ABSTRACT
Bordetella avium (BA) is a respiratory pathogen of particular importance for turkeys. Specific adherence and damage to the respiratory epithelia are crucial steps of the pathogenesis, but knowledge about the mechanisms and the variety of virulence in field strains is limited. We analysed 17 BA field strains regarding their in vitro virulence-associated properties in tracheal organ cultures (TOC) of turkey embryos, and their genetic diversity. The TOC adherence assay indicated that BA field strains differ considerably in their ability to adhere to the tracheal mucosa, while the TOC ciliostasis assay illustrated a high degree of diversity in ciliostatic effects. These two virulence-associated properties were associated with each other in the investigated strains. Three of the investigated strains displayed significantly (P > 0.05) lower in vitro virulence in comparison to other strains. Genetic diversity of BA strains was analysed by core genome multilocus sequence typing (cgMLST). We applied a cgMLST scheme comprising 2667 targets of the reference genome (77.3% of complete genome, BA strain 197N). The results showed a broad genetic diversity in BA field strains but did not demonstrate a correlation between sequence type and virulence-associated properties. The cgMLST analysis revealed that strains with less marked virulence-associated properties had a variety of mutations in the putative filamentous haemagglutinin gene. Likewise, amino acid sequence alignment indicated variations in the protein. The results from our study showed that both adherence and ciliostasis assay can be used for virulence characterization of BA. Variations in the filamentous haemagglutinin protein may be responsible for reduced virulence of BA field strains.
Introduction
Bordetella avium (BA) is a bacterial pathogen of poultry and belongs to the genus Bordetella, which contains fifteen species with varying host tropism. While the phylogenetically closely related species B. bronchiseptica, B. holmesii, B. parapertussis and B. pertussis are adapted to mammalian hosts, the more distantly related B. avium and B. hinzii are bird-associated. Regarding the remaining species (B. ansorpii, B. bronchialis, B. flabilis, B. muralis, B. petrii, B. sputigena, B. trematum, B. tumbae and B. tumulicola), host tropism, reservoir and pathogenic roles have not been extensively studied yet.
BA is widely disseminated in wild and domesticated birds (Raffel et al., Citation2002; Stenzel et al., Citation2017) in the United States and Europe, but commercially raised turkeys are the most affected host for clinical disease. Mainly young turkeys display respiratory clinical signs during avian bordetellosis (Hinz et al., Citation1978; Saif et al., Citation1980; Kersters et al., Citation1984). Upon infection, BA colonizes the upper respiratory tract and adheres specifically to the local ciliated epithelium (Gray et al., Citation1981; Temple et al., Citation1998; Miyamoto et al., Citation2011). The local infection induces ciliostasis, apoptosis and extrusion of ciliated cells from the epithelium (Miyamoto et al., Citation2011). Macroscopic and microscopic lesions are visible in the trachea of affected birds (Saif et al., Citation1981; Gray et al., Citation1983; Arp & Cheville, Citation1984; Arp & Fagerland, Citation1987). In vitro experiments in tracheal organ cultures (TOCs), in combination with in vivo experiments, suggested a strong correlation between the ability of BA to adhere to the ciliated tracheal epithelial cells and the ability to colonize the respiratory tracts of turkeys (Marshall et al., Citation1984; Temple et al., Citation1998). The pathogenesis of B. bronchiseptica shows broad similarities to that of B. pertussis, and both Bordetella species have strong tropism for the ciliated respiratory tissue of their respective host (Soane et al., Citation2000; Anderton et al., Citation2004). A common genetic and functional background for virulence of these species has been suggested, and genomic sequences have been subjected to comparative analysis of the species (Spears et al., Citation2003; Sebaihia et al., Citation2006).
Currently, only a few BA genome sequences are available for genomic analysis. The genome of 197N, a spontaneous nalidixic acid-resistant variant of strain 197, which was isolated from a diseased turkey in the United States (Saif et al., Citation1980; Gentry-Weeks et al., Citation1991), was the first to be sequenced (Sebaihia et al., Citation2006). The second genome to become available was that of Nh1210, a strain isolated from cockatiels in Brazil affected by the lockjaw syndrome (Moreno et al., Citation2015). In comparison to other Bordetella species, the genome of BA strain 197N is relatively small, with a length of approximately 3.73 Mbp, and has an overall nucleotide similarity of 97% with B. bronchiseptica, B. parapertussis and B. pertussis and an amino acid similarity of 75% for recognizable protein orthologues found in BA (Sebaihia et al., Citation2006). The classical, mammalian-associated Bordetella species have virulence genes showing a high degree of similarity to one another. These species share important mechanisms for host–pathogen interaction, including adhesins and toxins (Mattoo & Cherry, Citation2005), but homologues of some of these virulence factors could not be found in BA. The pertussis toxin, which significantly contributes to the pathogenesis of whooping cough induced by B. pertussis, as well as adenylate cyclase, which is an important virulence factor in B. bronchiseptica, B. parapertussis and B. pertussis, are not present in BA strain 197N (Sebaihia et al., Citation2006). The dermonecrotic toxin is encoded in the BA genome, but the predicted protein sequences show only low percentage of identity to its so-called ancestor species B. bronchiseptica (Sebaihia et al., Citation2006; Linz et al., Citation2016). Bordetella adhesins, such as filamentous haemagglutinin (FHA), are located on the bacterial surface. They play an important role in the adhesion process and the colonization of the host’s respiratory epithelium (van den Berg et al., Citation1999; Edwards et al., Citation2005). FhaB, the preprotein of FHA, is found in BA strain 197N but has only a low percentage of sequence similarity to the FhaB of B. bronchiseptica (Sebaihia et al., Citation2006; Linz et al., Citation2016). Likewise, BA strain 197N contains some FHA-like proteins not found in B. bronchiseptica (Sebaihia et al., Citation2006). It has been assumed that these differences in virulence factors represent adaptation to different host species (Sebaihia et al., Citation2006).
