ABSTRACT
In poultry and zoo birds, mass outbreaks of amyloid A (AA) amyloidosis are often reported, and horizontal transmission is considered as one of the causes. However, oral transmission of avian AA amyloidosis in nature has been unclear. In order to clarify the horizontal transmission of avian AA amyloidosis, basic research using an appropriate oral transmission model is necessary. In this study, we developed an oral transmission model of AA amyloidosis using quails, and assessed the oral transmission efficiency of AA amyloidosis in quails and mice. Young quails, adult quails, and young mice received inflammatory stimulation with lipopolysaccharide; simultaneously, homogeneous amyloid fibrils were orally or intravenously administered. By histological examination, induction of amyloidosis by oral or intravenous administration of amyloid was confirmed in all species. Furthermore, both quail and murine AA amyloidosis were orally transmitted in a dose-dependent manner. These results support the possibility of horizontal transmission of avian AA amyloidosis in nature. This model will be able to contribute to the elucidation of spontaneous horizontal transmission of avian AA amyloidosis in the future.
RESEARCH HIGHLIGHTS
Quail AA amyloidosis was orally transmitted in a dose-dependent manner.
Oral transmission was less efficient than intravenous transmission.
In-cage horizontal transmission did not occur during 4-week cohabitation.
Amyloid deposition in tissues of quail was grossly visible.
Introduction
Amyloid is generated by misfolding of a precursor protein having physiological functions. Amyloidosis is a generic term for diseases caused by amyloid deposition in systemic or localized tissue. Amyloidoses are classified into 36 types according to the precursor protein in humans (Benson et al., Citation2018). Amyloid A (AA) amyloidosis is one of the systemic amyloidoses and has been reported in various species including birds (Cowan, Citation1968; Jakob, Citation1971; Landman, Gruys, & Gielkens, Citation1998). The precursor protein of AA is serum amyloid A (SAA), which is an acute phase protein mainly synthesized in the liver in response to interleukin (IL)-1, IL-6 and tumour necrosis factor (TNF)-α elevations (Röcken & Shakespeare, Citation2002). During acute phase inflammation, SAA persists supersaturation and causes AA fibrillization. If inflammation persists due to chronic disorders, AA fibrils progressively deposit and develop AA amyloidosis. In the mouse model, AA amyloidosis can be induced by continuous inflammatory stimulations (Skinner et al., Citation1977; Kuroiwa et al., Citation2003). In this model, AA amyloidosis can be readily induced by administration of amyloid enhancing factor (AEF), and this phenomenon is called “transmission of AA amyloidosis” (Murakami et al., Citation2014). The AEF is thought to be composed of amyloid fibrils that act as a seed for amyloid formation. The AEF acts by various administration routes including oral administration (Cui et al., Citation2002). In the cheetah, amyloid fibrils are detected in feces of individuals suffering from AA amyloidosis (Zhang et al., Citation2008). Interestingly, IL-6-overexpressing transgenic mice orally administered with cheetah faecal amyloid fibrils develop amyloidosis. These reports indicate the possibility of animal AA amyloidosis outbreaks due to horizontal transmission.
Avian AA amyloidosis is occasionally observed in waterfowl and chickens accompanied by chronic inflammation such as bacterial infection (Cowan, Citation1968; Dias & Montau, Citation1994; Landman et al., Citation1998). As well as a murine model, avian AA amyloidosis can be experimentally induced by chronic inflammatory stimulation such as repetitive administration of casein or oil-emulsified bacteria (Druet & Janigan, Citation1966; Ling, Citation1992). In chicken and quail, AA amyloidosis can be induced by vaccination or continuous administration of lipopolysaccharide (LPS), respectively (Murakami et al., Citation2013; Nakayama et al., Citation2017). Furthermore, intravenous administration of amyloid fibrils causes transmission of chicken and quail AA amyloidoses (Murakami et al., Citation2013; Nakayama et al., Citation2017). In the chicken model, oral transmission of AA amyloidosis has also been demonstrated (Murakami et al., Citation2013). In poultry or zoo birds, mass outbreaks of AA amyloidosis are often reported, and horizontal transmission is considered as one of the causes (Murakami et al., Citation2013). In order to clarify the horizontal transmission of avian AA amyloidosis, basic research using an appropriate oral transmission model is necessary.
