ABSTRACT
We evaluated a blend of medium-chain fatty acids (MCFA), organic acids, and a polyphenol antioxidant on gut integrity. Eighty Ross Broilers were exposed to 20–22°C (control – normothermic) or to 35–39.5°C (heat stress) for eight hours a day for a period of 1 or 5 days. Birds were fed a standard diet, or a diet supplemented with the test blend. Thereafter, birds were euthanized, and intestinal sections were excised for morphological, morphometric and gene expression analyses. Blood samples were collected for glucose-6-phosphate dehydrogenase (G6PD), glutathione peroxidase (GSH-Px) activity and trolox equivalent antioxidant capacity (TEAC) determination. Heart and liver tissues were used to quantify the expression of heat shock proteins 60 and 70 (HSP60 and HSP70, respectively) and inhibitor of kappa light chain gene enhancer in B cells alpha (IKBA). The jejunum was the most sensitive intestinal section, where heat stress modulated the expression of HSP70, of the inflammatory markers IKBA, interleukin 8 (IL-8), interferon gamma (IFNγ), and toll-like receptor 4 (TLR4). Moreover, expression of tight junctions (CLDN1, ZO1 and ZO2) and nutrient transporters (PEPT1 and EAAT3) was modulated especially in the jejunum. In conclusion, the feed additive blend protected intestines during heat stress from the decrease in villus height and crypt depth, and from the increase in villus width. Especially in the jejunum, heat stress played an important role by modulating oxidative stress and inflammation, impairing gut integrity and nutrient transport, and such deleterious effects were alleviated by the feed additive blend.
RESEARCH HIGHLIGHTS
Jejunum is the most sensitive intestinal segment during heat stress.
Heat stress affects the expression of tight junctions and nutrient transporters.
Feed management helps to alleviate the disturbances caused by heat stress.
A blend of MCFA, organic acids and a polyphenol protects broilers under heat stress.
Introduction
Heat stress is a worldwide problem, resulting in compromised bird welfare and economic losses due to decreased meat production and even bird mortality (Lara and Rostagno, Citation2013). This problem is aggravated by global warming resulting in more extreme climates (Tanizawa et al., Citation2014). At the same time, genetic progress in broilers resulting in more rapid growth, and consequently higher heat production by the bird itself (Havenstein et al., Citation2003), increases the sensitivity of birds to high environmental temperatures.
Birds dissipate heat via evaporative cooling from the respiratory tract, i.e. panting (Marder and Arad, Citation1989), which induces dehydration associated with oxidative stress and subsequent damage of the intestinal epithelium (Gu et al., Citation2012). Impairment of the intestinal barrier occurs because of a disturbance in the tight junction network. This may result in diminished absorption of nutrients, increased risk of bacterial translocation, and activation of the inflammatory cascade (Dokladny et al., Citation2006). Feed supplementation with organic acids is one of the strategies of managing intestinal health by inhibiting bacterial growth via modulation of pH and microbiota in the intestinal tract. Medium-chain fatty acids (MCFA) decrease the host response to inflammation (Carlson et al., Citation2015) and are candidates for use as supplements in the feed to counteract the effects of heat stress. Oxidative stress in the intestines can be lowered by providing locally effective antioxidants, such as plant polyphenols. It is known that under thermal stress, plants increase the biosynthesis of phenolic compounds as an acclimation mechanism (Rivero et al., Citation2001). These phenolic compounds play an important role due to their antioxidant, anti-inflammatory, and antimicrobial activities, and increase glutathione peroxidase activity in heat-challenged broilers (Akbarian et al., Citation2016).
Based on the above-mentioned information, we hypothesized that a blend of MCFA, organic acids and a phenolic plant extract (Presan FY®) may support the intestinal health of broilers challenged with heat stress. Furthermore, we aimed to gain knowledge on the effects of heat stress, period of heat exposure (1 or 5 days; 8 consecutive hours per day), and diet on broiler intestinal function. To assess this, intestinal integrity and functioning were evaluated by morphological and morphometric analysis of the duodenum, jejunum and ileum. Furthermore, we measured the mRNA expression of markers for tight junction proteins (claudin 1 and 5 – CLDN1 and CLDN5; zona occludens 1 and 2 – ZO1 and ZO2), inflammation (inhibitor of kappa light chain gene enhancer in B cells alpha – IKBA; interleukin 8 – IL-8; interferon gamma – IFNγ; and toll-like receptors 2 and 4 – TLR2 and TLR4), transforming growth factor beta (TGFß), nutrient transporters (excitatory amino-acid transporter 3 – EEAT3; peptide transporter 1 – PEPT1; sodium-dependent glucose transporter – SGLT) and oxidative stress (Haem oxygenase – HMOX; Heat Shock Proteins 60 and 70 – HSP60 and HSP70) in the small intestine. The mRNA expression of stress indicators (HMOX, HSP60 and HSP70) was also measured in the liver and myocardium.
Materials and methods
Birds and treatments
Ross Broilers (n = 80) were obtained at the age of 14 days from a commercial broiler farm. Birds were housed in special bird units with a light programme of 16 h light (04:00–20:00) per 24 h. On day 17, the birds weighed an average of 956±13 g (ranging of 944–970 g) and were randomly allocated to treatments and adapted to the individual feed mixtures for a period of 7 days, when they reached a mean body weight of 1600±40 g. Thereafter, each group was exposed to normal (20–22°C) or elevated (35–39.5°C for 8 h a day) temperature programmes for a period of 1 or 5 days and euthanized immediately after exposure, i.e. at days 1 or 5 (under the same temperature schedule) for tissue and blood sampling. Feed and water were given ad libitum. The experimental protocol was established in line with the prerequisites of the use of birds in research (DIRECTIVE 2010/63/EU) and had been approved prior to the onset of the experimental trials by the Animal Welfare Committee of the University of Veterinary Medicine Hannover (competent ethics committee of the university) on the uses of animals in research according to BGBl. I S. 1105/2.
