 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
To protect layers, breeders and grandparents against damage by infectious bronchitis virus infections during the laying period, vaccination using live priming followed by a boost with inactivated IB vaccine is commonly used. For many IB variants, homologous live vaccines are not available for priming. Very little is known about the efficacy of priming with heterologous live IB vaccines (or combination of live IB vaccines) to induce broad IB protection in long-living chickens. In this study, the protection levels induced by vaccination programmes with only heterologous live priming by a Massachusetts vaccine and a 4/91 vaccine, only a multivalent inactivated vaccine that contained D1466 antigen and a combination of both, against a D1466 challenge were compared. The infection with infectious bronchitis virus D1466, a genotype II, lineage 1 virus, was able to cause serious damage to the unvaccinated laying hens resulting in respiratory signs, a long-lasting drop in egg production and loss of egg quality. All three vaccination programmes induced significant levels of protection against challenge with a pathogenic D1466 strain. Overall, the vaccination programme using the broad heterologous live priming and the inactivated vaccine provided high protection against the combination of egg drop and loss of egg quality. The results showed that this combination of heterologous live vaccines was able to increase the efficacy of the inactivated infectious bronchitis virus vaccine despite the very low antigenic relationship of both live vaccines with the challenge strain.
Introduction
Infectious bronchitis virus (IBV) is prevalent worldwide and is one of the most important viral infections causing clinical disease and performance drops in all types of chickens. IBV can cause respiratory problems, drops in egg production, poor egg shell and internal egg quality, drop in hatchability and day-old chick quality, nephritis and sometimes false layers (Jackwood & De Wit, Citation2013).
An important characteristic of IBV is that it has a high mutation rate and the number of types (serotypes or genotypes) that has been detected and reported is increasing over time. More and more countries have shown that multiple variant IBV strains circulate in their poultry industry (De Wit, Cook et al., Citation2011; Jackwood, Citation2012). For decades, vaccination against IBV has been an important tool to prevent IBV infection and its detrimental effects, but the prevalence of multiple variant IBV strains complicates the challenges and our ability to raise sufficient protection against all the different variants and upcoming new IBV variants. For many IBV variants, homologous live vaccines are not available. In young birds, it has been shown that vaccination with two antigenically distinct live-attenuated vaccines, such as Massachusetts (Mass or GI-1) and 4/91 (also known as 793B or GI-13) (Valastro et al., Citation2016), can result in a broad cross-protection against many different IBV types (Cook et al., Citation1999; Terregino et al., Citation2008; De Wit et al., Citation2015; Awad et al., Citation2015; De Wit et al., Citation2017) the so called ‘Protectotype’ approach. A meta-analysis on the data of 18 IBV vaccination-challenge trials, using live vaccines of six different serotypes and challenge strains of eight different serotypes, showed that not every variant requires its own homologous vaccine and that combinations of two heterologous vaccines provide more cross-protection than one heterologous live vaccine (De Wit et al., Citation2013).
