ABSTRACT
Histomonosis, or blackhead disease, is a well-known disease in turkeys that can cause high mortality, but outbreaks with lower losses are also observed. The disease is less fatal in chickens but is economically important due to reduced performance and its co-appearance with colibacillosis. The lack of specific prophylactic and therapeutic interventions has led to a re-emergence of the disease in recent years, mainly in turkeys, free-range layers and chicken parent stock.
Background
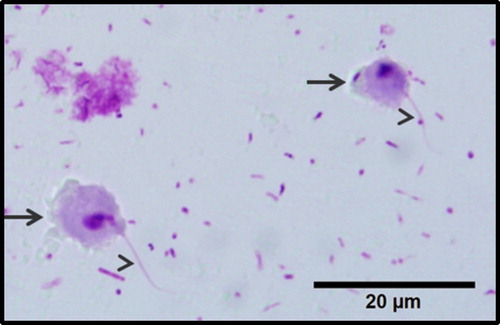
Histomonosis caused by Histomonas meleagridis () was first described in 1893 as a fatal disease in turkeys (Cushman, Citation1893). Soon afterwards, Chester & Robin (Citation1901) were able to reproduce the disease in chickens. The disease is reported in several countries indicating a wide spread of the parasite (McDougald, Citation2005). Due to intensive efforts in research, efficacious medication was implemented in the middle of the last century which led to the disappearance of the disease (Liebhart et al., Citation2017). The withdrawal of such drugs used for prophylactic and therapeutic purposes resulted in the re-emergence of the disease in recent years (Hess et al., Citation2015; Clark & Kimminau, Citation2017). Considering the importance in the field, the main focus is on turkeys due to the high mortality occasionally noticed. In contrast, chickens are very often described as a reservoir and asymptomatic carriers of the protozoan parasite. However, recent data from the field, together with experimental data, underline the importance of histomonosis in chicken layers and breeders (Grafl et al., Citation2011; van der Heijden & Landman, Citation2011; Liebhart et al., Citation2013; Dolka et al., Citation2015). Despite this, current research on the protozoan parasite and the disease is very limited, which might be due to several factors like difficulties in handling the parasite in vitro, which is also reflected by the great variation within experimental trials (Hauck & Hafez, Citation2013), different influences on the disease in the field and the extensive knowledge gained in the first half of the last century until development of efficacious medication. The lack of research is very obvious considering genetic data of the protozoan parasite, which also reflects the difficulties in establishing well-defined cultures.
Clinical signs and macroscopic lesions of histomonosis
In turkeys and chickens, apathy, depression, and ruffled feathers, together with decreased feed and water uptake, characterize clinical signs. A sulfur-coloured diarrhoea might be noticed in turkeys due to the rapid progression of the disease and the severe destruction of the liver leading to high mortality. Mortality in turkeys can vary from <10% up to the total loss of the flock. In layer chickens, a drop in egg production (up to 30%) was demonstrated experimentally (Liebhart et al., Citation2013). An increase of mortality (∼2%/week) lasting several weeks might be noticed in chickens, accompanied by a lower body weight. If pullets at the end of rearing are infected, egg shell quality as well as egg production decrease for several weeks and mortality stays above target values (Gerth et al., Citation1985).
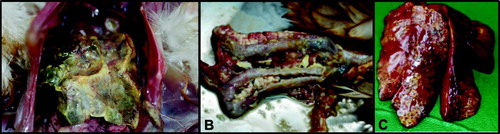
While prominent liver lesions are frequently present in turkeys, they are less obvious, or even absent, in chickens. Frequently, histomonosis in chickens appears together with an Escherichia coli infection characterized by salpingitis and peritonitis (). Therefore, it is advisable to perform a complete post mortem investigation with special focus on the caeca in case of colibacillosis. Histology can be used to differentiate histomonosis from coccidiosis.
Control
In Europe, North America and other parts of the world no specific treatment is available for use to treat the disease in chickens and turkeys. Since its initial description in 1962 by Lindquist (Citation1962), various experimental studies have demonstrated that paromomycin, an aminoglycoside antibiotic, effectively minimizes the disease outcome when given prophylactically but it contradicts efforts to reduce antimicrobials in the feed (Liebhart et al., Citation2017). In Europe, the “off-label” use of this antibiotic is sometimes practised in diseased chickens and turkeys. A dose of 12.5 mg/kg bodyweight has been administered in turkeys in recent outbreaks of histomonosis in Austrian turkey flocks with varying success which can be explained by the progression of the disease prior to medication (Sulejmanovic et al., Citation2017). Administration of the same dosage in experimental infections of broilers showed a reduction of lesions when applied at the day of challenge underlining the prophylactic action of the drug (Vereecken et al., Citation2015).
