ABSTRACT
In this study, the relative contribution of vertical transmission, within-farm transmission and between-farm transmission of Mycoplasma synoviae in layer pullet flocks was quantified using logistic regression analysis. Data from 311 Dutch pullet flocks, of which 172 (55%) were positive for M. synoviae, were included in the study. Also the M. synoviae status of the parent stock of these flocks was included. The M. synoviae status was determined with the M. synoviae rapid plate agglutination test. Data analysis showed that vertical transmission was the most important transmission route for M. synoviae in layers as is demonstrated by an odds ratio of 5.8 (P = 0.000). A positive association with M. synoviae infections was found for layer pullet flocks on a multi-house farm where at least one other flock was M. synoviae-positive compared to single-house farms (odds ratio 3.1, P = 0.022), while a negative association was found when no other M. synoviae-positive flocks were present (odds ratio = 0.2, P = 0.003). No association was found between M. synoviae status of pullet flocks and poultry farm density. Odds ratios were 0.54 (P = 0.288) and 0.34 (P = 0.073), respectively, for medium and highest poultry farm density compared to lowest poultry farm density. This is the first time that the relative contribution of horizontal and vertical transmission of M. synoviae has been quantified. These results can be extrapolated to M. synoviae control in general, and emphasize the importance of M. synoviae control in parent stock and practical channelling.
Introduction
Mycoplasma synoviae infections are able to cause significant economic losses in the commercial poultry industry. M. synoviae has been described as the aetiological agent of airsacculitis (Kleven et al., Citation1972, Citation1975), infectious synovitis (Morrow et al., Citation1990; Landman & Feberwee, Citation2001, Citation2012) and eggshell apex abnormalities (Feberwee et al., Citation2009; Catania et al., Citation2010, Citation2016; Gole et al., Citation2012). The clinical and economic relevance of M. synoviae increased during the past three decades considering the emergence of strains with joint tropism that cause arthritis (Morrow et al., Citation1990; Landman & Feberwee, Citation2001, Citation2012) and strains causing eggshell apex abnormalities and production drops (Feberwee et al., Citation2009; Catania et al., Citation2010; Gole et al., Citation2012). The increasing relevance of M. synoviae has contributed to the implementation of monitoring programmes for M. synoviae in the Netherlands and in other countries (Kamp, Citation2014; Anonymous, Citation2018).
Before the start of a control programme, information on the prevalence of M. synoviae in different poultry types and routes of transmission is required. In the Netherlands, two different studies were performed. In 2005, a study on the occurrence of M. synoviae in commercial Dutch poultry was performed, and this showed a prevalence of 73% and 25% in commercial layer farms and layer parent farms, respectively (Feberwee et al., Citation2008). In this study, which was performed in 2009, the M. synoviae prevalence in layer pullets and the relative contribution of vertical transmission, within-farm transmission and between-farm transmission for M. synoviae in layer pullet flocks were quantified. The results of this study are reported here.
Materials and methods
M. synoviae status of layer pullet flocks
A flock was defined as a group of birds of the same age in the same poultry house. Three hundred and eleven flocks were sampled. Information on associated risk factors was collected from the flock owners. Submissions from the obligatory Dutch Mycoplasma gallisepticum monitoring programme were used for this study which excluded layer pullet flocks originating from abroad. Layer pullet flocks hatched from imported eggs were not included as the status of foreign parent flocks was not available.
Three hundred and eleven layer pullet flocks were housed on a total of 104 different farms. Twenty-nine layer pullet flocks were housed on 26 different single-house farms, of which on three farms two sequential flocks were sampled. A total of 282 layer pullet flocks originated from 78 multi-house farms. Thirty-three farms had two houses, 22 farms had three houses, 16 farms had four houses, five farms had five houses and two farms had six houses. Moreover, eight out of 78 multi-house farms were multi-age farms and 29 out of 282 pullet flocks originated from these farms. Flock size ranged from 1600 to 64,600 birds with the 25th percentile, median and 75th percentile being 8435, 16,265 and 31,500 birds, respectively. All farms complied with the Dutch hygiene standardized biosecurity programme (IKB) standards. No vaccinations against M. synoviae were used and the history of antibiotic treatment programmes was not known.
