.x
- Nisbet, A.J., Meeusen, E.N., Gonzalez, J.F. & Piedrafita, D.M. (2016). Immunity to Haemonchus contortus and vaccine development. Advances in Parasitology, 93, 353–396. doi: 10.1016/bs.apar.2016.02.011
- Nunn, F., Bartley, K., Palarea-Albaladejo, J., Innocent, G.T., Turnbull, F., Wright, H.W. & Nisbet, A.J. (2019). A novel, high-welfare methodology for evaluating poultry red mite interventions in vivo. Veterinary Parasitology, 267, 42–46. doi: 10.1016/j.vetpar.2019.01.011
- Pastor-Fernandez, I., Kim, S., Billington, K., Bumstead, J., Marugan-Hernandez, V., Kuster, T., Ferguson, D.J.P., Vervelde, L., Blake, D.P. & Tomley, F.M. (2018). Development of cross-protective Eimeria-vectored vaccines based on apical membrane antigens. International Journal for Parasitology, 48, 505–518. doi: 10.1016/j.ijpara.2018.01.003
- Pritchard, J., Kuster, T., Sparagano, O. & Tomley, F. (2015). Understanding the biology and control of the poultry red mite Dermanyssus gallinae: a review. Avian Pathology, 44, 143–153. doi: 10.1080/03079457.2015.1030589
- Rand, K.N., Moore, T., Sriskantha, A., Spring, K., Tellam, R., Willadsen, P. & Cobon, G.S. (1989). Cloning and expression of a protective antigen from the cattle tick Boophilus microplus. Proceedings of the National Academy of Sciences of the United States of America, 86, 9657–9661. doi: 10.1073/pnas.86.24.9657
- Rappuoli, R., Bottomley, M.J., D'Oro, U., Finco, O. & De Gregorio, E. (2016). Reverse vaccinology 2.0: Human immunology instructs vaccine antigen design. Journal of Experimental Medicine, 213, 469–481. doi: 10.1084/jem.20151960
- Rodriguez, M., Penichet, M.L., Mouris, A.E., Labarta, V., Luaces, L.L., Rubiera, R., Cordovés, C., Sánchez, P.A., Ramos, E., Soto, A., Canales, M., Palenzuela, D., Triguero, A., Lleonart, R., Herrera, L. & de la Fuente, J. (1995). Control of Boophilus microplus populations in grazing cattle vaccinated with a recombinant Bm86 antigen preparation. Veterinary Parasitology, 57, 339–349. doi: 10.1016/0304-4017(94)00678-6
- Schmatz, D.M., Crane, M.S. & Murray, P.K. (1984). Purification of Eimeria sporozoites by DE-52 anion exchange chromatography. Journal of Protozoology, 31, 181–183. doi: 10.1111/j.1550-7408.1984.tb04314.x
- Shirley, M.W., Bushell, A.C., Bushell, J.E., McDonald, V. & Roberts, B. (1995). A live attenuated vaccine for the control of avian coccidiosis: trials in broiler breeders and replacement layer flocks in the United Kingdom. Veterinary Record, 137, 453–457. doi: 10.1136/vr.137.18.453
- Sigognault Flochlay, A., Thomas, E. & Sparagano, O. (2017). Poultry red mite (Dermanyssus gallinae) infestation: a broad impact parasitological disease that still remains a significant challenge for the egg-laying industry in Europe. Parasites & Vectors, 10, 357. doi: 10.1186/s13071-017-2292-4
- Sparagano, O.A., George, D.R., Harrington, D.W. & Giangaspero, A. (2014). Significance and control of the poultry red mite, Dermanyssus gallinae. Annual Review of Entomology, 59, 447–466. doi: 10.1146/annurev-ento-011613-162101
- Van Emous, R. (2017). Verwachtte schade bloedluis 21 miljoen euro.
- Willadsen, P. (2004). Anti-tick vaccines. Parasitology, 129, S367–S387. doi: 10.1017/S0031182003004657
- Williamson, A.L., Lecchi, P., Turk, B.E., Choe, Y., Hotez, P.J., McKerrow, J.H., Cantley, L.C., Sajid, M., Craik, C.S. & Loukas, A. (2004). A multi-enzyme cascade of hemoglobin proteolysis in the intestine of blood-feeding hookworms. Journal of Biological Chemistry, 279, 35950–35957. doi: 10.1074/jbc.M405842200
- Wright, H.W., Bartley, K., Huntley, J.F. & Nisbet, A.J. (2016). Characterisation of tropomyosin and paramyosin as vaccine candidate molecules for the poultry red mite, Dermanyssus gallinae. Parasites & Vectors, 9, 544. doi: 10.1186/s13071-016-1831-8
- Wright, H.W., Bartley, K., Nisbet, A.J., McDevitt, R.M., Sparks, N.H., Brocklehurst, S. & Huntley, J.F. (2009). The testing of antibodies raised against poultry red mite antigens in an in vitro feeding assay; preliminary screen for vaccine candidates. Experimental and Applied Acarology, 48, 81–91. doi: 10.1007/s10493-009-9243-5
