ABSTRACT
The assessment of bird-based welfare indicators plays an important role in the evaluation of bird welfare. The aim of the study was to histologically validate a visual scoring system for hock burn in broilers and to detect threshold values of a visual score to define welfare-relevant alterations in terms of mild lesions or ulcers of the hock. We collected 200 hocks of 39- to 42-day-old Ross 308 broilers after the slaughter process. Each hock was scored visually (“macro scores” 0–4) and evaluated histologically (“micro scores” 0–3), with high scores representing more severe lesions. Although we found a tendency for higher micro scores with increasing macro scores, an exact allocation of macro to micro scores was not possible. For example, macro score 1 could represent micro scores 1, 2 and 3, whereas macro scores 3 and 4 always represented micro score 3 (ulcer). The conditional probability of certain micro scores for given macro scores was estimated using a multinomial logistic regression model. Ulcer showed the highest probability at macro score 1, whereas mild lesions were not found to have an estimated highest probability at any macro score. The depth of inflammation of hock burn lesions increased with increasing macro scores up to macro score 3 with an average depth of 1019 µm. Visually more severe and deeper lesions were also histologically rated with higher scores. Thus, considering limitations, the herein validated macroscopic assessment scheme for hock burn allows an estimation of histological alterations in hocks of broilers.
RESEARCH HIGHLIGHTS
Histological validation of a visual assessment scheme for hock burn in broilers.
Tendency for higher micro scores with increasing macro scores.
Estimation of histological score via macro score possible with limitations.
Histological depth of inflammation increased with an increasing macro score.
Introduction
Hock burn is considered a contact dermatitis of the hock of broilers with a brown to black discolouration of the skin (Greene et al., Citation1985; Bessei, Citation2006). Hock burn lesions are of importance in the evaluation of bird welfare (Bessei, Citation2006), and in commercially raised flocks, 35% (fattening day 35; Bergmann et al., Citation2016) or even 88% (week 6; Kjaer et al., Citation2006) of the broilers in a flock may be affected by this type of dermatitis. Data concerning hock burn recorded at slaughter could replace measurements performed on-farm, and severe hock burn detected on-farm was found to predict impaired walking ability assessed by gait scores (de Jong et al., Citation2016). Hock burn lesions are potentially painful; birds with such lesions walk more slowly than birds with healthy hocks (McKeegan, Citation2010). Thus, this bird-based measure could be a useful indicator of flock health (Hepworth et al., Citation2011) and an indicator to assess and identify bird welfare problems (Saraiva et al., Citation2016).
The weight of broilers is a known risk factor for the occurrence of hock burn (Sørensen et al., Citation2000; Broom & Reefmann, Citation2005; Haslam et al., Citation2007; Hepworth et al., Citation2010; Bergmann et al., Citation2016; Saraiva et al., Citation2016; Louton, Bergmann et al., Citation2018). The gender of broilers (McKeegan, Citation2010) and the litter quality (Bessei, Citation2006; Allain et al., Citation2009; de Jong et al., Citation2014) also play a role in the development of contact dermatitis of the hock. Even though the choice of a suitable litter type and quality is essential for minimizing the risk of the development of hock burn (Bessei, Citation2006; Allain et al., Citation2009; de Jong et al., Citation2014; Jacob et al., Citation2016), it seems that, in comparison with contact dermatitis of the foot (foot pad dermatitis, FPD), the risk factor for hock burn is the weight of the broilers rather than the litter characteristics (McKeegan, Citation2010; Louton, Bergmann et al., Citation2018).