Biosecurity is of high importance to control the introduction of the pathogen into a flock and the infection. Preventing contact with the caecal worm Heterakis gallinarum, which acts as an efficient intermediate host, has a high priority. Because H. meleagridis changes the milieu in the caecum, hardly any nematodes are found during necropsy of turkeys suffering from blackhead disease. Therefore, the presence of H. gallinarum should be confirmed in faecal samples or in caeca of non-diseased birds from the same flock as well. Furthermore, it needs to be considered that the reproduction of the caecal worm is generally much lower in turkeys than in chickens (Lund & Chute, Citation1974).
Although a non-compromised gut barrier seems of high importance for the progression of histomonosis, controversial results are reported with regard to the interaction with Eimeria spp. Whereas the presence of Eimeria tenella can support the spread of the flagellate to the liver, E. adenoeides interfered negatively with the progression of histomonosis in a dose-dependent manner in the relevant host (McDougald & Hu, Citation2001; McDougald & Fuller, Citation2005). Vice versa, H. meleagridis negatively influences oocyte production of E. tenella due to the tissue damage, depending on the time points of infection (Chappel, Citation1973).
The aetiology of blackhead disease was questioned for a long time due to the yet unresolved interaction between the parasite and certain bacteria (Hess, Citation2017). It is well known that a certain composition of microbiota favours the infection, and gnotobiotic turkeys or chickens are insusceptible (Bradley & Reid, Citation1966; Hauck, Citation2017). Although this might bear options for manipulating the infection, an effective way to interrupt the infectious cycle has not yet been established. So far, it remains speculative whether influencing the microbiota is the mode of action of certain herbal products for which a positive effective is sometimes noticed in layer chickens. However, experimental data in turkeys revealed reciprocal in vitro and in vivo results, with the latter results indicating that those substances are largely ineffective (Liebhart et al., Citation2017). Very often colibacillosis is noticed in chickens following histomonosis, indicating a translocation of E. coli from the gut to internal organs most likely because of a damaged gut barrier (Paudel et al., Citation2018). The appearance of colibacillosis argues for an intervention with antimicrobials which faces general constraints for the use of drugs.
Vaccination as a new protection strategy was recently demonstrated in various experimental studies as highly efficacious (Liebhart et al., Citation2017). Considering the big hurdles of licencing new pharmaceuticals in poultry (Regmi et al., Citation2016), vaccination might be the preferred option keeping in mind that a live vaccine is needed which has to contain at least one bacterial strain in order to promote the growth of the parasite in vitro and to support infection in vivo. However, more investigations are needed prior to registration and a wider use in the field and to fully elucidate the mode of action (Mitra et al., Citation2018).
Outlook and perspectives
Due to the ban of efficacious drugs for prophylaxis and therapeutic purposes blackhead disease is a re-emerging disease in turkeys and chickens. The complicated nature of the pathogen, its epidemiology and the various influences on the pathogenesis of the disease need substantial efforts in order to develop a sophisticated protection strategy.
The interaction between H. meleagridis and the microbiota has hardly been studied and the true nature of the interaction between the protozoan parasite and certain bacteria is not yet resolved. This might be important to implement a targeted intervention in vivo but also to establish axenic cultures in vitro, which so far do not exist.
The epidemiology of the pathogen and the disease is poorly understood and very basic questions remain to be answered. This targets features like the introduction of H. meleagridis into a poultry house and its transmission within a flock. More detailed investigations should help to explain the huge variations in mortality and the manifestation of the parasite on certain farms. Furthermore, the absence of the intermediate vector H. gallinarum in caeca of diseased birds argues for a resistant form of the parasite as no true cysts have been detected so far. Whereas the infection of free-range chickens can be explained by low biosecurity, the appearance of blackhead disease in parent stock is much more difficult to resolve. Therefore, an intensive monitoring should be applied based upon PCR to detect the nucleic acid of the parasite, and ELISA for serological examinations to confirm infection. Special attention should be paid to trace H. gallinarum as histomonads survive for up to 3 years in the egg of the nematode.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bradley, R.E. & Reid, W.M. (1966). Histomonas meleagridis and several bacteria as agents of infectious enterohepatitis in gnotobiotic turkeys. Experimental Parasitology, 19, 91–101. doi: 10.1016/0014-4894(66)90057-9
- Chappel, L.R. (1973). The effect of Histomonas meleagridis on the development of Eimeria tenella. Journal of Parasitology, 59, 637–643. doi: 10.2307/3278854
- Chester, F.D. & Robin, A. (1901). Enterohepatitis or blackhead of fowls. Twelfth Annual Report Delaware Agricultural Station, 60–66.
- Clark, S. & Kimminau, E. (2017). Critical review: future control of blackhead disease (histomoniasis) in poultry. Avian Diseases, 61, 281–288. doi: 10.1637/11593-012517-ReviewR
- Cushman, S. (1893). The production of turkeys. Rhode Island Agricultural Experiment Station Bulletin, 25, 89–123.
- Dolka, B., Żbikowski, A., Dolka, I. & Szeleszczuk, P. (2015). Histomonosis – an existing problem in chicken flocks in Poland. Veterinary Research Communications, 39, 189–195. doi: 10.1007/s11259-015-9637-2
- Gerth, C., Rüdiger-Boesch, B., Schmidt, U., Mumme, J. & Friedhoff, K.T. (1985). Histomoniasis in pullet stock and its effect on later laying performance. Tierärztliche Praxis, 13, 519–527.