Information about the diversity of BA field strains regarding virulence is very limited. Comparative studies mainly concentrate on antibiotic resistance profiles showing some variety in resistance to antibiotic substances (Beach et al., Citation2012; Grespan et al., Citation2012). Furthermore, 10 BA strains isolated from cockatiels and one strain isolated from turkeys did not differ in virulence-associated properties, such as tracheal attachment or cytotoxic effects (Grespan et al., Citation2012).
The aim of the present study was to investigate the diversity of BA field strains in virulence-associated properties in an in vitro culture system of the upper respiratory tract of turkeys, and to gain an overview of the genetic diversity of putative BA virulence factors. The ability to adhere to the epithelial surface and the inhibiting effect on ciliary activity in TOCs were adopted as representative parameters for virulence of the strains. Finally, the results for 17 BA strains were correlated to their core genome multilocus sequence typing (cgMLST) data with a focus on potential differences in sequence types of adhesion-associated genes.
Materials and methods
Bacterial strains and preparation of inocula
Seventeen BA strains isolated from the respiratory tract of different poultry species () have been included in this study. The isolates were identified as BA by PCR which targets a part of the BA genome with so far unknown function (Register & Yersin, Citation2005). The sensitivity of this PCR assay was reported as 100%, based on an analysis of 72 BA isolates from diverse geographic locations and covering a time span of at least 25 years (Register & Yersin, Citation2005). Additionally, all strains were verified by matrix assisted laser desorption ionisation-time of flight mass spectrometry as described before (Marko et al., Citation2012). The main targets in the measurement range of this method are ribosomal proteins. For inoculation of TOCs of turkeys, BA strains were cultured for 24 h under aerobic conditions at 37.5°C on Columbia agar containing 7% (v/v) sheep blood (Columbia sheep blood agar; Oxoid Deutschland GmbH, Wesel, Germany). Bacteria were suspended in prewarmed Earle’s Salt Solution (ESS; Biochrom, Berlin, Germany) and the turbidity of the suspension was measured using a densitometer (Densimat, Biomérieux SA, Marcy-L’étoile, France). A suspension of McFarland-standard 3.1 was produced, which had been tested by serial dilutions to be equal to a bacterial concentration of 2 × 109 colony forming units (CFU)/ml. The bacterial suspension was diluted with 37°C warm ESS to the required concentrations of 2 × 107 and 2 × 105 CFU/ml. The number of CFU was verified immediately after inoculation by 10-fold serial dilutions of the inoculum and colony counting on Columbia sheep blood agar after an incubation time of 48 h.
Table 1. B. avium isolates used in this study.
Preparation of TOCs
TOCs were prepared from 26-day-old turkey embryos (Moorgut Kartzfehn, Bösel, Germany) as described previously (Petersen et al., Citation2012). TOCs were incubated for 2–6 days in an overhead shaker at 37.5°C. Prior to further treatment, ciliary activity was assessed using an inverted microscope (Zeiss, Oberkochen, Germany) and only TOCs with 100% ciliary activity were selected for the following experiments.
Adherence assay
The adherence assay was performed as previously described (Temple et al., Citation1998), with modifications. TOCs were washed three times with 2 ml ESS. Subsequently, each TOC was inoculated with 1 ml ESS with BA or without BA as a negative control. For each strain and each bacterial concentration, six replicates were prepared. For each experiment, three control TOCs were inoculated with 1 ml sterile ESS to exclude the possible effects of contamination. TOCs were incubated for 3 h under continuous overhead shaking at 37.5°C. The inocula were removed and each TOC was washed three times with 2 ml ESS for 2 min under continuous shaking. Subsequently, each TOC was placed in a new sterile 5 ml tube containing 1 ml PBS Dulbecco (Biochrom) with 1% (v/v) Triton X-100 (Sigma-Aldrich, Steinheim, Germany). TOCs were incubated for 1–2 h at 4–6°C and collected after 1 min mixing on a vortex mixer at the highest power. Supernatants were diluted in 10-fold steps in physiologic saline solution and plated out in duplicates on Columbia sheep blood agar. After 48 h of incubation at 37.5°C under aerobic conditions, colonies were counted and the numbers of CFU per TOC were calculated. The assays were repeated once, with a total of 12 TOCs tested for each strain and concentration combination. The results for these 12 TOCs, which were used per strain and bacterial concentration, were taken together for statistical analysis.
Ciliostasis assay
TOCs were washed three times with 2 ml ESS and inoculated with 1 ml ESS with BA at the two different CFU concentrations used for the adherence assay, with a total of 12 TOCs tested for each strain and concentration for each experiment. Three control TOCs were sham-inoculated with sterile ESS to exclude contamination of the cultures and unspecific ciliostasis. The experiment was performed with an infectious dose of 105 CFU/TOC for 48 h and 107 CFU/TOC for 24 h as a faster progression of ciliostasis was expected in the TOCs inoculated with the higher concentration of bacterial suspension. TOCs were incubated for 48 or 24 h at 37.5°C under continuous overhead shaking. Every 4 h, the ciliary activity of the tracheal epithelial cells was monitored semiquantitatively by using an inverted light microscope. Every TOC was divided virtually into 20 parts, being equivalent to 5% steps. Each of these sections was visually scored with 0–5 percentage points of ciliary activity, considering integrity of the epithelial surface and activity of the cilia. The final score of each TOC was built by the sum of the 20 parts and was rounded up or down to 5% accuracy.
