ABSTRACT
Three-week-old turkey poults were infected with pure lines of three species of Eimeria (E. adenoeides, E. gallopavonis, and E. meleagrimitis) recently isolated from commercial turkey farms. The lines had been propagated from a single oocyst and identified by species-specific PCR amplification of the mitochondrial cytochrome c oxidase subunit I (COI) gene. Five to six days after infection their intestines were removed and examined for the presence of intestinal lesions. A description and review of the pathology caused by these parasites is provided, and a scoring system developed by which the severity of the lesions can be evaluated. The system is similar to that described by Johnson, J. and Reid, W. M. [1970. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Experimental Parasitology, 28, 30–36] for chickens in which a score of zero to four is assigned to lesions of increasing severity. The intestinal lesions observed here, and their assigned scores, are supported by representative illustrations. It is hoped that they may prove a useful tool for evaluating the pathology caused by E. adenoeides, E. gallopavonis, and E. meleagrimitis in the turkey.
RESEARCH HIGHLIGHTS
A scoring system has been developed for intestinal lesions caused by three species of Eimeria that infect the turkey.
The lesions attributable to these species are illustrated.
Introduction
In 1970, a manuscript was published concerning a method by which the pathological consequences of infection with Eimeria species in the chicken could be quantified (Johnson & Reid, Citation1970). This procedure, that allocates a simple scoring system (0–4 scale) for different regions of the intestine that exhibit lesions of varying severity, has become one of the most frequently quoted references in the coccidiosis literature. It has proved of prime utility in evaluating the ability of various drugs to prevent infection and is a requirement for many regulatory authorities in order to obtain approval for their use (Holdsworth et al., Citation2004). It has also been widely employed to determine whether resistance has been acquired in field isolates of Eimeria to anticoccidial drugs. Although the disease coccidiosis in the turkey has much in common with its counterpart in the chicken, a consistent lesion scoring method similar to that described by Johnson and Reid is not available. This is partly a consequence of the general paucity of research concerned with the pathology of coccidiosis in the turkey compared with the extensive literature for the chicken (Chapman, Citation2008). Unfortunately, the strains of Eimeria in the turkey, upon which original pathological descriptions were based, no longer exist (e.g. Moore & Brown, Citation1951; Hawkins, Citation1952; Clarkson, Citation1958, Citation1959). Recent studies, utilizing classical and molecular methodology, have attempted to re-describe some of the more common and pathogenic species with descriptions of the pathology caused by infection thus providing a basis for both classification and further research (El-Sherry et al., Citation2014a, b, Citation2015, Citation2019). In this article we review what is known about the pathology caused by three species of Eimeria that infect the turkey (Eimeria adenoeides, Eimeria gallopavonis, and Eimeria meleagrimitis), describe the lesions caused by these species, illustrate them with colour photographs, and provide a scoring system by which the severity of these lesions may be evaluated.
Materials and methods
Birds and husbandry
Newly hatched (day-old) turkey poults (Nicholas breed) that had not been vaccinated against coccidiosis were utilized in the experiments. They were reared in brooder cages at a stocking density of 257 cm2/poult until they were 14 days of age. Poults were moved to grower cages at 2 weeks of age, where they were kept until the end of the experimental period. Husbandry and management followed Guidelines for the Care and Use of Animals in Agricultural Research (FASS, Citation2010) and all experiments were approved by the Institutional Animal Care Committee. All poults were fed an unmedicated, nutritionally adequate corn-soybean diet (NRC, Citation1994) and had free access to water.
