ABSTRACT
Dermanyssus gallinae is a haematophagous ectoparasite primarily known as a pest of domestic and wild birds. It occasionally feeds on a range of mammals, and, more importantly, is of growing concern in human medicine. This review highlights mite attacks on people working with poultry, and updates the increasing incidence of dermanyssosis in urban environments in Europe. Although several cases of dermanyssosis have been documented, there are a number of reasons why diagnosis of D. gallinae infestations in humans is likely to be underestimated. Firstly, medical specialists are not well aware of D. gallinae infestations in humans. There is also a lack of collaboration with specialists from other disciplines. The problem is compounded by misdiagnoses and by the lack of diagnostic tools. We review the literature on human dermanyssosis cases in Europe, and also provide information on the epidemiology, clinical, histo-pathological and immunological aspects of dermanyssosis. We stress the need for improved recognition of this challenging infestation in humans, and provide straightforward recommendations for health practitioners, starting with collection of the correct anamnestic information and including appropriate management methods for case recognition and resolution. Finally, we indicate the most urgent areas to be addressed by future research.
RESEARCH HIGHLIGHTS
Dermanyssus gallinae is of growing concern in human medicine.
Most physicians are not well aware of dermanyssosis in humans.
Bio-epidemiological and clinical aspects of this ectoparasitosis are highlighted.
Practical key actions for diagnosis and correct management of infestation in humans are provided.
Introduction
Dermanyssus gallinae (Arthropoda: Dermanyssidae) is a cosmopolitan haematophagous ectoparasite of birds. It is primarily a well-known pest of poultry farms worldwide, affecting over 80% of European poultry farms, with peaks above 90% in the Netherlands, Germany and Belgium (Mul et al., Citation2017). The infestation burden on caged laying hens can be up to 500,000 mites per bird in severe cases (Kilpinen, Citation2005); this causes extreme stress, associated with feather-pecking, increased self-grooming and cannibalism (Kilpinen, Citation2005; Mul et al., Citation2009), in addition to blood loss. As a consequence, the welfare, health and productivity of the birds are severely affected (Wójcik et al., Citation2000; Cosoroaba, Citation2001; Kilpinen, Citation2005). In addition, D. gallinae serves as a vector for a number of viral and bacterial avian pathogens (Valiente Moro et al., Citation2009; Circella et al., Citation2011; Chu et al., Citation2015; Sommer et al., Citation2006).
It also poses a threat to other birds, such as broilers, turkeys and ducks, and also to canaries, budgerigars and synanthropic birds typically found in urban centres (e.g. pigeons, sparrows, starlings, doves).
D. gallinae mites are temporary nocturnal visitors; they remain hidden in close proximity to their hosts during daylight hours, and move onto their hosts at nightfall in order to feed. The life cycle consists of the following stages: egg, larva, two nymphal stages, adult male and female. All legged stages, except larvae, feed on blood. The complete life cycle typically takes two weeks, but under ideal conditions (35 °C and relative humidity over 70%), it may require only one week (Sparagano et al., Citation2014).
Although D. gallinae is largely considered an avian-specific ectoparasite, when the natural host is absent hungry mites will occasionally feed on a range of mammals, i.e. cats (Grant, Citation1989; Di Palma et al., Citation2018), dogs (Declerq & Nachtegaele, Citation1993), gerbils (Lucky et al., Citation2001), other rodents (Kowal et al., Citation2014), horses (Mignon & Losson, Citation2008), and humans. They may attack any person working in infested poultry farms or living in an urban environment where there are synanthropic birds.
The most important point related to human infestation is that physicians are usually unfamiliar with the dermatitis caused by several zoonotic ectoparasites, including D. gallinae (Haag-Wackernagel, Citation2005; Cafiero et al., Citation2008; Collgros et al., Citation2013). It is actually very difficult to diagnose a D. gallinae infestation from the cutaneous reactions it causes in humans; since the reactions are uncharacteristic (Kavallari et al., Citation2018), infestations are often misdiagnosed. The lack of guidelines and/or recommendations, and the insufficient awareness of physicians/dermatologists with this infestation and with the eco-biology of this ectoparasite, can prevent them from managing affected patients correctly. Furthermore, misdiagnosis of an infestation, and the inevitable relapses, negatively affect patients’ quality of life (Dogramaci, Culha, & Ozçelik, Citation2010). The problem of misdiagnosis is also worrying due to the possible role of D. gallinae as a vector/reservoir of several zoonotic pathogens (De Luna et al., Citation2008; Circella et al., Citation2011; Boseret et al., Citation2013).
According to the recent strategic document provided by the tripartite agreement of FAO, OIE and WHO (Citation2017), the impact of D. gallinae on human health can be fully considered as a One Health issue.
This article is concerned with improvements in understanding dermanyssosis in humans. It aims to highlight salient aspects and key features of this ectoparasite, starting with its epidemiology by bringing order to the literature and updating, to the best of our knowledge, the case reports in Europe, and considering the clinical and diagnostic aspects involved. It provides physicians/dermatologists with practical information about the key actions needed for correct management of infestations in humans, and informs the scientific community about future research priorities to fill the gaps in the current knowledge of dermanyssosis.
Epidemiology and public health significance of D. gallinae in Europe
Given the high percentage of infected poultry farms (on average 83% of the European laying hen farms) (Mul et al., Citation2013) human dermanyssosis in this specific context can be regarded as “occupational cases” (Cafiero et al., Citation2011), while those related to synanthropic birds can be regarded as “urban cases” (). summarizes the cases of dermanyssosis (occupational and urban) documented to date.
Figure 1. Dermanyssosis cases in Europe (red dots: urban cases; yellow dots: occupational cases). (Colour online)
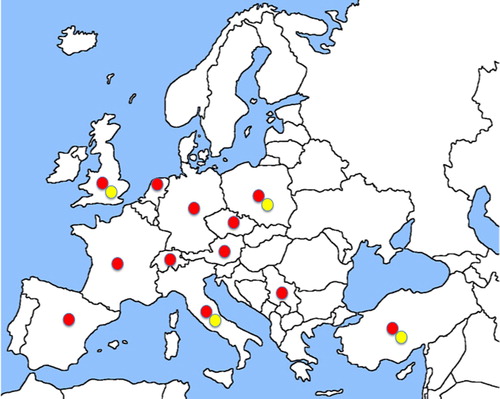
Table 1. Reports of Dermanyssus gallinae on humans in Europe
Occupational cases
Despite the high occurrence of dermanyssosis among poultry industry farmers, technicians and veterinarians working in infested farms (Camarda, personal communication, January 23, 2019), there are very few case-reports linked to poultry in the literature. Attacks on humans in poultry sheds occur when the infestation level in poultry farms is high, and when farmers, employees or visitors do not wear adequate protective clothing. D. gallinae bites can be considered a serious health hazard, and a source of discomfort and stress for all personnel working in poultry houses including amateurs. In these conditions, and given that the mite can bite in less than 1 s (Auger et al., Citation1979), there can be a major risk of attacks on humans handling birds and/or cages and collecting eggs (Rosen et al., Citation2002), or even visiting an infested farm. In some countries this has become a socio-economic problem for poultry workers, who demand three times the usual rate of pay before they are willing to work with D. gallinae-infested birds (Sahibi et al., Citation2008). Several occupational cases of human dermanyssosis have been registered worldwide (Sparagano et al., Citation2014).