Currently, oral transmission of avian AA amyloidosis is performed using the chicken model described above. However, chickens have the disadvantage of being large in size and require a large space, thus it is more difficult to conduct experiments using chickens in general laboratories. In contrast, quails can be kept in a smaller space (Kawashima et al., Citation2016) and are easily handled by their compliant nature. Additionally, in both chickens and quails, dose-dependent transmission of amyloidosis has not been demonstrated yet. In this study, we developed an oral transmission model of AA amyloidosis using quails, and assessed the oral transmission efficiency of AA amyloidosis in quails and mice.
Materials and methods
Animals
For quail experiments (experiments 1–3), fertilized eggs and adult quails that were around 50-week-old, and retired from breeding purposes, were obtained from Watanabe laboratory of the Tokyo University of Agriculture and Technology (TUAT). Fertilized eggs were hatched and grown up to 6 weeks of age. They were recorded as a crossbreed of WE strain (derived from Nisseiken, Tokyo, Japan) and NIES-L strain (derived from National Institute of Environmental Studies, Tsukuba, Japan), though the crossing ratio and the number of filial generations were not recorded. Six-week-old quails and adult quails were respectively referred to as young quail groups (19 males and 16 females, groups H to M) and adult quail groups (27 males and 29 females, groups A to G). Tap water and mashed feed (Toyohashi Feed Mills Co, Ltd, Aichi, Japan) were supplied without restriction.
For the murine experiment (experiment 4), 38 of the 6-week-old male ICR mice (obtained from Japan SLC, Hamamatsu, Japan) were used. Tap water from a bottle and powder feed (CRF-1, Charles River Laboratories, MA, USA) were supplied without restriction.
At necropsy in all experiments, animals were euthanized by exsanguination after deep anesthetization with 4% isoflurane (DS Pharma Animal Health Co, Ltd, Osaka, Japan). The research programmes were approved (28–100, 29–50, 29–51, 30–75, 30–96) by the Animal Care and Use Committee at TUAT and the research was performed according to the guidelines for animal experiments at TUAT.
Experiment 1: Oral transmission of AA amyloidosis in adult quails
Nine male and nine female adult quails were subjected to the experiment. The quails in all groups were intraperitoneally injected with 2 mg/kg body weight (BW) of LPS (O111: B4) (Sigma-Aldrich Japan, Tokyo, Japan) twice a week. Simultaneously with the first administration of LPS, the quails in group B were intravenously administered with 1 mg of quail AEF (qAEF) prepared using previously described methods (Nakayama et al., Citation2017). At the same time, the quails in group C were orally administered with 1 mg of qAEF. The quails in group A did not receive amyloid injection and were used as negative controls. All quails were sacrificed 4 weeks after the first administration of LPS.
Experiment 2: Examination of in-cage horizontal transmission of quail AA amyloidosis
Eighteen male and 21 female adult quails were subjected to the experiment. The quails in group D were intraperitoneally injected with 2 mg/kg BW of LPS twice a week. Simultaneously with the first administration of LPS, the quails in group D were intravenously administered with qAEF. The quails in group E were untreated and used as negative controls. After 3 weeks, quails in groups F and G were housed with groups D and E, respectively. Quails were housed in a ratio of two quails from groups D and E and three quails from groups F and G per cage. In all cages, the gender of the quails housed together was uniformed to male or female. Plastic sheets were placed in the bottom half of the cages to prevent faeces from falling off the floor mesh. This was expected to facilitate ingestion of faeces containing amyloid. Simultaneously with the introduction of quails from groups F and G, quails in groups D, F, and G were intraperitoneally injected with 2 mg/kg BW of LPS twice a week. All quails were sacrificed 4 weeks after the introduction of groups F and G.
