ABSTRACT
Helicobacter pullorum is an emerging zoonotic pathogen that causes gastroenteritis in chickens and inflammatory bowel disease in humans ingesting contaminated meat. However, the mechanism by which the bacterium causes disease is unclear. Type six secretion system (T6SS) plays a major role in bacterial pathogenesis and adaptation. Haemolysin coregulated protein (Hcp) plays a central role in the structure of the T6SS pilus and acts as effector protein in certain bacteria. In this study, H. pullorum isolated from 156 caecal samples of broiler chickens was screened for the presence of T6SS Hcp gene via PCR amplification. 30.7% of caecal and 18.3% of liver samples tested positive for presence of H. pullorum. From these positive samples, 29.7% possessed the T6SS gene. In bacterial co-culture experiments, significant loss of viability (81.6–39.1%) was observed for H. pullorum-infected hepatocytes and presence of Hcp did not contribute to the loss of cell viability. Nevertheless, infection of erythrocytes with Hcp-positive isolates was associated with greater haemolytic activity compared to infection with Hcp-negative isolates. Therefore, presence of T6SS could be indicative of virulent strains meriting further studies to characterize this virulence factor in H. pullorum infection.
Introduction
The poultry industry contributes to a large sum of the national Gross Domestic Product worldwide, especially in Asia, Europe and USA (Bester & Essack, Citation2008). In the last few decades the poultry industry has expanded enormously, as the lifestyle of people is leaning more towards white meat consumption, especially broiler chickens. This has led to a drastic increase in food-borne diseases disseminated by poultry birds (Jennings et al., Citation2011). While meat and meat products are considered important sources of human intestinal infections, the most common of which is infectious diarrhoea, it is a major challenge in the field of public health to deal with these zoonotic organisms (Flammer et al., Citation1999). Infectious diarrhoea is a major cause of morbidity and mortality throughout the world, particularly in children (Duggan et al., Citation1992). Salmonella enterica, Campylobacter spp., and Shiga toxin-producing Escherichia coli are the most common infectious intestinal pathogens associated with infectious diarrhoea. Contamination of food by these pathogens can occur at any stage of food processing, food packaging or during handling of uncooked meat. These bacteria can therefore be easily be transmitted from birds to humans causing enteric disease (Zhao et al., Citation2001). However, some studies have suggested involvement of a novel poultry-associated pathogen Helicobacter pullorum in causing human infections including diarrhoea (Stanley et al., Citation1994). H. pullorum is an enteric Helicobacter species, many of which are infectious pathogens (Hameed & Sender, Citation2011). This bacterium has been associated with inflammatory bowel disease (IBD), colitis, cholecystitis, liver problems and cirrhosis in humans, and vibrionic hepatitis in chickens (Stanley et al., Citation1994).
H. pullorum prevalence has been reported in various poultry birds in various regions of the world. The prevalence rate can vary from 4 to 100% depending on geographical region and farming practices, but data from Asia are lacking despite poultry contributing to a large chunk of the agricultural economy (Ceelen et al., Citation2005). Moreover, presence of putative Type six secretory system (T6SS) associated genes have been identified in whole genome sequences of H. pullorum poultry and clinical isolates (Borges et al., Citation2015) but their association with bacterial virulence or prevalence is currently unknown. T6SS in Campylobacter jejuni has been associated with bloody diarrhoea and therefore makes a significant contribution to bacterial virulence (Harrison et al., Citation2014). Haemolysin coregulatory protein (Hcp), a structural and effector protein of the T6SS, is considered a hallmark gene of T6SS function (Bleumink-Pluym et al., Citation2013). In this study, we have described the presence of H. pullorum in retail poultry in suburban Rawalpindi, Pakistan, and screened for the presence of a possible virulence determinant, hcp gene of the T6SS in isolates.
Materials and methods
Sample collection and transport
Fresh poultry samples were purchased from retail markets from Tramri, Chak shehzad in suburban Rawalpindi, Pakistan. The samples were collected during 2016–2017 and the study was approved by the COMSATS University Islamabad ethical review board (CUI/ERB/16/47).
Special care was taken to avoid cross contamination, by ethanol sterilization of tools used during slaughtering of the birds. The distal portions of caecum and liver were cut using surgical blades and transferred into Falcon tubes containing phosphate buffered saline (PBS) with sterile forceps. Liver and caecum samples from each bird were transferred separately and transferred within 2 h to the lab for analysis.