Library construction, genome sequencing and annotation
For BA genome sequencing, bacterial colonies were suspended in 70% ethanol. Total DNA was isolated for genome sequencing using the Zymo research Quick-DNATM Fecal/Soil Microbe Kit (Zymo Research, Irvine, CA, USA). Next Generation Sequencing was conducted using the Illumina MiSeq V3 (Illumina Inc., San Diego, CA, USA) platform. For sequencing with the MiSeq, shotgun and mate-pair libraries were both constructed for each strain using the NEBNext Ultra DNA Library Prep Kit E7370 (New England Biolabs GmbH, Frankfurt/Main, Germany) and Illumina Nextera Mate Pair Sample Preparation Kit FC-132-1001 (Illumina). Purification and size-selection were done with AMPure XP beads (Beckman Coulter, Brea, CA, USA) and controlled on a Fragment Analyzer (Advanced Analytical Technologies, Ankeny, IA, USA). Parts of the libraries were constructed using the Illumina TrueSeq DNA LT Sample Prep kit FC-121-2001 (Illumina) and sequenced using the MiSeq Reagent Kit v3 MS-102-3003 (2×300-cycle). Prior to sequencing, libraries were inspected with a Qubit® 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) and Fragment Analyzer (Advanced Analytical Technologies). MIRA 4 (Chevreux et al., Citation1999), A5-miseq (Coil et al., Citation2015), SPAdes 3 (Nurk et al., Citation2013) and CLC Genomics Workbench 8 (Qiagen, Venlo, Netherlands) were used for initial assemblies. For generating pseudo draft genomes, the resulting contigs were submitted to CONTIGuator (Galardini et al., Citation2011). Genes were annotated with the RAST pipeline (Aziz et al., Citation2008).
cgMLST analysis
To define a core genome for cgMLST (Mellmann et al., Citation2011), genome sequences of BA isolates 197N (GenBank accession number NC_010645) and Nh1210 (JWMK00000000.1), downloaded from National Center for Biotechnology Information, as well as sequence data of 45 BA field isolates from our data bank, were compared. The 45 BA isolates included the 17 isolates which were evaluated in the TOC assays. A genome-wide gene-by-gene comparison was performed using the cgMLST target definer function of the SeqSphere+ Software (Ridom GmbH, Münster, Germany) with default parameters as described previously (Ruppitsch et al., Citation2015) to determine the cgMLST gene set. The “hard defined core genome” approach was used and BA strain 197N (accession number NC 010645) served as the reference genome.
Putative virulence-related genes were selected for further analysis. The target genes were assessed for the occurrence of frame shifts and ambiguous nucleotides. Alleles for each gene were assigned automatically by the SeqSphere+ software to ensure unique nomenclature. The combination of all alleles in each strain formed an allelic profile that was used to generate minimum-spanning trees (MSTs) using the parameter “pairwise ignore missing values” during distance calculation.
Amino acid sequence alignment
Amino acid sequence alignment was done with the constraint-based alignment tool COBALT for multiple protein sequences alignment (https://www.ncbi.nlm.nih.gov/tools/cobalt/re_cobalt.cgi) (Papadopoulos & Agarwala, Citation2007). The BLAST programme (http://www.ncbi.nlm.nih.gov/BLAST/) was used for sequence homology searches.
Statistical analysis
Statistical analysis of adherence and ciliostasis assay data was performed using Statistix 10.0 software (Analytical Software, Tallahassee, FL, USA). As the data were not normally distributed, Kruskal–Wallis one-way ANOVA and Dunn’s all pairwise comparison as post hoc test were applied for the data sets of CFU and ciliary activity. P ≤ 0.05 was considered as significant difference when comparing different groups.
Accession numbers
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession numbers listed in .
Results
Adherence and ciliostasis assay
In order to assess differences in virulence-associated characteristics between the 17 BA strains examined here, adherence to TOCs () and the impact on functional activity of the ciliated tracheal epithelial cells () was determined. For each assay, two different BA inocula were tested to identify differences between strains showing high levels of virulence (105 CFU/TOC) and low or normal levels of virulence (107 CFU/TOC).
Figure 1. Adherence assay in TOCs. Number of adhered bacteria following inoculation with 105 CFU (A) and 107 CFU (B) of Bordetella avium per TOC. Bars represent the median (n = 12).
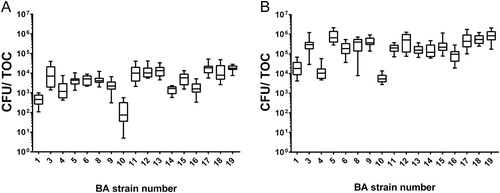
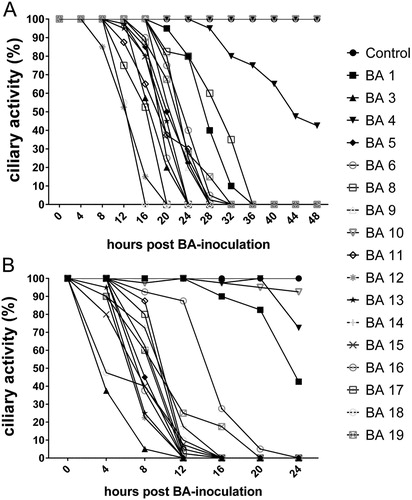
Figure 2. Ciliostasis assay in TOCs. Ciliary activity following inoculation with 105 CFU (A) and 107 CFU (B) of B. avium per TOC. Symbols represent the median (n = 12).
From every inoculated TOC, BA could be reisolated after the completion of the adherence and ciliostasis assay, while control TOCs remained sterile throughout the experiments (data not shown). Following inoculation with either of the bacterial concentrations, strains BA 1, BA 4 and BA 10 adhered in lower median numbers compared to the other strains, with BA 10 showing the lowest number of CFU ().
These trends could not be supported entirely by statistical analysis as BA 10 differed significantly only from strains BA 3, BA 5, BA 8, BA 11, BA 12, BA 15, BA 17, BA 18 and BA 19 in the adherence assays with either bacterial concentration (P ≤ 0.05). The difference from strains BA 6 and BA 13 was significant exclusively in the 105 CFU/ml-assay, and the difference from strain BA 9 was significant exclusively in the adherence assay applying the higher bacterial concentration of 107 CFU/ml (P ≤ 0.05, n = 12/group). Strains BA 1, BA 4, BA 14 and BA 16 did not differ significantly from BA 10 either in the adherence assay applying the lower bacterial concentration, or in the adherence assay applying the higher bacterial concentration (P > 0.05).