Experiment 3: Assessment of the oral transmission efficiency of AA amyloidosis in young quails
Nineteen male and 16 female young quails were subjected to the experiment. The quails in all groups were intraperitoneally injected with 2 mg/kg BW of LPS twice a week. Simultaneously with the first administration of LPS, the quails in groups I and J were intravenously administrated with qAEF. At the same time, the quails in groups K, L and M were orally administrated with qAEF. The dose of qAEF in each group is shown in (a). The quails in group H did not receive amyloid injection and were used as negative controls. The quails were sacrificed 4 weeks after the first administration of LPS.
Experiment 4: Assessment of the oral transmission efficiency of AA amyloidosis in mice
Thirty-eight male mice were subjected to the experiment. Mouse AEF (mAEF) was prepared using previously described methods (Ogawa et al., Citation2015) and serially diluted. All mice were intraperitoneally injected with 2 mg/kg BW of LPS twice a week. Simultaneously with the first administration of LPS, the mice in groups O to S were intravenously administered with mAEF. At the same time, the mice in groups T to Y were orally administered with mAEF. The dose of mAEF in each group is shown in (b). The mice in group N did not receive amyloid injection and were used as negative controls. All mice were sacrificed 4 weeks after the first administration of LPS.
Pathological examination
In experiments 1–3, liver, spleen, kidney, heart, lung, proventriculus, duodenum, small intestine, pancreas, caeca, pectoral muscle, and peritoneum of quails were macroscopically examined and collected at necropsy. In experiment 4, liver, spleen, kidney, heart, lung, stomach, small intestine, pancreas, caeca, and pectoral muscle of mice were collected at necropsy. All tissues were fixed in 10% neutral buffered formalin and then embedded in paraffin. For histopathological examination, 3-μm tissue sections were prepared and stained with haematoxylin and eosin (H&E) and Congo red (CR). The severity of amyloid deposition was quantified using CR-stained sections as follows: 0, no deposition; 1, mild deposition only in the vessel walls; 2, mild deposition in the vessel walls and interstitial tissues; 3, moderate deposition in the vessel walls and interstitial tissues; 4, severe deposition in the vessel walls and interstitial tissues. Each score was shown in the heat map of , and as follows: 0, white; 1, yellow; 2 orange-yellow; 3, orange; 4, red. For semiquantitative analysis of the severity of amyloid deposition for each individual, the arithmetic mean score of collected organs was calculated as the amyloid-index (AI) scores.
Statistical analysis
The AI scores of each group were analysed. In experiments 1–4, the numerical data consisting of two sample groups were analysed by a Mann–Whitney U test. In experiments 3 and 4, the numerical data were analysed by a Kruskal–Wallis ANOVA followed by Dunn's tests to compare between the negative control and AEF-treated groups. All analyses were performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA).
Results
Experiment 1: Oral transmission of AA amyloidosis in adult quails
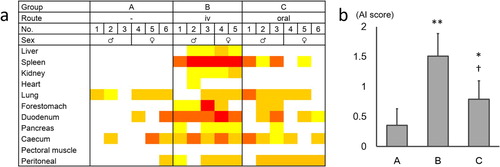
The degree of amyloid deposition of each organ and the mean AI score is shown in . The AI scores of groups B and C were significantly higher than the AI scores of group A. The AI score of group C was significantly lower than the AI scores of group B. In group A, amyloid deposition was observed mainly in the lung and intestinal tract. In groups B and C, moderate to severe amyloid deposition was observed in the spleen and intestinal tract.
Figure 1. The degree of amyloid deposition in experiment 1. (a) The degree of amyloid deposition is represented as: 4, red; 3, orange; 2, orange-yellow; 1, yellow; 0, white. (b) Mean AI scores of each group. The bars and error bars indicate the mean scores and standard deviation, respectively. *P < 0.05, **P < 0.01 vs. group A, †P < 0.05 vs. group B, by Mann–Whitney U test. Color online.