Serology
Sera were collected at the end of the rearing period at 15–17 weeks of age. For each flock, 25 serum samples were tested by M. synoviae Rapid Plate Agglutination (RPA) test (Soleil S. A. R. L., Cantenay Epinard, France). Positive and negative control sera were included in each run of the test. A pullet flock was regarded positive if at least two samples were positive in a 1:8 dilution. If only one sample was positive, the result was interpreted as dubious and these flocks were excluded from this study. If no sample was positive, the flock was regarded negative.
Criteria at risk for vertical transmission
In order to assess the contribution of vertical transmission as a source of M. synoviae infection for the layer pullet flocks, parent flocks of each layer pullet flock were identified. The M. synoviae status of Dutch layer breeding flocks was determined synchronously to the Dutch M. gallisepticum monitoring programme (PPE, Citation1993). In this programme, layer breeder flocks were monitored by serology on a four to eight week interval. Depending on the flock size, 24–60 birds (at least 1% of the flock) were sampled. The layer breeder flock was considered positive for M. synoviae if two or more serum samples showed agglutination at a dilution of 1:8 or higher in the M. synoviae RPA test. The status of the layer breeder flock was related to their offspring.
A layer pullet flock was regarded at risk of vertical transmission if at least one of its parent flocks was positive for M. synoviae regardless of the status of the other parent flocks. The status of a parent flock was determined based on the moment of its seroconversion. No risk of vertical transmission was expected if the status of all the parent flocks was negative. Flocks at risk for vertical transmission were compared to non-risk flocks.
Criteria at risk from within-farm transmission
To assess within-farm transmission of M. synoviae, each layer pullet flock was assigned to one of three groups: (1) single-house farm, (2) multi-house farm with at least one other seropositive layer pullet flock (at risk of within-farm transmission) and (3) multi-house farm without other seropositive flocks (not at risk of within-farm transmission). Flocks at risk of within-farm transmission and flocks not at risk of within-farm transmission were compared to single-house farms.
Criteria at risk from area poultry density
The locations of all poultry farms in the Netherlands, including layers, (broiler) reproduction flocks, turkeys and broilers, were established by geolocation based on the Dutch postal code and house number, and analysed in the Geographical Information System (MapInfo Professional version 6.5, MapInfo Corp. Troy, NY, USA); postal code and house number of farms were linked to geographical coordinates provided by MapInfo. Each flock was categorized as being located in an area with the lowest poultry farm density, an area with a medium poultry farm density or an area with the highest poultry farm density. The lowest, medium and highest poulry farm density were defined by the 33rd and 67th percentile of the number of farms in a 10 km radius around the farm. Lowest poultry farm density areas had less than 19 poultry farms within a 10 km radius, medium poultry farm density areas had 19–88 farms in a 10 km radius and highest poultry farm density areas had more than 88 farms in a 10 km radius. Flocks in areas with the highest and medium poultry farm densities were compared to farms in areas with the lowest poultry farm density.
Criteria for flock size
All flocks were grouped on flock size based on the 25th, 50th and 75th percentiles. Small flock size (≤ 8435 birds) were compared to small to medium flocks (8436–16,265 birds), medium to large flocks (16,266–31,500 birds) and large flocks (> 31,500 birds).
Statistical analysis
Data were collected and processed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). Correlation between the status of a layer pullet flock and associated risk factors was established by multivariable logistic regression using STATA 12.1 (Statacorp, College Station, TX, USA) and expressed as odds ratio (OR) and probability value. This method is designed to observe associations between multiple risk factors in such a way that the importance of each of the factors is corrected by the others. An odds ratio lower than 1 means a negative association between the case and the risk factor, an odds ratio of one indicates no relationship, and an odds ratio higher than one means a positive association. The more the odds ratio differs from 1 the stronger the association. Probability values for the odds ratios were calculated; a probability of 0.05 or lower was considered significant.
Results
The results of the serological M. synoviae status of layer pullet flocks, the quantification of the factors and their odds ratios are presented in . The risk assessment model had a pseudo R2 of 0.36 and goodness of fit (Prob > chi2) of 0.000.