Parasites
All three parasites used in this study were initially obtained from faecal samples collected from farms in the USA where turkey poults were raised commercially. Oocysts were harvested using standard methods (Shirley, Citation1995) and propagated in poults that had been reared in the absence of infection. A pure line of each species was obtained by isolating a single oocyst according to the procedure described by Shirley & Harvey (Citation1996) and subsequent propagation in naïve poults. Identity of each species was confirmed by species-specific PCR amplification of the mitochondrial cytochrome c oxidase subunit I (COI) gene according to procedures described by Rathinam et al. (Citation2015). In addition, the PCR product for each species was sequenced and subjected to BLAST analysis to confirm identity. For all three species, BLAST analysis of the sequence resulted in high identity (100% coverage and 100% identity) to respective GenBank sequences (KJ608415.1 for E. adenoeides; KJ608413.1 for E. gallopavonis, and KJ608414 for E. meleagrimitis).
Characterization of gross lesions
In order to characterize lesions, groups of 21-day-old poults (four replicates/group; eight poults/replicate) were inoculated with 0.05 − 4.0 × 105 sporulated oocysts of each species per bird. Control birds were sham inoculated with water. On days 5 and 6 after inoculation they were euthanized, and their intestines examined for the presence of lesions.
Results
Based upon examination of 256 poults infected with each species of Eimeria, a scoring system and a description for lesions of increasing severity were established (). The lesions evident in the intestines of selected poults are illustrated in . The infection doses and the average lesion scores observed for each dose are provided in . An increase in lesions was apparent with an increase in the dose for all three species. Of the two days when lesion scoring was performed, lesions were pronounced on day 5 for E. adenoeides and E. meleagrimitis and on day 6 for E. gallopavonis.
Figure 1. Appearance of macroscopic lesions of E. adenoeides in the caecal pouches of turkey poults observed five days post-inoculation. Pictures labelled a (score 0), b (score 1), c (score 2), d (score 3), and e (score 4) represent various grades of lesions. Picture e shows caecal plugs placed next to the caeca in which they were observed.
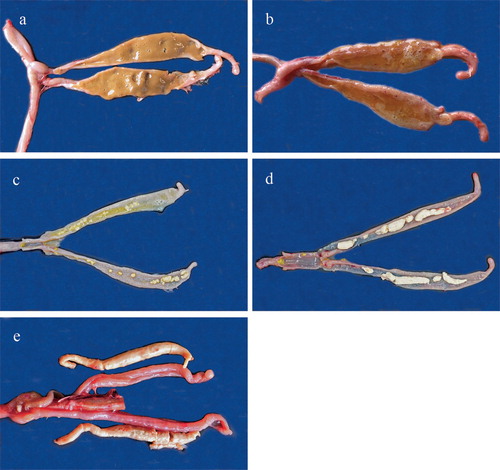
Figure 2. Appearance of macroscopic lesions of E. gallopavonis in the lower ileum and caecal neck of turkey poults observed 6 days following inoculation. Pictures labelled a (score 0), b (score 1), c (score 2), d (score 3), and e (score 4) represent various grades of lesions. Pictures b and c show ileum only. Arrow in b points to the small, white spots seen on ileal mucosa in score 1.
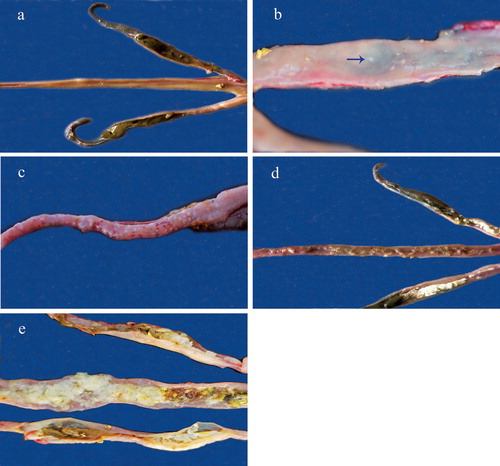
Figure 3. Appearance of macroscopic lesions of E. meleagrimitis in the duodenal mucosa of turkey poults observed 5 days following inoculation. Pictures labelled a (score 0), b (score 1), c (score 2), d (score 3), and e (score 4) represent various grades of lesions.