In Europe, although it is very common in the field, and a well-known pest in veterinary handbooks, there are few published records of poultry workers being attacked by D. gallinae (). However, cases have been recorded since the 1950s in Poland (Litwinski, Citation1955), and later in the UK (Rossiter, Citation1997), Italy (Pampiglione et al., Citation2001; Cafiero et al., Citation2011) and Turkey (Dogramaci et al., Citation2010; Şengül et al., Citation2017).
Case records concern both people living in rural areas, who rear free-range hens as a hobby (Pampiglione et al., Citation2001; Şengül et al., Citation2017), and poultry farmers (Rossiter, Citation1997; Dogramaci et al., Citation2010). Although there are several case reports, only one study has investigated the prevalence of cases involving poultry industry workers; a questionnaire among workers in infested poultry farms in Southern Italy revealed that 18% (11 of the 58 people interviewed) had experienced irritating and itchy skin eruptions (Cafiero et al., Citation2011).
Human infestation mostly occurs in daytime (when mites crawling off their preferential host, or hiding, are disturbed by human activities), and in spring-summer when climatic conditions are favourable to mites. However, given that temperatures in industrial farms are almost constant, infestations can occur in them throughout the year (Sparagano et al., Citation2014).
The importance of these mites in public health also stems from their role as reservoirs/vectors of zoonotic poultry pathogens, including Salmonella enterica (Pugliese et al., Citation2019), Erysipelothrix rhusiopathiae (Chirico et al., Citation2003; Valiente Moro et al., Citation2009; Huong et al., Citation2014), and the avian influenza A virus (Sommer et al., Citation2006). For these reasons, there has been wholehearted support for official recognition of D. gallinae as a zoonotic agent in all occupational safety regulations, and for recognition of this mite dermatitis as an occupational hazard for poultry industry workers (Cafiero et al., Citation2011).
Urban cases
Reports of dermanyssosis have become more frequent in recent years, particularly in residential contexts in association with common synanthropic birds, such as sparrows, starlings, doves, and mostly with feral pigeons (Columba livia). Feral pigeons are among the most successful avian settlers in our cities due to the abundance of available food and the absence of predators (Haag-Wackernagel, Citation2005). The ever-increasing pigeon populations build their nests close to homes (e.g. crevices and holes on the façades of buildings, behind external air-conditioner units, under eaves and in attics). When bird hosts are not available, mites search for alternative hosts and may migrate into nearby homes, where they bite humans. Most episodes of red mite dermatitis commonly occur in the late spring-early summer (Deoreo, Citation1958; Bellanger et al., Citation2008; Cafiero et al., Citation2013; Giangaspero et al., Citation2016). The seasonal occurrence of this infestation was extremely evident in an outbreak affecting five members of a family, who suffered recurrent pruritic skin lesions in April and May for four consecutive years before the parasites were observed and identified (Gavrilović et al., Citation2015). This seasonality reflects the red mite population peak; this is linked to the breeding season of birds, mostly pigeons, which peaks between April and June, when birds leave their nests once the chicks have fledged, and there are greater chances of human contact with mites. In fact, D. gallinae and Argas reflexus (sometimes in co-infestations) are the principal ectoparasites acquired by humans in urban environments from feral pigeons (Haag-Wackernagel, Citation2005; Haag-Wackernagel & Bircher, Citation2010); the occurrence of feral pigeon ectoparasites increases as the density of their host (i.e. feral pigeons) increases (Haag-Wackernagel, Citation1991).
In Europe, published/ascertained urban D. gallinae infestations have been recorded in 12 countries; these were more frequent in private homes/apartments (with more than 150 outbreaks recorded), but also in hospitals (six records) and offices/public buildings (12 records) ().
The urban cases of D. gallinae infestation have all been caused by mites migrating from synanthropic bird nests of pigeons, starlings or sparrows, except for a few cases: one caused by an accidental laboratory infestation in Switzerland (Haag-Wackernagel, Citation1988), and other cases caused by caged canaries in Netherlands (Frenken, Citation1965) and Italy (Cafiero et al., Citation2017) ().
The attacks can occur during daytime (mostly in workplaces) or at night (mostly in private homes).
The implications of these infestations in urban environments may well cause concern, since Chlamydia psittaci (Circella et al., Citation2011), and both Borrelia burgdorferi s.l. and Coxiella burnetii DNA, the agents of Lyme disease and Q Fever, respectively, have recently been detected in D. gallinae during three outbreaks of human dermatitis related to sparrow and pigeon nests (Raele et al., Citation2018). More importantly, Bartonella quintana DNA has been detected in Dermanyssus mites collected in an apartment during an outbreak of urban trench fever caused by B. quintana, which affected a family with high socio-economic status (Melter et al., Citation2012).
Clinical signs
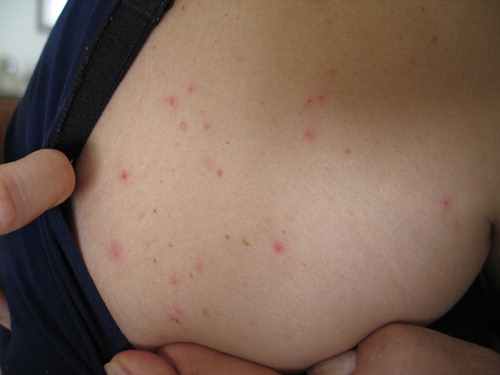
People attacked by D. gallinae mites present erythematous eruptions consisting of 1–3 mm papules () (Bardach, Citation1981; Collgros et al., Citation2013), sometimes with a visible central puncture mark or vesicles (Deoreo, Citation1958; Kowalska & Kupis, Citation1976; Haag-Wackernagel & Bircher, Citation2010; Cafiero et al., Citation2018); urticarioid manifestations have also been described (Rockwell, Citation1953; Deoreo, Citation1958; Sexton & Barton, Citation1975; Auger et al., Citation1979; Ahmed et al., Citation2018). Bites can be painful (Berndt, Citation1952) and skin lesions may occur in any area of the body, except in the interdigital spaces, genitals or skin folds; itching is commonly intense, with reported cutaneous excoriations due to scratching (Gavrilović et al., Citation2015).