The vast majority of papers reporting IBV vaccination-challenge experiments show results of trials with young chickens that are vaccinated during the first few weeks of life and challenged several weeks later and the level of protection is primarily measured in the respiratory tract (De Wit & Cook, Citation2014). The number of papers concerning IB vaccination-challenge trials with birds in lay is very limited as these experiments are very costly. For birds in lay, long-lasting protection is required after the application of the vaccines in the rearing period. In several experiments in the 1980s, it was shown that the highest level of protection against drops in egg production was achieved by a vaccination programme that consisted of a live priming and subsequent boost by an inactivated emulsion IB vaccine (Box et al., Citation1980; Box & Ellis, Citation1985; Box et al., Citation1988). The experiments of Box et al. showed a positive correlation between the level of haemagglutination inhibiting antibodies against the IBV challenge strain at the day of challenge (in most experiments a Mass strain, sometimes a D274 strain) and the level of protection against egg production drop. High antibody levels raised by inactivated vaccines alone did not correlate with a high level of protection (Box & Ellis, Citation1985). Muneer et al. (Citation1987) reported that boosting with an inactivated oil-emulsion vaccine containing a Mass antigen of layers that had been vaccinated earlier with two live vaccinations with a Mass vaccine did not result in protection against an Arkansas challenge at 52 weeks of age, but protection against a Mass challenge at 61 weeks was still significant. Only a few papers report the level of protection against heterologous challenges. Chousalkar et al. (Citation2010) reported that vaccination of pullets with live vaccines Vic S (GI-6 lineage) and A3 (GI-5 lineage) (Valastro et al., Citation2016) lowered the total viral RNA of the strain N1/88 (GIII genotype) post challenge at 25 weeks of age. De Wit et al. (Citation2019) reported the results of a vaccination-challenge experiment with layer pullets primed with IBV Mass and 793B vaccines prior to injection of inactivated vaccine containing Mass antigen. Adding the inactivated vaccine to the vaccination programme resulted in increased virus neutralizing antibody titres against the five IBV challenge strains and a significantly higher level of protection against drops in egg production following challenge.
With the increasing number of emerging IBV variants worldwide and the limited number of homologous vaccines, there is a need for more knowledge about how to achieve a high level of protection for laying birds against different IBV strains. One of the questions is whether there is a need for homologous live priming for the inactivated vaccine in order to achieve a high level of protection against egg drop and decrease in egg quality.
The long-lasting vaccination-challenge experiments for IBV in layers require special high level containment facilities and are very expensive to perform. In practice, this limits the number of groups that can be included in these studies compared to studies with young birds. De Wit et al. (Citation2019) showed that heterologous live priming using Mass and 793B vaccines prior to boosting with an inactivated vaccine with Mass antigen resulted in a higher level of neutralizing antibodies against seven serotypes of IBV than when the priming was done using two vaccinations with a live Mass vaccine. In this vaccination-challenge experiment in laying birds, we determined whether the efficacy of an inactivated vaccine, containing a homologous antigen to the challenge strain, was improved by live priming using a combination of heterologous vaccines which were both antigenically very different from the challenge virus. To achieve this, we used a D1466 challenge virus, a GII-1 virus that is genetically and antigenically very distinct from any live IBV vaccine (Davelaar et al., Citation1984; De Wit, Nieuwenhuisen-van Wilgen et al., Citation2011; Valastro et al., Citation2016). The D1466 variant (also called D212) was detected for the first time in The Netherlands in the late 1970s and has since then mainly been detected in layers and breeders (Davelaar et al., Citation1984; Worthington et al., Citation2008; Domanska-Blicharz et al., Citation2012). For D1466, live vaccines are no longer available. In the past, the available live D1466 vaccines induced a moderate level of homologous protection compared to vaccines of other serotypes (De Wit et al., Citation2013). In this study, the level of protection against clinical signs, ciliostasis, egg drop and egg abnormalities was determined.
Materials and methods
Virus cross-neutralization tests
Virus cross-neutralization tests were performed using strains M41, D1466 and 4/91 and their monospecific antisera. The sera had been raised in groups of 4–8-week-old specified pathogen free (SPF) White Leghorns at Royal GD, Deventer, The Netherlands. The antisera were pooled per virus and used in duplicate to determine the ability to neutralize a constant amount of 30–300 median tissue culture infectious doses (TCID50) of virus of each of the three serotypes. Each virus was mixed with two-fold dilutions of antisera and incubated for 1 h at 36–38°C. Subsequently, the virus-serum mixtures were transferred in duplicate to wells of microtitre plates which contained monolayers of primary chicken embryo kidney cells. The plates were placed in a CO2 incubator at 36–38°C for 72 h. The virus neutralization (VN) titres were expressed as the reciprocal of the highest dilution of serum that prevented cytopathic effects. The test was performed in the presence of appropriate controls.