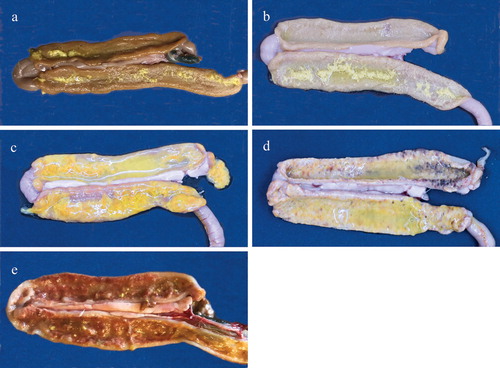
Table 1. Lesion scoring system for E. adenoeides.
Table 2. Lesion scoring system for E. gallopavonis.
Table 3. Lesion scoring system for E. meleagrimitis.
Table 4. Infection dose and corresponding average lesions observed 5 days post infection with E. adenoeides and E. meleagrimitis and 6 days post infection with E. gallopavonis.
Pathology and lesions of E. adenoeides
Lesions were primarily seen in the caeca and were mostly restricted to variations in the accumulation of caseous exudate and formation of plugs. Petechial haemorrhages were observed sporadically in the caecal mucosa but were not consistently correlated with any score. While the parasite develops in the lower ileum, in all the birds that were sampled, no discernible lesions were observed in the lower ileum and rectum (; (a)–(e)).
Pathology and lesions of E. gallopavonis
Lesions were primarily seen in the ileo-caecal junction, caecal neck, and rectum, and included petechiae and the presence of few to numerous white round spots on the ileal and rectal mucosa ((b,c)). In severe infections, lesions extended throughout the ileum all the way to the Meckel’s diverticulum with an accumulation of soft, white, cheesy caseous exudate that was occasionally mixed with orange or green mucoid content. The accumulation of caseous exudate in the caeca was confined to the caecal neck or proximal one-third of the caeca with the remainder of the caecal pouch unaffected (; (a)–(e)).
Pathology and lesions of E. meleagrimitis
Lesions were primarily seen in the duodenum and proximal parts of jejunum and included oedematous intestines with accumulation of mucus, formation of diphtheritic membranes, necrosis and sloughing. Petechial haemorrhages were observed sporadically in the duodenal mucosa but were not consistent (; (a)–(e)).
Discussion
Seven species of Eimeria are recognized from the turkey but, of these, the three included in this study (E. adenoeides, E. gallopavonis, and E. meleagrimitis) are considered the most pathogenic (Lund & Farr, Citation1965; Chapman, Citation2008). E. adenoeides develops in the rectum and caeca, E. gallopavonis in the ileum, rectum, and caeca, and E. meleagrimitis principally in the duodenum and jejunum extending in heavy infections to the lower intestine (Chapman, Citation2008). Their prevalence is difficult to determine because of the lack of clear-cut criteria for identification, but all three are considered to occur quite frequently in the field (Clarkson & Gentles, Citation1958; Edgar, Citation1986).
Pathology and lesions of E. adenoeides
The first detailed description of the pathological changes in the gut following infection with E. adenoeides included petechial haemorrhages in the lower small intestine, caeca and rectum, an accumulation of yellow–white mucoid material in the lower small intestine, and cheesy caseous clots in the caecum (Clarkson, Citation1958). According to Reid (Citation1972), pathology and lesions of E. adenoeides include congestion, oedema, petechiae, strands of mucus, blood, and a “cottage cheese-like” material in the caeca, lower small intestine and rectum. More recently, the lesions were described as white caseous plugs in the caecal pouches and limited petechial haemorrhage without the severe blood loss that is usually observed in caecal coccidiosis in the chicken (El-Sherry et al., Citation2014a). Similar pathology and lesions were seen in the present study.