Figure 2. Dermanyssus gallinae-dermatitis caused by mites migrating from a sparrows’ nest (original, M.A. Cafiero).
In the Italian survey of poultry farm workers, two of the 11 parasitized workers (18.18%) reported skin eruptions on their arms and hands, seven (63.63%) reported symptoms on their chests, and two (18.18%) on their legs. None of these workers usually wore personal protective equipment during their work, including egg collection, so that their skin was exposed, particularly on their hands and arms (Cafiero et al., Citation2011), as was also reported for poultry farmers in Israel (Rosen et al., Citation2002).
In addition, there are reports of intriguing cases of persistent infestation in unexpected sites, such as the ears (auditory meatus) in the UK (Rossiter, Citation1997), and on the scalp, as in the case of an Italian woman living in the country side (Pampiglione et al., Citation2001) and of two Turkish farmers (Dogramaci et al., Citation2010; Şengül et al., Citation2017). Surprisingly, all these cases involved elderly subjects (aged 60+). In all of the above-mentioned cases, symptoms occurred for as long as 9 months.
Urban cases document more severe clinical pictures, involving people attacked at night while sleeping at home, or else bedridden patients attacked in hospitals during the day. In these cases, skin lesions are particularly abundant on body areas covered by pyjamas, e.g. the trunk and limbs (Freeman & Kataria, Citation1969; Cafiero et al., Citation2018), or on the patient’s entire body; in an outbreak in a Hamburg hospital, one of the 12 parasitized patients suffered over 500 bites (Winkler, Citation1967). In these cases, cutaneous lesions are often described as grouped and more intense where clothes constrict the body, for example in relation to a belt (Cafiero et al., Citation2017) or under the breasts (Bellanger et al., Citation2008). When mites attack sleeping subjects, red spots (crushed mites or mite droppings) can be found on bedclothes and pillows (Regan et al., Citation1987; Cafiero et al., Citation2009).
By contrast, attacks in workplaces and public buildings occur during daylight hours. Patients also present intense itching, but their skin reactions are less severe and less numerous, almost always involving exposed body areas, mainly the arms and legs. Victims usually see mites crawling on their clothes or skin and/or on office furniture and usually refer to experiencing a biting/stinging sensation while they work (Fuentes et al., Citation2009; Giangaspero et al., Citation2016; Cafiero et al., Citation2017; Pezzi et al., Citation2017; Şengül et al., Citation2017).
Histopathological aspects
There are few studies of histological changes to the skin of animals and humans affected by D. gallinae-dermatitis.
In a study of poultry, hyperkeratosis, skin thickness and a focal loss of epidermis were observed in 90% of the birds examined, together with extremely numerous small focal lymphocytic infiltrations in all hens (Sokol & Rotkiewicz, Citation2010). Another study involving poultry reported severe subcutaneous oedema, congestion of the hypoderma, lymphocytic infiltration, necrosis of feather follicles at 24 h post-infestation (p.i.), and hyperkeratosis, parakeratosis, acanthosis and local epidermal hyperplasia at 72 h p.i. (Hobbenaghi et al., Citation2012).
A study of infested cats described a perivascular and interstitial inflammatory infiltrate (Di Palma et al., Citation2018).
In humans, histological examination of skin lesions caused by D. gallinae revealed a perivascular inflammatory skin reaction, and sometimes a superficial perivascular eosinophilic infiltration (Hidano & Asanuma, Citation1976; Kowalska & Kupis, Citation1976). Moreover, a modest spongiosis and focal parakeratosis were also found in a case of severe D. gallinae-related dermatitis (Cafiero, personal communication, February 10, 2019).
Immunological response
Unlike mammalian immunological responses to ectoparasitic arthropods, the humoral and cellular immune response of birds to D. gallinae (as for other Gamasida) are still poorly understood, and there is a complete lack of knowledge about human responses.
Arkle et al. (Citation2006) found significantly higher (P < 0.05) yolk IgY levels in hens’ eggs. However, no significant relationship was found between yolk IgY levels and D. gallinae population levels, or between serum and yolk IgY levels, although egg and serum samples were not collected from the same bird. Arkle (Citation2007) reported that the level of cytokine expression did not appear to be significantly correlated with D. gallinae levels in naturally infested commercial poultry, while numerical and significant associations between IgY and cytokine levels lacked consistency (although there was some suggestion of a relationship between IgY levels and IL-5 and IL-12α expression). These data would suggest that D. gallinae blood feeding stimulates a significant IgY immune response by the host; such immune response following natural infestation would allow older birds to better tolerate mite attacks in the presence of high IgY levels and lower side effects from these attacks compared to young chicks. Reports of host inflammation or skin damage following D. gallinae infestation of hosts (birds or humans) do not appear in the literature, possibly highlighting counter-immune reactions developed by the mites. Further hypotheses regarding the apparent lack of development of host immunity to D. gallinae include the following: (a) D. gallinae has a mechanism which modifies host immunity, making it less effective; (b) mite populations adapt to host immunity between the first attack and the later attacks (however it is unclear how mites could communicate within colonies and if the mite population diversity stays the same during the flock life) (Arkle et al., Citation2006).
Diagnosis
Medical texts and parasitology manuals rarely mention Mesostigmata mites in relation to human infestation. This means that most physicians are unfamiliar with the diagnosis of several less common mite-related forms of dermatitis, including the dermatitis caused by D. gallinae (Cafiero et al., Citation2008; Haag-Wackernagel & Bircher, Citation2010; Collgros et al., Citation2013).
This is extremely important, not only because misdiagnosis may lead to failure in the treatment of patients, but also because, as underlined above, D. gallinae may be a vector/reservoir of ascertained zoonotic agents (Chirico et al., Citation2003; Valiente Moro et al., Citation2009; Brännström et al., Citation2010; Circella et al., Citation2011; Melter et al., Citation2012; Raele et al., Citation2018).
Clinical recognition of dermatitis due to D. gallinae bites (described in the section above) is a challenging task. D. gallinae bites may be confused with urticarial atopic dermatitis, or with the dramatically common/widespread delusional ectoparasitosis caused by fragile psychological conditions (Lucky et al., Citation2001; Bellanger et al., Citation2008; Akdemir et al., Citation2009; Cafiero et al., Citation2013). They may also be confused with lesions caused by zoonotic mange (Sarcoptidae mite), baker’s itch (Acaridae mite), Cheyletiella, Trombicula and Cimex lectularius, if not arranged in linear arrays. Additionally, D. gallinae bites may be mistaken for those of avian mites in the Ornithonyssus genus (Acari: Macronyssidae); this mite group of zoonotic interest is similar to D. gallinae, but less common in Europe, and has a different relationship with the host (Cafiero et al., Citation2018).