Feed
The feed for the experimental period was delivered by Trouw Nutrition (Putten, the Netherlands) and consisted of a standard broiler feed that meets commercial practice recommendations. It was based on wheat, soybean meal, soybean oil, corn, minerals, amino acids and vitamins (see detailed composition at Supplementary Table 1). The standard feed was either not supplemented, or supplemented with a commercially available gut health additive (Presan FY®, Trouw Nutrition), which is based on MCFA (C8, C10 and C12), organic acids and a phenolic plant extract.
Sampling
As described above, the experimental protocol contained two distinct sampling points: 50% of the birds per group were euthanized immediately after 1-day heat challenge, and the remaining birds were euthanized after 5 days of heat stress. At both sampling points the body weight was recorded, and samples of liver, intestines and heart tissue were preserved for qRT-PCR analysis (described below). Blood samples were taken from each bird, after which plasma was collected and stored at −20°C for further analyses. Immediately after euthanasia, the small intestine was removed, and approximately 2-cm segments of the duodenum, jejunum and ileum were taken from the middle of each part. Two pieces per segment were collected, of which one piece was fixed in formalin 10% and embedded in paraffin for histology, and the other piece was snap-frozen in liquid nitrogen and stored at −80°C until RNA extraction for qRT-PCR.
Blood plasma analysis
Glucose-6-phosphate dehydrogenase (G6PD) concentration (U/g Hb) in total blood was measured with an assay kit from Trinity biotech (Wicklow, Ireland; no. 345-B). Plasma glutathione peroxidase (GSH-Px) activity (nmol NADP/min/ml) was measured by using a kit from Merck (Darmstadt, Germany; no. 353919). Plasma trolox equivalent antioxidant capacity (TEAC, mM) was measured by using an assay kit from Sigma (Saint Louis, MO, USA; no. CS0790).
Histological analyses and scores: morphology and morphometry
Four micrometer transverse sections were cut and every 10th section was kept and stained with haematoxylin–eosin. At least five samplings were performed for each tissue. Morphological and morphometrical analyses were performed as previously described by Santos et al. (Citation2015). In brief, the degree of mucosal damage was determined by applying the Chiu/Park scale (Quaedackers et al., Citation2000):
Degree 0: intact without visible damage;
Degree 1: damage in sub-epithelial space at villus tips;
Degree 2: extension of sub-epithelial space with moderate lifting;
Degree 3: massive lifting down the sides of villi with some denuded villi;
Degree 4: denuded villi with dilated capillaries:
Degree 5: disintegration of lamina propria;
Degree 6: crypt injury;
Degree 7: trans-mucosal infarction;
Degree 8: transmural infarction
To statistically compare the degrees among the treatments, a composite score per treatment was determined by averaging the score from each bird. For this, the percentage of villi with a specific degree was multiplied by its respective degree. This calculation was performed for each degree per treatment and the sum obtained was considered the composite score.
Morphometrical parameters were recorded using an image-analyzing system (Olympus, Tokyo, Japan), coupled to a microscope (Olympus). The villus height, top, middle and basal width of the villi, crypt depth, as well as epithelial cell, villi and serosa areas from morphologically normal villi were measured. A minimum of 15 villi per sample were measured. For this, villi having lamina propria were measured from the tip to base of the villi. Crypt depth was defined as the invagination between two villi. The number of villi per unit of serosa area and the mucosal (villus) surface area per unit serosa area were estimated.
Quantitative RT–PCR
RNA from samples of liver, heart and intestines was isolated using the SV Total RNA Isolation System (Promega, Madison, WI, USA) according to the manufacturer’s instructions, and total RNA was quantified by spectrophotometer (Nanodrop ND-1000, Thermo Scientific, Wilmington, DE, USA). Subsequently, 1 µg of extracted total RNA was reverse transcribed with the iScriptTM cDNA Synthesis kit (BIO-RAD, Hercules, CA, USA). The cDNA was diluted to a final concentration of 30 ng/µl. Primers, as presented in (Mott et al., Citation2008; Lorda-Diez et al., Citation2010; Osselaere et al., Citation2013), were commercially produced (Eurogentec, Maastricht, the Netherlands). The primers used were selected based on specificity and efficiency by qPCR analysis of dilution series of pooled cDNA at a temperature gradient (55–65°C) for primer-annealing and subsequent melting curve analysis. The reaction mixture for the qPCR containing 10 μl of the diluted cDNA was mixed with 15 μl iQSYBR Green Supermix (Bio Rad Laboratories Inc., USA), forward and reverse primers (final concentration of 0.4 pmol/μl for each primer) and sterile water according to the manufacturer's instructions. qPCR was performed using the MyIQ single-color real-time PCR detection system (Bio-rad) and MyiQ System Software Version 1.0.410 (Bio Rad Laboratories Inc., USA). Amplification efficiency was determined for each plate using linregPCR (Ruijter et al., Citation2009). Data were analysed using the efficiency-corrected Delta-Delta-Ct method (Pfaffl, Citation2001). The fold-change values of the genes of interest were normalized using the geometric mean of the fold-change values of two housekeeping genes. Housekeeping genes were tested for all the test conditions after which the most stable housekeeping genes were selected using the geNorm software. The most stable housekeeping genes had an M-value between 0.2 and 0.5. To determine if the inclusion of an additional housekeeping gene was required, the cut-off value for variation was set at 0.2. The fold-change values of the target genes were normalized using two housekeeping genes: hypoxanthine-guanine phosphoribosyl transferase (HPRT) and hexose-6-phosphate dehydrogenase (H6PD). The mRNA expression level of proteins involved in stress i.e. HMOX, HSP60 and HSP70 were evaluated in the liver, myocardium and intestines. Furthermore, mRNA expression of different markers in the duodenum, jejunum and ileum were measured: tight junction proteins (CLDN1, CLDN5, ZO1 and ZO2), inflammatory markers such as IKBA, IL-8, IFNγ, TLR2, TLR4, the marker of the transforming growth factor, TGFß, and the nutrient transporters EEAT3, PEPT1, and SGLT.