Pathology and lesions of E. gallopavonis
Although initially described by Hawkins (Citation1952), the first descriptions of the pathology and lesions produced following infection with E. gallopavonis include marked inflammatory changes in the lower small intestine, rectum, and proximal portions of caeca with accumulation of soft, white, cheesy material in the lumen of the affected organs, oedema, epithelial sloughing, and lymphocytic infiltration (Farr et al., Citation1961; Wehr et al., Citation1962; Lund & Farr, Citation1965; Reid, Citation1972). Wall thickening in the distal ileum and rectum, petechiae, clotted blood in the ileum, and caseous plugs in the caecal neck have been reported (Vrba & Pakandl, Citation2014). According to El-Sherry et al. (Citation2019), pathology and lesions caused by E. gallopavonis include necrosis, oedema, epithelial sloughing and white caseous material in the ileo-caecal junction, caecal neck and rectum. In heavy infections, the caseous material filled the lumen of the intestine from Meckel’s diverticulum through to the rectum. Many of these characteristics were observed in the present study.
Pathology and lesions of E. meleagrimitis
The first descriptions of pathology and lesions caused by E. meleagrimitis were oedema and congestion in the duodenum and upper small intestine with accumulation of brown necrotic material with streaks of blood (Hawkins, Citation1952; Clarkson & Gentles, Citation1958). Lesions described by Clarkson (Citation1959) were an enlarged, congested duodenum with presence of a red-brown necrotic core, sometimes extending into the upper small intestine. Hein (Citation1969) reported haemorrhagic enteritis, marked vascular congestion, and a mucosa covered with blood stained tissue debris in the proximal part of the small intestine. Similar descriptions were provided by Reid (Citation1972) and Joyner (Citation1973) including congestion and accumulation of a reddish brown mucoid necrotic core/exudate in the duodenum and jejunum. More recently, pathology and lesions caused by this species included necrosis, sloughing of the epithelium, ulceration, petechiae and mucoid casts mainly in the duodenum and jejunum (El-Sherry et al., Citation2014b). Another study reported petechiae, wall thickening, and a large amount of mucus in the duodenum and jejunum, as well as watery contents in the ileum (Vrba & Pakandl, Citation2014). Similar pathology and lesions were observed in this study.
Review of lesion scoring procedures for turkey coccidia
No lesion scoring system has been described for E. gallopavonis. Lesions in turkeys were evaluated following infection with a mixed coccidial culture based on a visual examination of intestinal damage, a score from 0 to 4 being assigned with 4 the most severe (Mathis, Citation1993). No descriptions of the actual lesions observed were provided. A procedure described by Abd El-Wahab et al. (Citation2013) for E. adenoeides relies on the number of petechial haemorrhages and thickening of the caecal mucosa but does not take into account the accumulation of caseous material in the pouches, which is a major pathological lesion for this species. There are a few resources that describe a five-point scoring scale for E. meleagrimitis (El-Sherry et al., Citation2014b; Immucox® Turkey Lesion Scores originally developed by ANSES, France). Whereas the Immucox® procedure relies mainly on the presence of pseudo-membranes and blood in the intestine and minimal changes in mucosa, especially in lower scores, the method proposed by El-Sherry et al. (Citation2014b) considers mucosal changes including congestion, the presence of petechiae, wall thickening, and necrosis.
Development of a standardized scoring system
Due to the lack of clear, standardized lesion score descriptions in the literature, a refined five-point scale scoring system with clear descriptions for different grades of pathology was developed based on observations from pathogenicity studies performed with pure cultures of E. adenoeides, E. gallopavonis, and E. meleagrimitis.