Experimental design
The experiment was conducted with the formal approval of the local animal welfare committee and registered according to the Dutch legislation. Eighteen-day-embryonated eggs originating from a healthy, well performing commercial brown egg layer (Isa Brown) breeding flock were purchased from a commercial hatchery, disinfected using formalin and hatched at GD (Animal Health). Post hatch, 225 female chicks were divided as they came to hand into five groups of 45 chickens each, and 10 chickens were bled for serum. Each group was housed in a separate isolator till 4 weeks of age. Groups P+B+Ch+ and P+B-Ch+ were primed (noted as P+) with two live heterologous IBV vaccines at days 1 and 14 according to . The other three groups remained unvaccinated in this period (non-primed, P-). From 4 weeks of age onwards, both live-vaccinated groups (P+B+Ch+ and P+B-Ch+) were housed together in a positive pressure room. The three non live-vaccinated groups (P-B-Ch+, P-B+Ch+, P-B+Ch-) were housed together in a second positive pressure room. The incoming air of both rooms was filtered using HEPA virus filters and stringent biosecurity was in place. At 12 weeks, birds of group P-B+Ch- were moved to a third positive pressure room. All hens of all groups were housed in individual laying cages from 12 weeks onwards. Birds of groups P-B+Ch+, P+B+Ch+, P-B+Ch- were boosted (coded as B+) with an inactivated vaccine coded IB3 (see later). At 24.5 weeks of age when the birds were at peak production, birds of groups P-B-Ch+, P-B+Ch+, P+B+Ch+, P+B-Ch+ were challenged with IBV D1466 (coded as Ch+). Birds of group P-B+Ch- remained unchallenged.
Table 1. Experimental design and results of the ciliostasis test at 5 days post D1466 challenge.
During the whole experiment, water and feed were supplied ad libitum and the lighting scheme was according to the advice of the breeding company.
IB vaccines and vaccination
Two live vaccines and one inactivated vaccine were used. Vaccine “Mass” was a live-attenuated monovalent vaccine of the Massachusetts (Mass) serotype (Nobilis IB MA5, Intervet International BV). Vaccine “4/91” was a live-attenuated monovalent vaccine (Nobilis IB 4/91, Intervet International BV) of the 793B serotype. The contents of a vial of each vaccine were reconstituted in oculo-nasal solvent and stored on melting ice until use. Both live vaccines were applied by eye drop (two drops of 0.05 ml per bird) at the day of hatch (Mass) or day 14 (4/91). The actual vaccine dose per bird, as determined by egg-titration of inocula, was 102.7 EID50/bird for Ma5 and 103.7 EID50/bird for IB 4/91.
The third vaccine was a multivalent inactivated oil-emulsion vaccine (Nobilis IB3/G/ND Intervet International BV, Boxmeer) administered at the manufacturer’s recommended dose of 0.5 ml by intramuscular injection. The multivalent inactivated vaccine contained antigens to three different IB serotypes M41, D274, D1466 together with Newcastle disease Clone 30 antigen and infectious bursal disease D78 antigen.
IBV challenge strain and challenge
The challenge strain D6830 of the D1466 serotype was isolated by GD Animal Health from a Dutch broiler breeder flock showing a drop in egg production. Each bird of groups P-B-Ch+, P-B+Ch+, P+B+Ch+ and P+B-Ch+ was challenged at 24.5 weeks of age by the administration of 0.5 ml by the intramuscular route and 0.5 ml by the intratracheal route, representing 106.1 EID50 of IBV D1466. Group P-B+Ch- was inoculated with a placebo (0.05 ml of negative allantoic fluid) by the intratracheal and intramuscular route.