Bacterial isolation and culture
Samples were processed for bacterial isolation on a sterile glass plate inside a biosafety cabinet (HF safe 1200, Heal Force). Chicken caeca were cut with sterile blades and washed once with PBS. The epithelial lining was scratched using a fresh blade and plated on Columbia agar (Oxoid, UK), supplemented with 5% defibrinated sheep blood and antibiotic cocktail (SR0147, Oxoid, UK). The supplement contains antibiotics (vancomycin, trimethoprim, cefsulodin and Amphotericin B) for selective growth. Liver samples were also cut, homogenized in PBS and plated. Plates were incubated under microaerophilic conditions (10% CO2, 5% O2, and 85% N2) provided by CampyGen sachets (Oxoid, UK) at 37°C for 48 h. Helicobacter colonies were phenotypically identified by their morphology as greyish-white colonies that were then selected and subcultured on selective media. Microscopic examination was used to confirm morphological characteristics specific of H. pullorum. Isolates were next subjected to Hippurate hydrolysis, catalase tests, oxidase tests for preliminary identification. (Ceelen et al., Citation2006). Since C. jejuni is able to hydrolyse hippurate, this test was used to differentiate H. pullorum as demonstrated previously (Atabay et al., Citation1998). All isolates were deposited in the CUI (COMSATS University Islamabad) Microbiology and Public Health Lab culture collection (Table S2).
Identification
DNA extraction was performed by the phenol–chloroform method using STE buffer (100 mM NaCl, 10 mM Tris-HCl pH 8.0 and 1 mM EDTA) for DNA resuspension and storage as described previously (http://campynet.vetinst.dk/Fla.htm). The quantity and quality of DNA was determined using NanoDrop spectrophotometer (Pearl, IMPLEN).
For identification, previously described Helicobacter genus-specific oligonucleotide primers, C97 and C05 (Beisele et al., Citation2011) and H. pullorum-specific oligonucleotide primers were used for species detection (Turk et al., Citation2012). Primer sequences are given in Supplementary Table S1. The cycling parameters used for Helicobacter genus PCR were: an initial denaturation at 94°C for 5 min, then 35 cycles at 94°C for 45 s annealing at 54°C for 45 s and extension at 72°C for 1 min, and then a final extension at 72°C for 5 min. The cycling parameters used for cytolethal distending toxin B (CdtB) screening were: an initial denaturation at 95°C for 2 min, then 35 cycles at 95°C for 45 s annealing at 50°C for 30 s and extension at 72°C for 45 s, and then a final extension at 72°C for 5 min. PCR products were resolved on a 1% agarose gel at 90 V, except cdtB PCR products which were resolved on a 2% agarose gel, and visualized under the gel documentation system GenoSens 1560 (Clinx Science Instruments Co., Ltd, Shanghai, China).
Virulence screening
In order to identify the presence of T6SS in our poultry isolates, H. pullorum-specific hcp primers were designed based on conserved H. pullorum putative hcp nucleotide sequences obtained from deposited H. pullorum genome sequences with the GenBank accession numbers ABQU00000000.1 (Human isolate), NZ_JNOA01000001.1, NZ_JNUR01000001.1 (poultry isolates). hcp sequences were aligned using a multiple sequence alignment tool, T-coffee for determining sequence homology and primers were designed manually within the conserved hcp sequences. Oligonucleotide properties were verified by OligoCalc (http://biotools.nubic.northwestern.edu/OligoCalc.html). The oligonucleotide primer sequences used for the screening of T6SS among the positive isolates of H. pullorum are included in Table S1. An initial denaturation at 95°C for 2 min, then 35 cycles on 95°C for 45 s, annealing at 54°C for 30 s and extension at 72°C for 45 s was performed, followed by a final extension at 72°C for 5 min.