In the ciliostasis assay, strains BA 1, BA 4 and BA 10 induced delayed and weak ciliostatic effect in comparison to the other strains (). The differences from the other strains were significant after 24 h inoculation with 107 CFU/ TOC (P ≤ 0.05). After 48 h inoculation with the low concentration of inoculum (105 CFU/TOC), only BA 4 and BA 10 differed significantly from the other strains.
In control TOCs, ciliary activity remained at 100% throughout the observation time of 24 or 48 h.
cgMLST/MLST analysis
Genome-wide gene-by-gene comparison of BA 197N, Nh1210 and 45 field isolates (17 of which had been tested in the adherence and ciliostasis assays) was performed to determine the cgMLST gene set. Whole genome sequence data of the 17 investigated strains in our study were submitted for release to NCBI databank. Biosample accession numbers are listed in . Using BA strain 197N (accession numbers NC_010645) as a reference genome, the cgMLST Target Definer created a cgMLST scheme comprising 2667 targets of the reference genome (77.3% of the complete genome). On average, 99.1% cgMLST genes were called successfully from 47 BA genomes. Based on the defined core genome, cgMLST and pairwise comparison of the strains revealed a minimum distance of 1–1330 allelic differences between the strains (). Among the 2667 target genes, we chose 119 putative virulence-related genes (Supplemental Table 1) for further MLST analysis. The MLST profiles of the 17 BA strains () which had been utilized for the in vitro assays in TOCs were included in the generation of an MST (). Based on the 119 putative virulence-related genes, pairwise comparison of the strains revealed a minimum distance of 1–71 allelic differences between the strains. Regarding the sequence types, the analysed BA strains appeared to cluster in at least two groups (): the first group (on the left-hand side of the distance tree) had at least 71 allelic differences in their sequence types in comparison to the second group. It included only five BA strains, and between these five strains the minimum distance amounted to 6–46 allelic differences. Most of the analysed strains formed the second group in the distance tree (on the right-hand side of the distance tree, ) and showed very limited variation in the sequence types of their core genomes. The lowest degree of difference, represented by variation in only one allele, was detected between strain BA 16 and BA 17 as well as between BA 16 and BA 4 (). The highest number of allele differences between two neighboured strains in the second group was 15.
Figure 3. MST based on cgMLST allelic profiles of 17 B. avium isolates. Each circle represents an allelic profile based on sequence analysis of 2667 genes. The strains with reduced virulence-associated properties are coloured in light grey, while strains with more marked virulence-associated properties are coloured in dark grey. The numbers on the connecting lines illustrate the number of target genes with differing alleles.
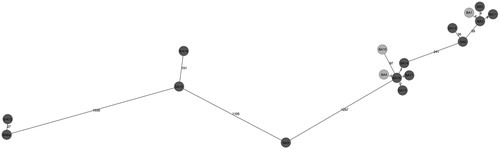
Figure 4. MST based on multilocus sequence typing (MLST) allelic profiles of 17 B. avium isolates. Each circle represents an allelic profile based on sequence analysis of 119 virulence-related genes. The strains with reduced virulence-associated properties are coloured in light grey, while strains with more marked virulence-associated properties are coloured in dark grey. The numbers on the connecting lines illustrate the number of target genes with differing alleles. Target genes differing between the strains with reduced virulence-associated properties and their next neighbour are listed in the table within the figure.
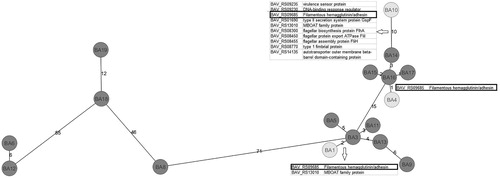
To gain more detailed insight into the allelic diversity among isolates with different virulent abilities, we analysed all pairwise allelic differences between isolates with weak and strong virulence-associated properties in TOCs. Strain BA 4 and its next neighbour, BA 16, differed in only one gene of 119 virulence-associated genes despite their different virulence-associated behaviour in the in vitro assays. The one single gene locus, which constitutes the sequence type variation between strain BA 4 and BA 16, was the locus for the putative FHA protein (BAV_RS09685). BA 1 and BA 3 differed in only two of the core genome genes. Of the two genes responsible for sequence type variation, one was again the locus of the putative FHA protein. The second gene locus was a fimbrial protein. Strain BA 10, finally, differed from its next neighbour BA 14 in 10 loci. The 10 loci with different alleles in BA 10 in comparison to BA 14 had been described to be related to signal transduction mechanisms, secretion proteins, membrane genesis, flagellae, fimbriae, autotransporters and the FHA protein. Additionally, BA 10 was lacking two genes for putative fimbrial proteins.
Amino acid sequence alignment
As the gene locus encoding the FHA protein (BAV_RS09685) was the only DNA section with substantial difference between the three less adherent strains in comparison to their next highly adherent neighbour strain, the corresponding protein was selected for further analysis. The amino acid sequence alignment of the putative FHA protein of the 17 BA isolates is shown in the supplemental materials (Supplementary Figure S1). In the highly conserved domains of the protein, some differences were detected in the amino acid sequences. These differences between the strains showed no direct correlation to the two virulence-associated properties assessed in the TOCs. In a region of the protein with unknown function (beginning with amino acid 2320), the sequences of the analysed isolates showed a higher degree of heterogeneity. A phylogenetic tree was constructed using the neighbour-joining method based on the COBALT multiple sequence alignment and the evolutionary distances were calculated by the proportional (p) distance model (). Phylogenetic neighbour-joining tree analysis indicated that low virulent BA 1 and BA 10 formed a unique branch in the tree. Furthermore, low virulent BA 4 is located on a branch very near to BA 1 and BA 10. No further correlation between the virulence-associated properties and the amino acid sequence alignment of the putative FHA was demonstrated.