Ciliostasis test
The level of protection of the trachea was determined using the ciliostasis test on five tracheal organ cultures (TOC) per chicken at 5 days post challenge (dpc) (De Wit et al., Citation2013). The tracheas were placed in Hank’s Minimum Essential Medium immediately after euthanizing the chickens by an intravenous injection of 0.1–0.2 ml of T61 (a mixture of embutramide, mebezoniumjodide and tetracainehydrochloride, MSD, Boxmeer, the Netherlands) and subsequent bleeding. Five tracheal rings (equally divided over the total length of the trachea) were cut and placed in the medium at 37°C. The level of ciliostasis for each TOC was determined independently by two technicians. The level of ciliostasis in each ring (TOC score) was expressed as 0 (all cilia beating), 1 (75–99% cilia beating), 2 (50–75% cilia beating), 3 (25–50% cilia beating), 4 (<25% cilia beating). One chicken could score between 0 and 20 (five rings from each trachea; maximum score 4). An individual chicken was recorded as protected against challenge if the ciliostasis score was less than 10 (modified from Cook et al., Citation1999; De Wit et al., Citation2013). For each group, a ciliostasis protection score (0–100%) was calculated by the formula:
Monitoring egg parameters
From 10 days before the challenge until the end of the experiment at 28 dpc egg production and egg quality were monitored daily for each hen individually. Each egg was checked for the following parameters: soft-shelled egg, thin-shelled egg, crack (including hair cracks), sandpaper shell, presence of a ring (ridging), double yolk and presence of internal blood or meat spots. The egg laying performance was expressed as the percentage of production/group/three-day period (100% = 1 egg/hen/day).
Statistical analysis
Effects of vaccination on egg laying performance and egg quality were assessed using Pearson’s chi-squared test. If differences were significant, pairwise comparisons of all five groups were performed using the proportion test for equal proportions.
For ciliostasis, the total score (CPS) over the five trachea rings of the ciliostasis test at 5 dpc was compared among groups P-B-Ch+, P-B+Ch+, P+B+Ch+ and P+B-Ch+ using the Kruskal–Wallis test at the 5% level. In addition, the BPS was compared among the four groups using a two-sided Fisher’s exact test. In case of a significant overall result at the 5% significance level, further pairwise tests between any two groups (including Group P-B+Ch-) were performed using Fisher’s exact tests, each at the 5% significance level.
Results
Virus cross-neutralization tests
The results of the two-way cross-neutralization tests using the IBV strains M41, D1466 and 4/91 and their antisera showed that all strains were of different serotypes (). The level of antigenic relationship between the strains was determined using the formula of Archetti and Horsfall (Citation1950). The antigenic cross-relationship between D1466 and M41 was 1%, the same as for D1466 and 4/91. The antigenic cross-relationship between 4/91 and M41 was 2%.
Table 2. Results of virus cross-neutralization tests using IBV strains D1466, M41 and 4/91.
Clinical signs post challenge
All challenged groups showed respiratory distress and head shaking from 1 till 10 dpc. From 6 dpc onwards clinical signs were decreasing and were gone at 10 dpc in all four challenged groups.
Ciliostasis test
The results of the ciliostasis tests at 5 dpc are presented in . All birds of the mock-challenged group P-B+Ch- showed 100% cilia movement at 5 dpc. The unvaccinated challenged group P-B-Ch+ showed a CPS of 24% and a BPS of 10%. A BPS of 10% was also seen in the other non-primed group P-B+Ch+. Both groups that had been primed with the live vaccines had a higher BPS of 40% and 50%. The CPS of the two groups were 39% and 51% showing that the vaccination programmes, in general, did not induce high levels of protection at the tracheal level. The statistical analyses on the total score of the ciliostasis test revealed no significant differences between the CPS levels of the four challenged groups (P = 0.259, Kruskal–Wallis test). The same was the case for the BPS of the four challenged groups (P = 0.160, Fisher’s exact test). The CPS and BPS scores of the non-challenged group were significantly different from the scores of the challenged groups.
Individual hens of group P-B-Ch+
The egg production of the individual hens of the non-vaccinated challenged group from 10 days before challenge until 28 dpc are listed in . The observed drop in average egg production was not caused by a drop in production of all hens, neither was the effect on the egg quality. Whereas 18 hens produced either 27 or 28 eggs in the 28-day interval post challenge, three hens stopped producing eggs completely between 5 and 8 dpc. Four hens produced 27 or 28 eggs without abnormalities. The number of eggs with internal egg shell abnormalities post challenge varied from 0 to 16 eggs per hen.