Viability assay
Cell viability was determined by live/dead staining using trypan blue. HepG2 cells (obtained from ATCC) were maintained in DMEM supplemented with 10% FCS at 37°C, 10% CO2. The following day medium was discarded and cells were washed three times with DMEM (antibiotic free medium) and finally 3 ml of DMEM medium was added in each well. Bacteria were harvested from fresh plates and suspended in DMEM. OD600 was adjusted to a bacterial count of 5 × 108 CFUs/ml (pre-determined via CFU counts of serially diluted bacterial suspension of 1 OD). One well of HepG2 cells was trypsinized and cell counts determined using a Neubauer chamber. The remaining cells were infected with bacterial isolates at 10–100 multiplicity of infection (MOI) (10-100 bacteria per hepatocyte) and incubated at standard conditions for 6 h.
Cells were trypsinized after incubation and trypan blue was mixed with the cell suspension at a ratio of 1:1. The cell suspension was loaded into a haemocytometer. Live and dead cells were counted under a light microscope. To observe cell morphology, adherent cells were directly exposed to trypan blue at a ratio of 1:1 and visualized under the light microscope.
Haemolytic activity
Sheep blood (obtained from NARC) was washed three times in 9 volumes of PBS. After each washing cells were pelleted by centrifugation at 150 × g for 5 min. Final pellet was diluted 1:9 in sterile PBS. Bacterial cells were harvested from one-day-old plates, washed in PBS and resuspended to a concentration of 5 × 108 bacteria per ml. An equal volume of bacterial cells were mixed with the erythrocytes and centrifuged to facilitate bacteria and erythrocyte contact. The pellet was incubated microaerobically at 42°C for 4 h. After incubation, the pellet was resuspended in cold PBS and centrifuged. Absorbance at 540 nm of the supernatant fraction was determined using a UV-vis spectrophotometer thermospectronic (Model: Genesys 10-S, USA).
Results and discussion
H. pullorum is an enterohepatic Helicobacter species that colonizes the caecum and liver of poultry chickens and can be transmitted to humans where it is associated with gastroenteritis, colitis and inflammatory bowel disease (Atabay et al., Citation1998). The bacterium has also been isolated from chickens presenting diarrhoea and vibrionic hepatitis (Stanley et al., Citation1994). H. pullorum is considered an important food-associated emerging zoonotic pathogen (Javed et al., Citation2017)
H. pullorum prevalence in poultry
In this study, liver and caecum of broiler chickens obtained from the retail market were analyzed for the presence of the bacterium using both culture and molecular methods for detection. Greyish-white colonies appearing on selective medium after two days of culture were selected and subcultured on selective medium and, after preliminary confirmation through biochemical tests, genus-specific PCR and H. pullorum-specific PCR were performed for identification. A total number of 156 retail poultry broiler samples (comprising of caeca and liver of 78 birds) were obtained from the retail market, Chak shahzad, and G-10/1 Islamabad. Seventy-one (91%) liver samples and 78 (100%) caecal samples were culture positive and exhibited typical morphological and phenotypic characteristics of H. pullorum. Seven liver samples showed no growth exhibiting colony or morphological features typical of H. pullorum on selective plates. Of these 149 isolates, 54 (57.4%) belonged to the Helicobacter genus predominantly detected from the caecal contents while 42.5% were liver isolates ((A), ). H. pullorum identification was based on PCR screening of CdtB (cytolethal distending toxin B) gene ((B)). Among 54 Helicobacter-positive isolates 37 (68.5%) were positive for H. pullorum (), the rest could possibly be H. canadensis, which shows similar growth characteristics and niche (Manfreda et al., Citation2011).
Figure 1. Gel electrogram of PCR screening of bacterial isolates. (A) Helicobacter genus screening, L = 1 Kb Generuler DNA ladder. Numbered lanes indicate PCR amplified product on 1% agarose gel. Lane 1 is the negative control, lane 2 is the positive control H. pullorum MIT strain, lanes 1–12 are H. pullorum isolates. (B) H. pullorum cdtB screening PCR; amplicons were loaded onto a 2% agarose gel. L = 1 Kb Generuler DNA ladder, numbered lanes indicate PCR amplified product from screened isolates. (C) Hcp screening PCR; amplified product was loaded onto a 1% agarose gel. L = 100 bp Generuler DNA ladder. Numbered lanes indicate PCR amplified product from screened H. pullorum isolates.