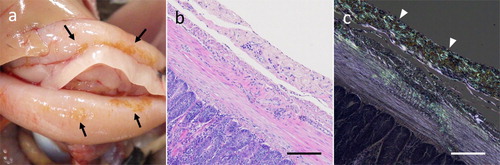
At necropsy, in some individuals, yellowish brown lesions were observed in the peritoneum including intestinal serosa ((a)). The yellow lesion was composed of amyloid deposits ((b)).
Figure 2. Macroscopic and histological features of amyloid deposition in quail. (a) Tan discoloured lesions were found in the serosa of the duodenum. (b, c) Histological image of serial sections of discoloured lesions. Severe amyloid deposition band in the serosal stroma appeared as an acidophilic nonstructural deposit by H&E (b), which stained with Congo red and showed green birefringence under polarized light (c). Bar = 100 μm. Color online.
Experiment 2: Examination of in-cage horizontal transmission of quail AA amyloidosis
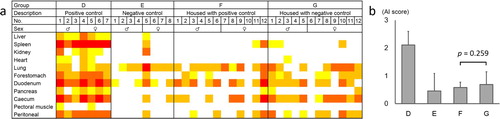
The degree of amyloid deposition in each organ and the mean AI score in experiment 2 is shown in . In quails from group D, amyloid deposition was observed in systemic organs. Although the AI scores of groups F (housed with D) and G (housed with E) were higher than that of group E, there was no significant difference in the AI scores between groups F and G. In other words, in-cage horizontal transmission of AA amyloidosis was not determined.
Figure 3. The degree of amyloid deposition in experiment 2. (a) The degree of amyloid deposition is represented as: 4, red; 3, orange; 2, orange-yellow; 1, yellow; 0, white. (b) Mean AI scores of each group. The bars and error bars indicate the mean scores and standard deviation, respectively. There was no significant difference between groups F and G by Mann–Whitney U test.
Experiment 3: Assessment of the oral transmission efficiency of AA amyloidosis in young quails
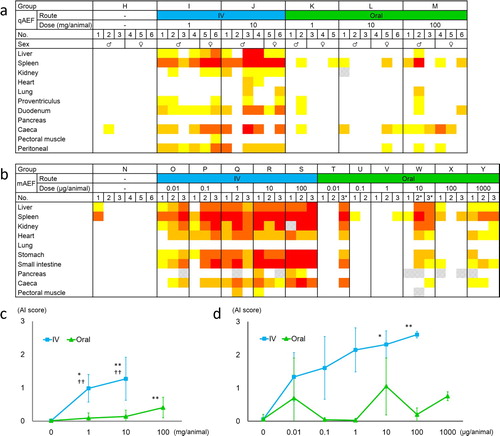
The degree of amyloid deposition in each organ and the mean AI score in experiment 3 are shown in (a) and (c). The quails in groups I to M showed splenic amyloid deposition which was not observed in group H. Significant elevation of mean AI scores compared with the control group was confirmed in groups I, J and M. The mean AI scores of groups I and J were significantly higher than those of groups K and L, respectively.
Figure 4. The degree of amyloid deposition in experiments 3 (a, c) and 4 (b, d). (a, b) The degree of amyloid deposition is represented as: 4, red; 3, orange; 2, orange-yellow; 1, yellow; 0, white; shading, not tested. IV, intravenous. *Injured individuals. (c, d) Mean AI scores of each group. The dots and error bars indicate the mean scores and standard deviation respectively. *P < 0.05, **P < 0.01 vs. negative control group, by Kruskal–Wallis ANOVA followed by Dunn's tests. ††P < 0.01 vs. oral administration group given the same dose, by Mann–Whitney U test.
Experiment 4: Assessment of the oral transmission efficiency of AA amyloidosis in mice
The degree of amyloid deposition of each organ and the mean AI score in experiment 4 are shown in (b) and (d). Amyloid deposition in systemic organs, mainly spleen, was observed in all intravenous mAEF-administration groups and the highest-dose oral mAEF-administration groups. Mean AI scores of groups R and S were significantly higher than that of the control group by Kruskal–Wallis ANOVA followed by Dunn's tests. Polydipsia and polyuria were observed in groups R and S. Among oral administration groups, three mice (T-3, W-2, and W-3) were seriously injured by fighting in the cage, and showed systemic amyloid deposition. While there was no significant difference in AI score among the groups receiving the same dose by Mann–Whitney U test, the mean AI score of the intravenous administration group was higher than that of the oral administration group.