The lesion scoring system described here is one criterion that may be of value in determining the severity of Eimeria infection in turkeys. While a dose-dependant positive trend of lesion scores was observed in our study, it is important to carry out a preliminary experiment to determine an optimal dose of oocysts if lesion score is to be a criterion for evaluating the severity of an infection. The lesions are illustrated to facilitate their identification. Johnson & Reid (Citation1970) recommend that lesion scoring be accompanied by microscopic examination of mucosal scrapings for the presence of life cycle stages, but it is doubtful whether this is actually carried out in most cases, because of the time-consuming work involved. The procedure is inherently subjective and should be performed by an experienced worker familiar with the pathology caused by the parasites and blinded to treatments. As in the chicken, the method is of particular use for evaluating the efficacy of anticoccidial drugs in preventing coccidiosis and establishing the occurrence of drug resistance, evaluating coccidiosis vaccine efficacy and routine monitoring of turkey coccidiosis in the field. Caution should be exercised in utilizing lesion scores in experiments concerned with immunity since lesions may be present in partially immune or completely immune chickens even though weight gain is not depressed and the lesions contain few or no parasites (references in Chapman et al., Citation2005). Some studies in chickens have demonstrated limitations to the use of lesions alone in assessing drug efficacy as there is a non-linear relationship between the magnitude of infection (oocyst dose) and lesion scores (Conway et al., Citation1990). However, used in conjunction with other criteria, such as weight gain during the acute phase of infection, lesion scoring has proved the most widely employed procedure for evaluating the deleterious effects of coccidiosis upon galliform birds.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abd El-Wahab, A., Visscher, C.F., Wolken, S., Reperant, L.M., Beineke, A., Beyerbach, M. & Kamphues, J. (2013). Outcome of an artificial coccidial infection in poults under the influence of floor heating. Poultry Science, 92, 629–637.
- Chapman, H.D. (2008). Coccidiosis in the turkey. Avian Pathology, 37, 205–223. doi: 10.1080/03079450802050689
- Chapman, H.D., Roberts, B., Shirley, M.W. & Williams, R.B. (2005). Guidelines for evaluating the efficacy and safety of live anticoccidial vaccines, and obtaining approval for their use in chickens and turkeys. Avian Pathology, 34, 279–290. doi: 10.1080/03079450500178378
- Clarkson, M.J. (1958). Life history and pathogenicity of Eimeria adenoeides Moore and Brown, 1951, in the turkey poult. Parasitology, 48, 70–88. doi: 10.1017/S0031182000021065
- Clarkson, M.J. (1959). The life history and pathogenicity of Eimeria meleagrimitis Tyzzer, 1929, in the turkey poult. Parasitology, 49, 70–82. doi: 10.1017/S0031182000026718
- Clarkson, M.J. & Gentles, M.A. (1958). Coccidiosis in turkeys. Veterinary Record, 70, 211–214.
- Conway, D.P., McKenzie, M.E. & Dayton, A.D. (1990). Relationship of coccidial lesion scores and weight gain in infections of Eimeria acervulina, E. maxima and E. tenella in broilers. Avian Pathology, 19, 489–496. doi: 10.1080/03079459008418702
- Edgar, S.A. (1986). Coccidiosis in Turkeys: Biology and Incidence. In L.R. McDougald, L.P. Joyner & P.L. Long (Eds.), Research in Avian coccidiosis, Proceedings of the Georgia Coccidiosis Conference, 1985 (pp. 116–123). Athens: University of Georgia.
- El-Sherry, S., Ogedengbe, M.E., Hafeez, M.A., Sayf-AI-Din, M., Gad, N. & Barta, J.R. (2014a). Re-description of a genetically typed, single oocyst line of the turkey coccidium, Eimeria adenoeides Moore and Brown, 1951. Parasitology Research, 113, 3993–4004. doi: 10.1007/s00436-014-4066-7
- El-Sherry, S., Rathinam, T., Hafeez, M.A., Ogedengbe, M.E., Chapman, H.D. & Barta, J.R. (2014b). Biological re-description of a genetically typed, single oocyst line of the turkey coccidium Eimeria meleagrimitis Tyzzer 1929. Parasitology Research, 113, 1135–1146. doi: 10.1007/s00436-014-3751-x
- El-Sherry, S., Ogedengbe, M.E., Hafeez, M.A., Sayf-Al-Din, M., Gad, N. & Barta, J.R. (2015). Sequence-based genotyping clarifies conflicting historical morphometric and biological data for 5 Eimeria species infecting turkeys. Poultry Science, 1–11.