Discussion
The present study demonstrates that the combination of ciliostasis and adherence assay in TOCs offers a useful tool for the characterization of BA field strains. As ciliostasis and epithelial damage have been described to play an important role in pathogenesis of avian bordetellosis (Gray et al., Citation1981, Citation1983; Arp & Fagerland, Citation1987; Miyamoto et al., Citation2011), as well as other Bordetella-induced diseases in various host species (Bemis et al., Citation1977; Sekiya et al., Citation1988; Chen et al., Citation1989; Soane et al., Citation2000; Anderton et al., Citation2004), we chose TOCs of turkeys as a model for BA infection. The adherence assay, which allows the quantification of the adhered bacteria, was described by Temple et al. (Citation1998). The ciliostasis assay in TOCs, as performed in our study, has not been described for BA-infection studies until now. We modified a protocol in which ciliostasis was investigated in tracheal explant cultures (Miyamoto et al., Citation2011), and referred to studies which evaluated virulence of other respiratory pathogens of turkeys (Naylor & Jones, Citation1994; Petersen et al., Citation2012; Hartmann et al., Citation2015; Sid et al., Citation2016) as well as other Bordetella species in TOCs of their respective mammalian host (Collier et al., Citation1977; Anderton et al., Citation2004).
It has been shown that there is a strong correlation between the ability of BA strains to adhere to the tracheal mucosa in TOCs on the one side, and the ability to colonize turkeys and to induce a respiratory disease on the other side (Marshall et al., Citation1984; Temple et al., Citation1998). Our results show that the ability to adhere to the tracheal mucosa and the capacity to induce ciliostasis are associated with each other. Furthermore, the characteristics of comparatively low adherence and weak ciliostatic effect were reproducible for two differently concentrated inocula (105 and 107 CFU/ml and TOC) in our study. Finally, strain BA 9, which was described in previous studies to induce severe disease in one-day-old turkey poults (Kersters et al., Citation1984), displayed marked virulence-associated properties in the TOC experiments. Although we have not performed comparative in vivo experiments here, our results support the hypothesis that the parameters of adherence and ciliostasis are meaningful criteria to estimate the in vivo virulence of BA strains. However, it is not clear whether ciliostasis is an effect of bacterial adherence to the host epithelium. Further research has to be conducted on the functional connection between adherence and ciliostasis in BA.
Using cgMLST analysis, a significant degree of genetic diversity was uncovered amongst the 17 BA field strains investigated here using the TOC assays. The highest number of allelic differences between two neighboured BA strains in the minimum-spanning tree was 1330 between strains BA 18 and BA 6 (). These results indicate that there is a broad genetic diversity in field strains within the species of BA, which has not been studied before. These findings are remarkable in view of the fact that only a limited number of isolates with a limited overall diversity regarding their geographic origin (most strains from German poultry flocks) were analysed in this study. The genetically most similar strains were BA 16, BA 17 and BA 4. Although epidemiological data are not available, it is worth noting that these strains had been isolated in two different years (2013 and 2016), from three different host species (goose, broiler chicken, and turkey ()). An MST using the MLST results () suggested that the BA strains clustered in two groups, which performed differently in the TOC analyses. All of the strains in the first group showed distinct virulence-associated properties in vitro, while in the second group, strains with lower and higher virulence were mixed. Strains BA 1, BA 4 and BA 10, which were those with weakest or no virulence-associated properties in vitro, were widely distributed throughout the second group of strains and had variable numbers of allelic differences compared with their respective closest neighbour. Consequently, a close relationship between the strains, which were found to have reduced virulence-associated properties, could not conclusively be visualized based on MLST data as these strains did not form a distinct group in our study. A correlation of sequence type and in vitro phenotype could therefore not be established. Applying our research protocol to the analysis of more BA strains from more diverse origin (preferably from all over the world) may shed more light on the question of genetic diversity of virulence factors of BA.
The MLST analysis enabled us to identify a potential factor for differences in virulence-associated properties. One purpose of the analysis was to find a common underlying factor for the low adherence rate and delayed ciliostatic effect. Therefore, we further investigated the genes which had different sequence types in the strains with low virulence-associated properties in comparison with their next neighbour with more marked virulence-associated properties. The only putative protein which was shown to have an allelic difference in all of the identified strains with low virulence-associated properties (BA 1, BA 4, BA 10) in comparison to their next neighbour was the putative FHA (BAV_RS09685). The results indicate that mutations in the FHA locus are a common feature of the low adhering and weak ciliostatic BA strains. These mutations are associated, and may be to some extent responsible for lower virulence-associated properties in TOCs. It has to be considered that only a selected number of genes, which were speculated to be virulence-associated genes, were included in this study. It is possible that some crucial genes for the differences in virulence-associated properties were not included in the MLST analysis. Differences in the phenotype may possibly also be associated with other genetic deviations, for instance with genes coding for metabolic features. However, due to the outstanding importance of adherence for the pathogenesis of avian bordetellosis, it is reasonable to focus on genes which are related to this kind of host pathogen interaction. As we defined a core genome comprising 77.3% of complete BA 197N reference genome, the probability of covering the important genes is high. Furthermore, it is known from the literature that FHA plays an important role in the pathogenesis of Bordetella infections as it is the major adhesin and haemagglutinin in the mammalian-adapted Bordetellae (Relman et al., Citation1989; Kimura et al., Citation1990; Locht et al., Citation1993; Cotter et al., Citation1998). It has been demonstrated in strain 197N that BA possesses an orthologue to the FHA protein in B. bronchiseptica (Spears et al., Citation2003; Sebaihia et al., Citation2006) and that FHA-negative mutants of that strain are attenuated in their ability to colonize the respiratory epithelium of the host (Cotter et al., Citation1998; Spears et al., Citation2003) although one research group reported that eliminating expression of FHA had no effect on binding to turkey tracheal rings (Spears et al., Citation2003). Notably, the sequence similarity between B. bronchiseptica FHA and BA 197N FHA is very low (Sebaihia et al., Citation2006; Linz et al., Citation2016) and the genetic organizations differ between these two species (Stibitz et al., Citation1988; Spears et al., Citation2003). Additionally, FHA of BA seems to have a different function as it is not responsible for haemagglutination (Spears et al., Citation2003). It has been shown that BA possesses two species-specific genes involved in haemagglutination activity and adhesion to the tracheal epithelium (Temple et al., Citation2010) and it was speculated that these genes partly substitute the FHA function. As comparative studies including BA field strains are lacking so far, function of FHA in BA strains is still controversially discussed; more precisely the question whether the FHA protein is of high relevance for the virulence of BA as is the case for the mammalian-adapted Bordetellae. Our study confirms that mutations in the FHA locus do indeed exist in BA field strains, and it suggests that these variations potentially have an impact on the virulence of the strains. We furthermore analysed the putative amino acid sequence of the FHA protein to overcome some limitations of the cgMLST method, which is not suitable for distinction between missense or nonsense and silent mutations. The amino acid sequence alignment revealed that the main sequence differences fall in a region of the protein with unknown function. Further analyses of the protein structure have to be conducted to assess if these mutations have an effect on the stability or activity of the protein and should be addressed in follow-up studies.