Table 1. Primers used for the quantification of genes of interest (GOI) and housekeeping gene (HKG) expression.
Statistical analysis
The data analysis for this paper was performed using SAS software, Version 9.1 of the SAS System (SAS Institute Inc., Cary, NC, USA). Data were compared using a 2 × 2 × 2 factorial design (Heat Stress × Diet × Day), where two-way ANOVA and LSmeans was applied. Differences were considered significant when P < 0.01, except for differences in gene expression, for which P < 0.001 was used.
Results
Bird inspection
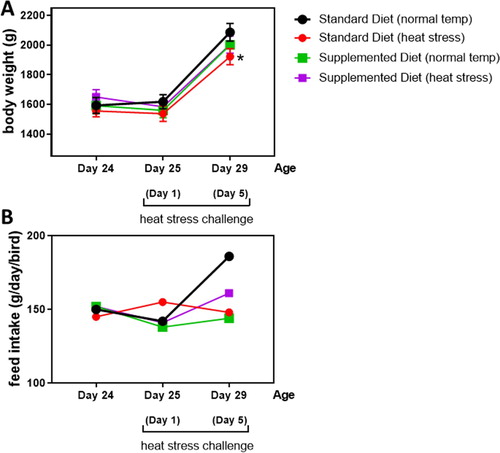
Birds were inspected every day for clinical signs of disease or discomfort. During heat exposure, the birds were mostly panting, with wing spreading and squatting close to the ground. Activity was regained immediately when room temperature fell below 30°C. After 1-day heat stress challenge, birds fed standard diet (body weight 1537 g) were numerically lighter than those receiving standard diet at normothermic conditions (body weight 1618 g), but no significant differences were observed among the groups. After 5 days of consecutive heat stress challenge, a significant decrease in body weight was observed in the birds fed standard diet (1922 g) when compared with those fed standard diet under normal temperature (2086 g; (a)). The mean body weight of the birds fed supplemented diet did not differ from any other treatment regardless of the presence (2003 g) or absence (2001 g) of heat stress challenge ((a)). Feed intake was measured for each group and the individual daily intake was estimated. Although no statistical analysis was possible, unchallenged birds fed the standard diet had the numerically highest feed intake ((b)).
Figure 1. Mean body weight and daily feed intake of the birds fed standard or supplemented diets at the start of the experiment (day 24) and after 1-day (25-days of age) or 5 days (29-days of age) heat stress challenges. *Differs significantly (P < 0.05) from birds fed standard feed under normal temperature.
Markers of cellular oxidative stress
Oxidative stress markers were assessed in plasma/blood samples (). There was a significant interaction of heat stress, diet and day on the G6PD levels, which were not affected by the diet or day of assessment alone. When birds were fed the standard diet, G6PD levels were significantly decreased in blood after one heat stress challenge (day 1) (1.59 U/g Hb) when compared to birds fed standard diet under normal temperature (6.03 U/g Hb), and such difference was maintained after 5 days heat stress. No changes were observed when birds were fed supplemented diets regardless of heat stress challenge, where G6PD levels were 4.19 and 3.44 U/g Hb at day 1, and 3.80 and 3.15 U/g Hb at day 5.
Table 2. Mean (± SEM) levels of glucose-6-phosphate dehydrogenase (G6PD), glutathione peroxidase (GSH-Px) activity and trolox equivalent antioxidant capacity (TEAC) in blood/plasma from birds fed with standard or supplemented diets under either heat stress challenge or normothermic conditions.
There was a significant interaction of heat stress, diet and day on the GSH-Px activity. A significant increase of GSH-Px activity (14.36 nmol/min/ml) was observed directly after 1-day of heat stress challenge in broilers fed standard diet compared to normothermic conditions (7.13 nmol/min/ml). However, after 5 days of heat stress, GSH-Px activity in plasma from birds fed standard diet decreased significantly (4.69 nmol/min/ml) when compared with 1-day heat stress. Birds fed supplemented diet presented GSH-Px activity similar to normothermic conditions, regardless of heat stress challenge ().
The TEAC levels were affected by the interaction of heat stress, diet and day. After 1-day of heat stress challenge, broilers fed standard diet presented a significant decrease in TEAC when compared to all the other treatments. After 5 days of heat stress, this difference was no longer observed. Again, birds fed supplemented diet presented TEAC levels similar to normothermic conditions, regardless of heat stress challenge ().
Morphological and morphometrical analyses of intestinal sections
Chiu/Park scale
All intestinal segments from birds at thermoneutral conditions were predominantly normal (50–80%) and, when presenting damage, this was limited to damage degree 1, i.e. impairment in the sub-epithelial space at villus tips, independent of the analysis period. However, 1- or 5-days heat stress challenge led to a significant increase in the severity (degree 2–3) of villus damage in duodenum and jejunum from birds fed standard diet. Birds fed supplemented diet and subjected to 1- or 5-days heat challenge showed the same duodenum villus damage levels as birds raised at normal temperature. However, the jejunum from birds fed supplemented diet and submitted to 1-day heat stress presented a significant increase in damage degree, followed by a decrease after 5 days of heat stress challenge ().
Table 3. Mean (± SEM) degree of villus damage in the duodenum, jejunum and ileum from non-stressed and heat stressed birds.
Morphometrical analysis
Duodenum
A significant decrease in the absorption surface area of the duodenum was observed, demonstrated by decreased villus height and villus width, in birds fed standard diet and submitted to heat stress for 1-day. After 5 days of heat stress, the villus width was significantly increased together with villus height and serosa width in challenged birds fed standard diet. Feeding birds a supplemented diet did not avoid decrease in villus length after 1-day heat stress, but avoided the decrease in villus width. After 5 days of heat stress, villus height from birds fed supplemented diet did not differ from those challenged birds fed standard diet ().
Table 4. Morphometrical analysis (mean ± SEM) of duodenum after 1-day or 5-days heat stress challenge.