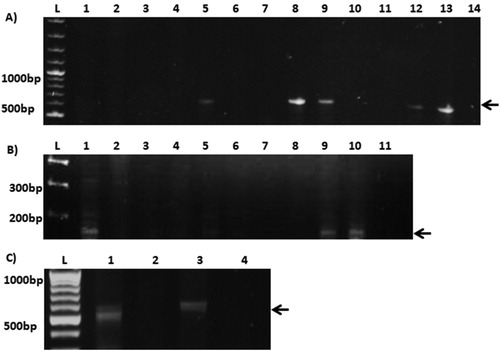
Table 1. Characterization of poultry isolates.
The prevalence of H. pullorum has been reported in a high number of birds worldwide. However, the overall prevalence rate in poultry seems to vary depending on geographical region. For instance, PCR-based identification showed 76.4% prevalence in turkeys in a Finnish study (Zanoni et al., Citation2011), whereas 23.5% fresh chicken meat tested by culture was positive for H. pullorum from different regions of Portugal (Borges et al., Citation2015). In contrast, molecular identification in liver and gastrointestinal tract samples of 110 broiler chickens showed lower prevalence rates in Belgium where 33.6% caecal, 31.8% colon, 10.9% jejunum and 4.6% liver samples tested positive for H. pullorum (Ceelen et al., Citation2006). Our study also shows similar prevalence rates in poultry birds in Pakistan with a greater bacterial burden in caeca (30.7%). At the same time, a high number of liver samples (18.3%) also tested positive for the bacterium. It must be noted that in our observation presence of the bacterium was not associated with any gross morphological anomalies of the liver or caecum or weight of the bird.
Presence of T6SS in H. pullorum
Despite the association of this bacterium with vibrionic hepatitis in chickens, and diarrhoea and colitis in humans the virulence mechanisms of H. pullorum have not been explored extensively. The cytotoxin, CdtB is the only well characterized virulence factor in the bacterium but it is present in all H. pullorum poultry isolates. This cytotoxin has been implicated in cellular oedema, cytoskeletal anomalies and G2/M cycle arrest in infected host cells (Young et al., Citation2000). In E.coli and Campylobacter, Cdt toxin can lead to IBS (inflammatory bowel syndrome) and excessive diarrhoea (Whitehouse et al., Citation1998). H. pullorum CdtB has also been shown to play a role in bacterial attachment and inflammation by stimulating the NF-KB pathway (Varon et al., Citation2009).
Recently it has been shown that presence of a functional Type six secretion system (T6SS) increases virulence of certain human and bird pathogens (Jani & Cotter, Citation2010). T6SS is a modification of phage tail like structure which helps to translocate the effector proteins to the host. Two important proteins associated with T6SS are Haemolysin coregulated protein (Hcp) and Valine glycine repeat (VgrG) (Pukatzki et al., Citation2009) which require each other for their proper function. Hcp plays a central role in the structural formation of the T6SS pilus, as well as acting as an effector protein in certain bacteria like E. coli, C. jejuni, and Vibrio cholera (Lien & Lai, Citation2017). In silico identification of T6SS genes has been performed in both H. pullorum avian and human genomes (Borges et al., Citation2015); however its prevalence in local strains has not been previously reported. Similarly, whether the presence of this secretion system confers added virulence properties to the bacterium remains to be seen. We therefore screened for T6SS gene Hcp in our isolates using hcp-specific primers designed for this purpose ((C)). Among 37 H. pullorum isolates only 11 (29.7%) possessed the hcp gene. Specifically, 36.37% of H. pullorum caecal isolates tested positive for hcp compared to 63.63% of liver isolates ().
H. pullorum affects host cell viability and presents enhanced haemolytic activity in hcp-positive isolates
To observe whether H. pullorum infection resulted in loss of host cell viability, human hepatocytes (HepG2) were infected with H. pullorum isolates at an MOI of 10 or 100. C. jejuni infection was used as a positive control which markedly reduced cell viability (6.3%) compared to the untreated control. In comparison infection with H. pullorum also resulted in a rapid loss of cell viability but to a lesser extent. This loss in cellular viability was not significantly influenced by presence of hcp- as hcp-negative isolates also induced loss of viability i.e. TH35 (81.6%) and TH37 (39.1%) to a similar extent as those possessing the hcp gene, i.e. TH4 (51.59%) and TH40 (51.52%). The reduction in cellular viability upon infection was much lower than that induced by C. jejuni; that is in case of TH35 a 75.3% difference in viability was observed compared to C. jejuni infected cells, while TH37, TH4 and TH40 showed differences of 32.8%, 45.29% and 45.22%, respectively ((A)). The T6SS genetic cluster in H. pullorum is conserved in chicken and human isolates, and similar in genetic organization to C. jejuni T6SS. However in contrast to C. jejuni T6SS, in H. pullorum it is organized in three blocks intercalated with genes of unknown function, two of which are inverted (Borges et al., Citation2015).