- Grafl, B., Liebhart, D., Windisch, M., Ibesich, C. & Hess, M. (2011). Seroprevalence of Histomonas meleagridis in pullets and laying hens determined by ELISA. The Veterinary Record, 168, 160. doi: 10.1136/vr.c6479
- Hauck, R. (2017). Interactions between parasites and the bacterial microbiota of chickens. Avian Diseases, 61, 428–436. doi: 10.1637/11675-051917-Review.1
- Hauck, R. & Hafez, H.M. (2013). Experimental infections with the protozoan parasite Histomonas meleagridis: a review. Parasitology Research, 112, 19–34. doi: 10.1007/s00436-012-3190-5
- Hess, M. (2017). Commensal or pathogen – a challenge to fulfil Koch’s postulates. British Poultry Science, 58, 1–12. doi: 10.1080/00071668.2016.1245849
- Hess, M., Liebhart, D., Bilic, I. & Ganas, P. (2015). Histomonas meleagridis – new insights into an old pathogen. Veterinary Parasitology, 208, 67–76. doi: 10.1016/j.vetpar.2014.12.018
- Liebhart, D., Ganas, P., Sulejmanovic, T. & Hess, M. (2017). Histomonosis in poultry: previous and current strategies for prevention and therapy. Avian Pathology, 46, 1–18. doi: 10.1080/03079457.2016.1229458
- Liebhart, D., Sulejmanovic, T., Grafl, B., Tichy, A. & Hess, M. (2013). Vaccination against histomonosis prevents a drop in egg production in layers following challenge. Avian Pathology, 42, 79–84. doi: 10.1080/03079457.2012.760841
- Lindquist, W.D. (1962). Some effects of paromomycin sulfate on blackhead in turkeys. American Journal of Veterinary Research, 23, 1053–1056.
- Lund, E.E. & Chute, A.M. (1974). The reproductive potential of Heterakis gallinarum in various species of galliform birds: implications for survival of H. gallinarum and Histomonas meleagridis to recent times. International Journal for Parasitology, 4, 455–461. doi: 10.1016/0020-7519(74)90061-7
- McDougald, L.R. (2005). Blackhead disease (histomoniasis) in poultry: a critical review. Avian Diseases, 49, 462–476. doi: 10.1637/7420-081005R.1
- McDougald, L.R. & Fuller, L. (2005). Blackhead disease in turkeys: direct transmission of Histomonas meleagridis from bird to bird in a laboratory model. Avian Diseases, 49, 328–331. doi: 10.1637/7257-081004R.1
- McDougald, L.R. & Hu, J. (2001). Blackhead disease (Histomonas meleagridis) aggravated in broiler chickens by concurrent infection with cecal coccidiosis (Eimeria tenella). Avian Diseases, 45, 307–312. doi: 10.2307/1592969
- Mitra, T. Kidane, F.A., Hess, M. & Liebhart D. (2018). Unravelling the immunity of poultry against the extracellular protozoan parasite Histomonas meleagridis, is a cornerstone for vaccine development: a review. Frontiers in Immunology 9, 2518 ID:91034.
- Paudel, S., Stessl, B., Fürst, C., Jandreski-Cvetkovic, D., Hess, M. & Hess, C. (2018). Identical genetic profiles of Escherichia coli isolates from the gut and systemic organs of chickens indicate systemic bacterial dissemination, most likely due to intestinal destruction caused by histomonosis. Avian Diseases, 62, 300–306. doi: 10.1637/11816-021818-Reg.1
- Regmi, P.R., Shaw, A.L., Hungerford, L.L., Messenheimer, J.R., Zhou, T., Pillai, P., Omer, A. & Gilbert, J.M. (2016). Regulatory considerations for the approval of drugs against histomoniasis (blackhead disease) in turkeys, chickens, and game birds in the United States. Avian Diseases, 60, 725–730. doi: 10.1637/11451-061516-Review.1
- Sulejmanovic, T., Liebhart, D., Magdefrau-Pollan, B., Sanglhuber, E.M., Wiesinger, E., Bilic, I. & Hess, M. (2017). Emergence of fatal histomonosis in meat turkey flocks in Austria from 2014 to 2016. Wiener Tierärztliche Monatsschrift 104, 277–287.
- van der Heijden, H.M.J.F. & Landman, W.J.M. (2011). High seroprevalence of Histomonas meleagridis in Dutch layer chickens. Avian Diseases, 55, 324–327. doi: 10.1637/9609-120610-ResNote.1
- Vereecken, M., Depondt, W., Geerinckx, M. & Gussem, K.d. (2015). Impact of an experimental Histomonas meleagridis infection in poultry and the efficacy of different dosages of paromomycin sulphate upon this infection. Proceedings of the XIXth Congress of the World Veterinary Poultry Association (p. 1100-1104). 07-11 September, Cape Town.