In conclusion, our investigations show that BA field strains vary significantly in some of their virulence-associated characteristics. For three strains, the abilities to adhere to the tracheal epithelium and to induce ciliostasis in TOCs were significantly reduced in comparison to the other investigated strains. Furthermore, cgMLST analysis demonstrated a high variation in sequence types of BA field strains. Until now, only limited genome sequence data of BA have been available. We sequenced 17 additional BA strains and showed that the BA genome is more diverse than previously thought. We could not confirm a correlation between sequence type and virulence-associated properties but identified mutations in the putative FHA as a potential candidate responsible for reduced virulence of BA field strains. Further investigations are needed to confirm the substantial role of FHA for virulence and the significance of other virulence factors for BA pathogenesis.
Supplemental Material
Download Zip (17 MB)Acknowledgements
The authors would like to thank Dr. Karsten Liere for preparing and uploading genome sequence data. Additionally, the authors thank RIPAC-LABOR GmbH, Potsdam, Germany and Heidemark GmbH, Veterinary Laboratory, Haldensleben for providing BA field strains.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Arne Jung http://orcid.org/0000-0002-9866-6841
Additional information
Funding
References
- Anderton, T.L., Maskell, D.J. & Preston, A. (2004). Ciliostasis is a key early event during colonization of canine tracheal tissue by Bordetella bronchiseptica. Microbiology, 150, 2843–2855. doi: 10.1099/mic.0.27283-0
- Arp, L.H. & Cheville, N.F. (1984). Tracheal lesions in young turkeys infected with Bordetella avium. The American Journal of Veterinary Research, 45, 2196–2200.
- Arp, L.H. & Fagerland, J.A. (1987). Ultrastructural pathology of Bordetella avium infection in turkeys. Veterinary Pathology, 24, 411–418. doi: 10.1177/030098588702400508
- Aziz, R.K., Bartels, D., Best, A.A., DeJongh, M., Disz, T., Edwards, R.A., Formsma, K., Gerdes, S., Glass, E.M., Kubal, M., Meyer, F., Olsen, G.J., Olson, R., Osterman, A.L., Overbeek, R.A., McNeil, L.K., Paarmann, D., Paczian, T., Parrello, B., Pusch, G.D., Reich, C., Stevens, R., Vassieva, O., Vonstein, V., Wilke, A. & Zagnitko, O. (2008). The RAST Server: rapid annotations using subsystems technology. BMC Genomics, 9, 75. doi: 10.1186/1471-2164-9-75
- Beach, N.M., Thompson, S., Mutnick, R., Brown, L., Kettig, G., Puffenbarger, R., Stockwell, S.B., Miyamoto, D. & Temple, L. (2012). Bordetella avium antibiotic resistance, novel enrichment culture, and antigenic characterization. Veterinary Microbiology, 160, 189–196. doi: 10.1016/j.vetmic.2012.05.026
- Bemis, D.A., Greisen, H.A. & Appel, M.J. (1977). Pathogenesis of canine bordetellosis. Journal of Infectious Diseases, 135, 753–762. doi: 10.1093/infdis/135.5.753
- Chen, W., Alley, M. & Manktelow, B. (1989). Experimental induction of pneumonia in mice with Bordetella parapertussis isolated from sheep. Journal of Comparative Pathology, 100, 77–89. doi: 10.1016/0021-9975(89)90092-3
- Chevreux, B., Wetter, T. & Suhai, S. (1999). Genome sequence assembly using trace signals and additional sequence information. German Conference on Bioinformatics (pp. 45–56). Hannover, Germany.
- Coil, D., Jospin, G. & Darling, A.E. (2015). A5-miseq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics (Oxford, England), 31, 587–589. doi: 10.1093/bioinformatics/btu661
- Collier, A.M., Peterson, L.P. & Baseman, J.B. (1977). Pathogenesis of infection with Bordetella pertussis in hamster tracheal organ culture. Journal of Infectious Diseases, 136, S196–S203. doi: 10.1093/infdis/136.Supplement.S196
- Cotter, P.A., Yuk, M.H., Mattoo, S., Akerley, B.J., Boschwitz, J., Relman, D.A. & Miller, J.F. (1998). Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infection and Immunity, 66, 5921–5929.
- Edwards, J.A., Groathouse, N.A. & Boitano, S. (2005). Bordetella bronchiseptica adherence to cilia is mediated by multiple adhesin factors and blocked by surfactant protein A. Infection and Immunity, 73, 3618–3626. doi: 10.1128/IAI.73.6.3618-3626.2005
- Galardini, M., Biondi, E.G. Bazzicalupo, M. & Mengoni, A. (2011). CONTIGuator: a bacterial genomes finishing tool for structural insights on draft genomes. Source Code for Biology and Medicine, 6, 11. doi: 10.1186/1751-0473-6-11
- Gentry-Weeks, C.R., Provence, D.L., Keith, J.M. & Curtiss, R. 3rd. (1991). Isolation and characterization of Bordetella avium phase variants. Infection and Immunity, 59, 4026–4033.