Figure 2. (A) H. pullorum infection affects host cell viability. Hepatocytes, HepG2 cells, were infected with C. jejuni (positive control) and T6SS-positive (TH4) and T6SS-negative (TH40) H. pullorum isolates. Top: % viability normalized to uninfected control. Viable and dead cells were counted from each well in three different fields of view and percentages of viable cells determined. Bottom: one representative trypan blue live/dead staining image from uninfected and infected wells. Arrows indicate dead cells; (B) Upper panel shows comparison of haemolytic activity of Hcp-positive (TH4L, TH40L, TH31C, TH39C, KH1, KH2, KH3, KH10, KH12, KH14 and KH21) and Hcp-negative (T3L, TH33C, TH35C and TH37L) H. pullorum isolates using sheep erythrocytes. SDS (1%) was used as a positive control. Lower panel, comparing the pooled haemolytic activity of T6SS-positive and T6SS-negative isolates respectively. Statistical analysis was performed using unpaired t-test. Results are expressed as mean of two independent experiments. *= P < 0.05, **= P < 0.005, ***= P < 0.0005.
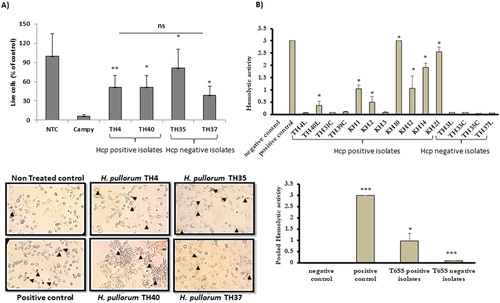
Both C. jejuni T6SS and H. hepaticus T6SS increase bacterial pathogenicity by enhancing the inflammatory response as well as influencing bacterial adhesion and invasion of host cells (Bartonickova et al., Citation2013). CDT and T6SS, apart from cellular attachment, also enhance the bacterium’s ability to colonize the hosts intestinal lining (Qumar et al., Citation2017). Furthermore, Hcp possesses haemolytic activity and has been shown to increase chances of having bloody diarrhoea in the case of C. jejuni (Harrison et al., Citation2014). To test whether H. pullorum Hcp presents similar haemolytic potential, sheep erythrocytes were infected with H. pullorum. After 6 h of infection, high haemolytic activity of infected cells was observed ((B)). Although great variability in haemolytic activity of all isolates tested was observed, the Hcp-positive isolates had significantly higher pooled haemolytic activity than Hcp-negative isolates, implicating H. pullorum Hcp as the probable potentiating factor.
A number of studies have suggested that Helicobacter species are present in the normal chicken microflora. The relative bacterial load of these pathogenic bacteria can be enhanced by decreased abundance of Lactobacillus and Corynebacterium and a higher abundance of Streptococcus and Ruminococcaceae in chickens (Kaakoush et al., Citation2014). Since the human gut, compared to that of chickens has a lower abundance of lactobacilli it may increase the likelihood of these pathogens causing disease in human hosts. The poultry birds obtained from the retail market in our study are transported from various farms in Punjab to Islamabad, therefore it is important to determine the source of the birds carrying these virulent strains. Further studies and routine microbiological screening of these birds are also necessary in order to curtail possible transmission to humans. CdtB and Hcp screening could be used as virulence markers for effective screening in farms.
Conclusion
This study shows that H. pullorum is frequently found in the liver and caecum of broiler chickens in Pakistan, and may be responsible for vibrionic hepatitis in chickens and colitis in humans. The presence of the T6SS gene hcp in a few isolates indicates highly virulent strains are present in poultry meat with the potential for causing severe disease. Therefore, the risk associated with infection by this zoonotic bacterium may be further confounded by high haemolytic activity observed in hcp-carrying isolates. This is the first study showing presence of T6SS in H. pullorum isolates and its possible role in pathogenesis.