Table 3. Egg production post D1466 challenge of the individual hens of the non-vaccinated group.
Eggs with blood and/or meat spots in the egg were produced from 3 dpc onwards. In total, 20 of these eggs were produced by 11 hens, varying from one to four per hen. Remarkably, eight of these 11 hens were hens that laid 27 or 28 eggs post challenge. In total 30 eggs with a ring were produced by seven hens from 5 dpc onwards, three of these hens produced 27 or 28 eggs post challenge. Four hens produced one or two eggs with a ring, three other hens produced five, seven or 12 eggs with a ring. Three hens produced both eggs with a ring and eggs with meat or blood spots (separate eggs).
Egg production of all groups
The egg production of all groups between 10 days before challenge until 28 dpc is shown in . The egg production in the mock-challenged group P-B+Ch- showed a small drop of 3.6% from 4–6 days after the mock challenge. The average drop in egg production during the 4 weeks post challenge (wpc) was 2.0%.
Figure 1. Three-day interval mean percentage of egg production post IBV D1466 challenge at day 0 in five groups of 30 layers vaccinated with different IB vaccination programmes using heterologous live priming and inactivated IBV vaccine.
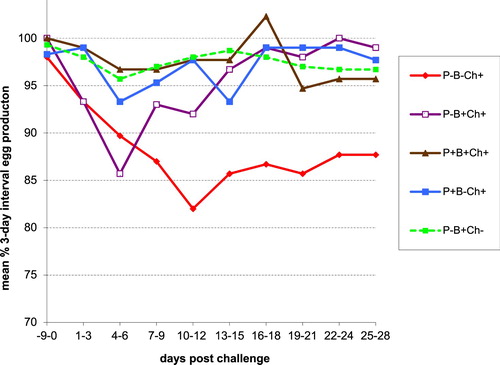
The unvaccinated and D1466 challenged group P-B-Ch+ showed a clear drop in egg production with a maximum of 16.0% at days 10–12 post challenge. The egg production was only partially recovered at the end of the experiment. The average drop in egg production during the 4 weeks was 10.9%.
Group P-B+Ch+ showed a maximum drop in egg production of 14.3% at 4–6 dpc, after which the egg production started to recover. The egg production was recovered completely at about 2.5 wpc. The average drop in egg production during the 4 weeks was 4.9%.
Group P+B-Ch+ showed a drop in egg production of 5.0% at 4–6 dpc. The egg production had recovered completely at about 2 wpc. The average drop in egg production during the 4 wpc was 1.1%.
Group P+B+Ch+ showed a maximum drop in egg production of 3.3% at 4–9 dpc. The egg production had recovered completely at about 2 wpc. The average drop in egg production in the 4 weeks post challenge 2.5%. One bird stopped laying eggs from day 18 onwards. This hen, appearing healthy until the end of the experiment, showed a soft-shelled egg in the abdomen at post mortem examination.
The levels of egg production post challenge (corrected for the starting point) differed significantly (P < 0.001). The loss of egg production from 1–28 dpc of group P-B-Ch+ was significantly higher than that of any other group. The loss of production of group P-B+Ch+ was significantly higher than that of vaccinated groups P+B-Ch+ and P+B+Ch+.
Egg quality disorders
An overview of the number of eggs with disorders in egg quality is listed in . The number of sandpaper eggs in group P-B+Ch+ was significantly higher (P < 0.05) compared to the other four groups. The number of sandpaper eggs in group P+B-Ch+ (P < 0.05) was significantly higher compared to the non-challenged group P-B+Ch-.
Table 4. The number of eggs with and without disorders produced in a 28-day interval post IBV D1466 challenge of five groups of 30 layers vaccinated with different programmes using heterologous live priming and inactivated IBV vaccine.