- Gray, J.G., Roberts, J.F., Dillman, R.C. & Simmons, D.G. (1981). Cytotoxic activity of pathogenic Alcaligenes faecalis in turkey tracheal organ cultures. The American Journal of Veterinary Research, 42, 2184–2186.
- Gray, J.G., Roberts, J.F., Dillman, R.C. & Simmons, D.G. (1983). Pathogenesis of change in the upper respiratory tracts of turkeys experimentally infected with an Alcaligenes faecalis isolate. Infection and Immunity, 42, 350–355.
- Grespan, A., Camera, O., Knobl, T., Gomes, C.R., Felizardo, M.R., Ferreira, T.S., Gobbi, D.D., Moreno, M., Sanches, A.A., Ferreira, C.S., Ferreira, A.J. & Moreno, A.M. (2012). Virulence and molecular aspects of Bordetella avium isolated from cockatiel chicks (Nymphicus hollandicus) in Brazil. Veterinary Microbiology, 160, 530–534. doi: 10.1016/j.vetmic.2012.06.023
- Hartmann, S., Sid, H. & Rautenschlein, S. (2015). Avian metapneumovirus infection of chicken and turkey tracheal organ cultures: comparison of virus–host interactions. Avian Pathology, 44, 480–489. doi: 10.1080/03079457.2015.1086974
- Hinz, K.H., Glunder, G. & Luders, H. (1978). Acute respiratory disease in turkey poults caused by Bordetella bronchiseptica-like bacteria. The Veterinary Record, 103, 262–263. doi: 10.1136/vr.103.12.262
- Kersters, K., Hinz, K.H., Hertle, A., Segers, P., Lievens, A., Siegmann, O. & Deley, J. (1984). Bordetella avium sp. nov., isolated from the respiratory tracts of turkeys and other birds. International Journal of Systematic Bacteriology, 34, 56–70. doi: 10.1099/00207713-34-1-56
- Kimura, A., Mountzouros, K.T., Relman, D.A., Falkow, S. & Cowell, J.L. (1990). Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infection and Immunity, 58, 7–16.
- Linz, B., Ivanov, Y.V., Preston, A., Brinkac, L., Parkhill, J., Kim, M., Harris, S.R., Goodfield, L.L., Fry, N.K., Gorringe, A.R., Nicholson, T.L., Register, K.B., Losada, L. & Harvill, E.T. (2016). Acquisition and loss of virulence-associated factors during genome evolution and speciation in three clades of Bordetella species. BMC Genomics, 17, 767. doi: 10.1186/s12864-016-3112-5
- Locht, C., Bertin, P., Menozzi, F.D. & Renauld, G. (1993). The filamentous haemagglutinin, a multifaceted adhesion produced by virulent Bordetella spp. Molecular Microbiology, 9, 653–660. doi: 10.1111/j.1365-2958.1993.tb01725.x
- Marko, D.C., Saffert, R.T., Cunningham, S.A., Hyman, J., Walsh, J., Arbefeville, S., Howard, W., Pruessner, J., Safwat, N., Cockerill, F.R., Bossler, A.D., Patel, R. & Richter, S.S. (2012). Evaluation of the Bruker Biotyper and Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry systems for identification of nonfermenting Gram-negative bacilli isolated from cultures from cystic fibrosis patients. Journal of Clinical Microbiology, 50, 2034–2039. doi: 10.1128/JCM.00330-12
- Marshall, D.R., Simmons, D.G. & Gray, J.G. (1984). Evidence for adherence-dependent cytotoxicity of Alcaligenes faecalis in turkey tracheal organ cultures. Avian Diseases, 28, 1007–1015. doi: 10.2307/1590277
- Mattoo, S. & Cherry, J.D. (2005). Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clinical Microbiology Reviews, 18, 326–382. doi: 10.1128/CMR.18.2.326-382.2005
- Mellmann, A., Harmsen, D., Cummings, C.A., Zentz, E.B., Leopold, S.R., Rico, A., Prior, K., Szczepanowski, R., Ji, Y., Zhang, W., McLaughlin, S.F., Henkhaus, J.K., Leopold, B., Bielaszewska, M., Prager, R., Brzoska, P.M., Moore, R.L., Guenther, S., Rothberg, J.M. & Karch, H. (2011). Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One, 6, e22751. doi: 10.1371/journal.pone.0022751
- Miyamoto, D.M., Ruff, K., Beach, N.M., Stockwell, S.B., Dorsey-Oresto, A., Masters, I. & Temple, L.M. (2011). Bordetella avium causes induction of apoptosis and nitric oxide synthase in turkey tracheal explant cultures. Microbes and Infection, 13, 871–879. doi: 10.1016/j.micinf.2011.04.011
- Moreno, L.Z., Knobl, T., Grespan, A.A., Felizardo, M.R., Gomes, C.R., Ferreira, T.S., Xavier de Oliveira, M.G., Myriantheus, L. & & Moreno, A.M. (2015). Draft genome sequence of Bordetella avium Nh1210, an outbreak strain of lockjaw syndrome. Genome Announcements, 3, 1–2.