Supplemental Material
Download MS Word (22.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Atabay, H.I., Corry, J.E. & On, S.L. (1998). Identification of unusual Campylobacter-like isolates from poultry products as Helicobacter pullorum. Journal of Applied Microbiology, 84, 1017–1024. doi: 10.1046/j.1365-2672.1998.00438.x
- Bartonickova, L., Sterzenbach, T., Nell, S., Kops, F., Schulze, J., Venzke, A., Brenneke, B., Bader, S., Gruber, A.D., Suerbaum, S. & Josenhans, C. (2013). Hcp and VgrG1 are secreted components of the Helicobacter hepaticus type VI secretion system and VgrG1 increases the bacterial colitogenic potential. Cellular Microbiology, 15, 992–1011. doi: 10.1111/cmi.12094
- Beisele, M., Shen, Z., Parry, N., Mobley, M., Taylor, N.S., Buckley, E., Abedin, M.Z., Dewhirst, F.E. & Fox, J.G. (2011). Helicobacter marmotae and novel Helicobacter and Campylobacter species isolated from the livers and intestines of prairie dogs. Journal of Medical Microbiology, 60, 1366–1374. doi: 10.1099/jmm.0.032144-0
- Bester, L.A. & Essack, S.Y. (2008). Prevalence of antibiotic resistance in Campylobacter isolates from commercial poultry suppliers in KwaZulu-Natal, South Africa. Journal of Antimicrobial Chemotherapy, 62, 1298–1300. doi: 10.1093/jac/dkn408
- Bleumink-Pluym, N.M.C., van Alphen, L.B., Bouwman, L.I., Wösten, M.M.S.M., van Putten, J.P.M. & Gaynor, E.C. (2013). Identification of a functional type VI secretion system in Campylobacter jejuni conferring capsule polysaccharide sensitive cytotoxicity. PLoS Pathogens, 9, e1003393. doi: 10.1371/journal.ppat.1003393
- Borges, V., Santos, A., Correia, C.B., Saraiva, M., Ménard, A., Vieira, L., Sampaio, D.A., Pinheiro, M., Gomes, J.P., Oleastro, M. & Dozois, C.M. (2015). Helicobacter pullorum isolated from fresh chicken meat: antibiotic resistance and genomic traits of an emerging foodborne pathogen. Applied and Environmental Microbiology, 81, 8155–8163. doi: 10.1128/AEM.02394-15
- Ceelen, L., Decostere, A., Verschraegen, G., Ducatelle, R. & Haesebrouck, F. (2005). Prevalence of Helicobacter pullorum among patients with gastrointestinal disease and clinically healthy persons. Journal of Clinical Microbiology, 43, 2984–2986. doi: 10.1128/JCM.43.6.2984-2986.2005
- Ceelen, L.M., Decostere, A., Van den Bulck, K., On, S.L., Baele, M., Ducatelle, R. & Haesebrouck, F. (2006). Helicobacter pullorum in chickens, Belgium. Emerging Infectious Diseases, 12, 263–267. doi: 10.3201/eid1202.050847
- Duggan, C., Santosham, M. & Glass, R.I. (1992). The management of acute diarrhea in children: oral rehydration, maintenance, and nutritional therapy. Centers for disease control and prevention. MMWR. Recommendations and Reports, 41, 1–20.
- Flammer, J., Haefliger, I.O., Orgul, S. & Resink, T. (1999). Vascular dysregulation: a principal risk factor for glaucomatous damage? Journal of Glaucoma, 8, 212–219. doi: 10.1097/00061198-199906000-00012
- Hameed, K.G.A. & Sender, G. (2011). Prevalence of Helicobacter pullorum in Egyptian hen’s eggs and in vitro susceptibility to different antimicrobial agents. Animal Science Papers and Reports, 29, 257–264.