As described by Pass (Citation1989), the skin structure in birds is generally similar to that in mammals, with an outer epidermis and an inner dermis. The epidermis varies in thickness, depending on the area of the body, and is thicker on the naked than the feathered parts of the legs. The outer epidermal layer contains keratinized, anuclear, flat cells and is thickest in the skin of scales on the legs and the plantar surface of the feet. Underneath this stratum corneum, Pass (Citation1989) pointed out the germinative layer consisting of the basal layer and stratum spinosum followed by the transitional layer, which corresponds to the stratum granulosum in mammals. In all parts of the bird skin, basal cells are based on a thin filamentous basal lamina attached to the dermis, building the so-called dermo-epidermal junction. First published histological examinations of hock burn lesions by Greene et al. (Citation1985) demonstrated that these lesions are acute inflammations related to necrosis of the epidermis and, in more severe lesions, the upper dermis. The authors observed erosions of the skin and stated that these were associated with basophilic debris in the stratum corneum and small vacuoles appearing in the epidermis. Furthermore, they observed an occasional appearance of heterophils throughout the epidermis, and hyperplasia of the epidermis in erosions. In ulcerated lesions, the authors observed an acute inflammation as the main feature. They described marked congestion of dermal capillaries and hyperplasia of the outer edge of the epidermis with complete destruction of the normal skin in the centre of the lesion; an eosinophilic mass with basophilic nuclear debris replaced the healthy skin in the centre of the lesion (Greene et al., Citation1985). However, the authors did not correlate these observations directly to macroscopic visual scoring systems. McKeegan (Citation2010) described a histological examination of visual scores of hock burn and FPD. The author observed hyperkeratosis and accumulation of cellular debris between scales in early-stage lesions and found acanthosis, hyperkeratosis and splitting of keratinized layers of the skin in moderate lesions. McKeegan (Citation2010) observed ulcers only in lesions of advanced scores and found a clear correlation between visual and histological scores. Recent publications focused on the histological validation of visual scoring systems for FPD in broilers (Michel et al., Citation2012; Heitmann et al., Citation2018) and turkeys (Mayne et al., Citation2006). A commonly used visual scoring system for hock burn is the scheme presented in the Welfare Quality® assessment protocol for poultry (Welfare Quality®, Citation2009). However, in contrast to contact dermatitis of the foot pads, a histological validation of visual assessment schemes for hock burn has not been published.
The References for the Implementation of the German Order on the Protection of Animals and the Keeping of Production Animals (Citation2011) propose a three-staged scoring system for the assessment of FPD, and the German state Lower Saxony published a decree that presents a four-staged scoring system for contact dermatitis of the foot pads (Ministry of Food, Agriculture and Consumer Protection of the German State Lower Saxony, Citation2014). In both governmental proposals, severe deep lesions are characterized by the presence of ulcers. Thus, the differentiation between mild lesions or erosions and ulcers seems to be important in the histological validation of contact dermatitis. Erosion is defined as circumscribed, superficial loss of epidermis, up to the stratum basale, which, by definition, is not affected in these types of lesions (de Gruyter, Citation2002). Ulcers are defined as loss of substance of the epidermis with the destruction of the stratum basale (Michel et al., Citation2012). To our knowledge, no standardized governmental scoring system to assess hock burn lesions is currently proposed in Germany or other European countries. However, because of their relevance for bird welfare mentioned in the first paragraph, the assessment of hock burn lesions could, additionally to FPD and other measures, serve as a suitable indicator of broiler health. According to a survey among slaughterhouses for poultry in the German-speaking territories (Austria, Switzerland, Germany), hock burn lesions are, additionally to foot pad lesions, already commonly assessed (Louton, Erhard & Wöhr Citation2018).
The aim of this study was to histologically validate a visual scoring system for hock burn in broilers and to detect the threshold value of a visual score to define welfare-relevant alterations in terms of mild lesions or ulcers of the hock. Furthermore, we assessed the relationship between the size of hock burns and the depth of the microscopically identified lesions. We hypothesized that lesions of a higher visual score (“macro score”) also have a higher histological score (“micro score”) and deeper inflammation than lesions of a lower macro score.
Materials and methods
Birds and Materials
The assessments at slaughter were performed at the slaughterhouse “Donautal Geflügelspezialitäten Zweigniederlassung der Lohmann & Co. KG” in Bogen, Germany. The hocks were from Ross 308 broilers aged 39–42 fattening days.
Inter-observer reliability test
For the inter-observer reliability test of the visual assessment, 250 hocks of Ross 308 broilers were collected after slaughter and assessed by five observers. Two scoring systems for a visual (macroscopic) assessment were tested, one relating to the size of the lesion, the other to the number of altered scales (). For the inter-observer reliability test of the microscopic assessment, 20 histological slides of hocks were assessed by three observers.
Table 1. Assessment of contact dermatitis of the hock of broilers by size (largest diameter) of the lesion or number of altered scales according to a modified Welfare Quality® (Citation2009) assessment scheme.
Visual assessment
For the visual assessment of hock burn and the assignment of a macro score, we primarily used two modified assessment schemes revised according to the Welfare Quality® assessment protocol for poultry (Welfare Quality®, Citation2009) (). After evaluation of the inter-observer reliability test results, the assessment scheme by which the definition of hock burn is based on the size of the lesion (scheme 1) was used for the further evaluation procedure of the project.
At the slaughter line, 200 hocks of broilers (with 40 hocks of each macro score) were collected for an individual assessment directly at the hock by one observer according to the modified scheme 1 of the Welfare Quality® assessment protocol for poultry (Welfare Quality®, Citation2009) (). After the visual assessment, the alterations of the hocks and the size of the hocks were measured (“manual size”) with a measuring gauge (Precise PS 7215, Burg-Wächter KG, Wetter, Germany). Each hock was photographed with a Sony Cyber-shot DSC-RX100 (Sony Europe Limited, Surrey, UK).