- El-Sherry, S., Ogedengbe, M.E., Hafeez, M.A., Sayf-Al-Din, M., Gad, N. & Barta, J.R. (2019). Cecal coccidiosis in turkeys: comparative biology of Eimeria species in the lower intestinal tract of turkeys using genetically typed, single oocyst-derived lines. Parasitology Research, 118, 583–598. doi: 10.1007/s00436-018-6147-5
- Farr, M.M., Wehr, E.E. & Shalkop, W.T. (1961). Pathogenicity of E. gallopavonis. The Virginia Journal of Science, 12, 150–151.
- FASS. (2010). Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching 3rd edn. Champaign, IL: Federation of Animal Science Societies.
- Hawkins, P.A. (1952). Coccidiosis in turkeys. Technical Bulletin 226. Michigan State College Agricultural Experiment Station, East Lansing, MI.
- Hein, H. (1969). Eimeria adenoeides and E. meleagrimitis: pathogenic effect in turkey poults. Experimental Parasitology, 24, 163–170. doi: 10.1016/0014-4894(69)90153-2
- Holdsworth, P.A., Conway, D.P., McKenzie, M.E., Dayton, A.D., Chapman, H.D., Mathis, G.F., Skinner, J.T., Mundt, H.-C. & Williams, R.B. (2004). World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Veterinary Parasitology, 121, 189–212. doi: 10.1016/j.vetpar.2004.03.006
- Johnson, J. & Reid, W.M. (1970). Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Experimental Parasitology, 28, 30–36. doi: 10.1016/0014-4894(70)90063-9
- Joyner, L.P. (1973). Coccidiosis in turkeys and its control. Folia Veterinaria Latina, 3, 110–123.
- Lund, E.E. & Farr, M.M. (1965). Coccidiosis of the Turkey. In H.E. Biester & L.H. Schwarte (Eds.), Diseases of Poultry 5th edn (pp. 1088–1093). Ames: Iowa State University Press.
- Mathis, G.F. (1993). Toxicity and acquisition of immunity to coccidia in turkeys medicated with anticoccidials. The Journal of Applied Poultry Research, 2, 239–244. doi: 10.1093/japr/2.3.239
- Moore, E.N. & Brown, J.A. (1951). A new coccidium pathogenic for turkeys, Eimeria adenoeides n. sp. (protozoa: Eimeriidae). The Cornell Veterinarian, 41, 124–135.
- NRC. (1994). Nutrient Requirements of Poultry. 9th rev. edn. Washington, DC: National Academies Press.
- Rathinam, T., Gadde, U. & Chapman, H.D. (2015). Molecular detection of field isolates of turkey Eimeria by polymerase chain reaction amplification of the cytochrome c oxidase I gene. Parasitology Research, 114, 2795–2799. doi: 10.1007/s00436-015-4546-4
- Reid, W.M. (1972). Turkey. In M.S. Hofstad, B.W. Calnek, C.F. Helmboldt, W.M. Reid & H.W. Yoder (Eds.), Diseases of Poultry 6th edn (pp. 977–986). Ames: Iowa State University Press.
- Shirley, M.W. (1995). Eimeria Species and Strains of Chicken. In J. Eckert, R. Brown, M.W. Shirley & P. Coudert (Eds.), Guidelines on Techniques in Coccidiosis Research (pp. 1–24). Luxembourg: Office for Official Publications of the European Communities.
- Shirley, M.W. & Harvey, D.A. (1996). Eimeria tenella: infection with a single sporocyst gives a clonal population. Parasitology, 112, 523–528. doi: 10.1017/S0031182000066099
- Vrba, V. & Pakandl, M. (2014). Coccidia of turkey: from isolation, characterisation and comparison to molecular phylogeny and molecular diagnostics. International Journal for Parasitology, 44, 985–1000. doi: 10.1016/j.ijpara.2014.06.004
- Wehr, E.E., Fahr, M.M. & Shalkop, W.T. (1962). Studies on pathogenicity of Eimeria gallopavonis to turkeys. Journal of Protozoology, 9, 8–9.