Jejunum
One-day heat stress resulted in a significant decrease of the jejunum absorption area (villus height), decrease in enterocyte proliferation (crypt depth), and increase of the villus and serosa width in birds fed standard diet. These effects were counteracted by the supplemented diet, except for serosa width that remained increased. After 5 days of heat stress, birds fed standard diet presented a significant increase in villus and serosa width and a decrease in crypt depth. Feeding birds with supplemented diet during these 5 days decreased crypt depth ().
Table 5. Morphometrical analysis (mean ± SEM) of jejunum after 1-day and 5-days heat stress challenge.
Ileum
Birds fed standard diet and challenged with 1-day heat stress presented a significantly increased serosa width, and this effect was not counteracted by the supplemented diet. Importantly, birds fed supplemented diet after 1-day at normothermic conditions, presented a significant decrease in villus height. After 5 days of heat stress, birds fed standard diet presented a significant decrease of the villus height and villus area:epithelial cells area ratio, as well as increased serosa width. A recovery in both villus height and serosa width was observed in birds challenged for 5 days when fed supplemented diet ().
Table 6. Morphometrical analysis (mean ± SEM) of ileum after 1-day or 5-days heat stress challenge.
Gene expression
Oxidative stress markers
All the three tested oxidative stress markers were regulated by the interaction heat stress × day × diet, regardless of the evaluated tissue. depicts data from liver and heart, while depicts data from the intestinal sections. After 1-day heat stress, birds fed standard diet presented HSP70 up-regulation in the liver, heart and jejunum. Similarly, HMOX was up-regulated in the heart and ileum and down-regulated in the jejunum. On the other hand, HSP60 was up-regulated in the liver and down-regulated in the jejunum from broilers fed standard diet and exposed for 1 day to heat stress. Such effects were counteracted by diet supplementation. After 5 days of heat stress HMOX was up-regulated only in the duodenal tissue, while HSP60 was up-regulated in the jejunum and ileum from broilers fed standard diet. Again, such effects were not observed when broilers were fed supplemented diet during heat stress challenge. Remarkably, broilers fed standard diets and exposed to 5 days of heat stress kept the up-regulated levels of HSP70 in the liver, heart, jejunum, and ileum. This time, diet supplementation was not sufficient to avoid HSP70 up-regulation in the jejunum after 5 days of heat stress challenge.
Table 7. Mean (± SEM) relative mRNA expression of genes encoding markers for oxidative stress (HMOX, HSP60, HSP70) in the liver and heart.
Table 8. Mean (± SEM) relative mRNA expression of genes encoding markers for oxidative stress (HMOX, HSP60, HSP70) in the intestinal sections.
Inflammatory markers
depicts data from liver and heart, while depict data from the intestinal sections. An interaction diet × day × heat stress was observed when assessing the expression of markers for inflammation in the present study. IKBA was up-regulated in both liver and heart from birds fed a standard diet and submitted to 1-day heat stress (). Neither heat stress or test diet affected the expression of inflammatory markers in the duodenum, except for the up-regulation of IKBA when the birds were fed supplemented diet at day 1 (). The most significant changes in the expression of inflammatory markers were observed in the jejunum (). For instance, after 1-day heat stress, broilers fed standard diet presented down-regulation of IKBA, IL-8, and TLR4. When broilers were fed standard diet and submitted to 5 days heat stress, the jejunum presented mRNA expression of IL-8 and TLR4 similar to unchallenged broilers, with only a down-regulation in IFNγ and up-regulation of IKBA. This down- and up-regulation of, respectively, IFNγ and IKBA was also observed in broilers fed supplemented diet (). Up-regulation of IL-8 and IFNγ was observed in the ileum from broilers fed standard diet submitted to 1 and 5 days heat stress, respectively ().
Table 9. Mean (± SEM) relative mRNA expression of IKBA in the liver and heart from broilers.
Table 10. Mean (± SEM) relative mRNA expression of genes encoding markers for inflammation (IKBA, IFNγ, IL8, TLR2, TLR4) in the duodenum.
Table 11. Mean (± SEM) relative mRNA expression of genes encoding markers for inflammation (IKBA, IFNγ, IL8, TLR2, TLR4) in the jejunum.
Table 12. Mean (± SEM) relative mRNA expression of genes encoding markers for inflammation (IKBA, IFNγ, IL8, TLR2, TLR4) in the ileum.
Tight junction proteins
depict data from duodenum, jejunum and ileum, respectively. The significant alterations in the expression of mRNA of tight junction proteins were mostly observed in the jejunum, with minor changes in the duodenum and ileum. The ZO1 up-regulation in the duodenum was observed after 5 days heat stress challenge in birds fed standard diet, or in birds fed supplemented diet under normothermic conditions (). After 5 days of heat stress being fed standard diet, CLDN1 was up-regulated in the jejunum. This up-regulation was also observed in the jejunum from broilers kept under normal temperature for 5 days and fed supplemented diet. Relative mRNA expression of both ZO1 and ZO2 was affected by heat stress only when this challenge was repeated for 5 days. Importantly, ZO1 was also up-regulated in the jejunum from broilers fed supplemented diet for 5 days under normal temperature (). In the ileum, ZO1 was up-regulated after 5 days heat stress regardless the diet, while and ZO2 was down-regulated at day 1 and up-regulated at day 5, regardless of the diet and temperature of exposure ().
Table 13. Mean (± SEM) relative mRNA expression of genes encoding markers for tight junctions (CLDN1, CLDN5, ZO1 and ZO2) in the duodenum.
Table 14. Mean (± SEM) relative mRNA expression of genes encoding markers for tight junctions (CLDN1, CLDN5, ZO1 and ZO2) in the jejunum.
Table 15. Mean (± SEM) relative mRNA expression of genes encoding markers for tight junctions (CLDN1, CLDN5, ZO1 and ZO2) in the ileum.