Discussion
In previous studies (Elliott-Bryant & Cathcart, Citation1998; Cathcart & Elliott-Bryant, Citation1999; Xing et al., Citation2001; Murakami et al., Citation2013), the induction of amyloidosis by oral administration of amyloid was defined as “oral transmission” of amyloidosis. In experiments 1 and 3, the mean AI scores in oral administration groups were significantly higher than control groups, indicating the experimental oral transmission of AA amyloidosis in adult and young quails. In adult quails, the AI scores of each group varied due to the spontaneous deposition of amyloid in the intestinal tract (Nakayama et al., Citation2017). In contrast, in young quails, there was no spontaneous amyloid deposition in the intestinal tract, thus young quails are considered to be a more useful model for oral transmission of AA amyloidosis, especially for dose-dependent evaluations. Since the quail is suitable for laboratory use, it is an effective model of avian AA amyloidosis in most laboratories. This model will be useful in clarifying the mechanism of oral transmission of avian AA amyloidosis in future.
In previous studies using mice, dose-dependent transmission of AA amyloidosis has already been reported (Cui et al., Citation2002), but it has not been reported in avian species. In this paper, we evaluated whether similar phenomena occurred in quails. As shown in experiments 3 and 4, dose-dependent intravenous and oral transmission of AA amyloidosis occurred in quails similar to mice. While the AEF dose per BW of group M (about 700–1000 mg/kg BW) was larger than that of group Y (about 25–33 mg/kg BW), it seems that amyloid deposition in group Y was more severe than in group M. Assuming that the efficiency of AA transmission is equivalent between mice and quails, it is considered that there is a difference in the inflammation caused by LPS between mouse and quail. In mice, the lethal dose by intraperitoneal LPS-injection is 25–60 mg/kg (Berczi et al., Citation1966), whereas it is 517 mg/kg in chickens; the chicken lethal LPS dose was used as a reference because there is no report of a lethal dose of LPS in quails (Adler & DaMassa, Citation1979). Therefore, the inflammatory stimulation by LPS given to quails in these experiments may have been inadequate to exacerbate systemic amyloidosis.
In both quails and mice, the groups with amyloid administration showed severe amyloid deposition in the spleen. As previously reported, splenic amyloid deposition is rarely observed in young quails without administration of AEF (Nakayama et al., Citation2017). In this study, splenic amyloid deposition was not observed in negative control quails from group H. Therefore, it is suggested that the AEF effect in young quails can be assessed by amyloid deposition in the spleen. Thus, splenic amyloid deposition in groups K, L and M was considered to be caused by oral administration of AEF.
As shown in , the amyloid deposition sites were observed macroscopically as a yellow lesion. In a previous study, we showed that amyloid arthropathy in chickens is macroscopically yellowish (Kobayashi et al., Citation2016). To our knowledge, there is no report on the macroscopic colour tone of amyloid deposition in tissues of mammals, but, at least in AA amyloidosis in quails and chickens, the amyloid deposition site can be determined macroscopically. In recent years, structure-specific intrinsic fluorescence of amyloid fibrils has been reported (Shaham-Niv et al., Citation2018). It is possible that the avian AA fibril has inherent optical properties. Future research would be necessary to determine whether the quail amyloid per se appears yellow or if it is derived from other components such as amyloid signature protein.