D. gallinae-related dermatitis can be diagnosed by using dermoscopy and reflectance confocal microscopy. However, this tool is helpful only when the mites are present on the patient’s skin, although this must be considered a rather rare circumstance (Navarrete-Dechent & Uribe, Citation2018). Dermoscopy evaluation of D. gallinae-induced dermatitis also reveals dilated vessels on an erythematous background in correspondence to the macules and papules, and reflectance confocal microscopy demonstrates the presence of intraepidermal vesicles (Cinotti et al., Citation2015).
Currently, D. gallinae-related attacks on humans can be addressed only via different perspectives/approaches, which require:
greatly improved awareness of the problem among medical doctors;
enhanced knowledge of D. gallinae taxonomy and eco-biological aspects;
closer collaboration of doctors with entomologists/acarologists/veterinarians.
Given these starting points, there are some important actions doctors should take when faced with suspected pruritic dermatitis caused by D. gallinae.
A series of straightforward recommendations/instructions is listed below, beginning with the collection of the correct anamnestic information and including appropriate/accurate management methods to enable case resolution.
Practical recommendations to physicians
Dermanyssus gallinae should always be suspected when unexplained, recurrent dermatitis occurs in humans.
Detailed personal and environmental anamnesis
In addition to collecting the usual anamnestic data, physicians should ask patients about where they live (i.e. in the countryside, city, etc.), their occupation and their lifestyle. They should also ask patients for details about the onset of pruritus: the place, season and time of day it appeared; the recurrence of the symptoms; whether other family members (including their pets) have/had the same symptoms; whether they live close to a poultry farm; and whether there are pet birds and/or bird nests near their home/workplace.
Site inspection
For poultry farm workers, it may be relatively simple to predict and identify the cause of their dermatitis, given the prevalence of D. gallinae in rearing systems, but in an urban context, it is crucial to have a good knowledge of D. gallinae biology/ecology in order to search for the mites. It is strongly recommended that inspections (or requests for inspections) be made for adult stages (which are visible to the naked eye) in the environment where an infestation is suspected. It is important to know that mites are attracted by warm hiding places simulating the body temperature of birds. In apartments, offices and public buildings, the places to inspect are under bed covers, electrical devices. In stand-by mode (e.g. laptop computers, television and radio clocks), and all other sites generating heat. Balconies, attics, eaves, windows and holes near the building must be also inspected for the presence of active or, more probably, abandoned birds’ nests ().
Environmental sample collection
Having obtained the consent of the house/building owner/s, dust samples must be taken near/under windows, beds, furniture and desks, where people usually rest or work. The dust must be brushed into a transparent bag, then sealed and labelled with the collection site, date and time. If noticed in a room, arthropod specimens can be collected using scotch tape (). Samples have to be examined under a microscope and we strongly recommend that the specimens be correctly identified, since correct identification is the first requirement before applying control methods.
Identification
D. gallinae is relatively small at the adult stage (0.5–1 mm long), with long legs and a greyish-white body that becomes reddish-brown when engorged (). However, since cases of dermatitis caused by the avian mites Ornithonyssus bursa (Castelli et al., Citation2015; Mentz et al., Citation2015; Bassini-Silva et al., Citation2019) and Ornithonyssus sylviarum (Orton et al., Citation2000; Cafiero et al., Citation2018), or by the rodent mite Ornithonyssus bacoti (Beck & Fölster-Holst, Citation2009; Cafiero et al., Citation2016) have been recorded in humans even recently, there may be some confusion over identification of the mite responsible.
Firstly, fresh (or, less efficiently, frozen) specimens must be macerated in lactophenol for one week at 45 °C on a hot plate, and then mounted on slides with Hoyer’s medium for light microscopy (LM) observations (Di Palma et al., Citation2012).
D. gallinae can be identified according to the following principal morphological characters (Di Palma et al., Citation2012; Giangaspero et al., Citation2016):
the dorsal surface has a shield with prominent lateral margins tapering towards the rear, but these do not reach the distal end of the body and are truncated at the end.
the ventral side has a sternal plate much wider than it is long and bears two pairs of bristles, with a third pair situated posteriorly and apart from the first two pairs.
the posteriorly rounded genitoventral shield bears one pair of setae.
the rounded or D-shaped anal plate bears three characteristic setae.
hair-like chelicerae (when extended).
chelicerae bases look like wine glasses upside down in the middle of the body when chelicerae are retracted.
Ornithonyssus spp. has different features (see Di Palma et al., Citation2012 and Giangaspero et al., Citation2016 for morphological details on this genus).
Molecular identification
It has been demonstrated that D. gallinae is a complex of species, which includes D. gallinae L1 (a cryptic species related to synanthropic birds) and D. gallinae sensu stricto (associated with poultry farms and chickens). Since these are morphologically indistinguishable (Roy & Buronfosse, Citation2011), it would be helpful to analyze the DNA of specimens from the environment to understand the source of attacks on humans.
Treatment of patients and the environment
Diagnosis of D. gallinae dermatitis is challenging, and misdiagnosis can lead to use of antihistamines and steroids to relieve symptoms, sometimes in combination with anti-parasite shampoos, antibiotics and tranquillizers. However, if symptoms are caused by D. gallinae infestation, the clinical signs usually return when treatments end.
If physicians suspect and/or confirm dermanyssosis in urban outbreaks, the following measures will achieve complete regression of the symptoms, and no evidence of mites or dermatitis will appear in the follow-up period: (i) patient showering extensively and washing their clothes at 60 °C; (ii) removal of the mite source (abandoned birds’ nest); (iii) intensive vacuum cleaning, removal of the vacuum bag which needs to be packed in a sealed plastic bag and thrown away outside in a contained bin; (iv) disinfestation of the infested areas using pyrethroids: (v) steam cleaning or washing of textiles (curtains, carpets, cushions) at 60 °C and then preferably dried with an automated laundry drier. Textiles which cannot be washed at 60 °C should be placed in a plastic bag for a day together with anti-moth balls releasing an acaricide product.
In the case of occupational dermanyssosis, the problem of farm infestation requires intervention using an integrated pest management (Sparagano et al., Citation2014; Mul, Citation2017). Moreover, physicians should remind their patients that all poultry workers must be protected by suitable protective clothing, before entering poultry houses.
Conclusions and future research perspectives
Dermatitis due to Dermanyssus mites is an increasing but neglected problem. It features as an occupational hazard for poultry workers due to the great prevalence of infested poultry farms, and also appears in urban contexts due to the worrying spread of synanthropic birds to megacities worldwide (George et al., Citation2015; Kavallari et al., Citation2018).
Medical specialists should always include D. gallinae in their differential diagnosis of patients presenting pruritic dermatitis of unknown aetiology.