Growth factor and glucose, amino acid and peptide transporters
depict data from duodenum, jejunum and ileum, respectively. The mRNA expression of TGFβ was not affected during the present study. In the duodenum, PEPT1 was up-regulated when broilers fed standard diet were submitted to 5 days heat stress. This effect was not observed at 1-day heat stress exposure, which was characterized by the up-regulation of SGLT regardless of the diet or housing temperature (). PEPT1 was down-regulated while EEA3 was up-regulated in the jejunum from broilers fed standard diet and submitted to 1-day heat stress. After 5 days of heat stress no effect was observed on PEPT1 expression in the jejunum. However, EAAT3 remained up-regulated including in the jejunum from heat-stressed broilers fed supplemented diet (). Expression of nutrient factors was not affected in the ileum ().
Table 16. Mean (± SEM) relative mRNA expression of genes encoding markers for growth factor (TGFβ) and nutrient transporters (PEPT1, SGLT and EAA3) in the duodenum.
Table 17. Mean (± SEM) relative mRNA expression of genes encoding markers for growth factor (TGFβ) and nutrient transporters (PEPT1, SGLT and EAA3) in the jejunum.
Table 18. Mean (± SEM) relative mRNA expression of genes encoding markers for growth factor (TGFβ) and nutrient transporters (PEPT1, SGLT and EAA3) in the ileum.
Discussion
We have characterized the intestinal responses to heat stress at two different time-points, immediately after exposure of broiler chickens to a single challenge (day 1) and after 5 days heat stress challenge (day 5). Furthermore, two different feed conditions were tested: standard feed or the same feed supplemented with a commercial gut health additive rich in MCFA, organic acids and a phenolic compound from a plant extract (Presan FY). The decreases in feed intake and body weight gain due to heat stress were expected and observed. However, due to the number of birds used, these are only indicative parameters. The focus of this study was to evaluate the effect of diet, day of challenge, and heat stress on selected functional blood parameters, gut morphology and mRNA expression of markers of oxidative stress, inflammation, tight junction proteins and transporters of glucose, amino acids and peptides.
The regulatory enzyme G6PD, present in red blood cells, is essential for providing the major part of NADPH, which promotes glutathione regeneration, protecting the cells against oxidative damage (Zhang et al., Citation2016). This enzyme is the only NADPH-producing enzyme activated due to oxidative stress (Filosa et al., Citation2003). After 1-day heat stress challenge, increased activity of GSH-PX in response to oxidative stress was observed, demanding a higher production of NADPH via G6PD. It seems that after 1-day of heat stress, the G6PD activity was already depleted as its activity was decreased. This decrease also remained during 5 days of heat stress, decreasing the source of NADPH for GSH-Px activity. This may explain why GSH-Px increased after 1-day heat stress with a subsequent decrease after 5 days of heat stress. Interestingly, the TEAC returned to normal levels, suggesting that the bird counteracted oxidative stress via non-enzymatic pathways. These blood and plasma alterations were not observed in broilers fed supplemented diets and submitted to heat stress. This effect may be attributed to the presence of a polyphenol in the tested additive. Indeed, it was shown before that feed supplementation with polyphenols improved the redox capacity in broilers (Dong et al., Citation2015), and resulted in an increased total number of red blood in heat stress challenged broilers (Aengwanich & Suttajit, Citation2013).
Morphological and morphometrical analyses of the intestinal tract confirmed that jejunum was the most sensitive intestinal section to heat stress followed by duodenum and ileum, as reported before (Santos et al., Citation2015). These findings may reflect a higher sensitivity of the jejunum to heat stress associated with a decreased cell proliferation of the intestinal cells. Self-renewing of the villi depends on the proliferation of the stem cells present in the crypt, which replace apoptotic cells in the villus (Tan & Barker, Citation2014). In a recent study, Xiaofang et al. (Citation2018) showed that intestinal cell proliferation is decreased in the jejunum, but not in the duodenum or ileum from broilers exposed to chronic heat stress (7 days). To compensate villus shortening, morphological changes such as an increase in villus width were observed, as intestinal villi can rapidly adapt their morphology and function with the aim to maintain the absorption of nutrients (Incharoen, Citation2013). These alterations were observed in the jejunum after 1 and 5 days of heat stress, and in the duodenum after 5 days of heat stress. It was remarkable that duodenal villus width decreased after 1-day of heat stress challenge, but increased after 5 days of heat stress. These variations show that intestinal segments react differently to heat stress, and parameters other than morphology should also be taken into account when evaluating heat stress and nutritional management for improving the entire intestinal function. Previous experiments had shown that a combination of organic acids with MCFA increases villus height and crypt depth without increasing villus width (Baltic et al., Citation2017). It is therefore assumed that also in the current experiments the blend of organics acids and MCFA contributed to the maintenance of intestinal functions by supporting enterocyte proliferation under the conditions of heat stress. The mRNA expression of oxidative stress markers helped to evaluate the effects of heat stress on the liver, myocardium and intestines in more detail. Heat shock proteins (HSPs) are chaperones involved in the maintenance of protein structure in cells under stress. HSP70 enhances cell thermo-tolerance by stabilizing hydrophobic regions in proteins avoiding their denaturation, while HSP60 is mostly involved in protein folding and cyto-protection against apoptosis and necrosis (Takada et al., Citation2010). It is also known that HSPs are involved in cellular defence mechanisms during adverse conditions such as exposure to heat (Parsell & Lindquist, Citation1993). Therefore, increased HSP level makes the bird more resistant to heat stress or different stressors. Not surprisingly, the most remarkable findings in the present study were related to HSP70 expression, which was up-regulated in the liver, heart and jejunum of broilers fed standard diet and exposed to heat stress for 1-day. After 5 days of challenge, HSP70 was also up-regulated in the ileum. The absence of HSP70 up-regulation in challenged birds fed supplemented diet indicates that these birds were not suffering sufficient stress conditions to activate the HSPs. HMOX was up-regulated in the ileum and down-regulated in the jejunum. It has been shown that HMOX promotes cell resistance against oxidative stress (He et al., Citation2001), explaining the greater sensitivity of the jejunum to heat stress than the duodenum and ileum.