Table 1. Serological M. synoviae status of layer pullet flocks and associated risk factors and their odds-ratios.
M. synoviae prevalence in layer pullet flocks
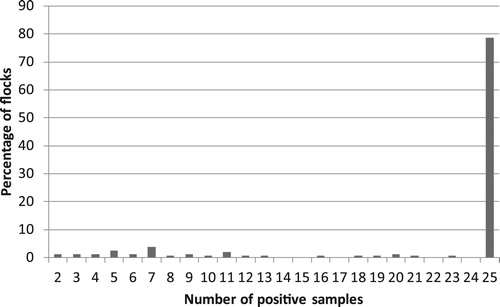
Out of the 311 layer pullet flocks that entered the study, 172 (55%) were positive for M. synoviae. The number of positive samples in each of these flocks is given in . The majority of flocks (78%) had positive reactions only, while 17% of the flocks had less than 15 positive samples.
Contribution of vertical transmission
In total, 113 out of 311 layer pullet flocks were at risk for vertical transmission. Of these 113, 95 flocks (84%) were M. synoviae seropositive at the end of the rearing period (). From the remaining 198 non-risk flocks, 77 (39%) were M. synoviae seropositive at the end of the rearing period while 121 (61%) remained negative. The odds ratio for layer pullet flocks to become M. synoviae positive when the flock contained offspring of M. synoviae positive parents, compared to flocks of which all parents were M. synoviae-negative was 5.8 (P = 0.000).
Contribution of within-farm transmission
Twenty-nine layer pullet flocks were housed on 26 single-house farms of which 13 (44%) were M. synoviae seropositive at the end of the rearing period (). One hundred and ninety-five layer pullet flocks were housed on multi-house farms and at risk of within-farm transmission. Of these 195 flocks, 150 flocks (77%) became M. synoviae seropositive while 45 flocks (23%) remained negative. The remaining 87 layer pullet flocks were housed on a multi-house farm and were not at risk of within-farm transmission, nine of these (10%) were seropositive for M. synoviae while 78 (90%) were negative. The positive flocks were the only positive flock at the farm. Odds ratios for flocks on multi-house layer pullet farms to become positive for M. synoviae, compared to single-house farms, were 3.1 (P = 0.022) and 0.2 (P = 0.003) for flocks at risk of within-farm transmission and flocks not at risk of within-farm transmission, respectively.
The M. synoviae status on multi-age farms was determined and denoted in . All flocks on multi-age farms but one were in the multi-house at risk group and no significant risk of multi-age farming was found when corrected for within-farm transmission. Multi-age farm management was therefore omitted from the final model.
Contribution of poultry farm density
Thirty-four layer pullet flocks were located in areas with the lowest poultry farm density, 137 flocks were located in areas with a medium poultry farm density and the remaining 140 flocks were located in areas with the highest poultry farm density (). Of the 34 flocks in the area with the lowest poultry farm density, 29 (85%) were M. synoviae seropositive at the end of the rearing period. In areas with a medium poultry density, 79 of 137 flocks (58%) were seropositive; and in areas with the highest poultry density 67 of 140 layer pullet flocks (46%) were seropositive at the end of rearing. The odds ratios for seroconversion in areas with the medium and highest poultry densities compared to the area with the lowest poultry density were 0.54 (P = 0.288) and 0.34 (P = 0.073), respectively.
Contribution of flock size
Seventy-eight flocks were small (≤ 8435), of which 34 (44%) were positive for M. synoviae. Of the 79 small to medium flocks, 40 (51%) were positive. Of the 76 medium to large flocks, 44 (58%) were positive. Of the 78 largest flocks, 57 (69%) were positive. Despite an increase in prevalence with larger flocks, and a significant odds ratio of 1.4 (P = 0.002) for M. synoviae in large flocks compared to small flocks, the effect of flock size was negligible in the multivariate analysis and it was not included in the final model.
Discussion
The prevalence of M. synoviae in Dutch layer pullet flocks was estimated at 55%. The most important transmission route for M. synoviae in layer pullet flocks identified in this study was vertical transmission followed by within-farm transmission, while there was no increased risk of between-farm horizontal transmission as reflected by poultry density. This is the first time that the relative importance of transmission routes of M. synoviae into layer pullet flocks has been quantified.