The widespread circulation of D. gallinae in humans indicates the need for targeted actions at different levels to provide better understanding of this mite, allowing researchers to fill the gaps in current scientific knowledge and enabling the development of strategies for recognition/diagnosis and control.
Therefore, we suggest that health service practitioners (physicians, medical doctors, dermatologists and occupational doctors) receive the above-mentioned recommendations. This could help to raise their awareness of the role of these ectoparasites in human health, allowing them to suspect and recognize D. gallinae infestations in humans, thus increasing the number of case reports.
In addition, there is a need for research with the following aims:
to carefully describe the symptoms and skin reactions of red mite dermatitis, although they may overlap other diseases;
to investigate the development of the lesions and haematological parameters (if any) over time;
to investigate the effects of D. gallinae on the human immune and dermal systems;
to uncover possible immunological host markers for setting up diagnostic tools.
All these efforts are crucial for the future development of a reliable diagnostic protocol that will make it possible to avoid using the wrong treatment products, which can cause side effects and damage patients’ health.
Finally, it is of paramount importance for treatment of this disease, as for other zoonotic infestations, that clinicians, parasitologists, microbiologists, veterinarians, epidemiologists and environmental scientists work together according to the “One Health” approach, which is often mentioned but too rarely put into practice.
This article, which is included in the ‘Research update on Dermanyssus gallinae, the poultry red mite’ supplement, is sponsored and supported by Coventry University.
Acknowledgements
We wish to thank EU COST Action FA1404 “Improving current understanding and research for sustainable control of the poultry red mite Dermanyssus gallinae (COREMI)” for supporting this article.
Disclosure statement
Authors declare to have no conflict of interest.
ORCID
Antonio Camarda http://orcid.org/0000-0002-3961-585X
Additional information
Funding
References
- Ahmed, N., El-Kady, A., Abd Elmaged, W. & Almatary, A. (2018). Dermanyssus gallinae (Acari: Dermanyssidae) a cause of recurrent papular urticaria diagnosed by light and electron microscopy. Parasitologists United Journal, 11, 112–118. doi: 10.21608/PUJ.2018.16320
- Akdemir, C., Gulcan, E. & Tanritanir, P. (2009). Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Türkiye Parazitoloji Dergisi, 33, 242–244.
- Arkle, S. (2007). Development of a vaccine against the poultry red mite (Dermanyssus gallinae). PhD Thesis. Newcastle University, Newcastle, United Kingdom.
- Arkle, S., Guy, J.H. & Sparagano, O. (2006). Immunological effects and productivity variation of red mite (Dermanyssus gallinae) on laying hens- implication for egg production and quality. World's Poultry Science Journal, 62, 249–257. doi: 10.1079/WPS200594
- Auger, P., Nantel, J., Meunier, N., Harrison, R.J., Loiselle, R. & Gyorkos, T.W. (1979). Skin acariasis caused by Dermanyssus gallinae (de Geer): an in-hospital outbreak. Canadian Medical Association Journal, 120, 700–703.
- Bardach, H. (1981). Acaraiasis due to Dermanyssus gallinae (gamasoidosis) in Vienna. H&G Zeitschrift für Hautkrankheiten, 56, 21–26.
- Bassini-Silva, R., Jacinavicius, F., Hernandes, F., Ochoa, R., Bauchan, G., Dowling, A. & Barros-Battesti, D. (2019). Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Revista Brasileira De Parasitologia Veterinária, 28, 134–139. doi: 10.1590/s1984-296120180097
- Beck, W. & Fölster-Holst, R. (2009). Tropical rat mites (Ornithonyssus bacoti) - serious ectoparasites. Journal Der Deutschen Dermatologischen Gesellschaft, 7, 667–670.
- Bellanger, A.P., Bories, C., Foulet, F., Bretagne, S. & Botterel, F. (2008). Nosocomial dermatitis caused by Dermanyssus gallinae. Infection Control & Hospital Epidemiology, 29, 282–283. doi: 10.1086/528815
- Berndt, W.L. (1952). The chicken mite attacking children. Journal of Economic Entomology, 45, 1098. doi: 10.1093/jee/45.6.1098a
- Boseret, G., Losson, B., Mainil, J., Thiry, E. & Saegerman, C. (2013). Zoonoses in pet birds: review and perspectives. Veterinary Research, 44, 36. doi: 10.1186/1297-9716-44-36
- Brännström, S., Hansson, I. & Chirico, J. (2010). Experimental study on possible transmission of the bacterium Erysipelothrix rhusiopathiae to chickens by the poultry red mite. Dermanyssus gallinae. Experimental and Applied Acarology, 50, 299–307. doi: 10.1007/s10493-009-9317-4
- Buijs, J. (2009). Arthropods that annoy Amsterdam people. Proceedings of the Netherlands Entomological Society Meeting, 20, 33–44.
- Cafiero, M.A., Camarda, A., Circella, E., Galante, D. & Lomuto, M. (2009). An urban outbreak of red mite dermatitis in Italy. International Journal of Dermatology, 48, 1119–1121. doi: 10.1111/j.1365-4632.2009.04107.x
- Cafiero, M.A., Camarda, A., Circella, E., Santagada, G., Schino, G. & Lomuto, M. (2008). Pseudoscabies caused by Dermanyssus gallinae in Italian city dwellers: a new setting for an old dermatitis. Journal of the European Academy of Dermatology and Venereology, 22, 1382–1383. doi: 10.1111/j.1468-3083.2008.02645.x
- Cafiero, M.A., Camarda, A., Galante, D., Mancini, G., Circella, E., Cavaliere, N., Santagagada, G., Caiazzo, M. & Lomuto, M. (2013). Outbreaks of red mite (Dermanyssus gallinae) dermatitis in city-dwellers. In M. Shoja, P.S. Agutter, R.S. Tubbs, M. Ghanei, K. Ghabili, A. Harris & M. Loukas (Eds.), Hypotheses in clinical Medicine (pp. 413–424). Hauppauge, NY: Nova Science Publishers, Inc.
- Cafiero, M.A., Circella, E., Santagada, G., Parisi, A., Lomuto, M. & Camarda, A. (2007). Infestazione da Dermanyssus gallinae nell’uomo, un problema di igiene Urbana. ODV-Obiettivi &Documenti Veterinari, 6, 41–45.
- Cafiero, M.A., Galante, D., Camarda, A., Giangaspero, A. & Sparagano, O. (2011). Why dermanyssosis should be listed as an occupational hazard. Occupational and Environmental Medicine, 68, 628. doi: 10.1136/oemed-2011-100002
- Cafiero, M.A., Galante, D., Raele, D., Nardella, M., Piccirilli, E. & Lomuto, M. (2017). Outbreaks of Dermanyssus gallinae (Acari, Mesostigmata) related dermatitis in humans in public and private residences, in Italy (2001–2017): an expanding skin affliction. Journal of Clinical Case Reports, 7, 1035.