The number of rings was significantly higher in groups P-B-Ch+ and P-B+Ch+ (P < 0.05). The numbers of meat or blood spots in the egg were significantly higher in groups P-B-Ch+, P-B+Ch+ and P+B-Ch+ (P < 0.05). There were no statistically significant differences between the four challenged groups and the unchallenged group in the number of soft-shelled eggs, thin-shelled eggs, cracks and the number of double yolks. The highest number of eggs with one or more disorders was found in group P-B+Ch+ () (P < 0.05 with all other groups) and the lowest numbers in groups P-B+Ch- and P+B+Ch+ (P < 0.05 compared to the other groups).
Discussion
In this study, the level of protection against an IBV D1466 challenge in layers was compared using vaccination programmes with only heterologous live priming by a Mass vaccine and a 4/91 vaccine, only an inactivated vaccine (IB3) that contained an M41, D274 and D1466 antigen, or the combination of both. The cross-neutralization tests confirmed the very low antigenic similarity between the Mass, 4/91 and D1466 strains (Davelaar et al., Citation1984; Cook et al., Citation1996; Cook et al., Citation1999).
The D1466 challenge strain D6830 caused a significant drop in egg production of 16% in the unvaccinated controls. Four weeks after challenge, the egg production was still 10% lower than at the day of challenge. There were remarkable differences between the responses of the hens to the challenge. Six of the 30 challenged hens produced 28 normal eggs in the 28 days post challenge despite the respiratory signs post challenge, three other hens completely stopped laying eggs. The challenge also caused significantly more disorders in the quality of the eggs that were still produced, again not equally divided over the birds. The percentages of rings (4.0%), and blood or meat spots inside the egg (2.7%) were significantly increased compared to the mock-challenged group. Per bird, the percentage of rings varied from 0% to 46%, the percentage of eggs with the blood or meat spots from 0% to 15%.
None of the three vaccination programmes was able to prevent respiratory distress and head shaking from 1–10 dpc; the signs were comparable in all four challenged groups. The ciliostasis protection scores of the four challenged groups varied from 24% to 51%, the binominal protection scores varied from 10% to 50%. The CPS and BPS values of the different groups did not differ significantly from each other, but higher values of CPS were found in the groups that had received a live priming (P+B-Ch+; 39%), the inactivated vaccine with the homologous D1466 antigen (38%) or both (P+B+Ch+; 51%). The potential of the combination of Mass and 4/91 vaccines to induce a certain level of cross-protection against D1466 in young birds has been reported by Cook et al. (Citation1999), who found a CPS of 59% in young birds that had been challenged with D1466 at 5 weeks of age after receiving the same live vaccination programme as in this present study. Although the CPS of the P+B+Ch+ and the study of Cook et al. (Citation1999) are similar, the mechanism behind the partial protection might be different as the interval between vaccinations and challenge in the two studies was over 22 and 3 weeks, respectively. It has been reported before by a meta-analysis (De Wit et al., Citation2013) that layers and breeders in which a boost had taken place with an inactivated vaccine showed a higher CPS than did birds that had only been vaccinated with live vaccines.
The loss of egg production between 1 and 28 dpc of group P-B-Ch+ was significantly higher than that of any of the three vaccinated groups. The egg production in group P-B+Ch+ showed a maximum drop of 14.3%, similar to the 16.0% of the non-vaccinated group P-B-Ch+, but the egg production of group P-B+Ch+ recovered much better. The loss of production of group P-B+Ch+ was significantly higher than that of vaccinated groups P+B-Ch+ and P+B+Ch+. The maximum drop was 5.0% and the recovery was within 2 wpc, showing the value of the heterologous live vaccinations compared to the use of the inactivated vaccine alone. The egg production in group P+B+Ch+ showed a maximum of 3.3% and a recovery of around 2 wpc. Although the egg production of group P+B+Ch+ was not significantly higher than that of group P+B-Ch+, the difference in egg quality was significant between the two groups. Whereas none of the seven egg quality parameters measured in group P+B+Ch+ showed a significant difference from the non-challenged group P-B+Ch-, group P+B-Ch+ had significantly more sandpaper eggs and eggs with an internal blood or meat spot. Overall, of the challenged groups, only group P+B+Ch+ had no significant difference in the number of eggs with one or more abnormalities, in comparison to the non-challenged group. This was the same for the combination of the drop in egg production and loss of egg quality as a representative of the detrimental effect of an IBV infection in the field.