Heat stress modulated inflammatory markers in the tissues. IKBA was up-regulated in the heart and liver of broilers fed standard diet and exposed to heat stress for 1-day, and in the jejunum from those birds challenged with 5 days heat stress. Such up-regulation was even higher in the jejunum from birds fed supplemented diet. Up-regulation of IKBA depicts the down-regulation of NFκB, a driver of inflammation, which is commonly activated shortly after heat stress (Nivon et al., Citation2012). It seems that the birds were able to cope with the stress in vital organs such heart and liver during stress, but not in the jejunum after 5 days challenge, where IKBA was up-regulated only when broilers were fed supplemented diet. The supplement tested in the present study is composed of MCFA, organic acids and plant extracts rich in polyphenols, all of them able to suppress NFκB signalling (Sahin et al., Citation2010; Kim et al., Citation2014; Carlson et al., Citation2015; Burt et al., Citation2016; Ebrahimzadeh et al., Citation2018), which was confirmed herein by the up-regulation of IKBA, a NFκB suppressor. This is also confirmed by the up-regulation of IKBA in the duodenum of birds fed supplemented diet, regardless of the stress challenge. Heat stress did not lead to the modulation of inflammatory markers in the duodenum and ileum to the same extent as in the jejunum. These findings are in line with the results of the morphological analysis showing that duodenum and ileum were the less sensitive intestinal sections to heat stress. In the jejunum a down-regulation of TLR4 and IL-8 immediately after 1-day heat stress challenge was observed, followed by a decrease in IFNγ mRNA expression after 5 days heat stress. This effect was observed in all challenged groups, regardless of the diet provided. TLR4 can be down-regulated after acute heat stress and is indicative of innate immune system disturbances under conditions of stress (Du et al., Citation2012). Also, as a response to stress, IL-8 enhances cell proliferation mainly in the crypts to stimulate the repair of intestinal injury (Zachrisson et al., Citation2001). This mechanism probably explains its up-regulation in the ileum and not in the jejunum, the most heat stress-sensitive intestinal section. Chronic heat stress leads to a decrease in the natural killer and TH1 cells (Nagai & Iriki, Citation2001), which are the main producers of IFNγ, explaining its down-regulation after 5-day heat stress. The inability of the supplemented diet to prevent IFNγ down-regulation can be explained by the fact that this blend does not act non-specifically against all inflammatory pathways.
Tight junctions are the first line barrier against the passage of luminal toxic compounds and pathogens into the circulation by preventing intestinal paracellular transport (Ulluwishewa et al., Citation2011). These junctions are mainly transmembrane protein complexes of claudins (CLDNs) and the cytosolic proteins zonula occludens, e.g. ZO1 and ZO2. One-day of heat stress was not enough to modulate the expression of these tight junctions. However, after 5 days of heat stress, birds fed standard diet presented an up-regulation of CDLN1 and ZO1 in the jejunum. The same up-regulation was observed in the jejunum of birds fed 5 days supplemented diet under normothermic conditions. Once more, these results confirm that the jejunum is the most sensitive intestinal section to heat stress (Santos et al., Citation2015; Varasteh et al., Citation2015). This up-regulation is most likely an adaptation/nutritional strategy to avoid intestinal leakage. It is important to note that tight junctions are dynamic structures interacting not only with antigens and pathogens but also with nutrients, ions and water (Ulluwishewa et al., Citation2011). Therefore, fluctuation in expression and assembly of tight junctions due to stress, as well as feed supplementation, is expected. A decrease in mRNA expression is generally observed under optimal conditions of gut health with low regenerative needs, whereas an increase is indicative for epithelial cell injury and tissue repair.
Not only the intestinal barrier is affected by heat stress, but its effect on growth factors and transporters of glucose, amino acids and peptides should be considered. Transport of di- and tri-peptides from the intestinal lumen into the enterocytes is performed by the peptide transporter 1 (PEPT1), which is most highly expressed in the duodenum, but also by the jejunum and ileum (Gilbert et al., Citation2007). Under feed restriction, PEPT1 is up regulated as means to uptake any available nutrient in the intestinal lumen (Madsen & Wong, Citation2011). PEPT1 down-regulation in the jejunum, on the other hand, may indicate the inability of the jejunum to adapt to heat stress with the same efficiency as the duodenum. The up-regulation of the amino acid transporter in the jejunum, therefore, appears as an alternative to cover the PEPT1 down-regulation. Glucose is absorbed across the apical sodium-dependent glucose transporter 1 (SGLT) system, which was also assessed by mRNA expression. Garriga et al. (Citation2006) previously reported the increase in apical glucose transport as an adaptation to heat stress. In the present study, not only one episode of heat stress increased SGLT expression in the duodenum, but also when broilers were fed a supplemented diet under thermoneutral conditions. This is also in line with a previous study showing an increased SGLT mRNA expression in rats fed a diet rich in medium-chain triacylglycerols (Yasutake et al., Citation1995). However, its interaction with MCFA remains to be understood.
In conclusion, our results indicate that duodenum, jejunum and ileum respond negatively to heat stress, but the jejunum remains the most sensitive intestinal section. Although morphological adaptation in the intestines is observed, a transient functional impairment and redox imbalance will certainly affect bird production and resilience. Feed management and the application of targeted feed additives appears to mitigate, or at least alleviate, functional alterations caused by heat stress. Feed enriched with a blend of MCFA, organic acids and a plant polyphenol has proven to be efficient in preventing heat stress-induced disturbances in redox balance, villus morphology and morphometry, as well as improving the integrity of tight junctions and nutrient transporters.
Disclosure statement
Presan® is a product of Nutreco; AA, PR, TvK, and CS are or were employees of Nutreco; others report no conflict of interest.