- Naylor, C. & Jones, R. (1994). Demonstration of a virulent subpopulation in a prototype live attenuated turkey rhinotracheitis vaccine. Vaccine, 12, 1225–1230. doi: 10.1016/0264-410X(94)90248-8
- Nurk, S., Bankevich, A., Antipov, D., Gurevich, A.A., Korobeynikov, A., Lapidus, A., Prjibelski, A.D., Pyshkin, A., Sirotkin, A. & Sirotkin, Y. (2013). Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. Journal of Computational Biology, 20, 714–737. doi: 10.1089/cmb.2013.0084
- Papadopoulos, J.S. & Agarwala, R. (2007). COBALT: constraint-based alignment tool for multiple protein sequences. Bioinformatics (Oxford, England), 23, 1073–1079. doi: 10.1093/bioinformatics/btm076
- Petersen, H., Matrosovich, M., Pleschka, S. & Rautenschlein, S. (2012). Replication and adaptive mutations of low pathogenic avian influenza viruses in tracheal organ cultures of different avian species. PLoS One, 7, e42260, 1–12. doi: 10.1371/journal.pone.0042260
- Raffel, T.R., Register, K.B., Marks, S.A. & Temple, L. (2002). Prevalence of Bordetella avium infection in selected wild and domesticated birds in the eastern USA. Journal of Wildlife Diseases, 38, 40–46. doi: 10.7589/0090-3558-38.1.40
- Register, K.B. & Yersin, A.G. (2005). Analytical verification of a PCR assay for identification of Bordetella avium. Journal of Clinical Microbiology, 43, 5567–5573. doi: 10.1128/JCM.43.11.5567-5573.2005
- Relman, D.A., Domenighini, M., Tuomanen, E., Rappuoli, R. & Falkow, S. (1989). Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proceedings of the National Academy of Sciences of the United States of America, 86, 2637–2641. doi: 10.1073/pnas.86.8.2637
- Ruppitsch, W., Pietzka, A., Prior, K., Bletz, S., Fernandez, H.L., Allerberger, F., Harmsen, D. & Mellmann, A. (2015). Defining and evaluating a core genome multilocus sequence typing scheme for whole-genome sequence-based typing of Listeria monocytogenes. Journal of Clinical Microbiology, 53, 2869–2876. doi: 10.1128/JCM.01193-15
- Saif, Y.M., Moorhead, P.D., Dearth, R.N. & Jackwood, D.J. (1980). Observations on Alcaligenes faecalis infection in turkeys. Avian Diseases, 24, 665–684. doi: 10.2307/1589804
- Saif, Y.M., Moorhead, P.D. & Whitmoyer, R.E. (1981). Scanning electron microscopy of tracheas from turkey poults infected with Alcaligenes faecalis. Avian Diseases, 25, 730–735. doi: 10.2307/1590004
- Sebaihia, M., Preston, A., Maskell, D.J., Kuzmiak, H., Connell, T.D., King, N.D., Orndorff, P.E., Miyamoto, D.M., Thomson, N.R., Harris, D., Goble, A., Lord, A., Murphy, L., Quail, M.A., Rutter, S., Squares, R., Squares, S., Woodward, J., Parkhill, J. & Temple, L.M. (2006). Comparison of the genome sequence of the poultry pathogen Bordetella avium with those of B. bronchiseptica, B. pertussis, and B. parapertussis reveals extensive diversity in surface structures associated with host interaction. Journal of Bacteriology, 188, 6002–6015. doi: 10.1128/JB.01927-05
- Sekiya, K., Futaesaku, Y. & Nakase, Y. (1988). Electron microscopic observations on tracheal epithelia of mice infected with Bordetella bronchiseptica. Microbiology and Immunology, 32, 461–472. doi: 10.1111/j.1348-0421.1988.tb01406.x
- Sid, H., Hartmann, S., Petersen, H., Ryll, M. & Rautenschlein, S. (2016). Mycoplasma gallisepticum modifies the pathogenesis of influenza A virus in the avian tracheal epithelium. International Journal of Medical Microbiology, 306, 174–186. doi: 10.1016/j.ijmm.2016.04.001
- Soane, M.C., Jackson, A., Maskell, D., Allen, A., Keig, P., Dewar, A., Dougan, G. & Wilson, R. (2000). Interaction of Bordetella pertussis with human respiratory mucosa in vitro. Respiratory Medicine, 94, 791–799. doi: 10.1053/rmed.2000.0823
- Spears, P.A., Temple, L.M., Miyamoto, D.M., Maskell, D.J. & Orndorff, P.E. (2003). Unexpected similarities between Bordetella avium and other pathogenic Bordetellae. Infection and Immunity, 71, 2591–2597. doi: 10.1128/IAI.71.5.2591-2597.2003
- Stenzel, T., Pestka, D., Tykalowski, B., Smialek, M., Koncicki, A. & Bancerz-Kisiel, A. (2017). Detection of Bordetella avium by TaqMan real-time PCR in tracheal swabs from wildlife birds. Polish Journal of Veterinary Sciences, 20, 31–36. doi: 10.1515/pjvs-2017-0005
- Stibitz, S., Weiss, A.A. & Falkow, S. (1988). Genetic analysis of a region of the Bordetella pertussis chromosome encoding filamentous hemagglutinin and the pleiotropic regulatory locus vir. Journal of Bacteriology, 170, 2904–2913. doi: 10.1128/jb.170.7.2904-2913.1988
- Temple, L.M., Miyamoto, D.M., Mehta, M., Capitini, C.M., Von Stetina, S., Barnes, H.J., Christensen, V.L., Horton, J.R., Spears, P.A. & Orndorff, P.E. (2010). Identification and characterization of two Bordetella avium gene products required for hemagglutination. Infection and Immunity, 78, 2370–2376. doi: 10.1128/IAI.00140-10
- Temple, L.M., Weiss, A.A., Walker, K.E., Barnes, H.J., Christensen, V.L., Miyamoto, D.M., Shelton, C.B. & Orndorff, P.E. (1998). Bordetella avium virulence measured in vivo and in vitro. Infection and Immunity, 66, 5244–5251.
- van den Berg, B.M., Beekhuizen, H., Willems, R.J., Mooi, F.R. & van Furth, R. (1999). Role of Bordetella pertussis virulence factors in adherence to epithelial cell lines derived from the human respiratory tract. Infection and Immunity, 67, 1056–1062.