In summary, this study shows that IBV D1466, a genotype II, lineage 1 virus, is able to cause serious damage to unvaccinated laying hens resulting in respiratory signs, a long-lasting drop in egg production and loss of egg quality. Vaccination programmes with only heterologous live priming by a Mass vaccine and a 4/91 vaccine, only an inactivated vaccine that contained a D1466 antigen and a combination of both all induced a significant level of protection against challenge with a pathogenic D1466 strain. Overall, the vaccination programme using the broad heterologous live priming and the inactivated vaccine provided high protection against the combination of egg drop and loss of egg quality. The results showed that this combination of heterologous live vaccines was able to increase the efficacy of the inactivated IBV vaccine despite the very low antigenic relationship of both live vaccines with the challenge strain.
Acknowledgement
The authors want to express their gratitude to Johan Welner, Machiel Esmann and Piet Vos for their help during this long-lasting study.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
J. J. (Sjaak) de Wit http://orcid.org/0000-0002-3459-1000
Additional information
Funding
References
- Archetti, I. & Horsfall, F. L., Jr. (1950). Persistent antigenic variation of influenza A viruses after incomplete neutralization in ovo with heterologous immune serum. Journal of Experimental Medicine, 92, 441–462. doi: 10.1084/jem.92.5.441
- Awad, F., Forrester, A., Baylis, M., Lemiere, S., Ganapathy, K., Hussien, H.A. & Capua, I. (2015). Protection conferred by live infectious bronchitis vaccine viruses against variant Middle East IS/885/00-like and IS/1494/06-like isolates in commercial broiler chicks. Veterinary Record Open, 2. doi: 10.1136/vetreco-2014-000111
- Box, P.G., Beresford, A.V. & Roberts, B. (1980). Protection of laying hens against infectious bronchitis with inactivated emulsion vaccines. Veterinary Record, 106, 264–268. doi: 10.1136/vr.106.12.264
- Box, P.G. & Ellis, K.R. (1985). Infectious bronchitis in laying hens: interference with response to emulsion vaccine by attenuated live vaccine. Avian Pathology, 14, 9–22. doi: 10.1080/03079458508436204
- Box, P.G., Holmes, H.C., Finney, P.M. & Froymann, R. (1988). Infectious bronchitis in laying hens: the relationship between haemagglutination inhibition antibody levels and resistance to experimental challenge. Avian Pathology, 17, 349–361. doi: 10.1080/03079458808436453
- Chousalkar, K.K., Cheetham, B.F. & Roberts, J.R. (2010). Detection of infectious bronchitis virus strain N1/88 from the oviduct and feces of experimentally infected vaccinated and unvaccinated hens. Poultry Science, 89, 1603–1608. doi: 10.3382/ps.2010-00685
- Cook, J.K.A., Orbell, S.J., Woods, M.A. & Huggins, M.B. (1996). A survey of the presence of a new infectious bronchitis virus designated 4/91 (793B). Veterinary Record, 138, 178–180. doi: 10.1136/vr.138.8.178
- Cook, J.K.A., Orbell, S.J., Woods, M.A. & Huggins, M.B. (1999). Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathology, 28, 477–485. doi: 10.1080/03079459994506
- Davelaar, F.G., Kouwenhoven, B. & Burger, A.G. (1984). Occurrence and significance of infectious bronchitis virus variant strains in egg and broiler production in the Netherlands. Veterinary Quarterly, 6, 114–120. doi: 10.1080/01652176.1984.9693924