Three hundred and eleven layer pullet flocks represent a third of the total number of layer pullet flocks in the Netherlands in a year. The test of choice for this monitoring study was the M. synoviae RPA test as this test was shown to have a comparable specificity and sensitivity to the RPA test used in previous research in which an RPA test from another origin was compared with culture, PCR test and different commercial ELISAs (Feberwee et al., Citation2005). Different criteria were used for the monitoring of layer pullet flocks and parent flocks. Layer pullet flocks were sampled at the end of the rearing period, when a high prevalence was expected. This resulted in a lower number of samples (25) which will detect antibodies at approximately 12.5% prevalence at 95% confidence (Thrusfield et al., Citation2001). In parent flocks, the moment of seroconversion was an important parameter. Therefore, the number of samples was increased to 1% of the flock, with a maximum of 60, resulting in a 95% chance of detection of antibodies at a seroprevalence of 5% (Thrusfield et al., Citation2001). The test interpretation was adjusted for higher specificity. An over- or under-diagnosis of M. synoviae can, however, not be completely excluded (Feberwee et al., Citation2008). The history of antibiotic use on the included farms was not known. Use of antibiotics may suppress the antibody response and give an underestimation of the number of positive flocks and, therefore, an underestimation of the odds ratios representing positive associations (Stanley et al., Citation2001). An underdiagnosis of late infections during the rearing period may have led to an underestimation of the importance of horizontal transmission in this study.
The study was performed with flocks, rather than farms, as an epidemiological unit. Under common Dutch practice, there is no one-on-one relationship between breeder flock and layer pullet flock, or layer pullet flock and layer production flock. Therefore, each layer pullet flock on a farm may originate from a different parent flock. Subsequently, it is common that flocks from a pullet farm, or even from a single-house, are placed on different layer production farms. Therefore, an approach on flock level was expected to be more representative than a farm-level approach. Furthermore, by using flocks as an epidemiological unit, both vertical transmission and the importance of within-farm transmission could be studied.
Both the Pseudo R2 and goodness of fit showed that the model fitted the data. Therefore, the results can be extrapolated to the whole population. Based on the multivariate analysis, flock size can be excluded as a relevant factor. Several factors were omitted from the study. Farm hygiene level was not taken into account because a certified standard biosecurity programme was applied by all farms. M. synoviae history of the farms was not available.
This study found an odds ratio of 5.8 (P = 0.000) between M. synoviae-positive layer pullet flocks and the associated M. synoviae-positive parent flocks. This implies that vertical transmission is an important transmission route of M. synoviae to layer pullet flocks in the Netherlands. Vertical transmission of M. synoviae has been well documented (Carnaghan, Citation1961; MacOwan et al., Citation1984) and the importance of M. synoviae-negative parent stock has been stressed before but has not been quantified (Kleven, Citation2008; Cobb, Citation2011). In the current study, layer pullet flocks were sampled at the end of the rearing period. Although in 78% of the flocks all samples were positive, no differentiation could be made between horizontal and vertical transmission based on the moment of seroconversion. An earlier sampling of the flocks, and genetic comparison of isolates of parent flocks and offspring, would contribute to confirmation of this finding. Nevertheless, vertical transmission had the strongest association with M. synoviae-positive layer pullet flocks in this study. The M. synoviae prevalence in layer parent stock in this study was 26% (10 out of 38 farms were positive) and this, together with mixing progeny from different parent flocks, likely contributed highly to the importance of vertical transmission. If the M. synoviae prevalence in parent stock is reduced, the impact of vertical transmission on the M. synoviae prevalence of layer pullet flocks will most likely decrease and the importance of other factors will increase.
Within-farm transmission was shown to be a significant factor for getting infected (OR = 3.1, P = 0.003). Moreover, a significant negative association between M. synoviae infections and flocks not at risk of within-farm transmission was found (OR = 0.2, P = 0.000). The low number of single-house farms and major contribution of vertical transmission probably reduced the relative importance of within-farm transmission in our study. Nevertheless, the results imply that within-farm transmission might be an important transmission route for M. synoviae. Within-farm transmission might be especially important on farms with multi age management. Because of the limited number of flocks at multi-age farms in the study, and confounding with flocks at risk of within-farm transmission, the risk of multi-age flock management was excluded from the final model.