- Cafiero, M.A., Raele, D., Mancini, G. & Galante, D. (2016). Dermatitis by tropical rat mite, Ornithonyssus bacoti (Mesostigmata, Macronyssidae) in city-dwellers: a diagnostic challenge. Journal of the European Academy of Dermatology and Venereology, 30, 1231–1233. doi: 10.1111/jdv.13162
- Cafiero, M.A., Viviano, E., Lomuto, M., Raele, D.A., Galante, D. & Castelli, E. (2018). Dermatitis due to Mesostigmatic mites (Dermanyssus gallinae, Ornithonyssus [O.] bacoti, O. bursa, O. sylviarum) in residential settings. Journal Der Deutschen Dermatologischen Gesellschaft, 16, 904–906.
- Castelli, E., Viviano, E., Torina, A., Caputo, V. & Bongiorno, M. (2015). Avian mite dermatitis: an Italian case indicating the establishment and spread of Ornithonyssus bursa (Acari: Gamasida: Macronyssidae) (Berlese, 1888) in Europe. International Journal of Dermatology, 54, 795–799. doi: 10.1111/ijd.12739
- Chirico, J., Eriksson, H., Fossum, O. & Jansson, D. (2003). The poultry red mite, Dermanyssus gallinae, a potential vector of Erysipelothrix rhusiopathiae causing erysipelas in hens. Medical and Veterinary Entomology, 17, 232–234. doi: 10.1046/j.1365-2915.2003.00428.x
- Chu, T., Murano, T., Uno, Y., Usui, T. & Yamaguchi, T. (2015). Molecular epidemiological characterization of poultry red mite, Dermanyssus gallinae, in Japan. Journal of Veterinary Medical Science, 77, 1397–1403. doi: 10.1292/jvms.15-0203
- Cinotti, E., Labeille, B., Bernigaud, C., Fang, F., Chol, C. & Chermette, R. (2015). Dermoscopy and confocal microscopy for in vivo detection and characterization of Dermanyssus gallinae mite. Journal of The American Academy of Dermatology, 73, e15–e16. doi: 10.1016/j.jaad.2015.03.022
- Circella, E., Pugliese, N., Todisco, G., Cafiero, M., Sparagano, O. & Camarda, A. (2011). Chlamydia psittaci infection in canaries heavily infested by Dermanyssus gallinae. Experimental and Applied Acarology, 55, 329–338. doi: 10.1007/s10493-011-9478-9
- Collgros, H., Iglesias-Sancho, M., Aldunce, M., Expósito-Serrano, V., Fischer, C., Lamas, N. & Umbert-Millet, P. (2013). Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clinical and Experimental Dermatology, 38, 374–377. doi: 10.1111/j.1365-2230.2012.04434.x
- Cosoroaba, I. (2001). Massive Dermanyssus gallinae invasion in battery-husbandry raised fowls. Revue de Médecine Vétérinaire, 152, 89–96.
- De Luna, C., Arkle, S., Harrington, D., George, D., Guy, J. & Sparagano, O. (2008). The poultry red mite Dermanyssus gallinae as a potential carrier of vector-borne diseases. Annals of The New York Academy of Sciences, 1149, 255–258. doi: 10.1196/annals.1428.085
- Declerq, J. & Nachtegaele, L. (1993). Dermanyssus gallinae infestation in a dog. Canine Practice, 18, 34–36.
- Deoreo, G.A. (1958). Pigeons acting as vector in acariasis caused by Dermanyssus gallinae (De Geer, 1778). Archives of Dermatological Research, 77, 422–429. doi: 10.1001/archderm.1958.01560040046007
- Di Palma, A., Giangaspero, A., Cafiero, M. & Germinara, G. (2012). A gallery of the key characters to ease identification of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) and allow differentiation from Ornithonyssus sylviarum (Acari: Gamasida: Macronyssidae). Parasites & Vectors, 5, 104. doi: 10.1186/1756-3305-5-104
- Di Palma, A., Leone, F., Albanese, F. & Beccati, M. (2018). A case report of Dermanyssus gallinae infestation in three cats. Veterinary Dermatology, 29, 348–e124. doi: 10.1111/vde.12547
- Diederen, B., Loomans, H. & Berg, H. (2006). Jeuk, huidafwijkingen en een dode duif naast het slaapkamerraam. Huisarts & Wetenschap, 49, 213–215. doi: 10.1007/BF03084708
- Dogramaci, A.C., Culha, G. & Ozçelik, S. (2010). Dermanyssus gallinae infestation: an unusual cause of scalp pruritus treated with permethrin shampoo. Journal of Dermatological Treatment, 21, 319–321. doi: 10.3109/09546630903287437
- FAO, OIE & WHO. (2017). The Tripartite’s Commitment Providing multi-sectoral, collaborative leadership in addressing health challenges. Retrieved from https://www.who.int/zoonoses/tripartite_oct2017.pdf?ua=1.
- Ferri, M. (2007). Infestazioni umane causate da Acaro rosso Dermanyssus gallinae, e altri acari ematofagi, collegate a colonie di colombi randagi. Convegno SIMVP, Torino, Venezia, Pesaro. Retrieved from https://docplayer.it/5981162-Infestazioni-umane-causate-da-acaro-rosso-dermanyssus-gallinae-e-altri-acari-ematofagi-collegate-a-colonie-di-colombi-randagi.html.
- Freeman, E. & Kataria, M.S. (1969). Poultry mite infestation. British Medical Journal, 4, 53. doi: 10.1136/bmj.4.5674.53
- Frenken, J.H. (1965). Dermanyssus gallinae (D. Avium). Strophulus or “insect” bites. Diet or D.D.T. Acta Leidensia, 33-34, 68–77.
- Fuentes, M.V., Saintz-Elipe, S., Saez-Duran, S. & Galn-Puchades, M.T. (2009). Human ectoparasitism due to the poultry red mite, Dermanyssus gallinae, in the city of Valencia (Spain) and its surroundings. Revista Ibero-latinoamericana de Parasitologia, 68, 188–191.
- Galante, D., Lomuto, M., Totaro, G., Mancini, C., Nardella La Porta, C. & Cafiero, M.A. (2011). A nosocomial outbreak of red mite (Dermanyssus gallinae) dermatitis in Italy. 21st ECCMID 27th ICC Milan, Italy 7-10 May 2011.
- Gavrilović, P., Kecman, V. & Jovanović, M. (2015). Diagnosis of skin lesions caused by Dermanyssus gallinae in five patients. International Journal of Dermatology, 54, 207–210. doi: 10.1111/ijd.12009
- Gelati, A., Calzolari, F.M. & Ferri, M. (2007). Infestazione umana da Acaro rosso Dermanyssus gallinae (De Geer); Igiene degli alimenti, 24, 1, Gennaio-Febbraio 2007, 46–48.