ORCID
Regiane R. Santos http://orcid.org/0000-0001-7030-6097
References
- Aengwanich, W. & Suttajit, M. (2013). Effect of polyphenols extracted from tamarind (Tamarindus indica L) seed coat on pathophysiological changes and red blood cell glutathione peroxidase activity in heat stressed broilers. International Journal of Biometeorology, 57, 137–143. doi: 10.1007/s00484-012-0540-z
- Akbarian, A., Michiels, J., Degroote, J., Majdeddin, M., Golian, A. & De Smet, S. (2016). Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. Journal of Animal Science and Biotechnology, 7, 37. doi: 10.1186/s40104-016-0097-5
- Baltic, B., Starcevic, M., Dordevic, J., Mrdovic, B. & Markovic, R. (2017). Importance of medium chain fatty acids in animal nutrition. IOP Conf. Ser. In. Earth Environmental Science, 85, 012048.
- Burt, S.A., Adolfse, S.J.M., Ahad, D.S.A., Tersteeg-Zijderveld, M.H.G., Post, J.A., Bruggemann, H. & Santos, R.R. (2016). Cinnamaldehyde, carvacrol and organic acids affect gene expression of selected oxidative stress and inflammation markers in IPEC-J2 cells exposed to Salmonella typhimurium. Phytotherapy Research, 30, 1988–2000. doi: 10.1002/ptr.5705
- Carlson, S.J., Nandivada, P., Chang, M.I., Mitchell, P.D., O’Loughlin, A., Cowan, E., Gura, K.M., Nose, V., Bistrian, B.R. & Puder, M. (2015). The addition of medium-chain triglycerides to a purified fish oil-based diet alters inflammatory profiles in mice. Metabolism, 64, 274–282. doi: 10.1016/j.metabol.2014.10.005
- Dokladny, K., Moseley, P.L. & Ma, T.Y. (2006). Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. American Journal of Physiology, Gastrointestinal, and Liver Physiology, 290, G204–G212. doi: 10.1152/ajpgi.00401.2005
- Dong, S., Li, H., Gasco, L., Xiong, Y., Guo, K.J. & Zoccarato, I. (2015). Antioxidative activity of the polyphenols from the involucres of Castanea mollissima Blume and their mitigating effects on heat stress. Poultry Science, 94, 1096–1104. doi: 10.3382/ps/pev101
- Du, Q., Min, S., Chen, L.Y., Ma, Y.D., Guo, X.L., Wang, Z. & Wang, Z.G. (2012). Major stress hormones suppress the response of macrophages through down-regulation of TLR2 and TLR4. Journal of Surgical Research, 173, 354–361. doi: 10.1016/j.jss.2010.10.016
- Ebrahimzadeh, S.K., Navidshad, B., Farhoomand, P. & Aghjehgheshlagh, F.M. (2018). Effects of grape pomace and vitamin E on performance, antioxidant status, immune response, gut morphology and histopathological responses in broiler chickens. South African Journal of Animal Science, 48, 324–336. doi: 10.4314/sajas.v48i2.13
- Filosa, S., Fico, A., Paglialunga, F., Balestrieri, M., Crooke, A., Verde, P., Abrescia, P., Bautista, J.M. & Martini, G. (2003). Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochemistry Journal, 370, 935–943. doi: 10.1042/bj20021614
- Garriga, C., Hunter, R.R., Amat, C., Planas, J.M., Mitchell, M.A. & Moretó, M. (2006). Heat stress increases apical glucose transport in the chicken jejunum. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 290, R195–R201. doi: 10.1152/ajpregu.00393.2005
- Gilbert, E.R., Li, H., Emmerson, D.A., Webb Jr K.E. & Wong, E.A. (2007). Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poultry Science, 86, 1739–1753. doi: 10.1093/ps/86.8.1739
- Gu, X.H., Hao, Y. & Wang, X.L. (2012). Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. intestinal oxidative stress. Poultry Science, 91, 790–799. doi: 10.3382/ps.2011-01628
- Havenstein, G.B., Ferket, P.R., & Qureshi, M.A. (2003). Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poultry Science, 82, 1500–1508. doi: 10.1093/ps/82.10.1500
- He, C.H., Gong, P., Hu, B., Stewart, D., Choi, M.E., Choi, A.M. & Alam, J. (2001). Identification of activating transcription factor 4 (ATF4) as a Nrf2 interacting protein: Implication for heme oxygenase-1 gene regulation. Journal of Biology and Chemistry, 276, 20858–20865. doi: 10.1074/jbc.M101198200
- Incharoen, T. (2013). Histological adaptations of the gastrointestinal tract of broilers fed diets containing insoluble fiber from rice hull meal. American Journal of Animal and Veterinary Sciences, 8, 79–88. doi: 10.3844/ajavsp.2013.79.88
- Kim, H.J., Yoon, H.J., Kim, S.Y. & Yoon, Y.R. (2014). A medium-chain fatty acid, capric acid, inhibits RANKL-induced osteoclast differentiation via the suppression of NFKB signaling and blocks cytoskeletal organization and survival in mature osteoclasts. Molecular Cell, 37, 598–604. doi: 10.14348/molcells.2014.0153
- Lara, L.J. & Rostagno M.H. (2013). Impact of heat stress on poultry production. Animal, 3, 356–369. doi: 10.3390/ani3020356
- Lorda-Diez, C.I., Montero, J.A., Garcia-Porrero, J.A. & Hurle, J.M. (2010). Tgfβ2 and 3 are coexpressed with their extracellular regulator Ltbp1 in the early limb bud and modulate mesodermal outgrowth and BMP signaling in chicken embryos. BMC Developmental Biology, 10, 69. doi: 10.1186/1471-213X-10-69
- Madsen, S.L. & Wong, E.A. (2011). Expression of the chicken peptide transporter 1 and the peroxisome proliferator-activated receptor α following feed restriction and subsequent refeeding. Poultry Science, 90, 2295–2300. doi: 10.3382/ps.2010-01173