- De Wit, J.J., Boelm, G.J., van Gerwe, T.J.W.M. & Swart, W.A.J.M. (2013). The required sample size in vaccination-challenge experiments with infectious bronchitis virus, a meta-analysis. Avian Pathology, 42, 9–16. doi: 10.1080/03079457.2012.751485
- De Wit, J.J., Brandao, P., Torres, C.A., Koopman, R. & Villarreal, L.Y. (2015). Increased level of protection of respiratory tract and kidney by combining different infectious bronchitis virus vaccines against challenge with nephropathogenic Brazilian genotype subcluster 4 strains. Avian Pathology, 44, 352–357. doi: 10.1080/03079457.2015.1058916
- De Wit, J.J. & Cook, J.K.A. (2014). Factors influencing the outcome of infectious bronchitis vaccination and challenge experiments. Avian Pathology, 43, 485–497. doi: 10.1080/03079457.2014.974504
- De Wit, J.J., Cook, J.K.A. & van der Heijden, H.M.J.F. (2011). Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathology, 40, 223–235. doi: 10.1080/03079457.2011.566260
- De Wit, J.J., Dijkman, R., Guerrero, P., Calvo, J., Gonzalez, A. & Hidalgo, H. (2017). Variability in biological behaviour, pathogenicity, protectotype and induction of virus neutralizing antibodies by different vaccination programmes to infectious bronchitis virus genotype Q1 strains from Chile. Avian Pathology, 46, 666–675. doi: 10.1080/03079457.2017.1346782
- De Wit, J.J., Malo, A. & Cook, J.K.A. (2019). Induction of IBV strain-specific neutralizing antibodies and broad spectrum protection in layer pullets primed with IBV Massachusetts (Mass) and 793B vaccines prior to injection of inactivated vaccine containing Mass antigen. Avian Pathology, 48, 135–147. doi: 10.1080/03079457.2018.1556778
- De Wit, J.J., Nieuwenhuisen-van Wilgen, J., Hoogkamer, A., Van de Sande, H., Zuidam, G.J. & Fabri, T.H.F. (2011). Induction of cystic oviducts and protection against early challenge with infectious bronchitis virus serotype D388 (genotype QX) by maternally derived antibodies and by early vaccination. Avian Pathology, 40, 463–471. doi: 10.1080/03079457.2011.599060
- Domanska-Blicharz, K., Lisowska, A., Jatczak, J., Mamczur, J. & Minta, Z. (2012). D1466-like genotype of infectious bronchitis virus responsible for a new epidemic in chickens in Poland. Veterinary Record, 171, 351. doi: 10.1136/vr.100888
- Jackwood, M.W. (2012). Review of infectious bronchitis virus around the world. Avian Diseases, 56, 634–641. doi: 10.1637/10227-043012-Review.1
- Jackwood, M.W. & De Wit, S. (2013). Infectious bronchitis. In G. Swayne, N. McDougald & N. Suarez (Eds.), Diseases of poultry (13th ed., pp. 117–135). Ames: Blackwell Publishing Professional.
- Muneer, M.A., Newman, J.A., Halvorson, D.A., Sivanandan, V. & Coon, C.N. (1987). Effects of avian infectious bronchitis virus (Arkansas strain) on vaccinated laying chickens. Avian Diseases, 31, 820–828. doi: 10.2307/1591038
- Terregino, C., Toffan, A., Beato, M.S., De Nardi, R., Vascellari, M., Meini, A., Ortali, G., Mancin, M. & Capua, I. (2008). Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathology, 37, 487–493. doi: 10.1080/03079450802356938
- Valastro, V., Holmes, E.C., Britton, P., Fusaroa, A., Jackwood, M.W., Cattoli, G. & Monne, I. (2016). S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification. Infection, Genetics and Evolution, 39, 349–364. doi: 10.1016/j.meegid.2016.02.015
- Worthington, K.J., Currie, R.J. & Jones, R.C. (2008). A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathology, 37, 247–257. doi: 10.1080/03079450801986529