- George, D., Finn, R., Graham, K., Mul, M., Maurer, V., Moro, C. & Sparagano, O. (2015). Should the poultry red mite Dermanyssus gallinae be of wider concern for veterinary and medical science? Parasites & Vectors, 8, 158. doi: 10.1186/s13071-015-0765-x
- Giangaspero, A., Camarda, A., Cafiero, M.A., Marangi, M. & Sparagano, O. (2016). Dermanyssus gallinae: a never-ending story for the poultry industry and public health. Nova Acta Leopoldina, 411, 1–17.
- Grant, D. (1989). Parasitic skin diseases in cats. Journal of Small Animal Practice, 30, 250–254. doi: 10.1111/j.1748-5827.1989.tb01553.x
- Haag-Wackernagel, D. (1988). Brutende Strassentauben als Ursache einer Invasion von Dermanyssus gallinae (de Geer, 1778). Prakt Schdlingsbekampfer, 8, 174–188.
- Haag-Wackernagel, D. (1991). Population density as a regulator of mortality among eggs and nestlings of feral pigeons (Columbia livia domestica) in Basel, Switzerland. In J. Pinowski, et al. (Ed.), Nestling mortality of granivorous birds due to microorganisms and toxic substances (pp. 21–31). Warsaw: Polish Scientific Publishers.
- Haag-Wackernagel, D. (2005). Parasites from feral pigeons as a health hazard for humans. Annals of Applied Biology, 147, 203–210. doi: 10.1111/j.1744-7348.2005.00029.x
- Haag-Wackernagel, D. & Bircher, A.J. (2010). Ectoparasites from feral pigeons affecting humans. Dermatology (Basel, Switzerland), 220, 82–92. doi: 10.1159/000266039
- Hidano, A. & Asanuma, K. (1976). Letter: Acariasis caused by bird mites. Archives of Dermatology, 112, 881–882. doi: 10.1001/archderm.1976.01630300078023
- Hobbenaghi, R., Tavassoli, M., Alimehr, M., Shokrpoor, S. & Ghorbanzadeghan, M. (2012). Histopathological study of the mite biting (Dermanyssus gallinae) in poultry skin. Veterinary Research Forum, 3, 205–208.
- Huong, C.T., Murano, T., Uno, Y., Usui, T. & Yamaguchi T. (2014). Molecular detection of avian pathogens in poultry red mite (Dermanyssus gallinae) collected in chicken farms. The Journal of Veterinary Medical Science, 76, 1583–1587. doi: 10.1292/jvms.14-0253
- Kavallari, A., Küster, T., Papadopoulos, E., Hondema, L., Øines, Ø, Skov, J., Sparagano, O. & Tiligada, E. (2018). Avian mite dermatitis: diagnostic challenges and unmet needs. Parasite Immunology, 40, e12539. doi: 10.1111/pim.12539
- Kilpinen, O. (2005). How to obtain a bloodmeal without being eaten by a host: the case of poultry red mite, Dermanyssus gallinae. Physiological Entomology, 30, 232–240. doi: 10.1111/j.1365-3032.2005.00452.x
- Kowal, J., Nosal, P., Niedziółka, R. & Kornaś, S. (2014). Presence of blood-sucking mesostigmatic mites in rodents and birds kept in pet stores in the Cracow area, Poland. Annals of Parasitology, 60, 61–64.
- Kowalska, M. & Kupis, B. (1976). Gamasoidosis (gamasidiosis)-not infrequent skin reactions, frequently unrecognized. Bulletin of Polish Medical Science and History, 15-16, 391–394.
- Litwinski, T. (1955). Report of the Wroclaw Section of the P.T.D. from May 30, 1953 Przeglad Dermatologiczny Wenereen, 5,72.
- Lucky, A.W., Sayers, C., Argus, J.D. & Lucky, A. (2001). Avian mite bites acquired from a new source - pet gerbils: report of 2 cases and review of the literature. Archives of Dermatology, 137, 167–170.
- Mancini, G., Galante, D. & Cafiero, M.A. (2012). An urban outbreak of severe dermatitis by the red mite, Dermanyssus gallinae De Geer, 1778 (Mesostigmata: Dermanyssidae) in Southern Italy, Apulia region. XXVII Congresso Nazionale della Società Italiana di Parassitologia (SoIPa), Alghero 26–29 giugno 2012 p. 328.
- Melter, O., Arvand, M., Votypka, J. & Hulinska, D. (2012). Bartonella quintana transmission from mite to family with high socio-economic status. Emerging Infectious Diseases Journal, 18, 163–165. doi: 10.3201/eid1801.110186
- Mentz, M.B., Silva, G.L.D. & Silva, C.E. (2015). Dermatitis caused by the tropical fowl mite Ornithonyssus bursa (Berlese) (Acari: Macronyssidae): a case report in humans. Revista da Sociedade Brasileira de Medicina Tropical, 48, 786–788. doi: 10.1590/0037-8682-0170-2015
- Mignon, B. & Losson, B. (2008). Dermatitis in a horse associated with the poultry mite (Dermanyssus gallinae). Veterinary Dermatology, 19, 38–43.
- Mul, M., Bens, H. & Odink-Schrijver, I. (2013). The Poultry Red Mite Dermanyssus gallinae (De Geer, 1778). A small pest that packs a big punch. Factsheet. Wageningen Livestock Research. In: https://www.researchgate.net/publication/258553789_Fact_sheet_Poultry_Red_Mite_in_Europe. Accessed 18 march 2018.
- Mul, M., Van Niekerk, T., Chirico, J., Maurer, V., Kilpinen, O., Sparagano, O., Thind, B., Zoons, J., Moore, D., Bell, B., Gjevre, A. & Chauve, C. (2009). Control methods for Dermanyssus gallinae in systems for laying hens: results of an international seminar. World's Poultry Science Journal, 65, 589–600. doi: 10.1017/S0043933909000403
- Mul, M., van Riel, J., Roy, L., Zoons, J., André, G., George, D., Meerburg, B.G., Dicke, M., van Mourik, S. & Groot Koerkamp, P.W.G. (2017). Development of a model forecasting Dermanyssus gallinae’s population dynamics for advancing integrated pest management in laying hen facilities. Veterinary Parasitology, 245, 128–140. doi: 10.1016/j.vetpar.2017.07.027
- Mul, M.F. (2017). Advancing Integrated Pest Management for Dermanyssus gallinae in laying hen facilities. (PhD thesis), Wageningen University, Wageningen, NL 194 pages ISBN: 978-94-6343-003-6. http://dx.doi.org/10.18174/394911.