The finding that the poultry farm density in an area was not an important risk factor for M. synoviae transmission to layer pullet flocks was not expected. The categories of poultry farm density were relative, whereas the lowest poultry farm density was a maximum of 19 farms per square km. The chance of horizontal transmission between farms in areas with a much lower density may be even lower. No information on the distance of airborne transmission of M. synoviae is available, and a radius of 10 km was chosen based on measurements on Mycoplasma hyopneumoniae (Otake et al., Citation2010). M. synoviae has been found in the dust (Marois et al., Citation2000) which may cause airborne transmission on shorter distances as indicated by endotoxin measurement (Jonges et al., Citation2015). More information is required on the distance of airborne transmission to further investigate its importance.
The M. synoviae prevalence in layers was not reassessed in this study. However, from these numbers, it is clear that M. synoviae infection during the rearing period has a major contribution to the prevalence in production layers because production flocks are already positive when they arrive at the farm. In order to set up a control programme for M. synoviae, it is important to know the prevalence of this mycoplasma species in different poultry types, and the most important routes of transmission. In this study, it was shown that the prevalence of M. synoviae in layer pullet stock was 55% and that M. synoviae-infected parent stock represents the most important risk for infection, followed by within-farm horizontal transmission. Although this analysis is performed on data from the Dutch poultry industry, M. synoviae infections in parent stock and multi-age housing occur worldwide, and therefore the results can be extrapolated to other countries and integrations alike. The results of this study emphasize the importance of M. synoviae control in parent stock, and channelling of M. synoviae-positive flocks.
Acknowledgement
The authors want to thank Anouk Veldhuis for her assistance with the statistics.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
J. J. de Wit http://orcid.org/0000-0002-3459-1000
Additional information
Funding
References
- Anonymous. (2018). National poultry improvement plan and auxiliary provision as of July 19, 2018. Department of Agriculture, 1–156. Washington DC, USA: Government Printing Office.
- Carnaghan, R.B.A. (1961). Egg transmission of infectious synovitis. Journal of Comparative Pathology and Therapeutics, 71, 279–285. doi: 10.1016/S0368-1742(61)80034-9
- Catania, S., Bilato, D., Gobbo, F., Granato, A., Terregino, C., Iob, L. & Nicholas, R.A. (2010). Treatment of eggshell abnormalities and reduced egg production caused by Mycoplasma synoviae infection. Avian Diseases, 54, 961–964. doi: 10.1637/9121-110309-Case.1
- Catania, S., Gobbo, F., Bilato, D., Gagliazzo, L., Moronato, M.L., Terregino, C., Bradbury, J.M. & Ramirez, A.S. (2016). Two strains of Mycoplasma synoviae from chicken flocks on the same layer farm differ in their ability to produce eggshell apex abnormality. Veterinary Microbiology, 193, 60–66. doi: 10.1016/j.vetmic.2016.08.007
- Cobb, S.P. (2011). The spread of pathogens through trade in poultry hatching eggs: overview and recent developments. Revue Scientifique et Technique de l'OIE, 30, 165–175. doi: 10.20506/rst.30.1.2025
- Feberwee, A., Mekkes, D.R., de Wit, J.J., Hartman, E.G. & Pijpers, A. (2005). Comparison of culture, PCR, and different serologic tests for detection of Mycoplasma gallisepticum and Mycoplasma synoviae infections. Avian Diseases, 49, 260–268. doi: 10.1637/7274-090804R
- Feberwee, A., de Vries, T.S. & Landman, W.J. (2008). Seroprevalence of Mycoplasma synoviae in Dutch commercial poultry farms. Avian Pathology, 37, 629–633. doi: 10.1080/03079450802484987
- Feberwee, A., de Wit, J.J. & Landman, W.J. (2009). Induction of eggshell apex abnormalities by Mycoplasma synoviae: field and experimental studies. Avian Pathology, 38, 77–85. doi: 10.1080/03079450802662772
- Gole, V.C., Chousalkar, K.K. & Roberts, J.R. (2012). Prevalence of antibodies to Mycoplasma synoviae in laying hens and possible effects on egg shell quality. Preventive Veterinary Medicine, 106, 75–78. doi: 10.1016/j.prevetmed.2012.02.018
- Jonges, M., van Leuken, J., Wouters, I., Koch, G., Meijer, A. & Koopmans, M. (2015). Wind-mediated spread of low-pathogenic avian influenza virus into the environment during outbreaks at commercial poultry farms. PLoS One, 10, e0125401. doi: 10.1371/journal.pone.0125401
- Kamp, H.J.G. (2014). Regeling van de Minister van Economische Zaken van 10 December 2014, nr WJZ/14139630, houdende wijziging van diverse regelingen in verband met de opheffing van bedrijfslichamen en de overname van taken. Staatscourant 35166, Ministerie van Economische zaken, ‘s Gravenhage.