- Harrison, J.W., Dung, T.T., Siddiqui, F., Korbrisate, S., Bukhari, H., Tra, M.P. & Champion, O.L. (2014). Identification of possible virulence marker from Campylobacter jejuni isolates. Emerging Infectious Diseases, 20, 1026–1029. doi: 10.3201/eid2006.130635
- Jani, A.J. & Cotter, P.A. (2010). Type VI secretion: not just for pathogenesis anymore. Cell Host & Microbe, 8, 2–6. doi: 10.1016/j.chom.2010.06.012
- Javed, S., Gul, F., Javed, K. & Bokhari, H. (2017). Helicobacter pullorum: an emerging zoonotic pathogen. Frontiers in Microbiology, 8, 604. doi: 10.3389/fmicb.2017.00604
- Jennings, J.L., Sait, L.C., Perrett, C.A., Foster, C., Williams, L.K., Humphrey, T.J. & Cogan, T.A. (2011). Campylobacter jejuni is associated with, but not sufficient to cause vibrionic hepatitis in chickens. Veterinary Microbiology, 149, 193–199. doi: 10.1016/j.vetmic.2010.11.005
- Kaakoush, N.O., Sodhi, N., Chenu, J.W., Cox, J.M., Riordan, S.M. & Mitchell, H.M. (2014). The interplay between Campylobacter and Helicobacter species and other gastrointestinal microbiota of commercial broiler chickens. Gut Pathogens, 6, 18. doi: 10.1186/1757-4749-6-18
- Lien, Y.W. & Lai, E.M. (2017). Type VI secretion effectors: methodologies and biology. Frontiers in Cellular and Infection Microbiology, 7, 254. doi: 10.3389/fcimb.2017.00254
- Manfreda, G., Parisi, A., Lucchi, A., Zanoni, R.G. & De Cesare, A. (2011). Prevalence of Helicobacter pullorum in conventional, organic, and free-range broilers and typing of isolates. Applied and Environmental Microbiology, 77, 479–484. doi: 10.1128/AEM.01712-10
- Pukatzki, S., McAuley, S.B. & Miyata, S.T. (2009). The type VI secretion system: translocation of effectors and effector-domains. Current Opinion in Microbiology, 12, 11–17. doi: 10.1016/j.mib.2008.11.010
- Qumar, S., Majid, M., Kumar, N., Tiwari, S.K., Semmler, T., Devi, S. & Ahmed, N. (2017). Genome dynamics and molecular infection epidemiology of multidrug-resistant Helicobacter pullorum isolates obtained from broiler and free-range chickens in India. Applied and Environmental Microbiology, 83, 1.
- Stanley, J., Linton, D., Burnens, A.P., Dewhirst, F.E., On, S.L.W., Porter, A., Owen, R.J. & Costas, M. (1994). Helicobacter pullorum sp. nov.-genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritis. Microbiology, 140, 3441–3449. doi: 10.1099/13500872-140-12-3441
- Turk, M.L., Cacioppo, L.D., Ge, Z., Shen, Z., Whary, M.T., Parry, N. & Fox, J.G. (2012). Persistent Helicobacter pullorum colonization in C57BL/6NTac mice: a new mouse model for an emerging zoonosis. Journal of Medical Microbiology, 61, 720–728. doi: 10.1099/jmm.0.040055-0
- Varon, C., Duriez, A., Lehours, P., Ménard, A., Layé, S., Zerbib, F. & Laharie, D. (2009). Study of Helicobacter pullorum proinflammatory properties on human epithelial cells in vitro. Gut, 58, 629–635. doi: 10.1136/gut.2007.144501
- Whitehouse, C.A., Balbo, P.B., Pesci, E.C., Cottle, D.L., Mirabito, P.M. & Pickett, C.L. (1998). Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infection and Immunity, 66, 1934–1940.
- Young, V.B., Knox, K.A. & Schauer, D.B. (2000). Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infection and Immunity, 68, 184–191. doi: 10.1128/IAI.68.1.184-191.2000
- Zanoni, R.G., Piva, S., Rossi, M., Pasquali, F., Lucchi, A., De Cesare, A. & Manfreda, G. (2011). Occurrence of Helicobacter pullorum in turkeys. Veterinary Microbiology, 149, 492–496. doi: 10.1016/j.vetmic.2010.11.013
- Zhao, C., Ge, B., De Villena, J., Sudler, R., Yeh, E., Zhao, S. & Meng, J. (2001). Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the greater Washington, D.C., area. Applied and Environmental Microbiology, 67, 5431–5436. doi: 10.1128/AEM.67.12.5431-5436.2001