- Navarrete-Dechent, C. & Uribe, P. (2018). A case of gamasoidosis caused by Dermanyssus gallinae, misdiagnosed as delusional parasitosis. Clinical and Experimental Dermatology, 43, 950–952. doi: 10.1111/ced.13687
- Neill, S.M., Monk, B.E. & Pembroke, A.C. (1985). Gamasoidosis - avian mite dermatitis (Dermanyssus gallinae). British Journal of Dermatology, 113, 44. doi: 10.1111/j.1365-2133.1985.tb13013.x
- Orton, D.I., Warren, L.J. & Wilkinson, J.D. (2000). Avian mite dermatitis. Clinical and Experimental Dermatology, 25, 129–131. doi: 10.1046/j.1365-2230.2000.00594.x
- Pampiglione, S., Pampiglione, G., Pagani, M. & Rivasi, F. (2001). Persistent scalp infestation by Dermanyssus gallinae in an Emilian country-woman. Parassitologia, 43, 113–115.
- Pezzi, M., Leis, M., Chicca, M. & Roy, L. (2017). Gamasoidosis caused by the special lineage L1 of Dermanyssus gallinae (Acarina: Dermanyssidae): a case of heavy infestation in a public place in Italy. Parasitology International, 66, 666–670. doi: 10.1016/j.parint.2017.05.001
- Prins, M., Go, I.H. & Van Dooren-Greebe, R.J. (1996). Parasitic pruritus: bird mite zoonosis. Nederlands Tijdschrift Voor Geneeskunde, 140, 2550–2552.
- Pugliese, N., Circella, E., Marino, M., De Virgilio, C., Cocciolo, G., Lozito, P., Cafiero, M.A. & Camarda, A. (2019). Circulation dynamics of Salmonella enterica subsp. enterica ser. Gallinarum biovar Gallinarum in a poultry farm infested by Dermanyssus gallinae. Medical and Veterinary Entomology, 33, 162–170. doi: 10.1111/mve.12333
- Raele, D.A., Galante, D., Pugliese, N., La Salandra, G., Lomuto, M. & Cafiero, M.A. (2018). First report of Coxiella burnetii and Borrelia burgdorferi sensu lato in poultry red mites, Dermanyssus gallinae (Mesostigmata, Acari), related to urban outbreaks of dermatitis in Italy. New Microbes and New Infections, 23, 103–109. doi: 10.1016/j.nmni.2018.01.004
- Reed, J., Hewitt, M. & Barrow, G.I. (1969). Mite infestation. British Medical Journal, 4, 622–623. doi: 10.1136/bmj.4.5683.622-c
- Regan, A.M., Metersky, M.L. & Craven, D.E. (1987). Nosocomial dermatitis and pruritus caused by a pigeon mite infestation. Archives of Internal Medicine, 147, 2185–2187. doi: 10.1001/archinte.1987.00370120121021
- Rockwell, E.M. (1953). Dermatitis due to Dermanyssus gallinae of pigeons. Archives of Dermatology and Syphilology, 68, 82. doi: 10.1001/archderm.1953.01540070085012
- Rosen, S., Yeruham, I. & Braverman, Y. (2002). Dermatitis in humans associated with the mites Pyemotes tritici, Dermanyssus gallinae, Ornithonyssus bacoti and Androlaelaps casalis in Israel. Medical and Veterinary Entomology, 16, 442–444. doi: 10.1046/j.1365-2915.2002.00386.x
- Rossiter, A. (1997). Occupational otitis externa in chicken catchers. Journal of Laryngology & Otology, 111, 366–367. doi: 10.1017/S0022215100137338
- Roy, L. & Buronfosse, T. (2011). Using mitochondrial and nuclear sequence data for disentangling population structure in complex pest species: a case study with Dermanyssus gallinae. PLOS One, 6, e22305. doi: 10.1371/journal.pone.0022305
- Sahibi, H., Sparagano, O. & Rhalem, A. (2008). Dermanyssus gallinae: Acari parasite highly aggressive but still ignored in Morocco. In: BSP spring, trypanosomiasis/leishmaniasis and malaria meetings. Newcastle Upon Tyne, 173 pp.
- Schrafl, A. (1930). Taubenkratze. Scheweiz Med Wochenschr 38:900 cit in Haag-Wackernagel D. (2005). Parasites from feral pigeons as a health hazard for humans. Annals of Applied Biology, 147, 203–210.
- Şengül, M., Kaçar, N., Karaca, M., Öner, S.Z. & Ergin, Ç. (2017). A case with scalp pruritus caused by Dermanysus gallinae (order: Mesostigmata). Mikrobiyoloji Bülteni, 51, 293–298. doi: 10.5578/mb.57351
- Sexton, D.J. & Barton, H. (1975). Bird-mite infestation in a university hospital. The Lancet, 7504, 445. doi: 10.1016/S0140-6736(75)91506-8
- Skierska, B. (1968). A case of mass invasion of bird-ticks, Dermanyssus gallinae (Redi, 1674) (Gamasoidea, Dermanyssidae) in humans. Wiadomości Parazytologiczne, 14, 322–326.
- Sokol, R. & Rotkiewicz, T. (2010). Histopathological change of the skin in hens infested with Dermanyssus gallinae. Polish Journal of Veterinary Sciences, 13, 385–387.
- Sommer, D., Heffels-Redmann, U., Köhler, K., Lierz, M. & Kaleta, E.F. (2006). Role of the poultry red mite (Dermanyssus gallinae) in the transmission of avian influenza A virus. Tierarztliche Praxis Ausgabe G: Grosstiere - Nutztiere, 44, 26–33.
- Sparagano, O., George, D., Harrington, D. & Giangaspero, A. (2014). Significance and control of the poultry red mite, Dermanyssus gallinae. Annual Review of Entomology, 59, 447–466. doi: 10.1146/annurev-ento-011613-162101
- Valiente Moro, C., De Luna, C., Tod, A., Guy, J., Sparagano, O. & Zenner, L. (2009). The poultry red mite (Dermanyssus gallinae): a potential vector of pathogenic agents. Experimental and Applied Acarology, 48, 93–104. doi: 10.1007/s10493-009-9248-0
- Wegner, Z. (1973). Two new cases of people attacked by parasitic bird mites in Poland. Wiadomości Parazytologiczne, 19, 187.
- Winkler, A. (1967). Endemie durch Vogelmilben (Dermanyssus gallinae) in einem Krankenhaus. Dermatologische Wochenschrift, 16, 458–459.
- Wójcik, A.R., Grygon-Franckiewicz, B., Zbikowska, E. & Wasielewski, L. (2000). Invasion of Dermanyssus gallinae (De Geer, 1778) in poultry farms in the Toruń region. Wiadomości Parazytologiczne, 46, 511–515.