- Marder, J. & Arad, Z. (1989). Panting and acid-base regulation in heat stressed birds. Comparative Biochemistry & Physiology Part A: Molecular and Integrative Physiology, 94, 395–400. doi: 10.1016/0300-9629(89)90112-6
- Mott, C.R., Siegel, P.B., Webb Jr K.E. & Wong, E.A. (2008). Gene expression of nutrient transporters in the small intestine of chickens from lines divergently selected for high or low juvenile body weight. Poultry Science, 87, 2215–2224. doi: 10.3382/ps.2008-00101
- Nagai, M. & Iriki, M. (2001). Changes in immune activities by heat stress. Thermotherapy for Neoplasia, Inflammation and Pain, 1, 266–270. doi: 10.1007/978-4-431-67035-3_30
- Nivon, M., Abou-Samra, M., Richet, E., Guyot, B., Arrigo, A.P. & Kretz-Remy, C. (2012). NF-kB regulates protein quality control after heat stress through modulation of the BAG3-HspB8 complex. Journal of Cell Science, 125, 1141–1151. doi: 10.1242/jcs.091041
- Osselaere, A., Santos, R., Hautekiet, V., De Backer, P., Chiers, K., Ducatelle, R. & Croubels, S. (2013). Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS One, 8, e69014. doi: 10.1371/journal.pone.0069014
- Parsell, D.A. & Lindquist, S. (1993). The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annual Review of Genetics, 27, 437–496. doi: 10.1146/annurev.ge.27.120193.002253
- Pfaffl, M.W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research, 29, e45. doi: 10.1093/nar/29.9.e45
- Quaedackers, J.S., Beuk, R.J., Bennet, L., Charlton, A., Oude Egbrink, M.G., Gunn, A.J. & Heineman, E. (2000). An evaluation of methods for grading histologic injury following ischemia/reperfusion of the small bowel. Transplant Proceedings, 32, 1307–1310. doi: 10.1016/S0041-1345(00)01238-0
- Rivero, R.M., Ruiz, J.M., Garcia, P.C., Lopez-Lefebre, L.R., Sanchez, E. & Romero, L. (2001). Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Science, 160, 315–321. doi: 10.1016/S0168-9452(00)00395-2
- Ruijter, J.M., Ramakers, C., Hoogaars, W.M., Karlen, Y., Bakker, O., Van den Hoff, M.J. & Moorman, A.F. (2009). Amplification efficiency: linking baseline and bias in the analysis of quantitative qPCR. Nucleic Acids Research, 37, e45. doi: 10.1093/nar/gkp045
- Sahin, K., Orhan, C., Tuzcu, M., Ali, S., Sahin, N. & Hayirli, A. (2010). Epigallocatechin-3-gallate prevents lipid peroxidation and enhances antioxidant defense system via modulating hepatic nuclear transcription factors in heat stressed quails. Poultry Science, 89, 2251–2258. doi: 10.3382/ps.2010-00749
- Santos, R.R., Awati, A., Roubos-van den Hil, P., Tersteeg-Zijderveld, M.H.G., Koolmees, P.A. & Fink-Gremmels, J. (2015). Quantitative histo-morphometric analysis of heat stress related damage in the small intestines of broiler chickens. Avian Pathology, 44, 19–22. doi: 10.1080/03079457.2014.988122
- Takada, M., Otaka, M., Takahashi, T., Izumi, Y., Tamaki, K., Shibuya, T., Sakamoto, N., Osada, T., Yamamoto, S., Ishida, R., Odashima, M., Itoh, H. & Watanabe, S. (2010). Overexpression of a 60-kDa heat shock protein enhances cytoprotective function of small intestinal epithelial cells. Life Science, 86, 499–504. doi: 10.1016/j.lfs.2010.02.010
- Tan, D.W. & Barker, N. (2014). Intestinal stem cells and their defining niche. Current Topics in Developmental Biology, 107, 77–107. doi: 10.1016/B978-0-12-416022-4.00003-2
- Tanizawa, H., Shiraishi, J., Tsudzuki, M. & Bungo, T. (2014). Effect of short-term thermal conditioning on physiological and behavioral responses to subsequent acute heat exposure in chicks. Journal of Poultry Science, 51, 80–86. doi: 10.2141/jpsa.0130040
- Ulluwishewa, D., Anderson, R.C., McNabb, W.C., Moughan, P.J., Wells, J.M. & Roy, N.C. (2011). Regulation of tight junction permeability by intestinal bacteria and dietary components. Journal of Nutrition, 141, 769–776. doi: 10.3945/jn.110.135657
- Varasteh, S., Braber, S., Akbari, P., Garssen, J. & Fink-Gremmels, J. (2015). Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto oligosaccharides. PLoS One, 10, e0138975. doi: 10.1371/journal.pone.0138975
- Xiaofang, H., Lu, Z., Ma, B., Zhang, L., Li, J., Jiang, Y., Zhou, G. & Gao, F. 2018. Chronic heat stress damages small intestinal epithelium associated with AMPK pathway in broilers. Journal of Agriculture and Food Chemistry, 66, 7301–7309. doi: 10.1021/acs.jafc.8b02145
- Yasutake, H., Goda, T. & Takase, S. (1995). Dietary regulation of sucrase-isomaltase gene expression in rat jejunum. Biochimica et Biophysica Acta, 1243, 270–276. doi: 10.1016/0304-4165(94)00143-L
- Zachrisson, K., Neopikhanov, V., Wretlind, B. & Uribe, A. (2001). Mitogenic action of tumour necrosis factor-alpha and interleukin-8 on explants of human duodenal mucosa. Cytokine. 15, 148–155. doi: 10.1006/cyto.2001.0917
- Zhang, J., Cao, M., Yang, W., Sun, F., Xu, C., Yin, L. & Pu, Y. (2016). Inhibition of glucose-6-phosphate dehydrogenase could enhance 1,4-benzoquinone-induced oxidative damage in K562 cells. Oxidative Medicine and Cellular Longevity, 2016, 3912515.