- Kleven, S.H., King, D.D. & Anderson, D.P. (1972). Airsacculitis in broilers from Mycoplasma synoviae: effect on air-sac lesions of vaccinating with infectious bronchitis and Newcastle virus. Avian Diseases, 16, 915–924. doi: 10.2307/1588772
- Kleven, S.H., Fletcher, O.J. & Davis, R.B. (1975). Influence of strain of Mycoplasma synoviae and route of infection on development of synovitis or airsacculitis in broilers. Avian Diseases, 19, 126–135. doi: 10.2307/1588963
- Kleven, S.H. (2008). Control of avian mycoplasma infections in commercial poultry. Avian Diseases, 52, 367–374. doi: 10.1637/8323-041808-Review.1
- Landman, W.J. & Feberwee, A. (2001). Field studies on the association between amyloid arthropathy and Mycoplasma synoviae infection, and experimental reproduction of the condition in brown layers. Avian Pathology, 30, 629–639. doi: 10.1080/03079450120092125
- Landman, W.J. & Feberwee, A. (2012). Longitudinal field study on the occurrence of Mycoplasma synoviae in Dutch turkey flocks with lameness and experimental induction of the condition. Avian Pathology, 41, 141–149. doi: 10.1080/03079457.2011.652595
- MacOwan, K.J., Atkinson, M.J., Bell, M.A., Brand, T.F. & Randall, C.J. (1984). Egg transmission of a respiratory isolate of Mycoplasma synoviae and infection of the chicken embryo. Avian Pathology, 13, 51–58. doi: 10.1080/03079458408418507
- Marois, C., Dufour-Gesbert, F. & Kempf, I. (2000). Detection of Mycoplasma synoviae in poultry environment samples by culture and polymerase chain reaction. Veterinary Microbiology, 73, 311–318. doi: 10.1016/S0378-1135(00)00178-4
- Morrow, C.J., Bell, I.G., Walker, S.B., Markham, P.F., Thorp, B.H. & Whithear, K.G. (1990). Isolation of Mycoplasma synoviae from infectious synovitis of chickens. Australian Veterinary Journal, 67, 121–124. doi: 10.1111/j.1751-0813.1990.tb07726.x
- Otake, S., Dee, S., Corzo, C., Oliveira, S. & Deen, J. (2010). Long-distance airborne transport of infectious PRRSV and Mycoplasma hyopneumoniae from a swine population infected with multiple viral variants. Veterinary Microbiology, 145, 198–208. doi: 10.1016/j.vetmic.2010.03.028
- PPE. (1993). Besluit onderzoek Mycoplasma gallisepticum en Mycoplasma meleagridis 1993.
- Stanley, W.A., Hofacre, C.L., Speksnijder, G., Kleven, S.H. & Aggrey, S.E. (2001). Monitoring Mycoplasma gallisepticum and Mycoplasma synoviae infection in breeder chickens after treatment with enrofloxacin. Avian Diseases, 45, 534–539. doi: 10.2307/1593001
- Thrusfield, M., Ortega, C., de Blas, I., Noordhuizen, J.P. & Frankena, K. (2001). WIN EPISCOPE 2.0: improved epidemiological software for veterinary medicine. The Veterinary Record, 148, 567–572. doi: 10.1136/vr.148.18.567