In this study, we developed an experimental quail model of oral transmissible AA amyloidosis, indicating that oral transmission of avian AA amyloidosis may occur in various avian species, not only in chickens. In birds, outbreaks of amyloidosis have been reported sporadically (Landman et al., Citation1998). In cheetahs, the outbreak of AA amyloidosis was thought to be due to horizontal transmission by coprophagy (Zhang et al., Citation2008). Considering that experimental oral transmission of avian AA amyloidosis occurs similarly to the mouse model, there is a possibility that horizontal transmission of avian AA amyloidosis may occur in nature. On the other hand, in experiment 2, in-cage horizontal transmission of quail AA amyloidosis did not occur within 4 weeks. In experiment 3, the transmission efficiency of AA amyloidosis in quail was dose-dependent, but oral administration of AEF to induce quail amyloidosis required a far higher dose than intravenous administration. Therefore, even if amyloid was excreted in faeces, it is considered that the amount of amyloid intake was insufficient to cause horizontal transmission in only 4 weeks. However, unlike in this study, captive birds, such as zoo birds, live in the same place for many years. Therefore, if amyloid is present in the environment, captive birds are thought to ingest environmental amyloid for a long period. In the experimental mouse model, the AEF once taken in the body continues to accumulate for a long time, triggering the onset of AA amyloidosis (Lundmark et al., Citation2002). Therefore, environmental amyloid, which has been ingested and accumulated in birds over a long period of time, may trigger the development of AA amyloidosis. Unlike chickens and ducks, quails allow us to verify long-term experiments in the laboratory. By conducting long-term intake experiments using quail in the future, we will be able to approach the elucidation of the horizontal transmission mechanism of avian AA amyloidosis.
Acknowledgements
We are greatly indebted to Prof. Gen Watanabe (Tokyo University of Agriculture and Technology) for providing retired quails and fertilized eggs. We also thank colleagues of laboratory of Veterinary Toxicology for support on rearing of quails, and useful comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adler, H.E. & DaMassa, A.J. (1979). Toxicity of endotoxin to chicks. Avian Diseases, 23, 174–178. doi: 10.2307/1589684
- Benson, M.D., Buxbaum, J.N., Eisenberg, D.S., Merlini, G., Saraiva, M.J.M., Sekijima, Y., Sipe, J.D. & Westermark, P. (2018). Amyloid nomenclature 2018: recommendations by the International Society of amyloidosis (ISA) nomenclature committee. Amyloid, 25, 215–219. doi: 10.1080/13506129.2018.1549825
- Berczi, I., Bertók, L. & Bereznai, T. (1966). Comparative studies on the toxicity of Escherichia coli lipopolysaccharide endotoxin in various animal species. Canadian Journal of Microbiology, 12, 1070–1071. doi: 10.1139/m66-143
- Cathcart, E.S. & Elliott-Bryant, R. (1999). Diet, amyloid enhancing factor (AEF) and amyloidogenesis: an hypothesis. Amyloid, 6, 107–113. doi: 10.3109/13506129909007310
- Cowan, D.F. (1968). Avian amyloidosis. I. General incidence in zoo birds. Pathologia Veterinaria, 5, 51–58.
- Cui, D., Kawano, H., Takahashi, M., Hoshii, Y., Setoguchi, M., Gondo, T. & Ishihara, T. (2002). Acceleration of murine AA amyloidosis by oral administration of amyloid fibrils extracted from different species. Pathology International, 52, 40–45. doi: 10.1046/j.1440-1827.2002.01309.x
- Dias, J.L. & Montau, R.J. (1994). Staphylococcosis in captive exotic waterfowl. Avian Pathology, 23, 659–669. doi: 10.1080/03079459408419035
- Druet, R.L. & Janigan, D.T. (1966). Experimental amyloidosis. Amyloid induction with a soluble protein antigen in intact, bursectomized and thymectomized chickens. American Journal of Pathology, 49, 1103–1123.
- Elliott-Bryant, R. & Cathcart, E.S. (1998). Amyloid enhancing factor and dietary transmission in accelerated amyloid A amyloidosis. Clinical Immunology and Immunopathology, 88, 65–69. doi: 10.1006/clin.1998.4555
- Jakob, W. (1971). Spontaneous amyloidosis of mammals. Veterinary Pathology, 8, 292–306. doi: 10.1177/030098587100800402
- Kawashima, T., Ahmed, W.M., Nagino, K., Ubuka, T. & Tsutsui, K. (2016). Avian test battery for the evaluation of developmental abnormalities of neuro- and reproductive systems. Frontiers in Neuroscience, 10, 296. doi: 10.3389/fnins.2016.00296
- Kobayashi, N., Murakami, T., Sakai, H., Yamaguchi, Y., Fukushi, H. & Yanai, T. (2016). Chicken amyloid arthropathy caused by Mycoplasma synoviae infection in Japan. Journal of Veterinary Science & Medical Diagnosis, 5. doi: 10.4172/2325-9590.1000211
- Kuroiwa, M., Aoki, K. & Izumiyama, N. (2003). Histological study of experimental murine AA amyloidosis. Journal of Electron Microscopy, 52, 407–413. doi: 10.1093/jmicro/52.4.407
- Landman, W.J., Gruys, E. & Gielkens, A.L. (1998). Avian amyloidosis. Avian Pathology, 27, 437–449. doi: 10.1080/03079459808419367
- Ling, Y. (1992). Experimental production of amyloidosis in ducks. Avian Pathology, 21, 141–145. doi: 10.1080/03079459208418827
- Lundmark, K., Westermark, G.T., Nyström, S., Murphy, C.L., Solomon, A. & Westermark, P. (2002). Transmissibility of systemic amyloidosis by a prion-like mechanism. Proceedings of the National Academy of Sciences of the United States of America, 99, 6979–6984. doi: 10.1073/pnas.092205999
- Murakami, T., Ishiguro, N. & Higuchi, K. (2014). Transmission of systemic AA amyloidosis in animals. Veterinary Pathology, 51, 363–371. doi: 10.1177/0300985813511128
- Murakami, T., Muhammad, N., Inoshima, Y., Yanai, T., Goryo, M. & Ishiguro, N. (2013). Experimental induction and oral transmission of avian AA amyloidosis in vaccinated white hens. Amyloid, 20, 80–85. doi: 10.3109/13506129.2013.783474
- Nakayama, Y., Kamiie, J., Watanabe, G., Suzuki, K. & Murakami, T. (2017). Spontaneous, experimentally induced, and transmissible AA amyloidosis in Japanese quail (Coturnix japonica). Veterinary Pathology, 54, 912–921. doi: 10.1177/0300985817723692
- Ogawa, S., Murakami, T., Inoshima, Y. & Ishiguro, N. (2015). Effect of heating on the stability of amyloid A (AA) fibrils and the intra- and cross-species transmission of AA amyloidosis. Amyloid, 22, 236–243. doi: 10.3109/13506129.2015.1095735
- Röcken, C. & Shakespeare, A. (2002). Pathology, diagnosis and pathogenesis of AA amyloidosis. Virchows Archiv, 440, 111–122. doi: 10.1007/s00428-001-0582-9
- Shaham-Niv, S., Arnon, Z.A., Sade, D., Lichtenstein, A., Shirshin, E.A., Kolusheva, S. & Gazit, E. (2018). Intrinsic fluorescence of metabolite amyloids allows label-free monitoring of their formation and dynamics in live cells. Angewandte Chemie International Edition, 57, 12444–12447. doi: 10.1002/anie.201806565
- Skinner, M., Shirahama, T., Benson, M.D. & Cohen, A.S. (1977). Murine amyloid protein AA in casein-induced experimental amyloidosis. Laboratory Investigation, 36, 420–427.
- Xing, Y., Nakamura, A., Chiba, T., Kogishi, K., Matsushita, T., Li, F., Guo, Z., Hosokawa, M., Mori, M. & Higuchi, K. (2001). Transmission of mouse senile amyloidosis. Laboratory Investigation, 81, 493–499. doi: 10.1038/labinvest.3780257
- Zhang, B., Une, Y., Fu, X., Yan, J., Ge, F., Yao, J., Sawashita, J., Mori, M., Tomozawa, H., Kametani, F. & Higuchi, K. (2008). Fecal transmission of AA amyloidosis in the cheetah contributes to high incidence of disease. Proceedings of the National Academy of Sciences of the United States of America, 105, 7263–7268. doi: 10.1073/pnas.0800367105