To evaluate whether a visual differentiation of deep versus superficial lesions was possible, the lesions were re-assessed by assigning one of two macro scores (visually superficial or visually deep). In a blinded setting, all photographs of the 200 hocks were scored as “no lesion”, “superficial lesion” or “deep lesion”. Lesions were considered deep when a separation of the epidermal layers was macroscopically visible or a loss of epidermal substance was evident; discolourations and lesions in which the epidermis was not visually separated were scored as superficial lesions. Examples of superficial and deep lesions are presented in Supplementary a and 1b.
Histological assessment
Veterinarian pathologists of the Bavarian Animal Health Service in Poing, Germany, performed the histological assessment in a blinded setting. They used a modified histological assessment scheme published by Michel et al. (Citation2012) for FPD ((a)–(d)). The legs of the broilers were cut approximately 5 cm above and below the hock, and the hocks were then used for the histological examination. A skin sample of each hock, including underlying tendon sheath, was collected and fixed by immersion in 10% neutral-buffered formalin for a minimum of 24 h. After fixation, samples were cut in cross direction to the epidermal surface, in case of a lesion across its most affected location, then dehydrated, embedded in paraffin wax, sectioned at ∼4 μm and stained with haematoxylin and eosin.
Figure 1. Adapted histological scores (micro scores) for hock lesions in broilers according to a modified histological assessment scheme originally used by Michel et al. (Citation2012) for FPD with respective definitions and exemplary pictures of histological slides. (a) Score 0 = no lesion. Physiological structure of epidermis. (b) Score 1 = mild/early-stage lesion. Mild to moderate hyperplasia and/or hyperkeratosis of the epidermis; superficial dermal congestion and oedema, variable and mild inflammatory infiltration. (c) Score 2 = moderate/superficial lesion. Marked hyperplasia and hyperkeratosis of the epidermis, abundant heterophilic epidermal exocytosis with pustule formation; congestion in the superficial dermis with inflammatory infiltration. (d) Score 3 = severe/deep lesion. Ulcer (full-thickness necrosis of the epidermis, replaced by necrotic and suppurative material). Score 2 lesions at the ulcer margins. (e) Tendon sheath (score: present/not present). Inflammation of the tendon sheath. (f) Thickness of physiological dermis, measured from the outer line of the stratum corneum to stratum basale. (g) Thickness of inflammation, measured at the thickest point of inflammation from the outer line to the demarcation line of the inflammation.
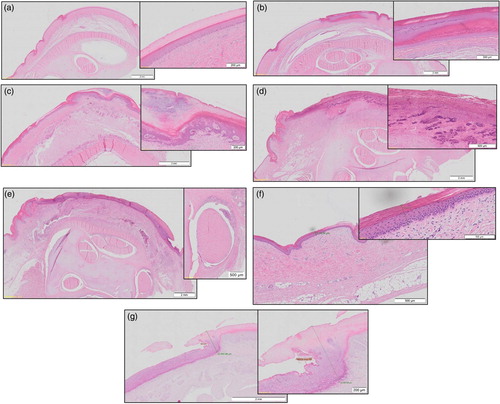
In the assessment scheme used, micro score 0 represents healthy, physiological skin ((a)), micro score 1 is characterized by mild hyperplasia and superficial inflammation ((b)), micro score 2 is classified as marked hyperplasia and inflammation of the epidermis ((c)) and micro score 3 represents an ulcer ((d)). For further analyses, micro scores 1 and 2 were summarized to represent “mild lesions” in terms of bird health. We also documented whether the tendon sheath was affected by the inflammation ((e)). After the assessment, the microscopic slides were scanned with an Olympus BX51 microscope (Olympus, Tokyo, Japan) to digitalize the samples and to measure the microscopic depth of inflammation. Using the programme Olympus VS-ASW (Olympus), the thickness of the dermis without alteration ((f)) and the depth of inflammation of the histological alteration at its deepest point ((g)) were measured.
Statistical methods
The inter-observer agreement was tested using the prevalence-adjusted and bias-adjusted kappa (PABAK) calculation by Byrt et al. (Citation1993). When more than two categories are to be assessed, the calculation is performed as follows (Gunnarsson et al., Citation2000): PABAK = (kp0 − 1)/(k − 1), where k represents the number of assessed categories (score of hock burn) and p0 the proportion of matching scores between the observers.
To assess the agreement between microscopic and macroscopic scoring, the conditional probabilities of all microscopic categories given the macroscopic categories were estimated by using multinomial regression models. To examine the relationship between macro score and inflammation depth, a Hurdle-Gamma regression model was used. In this model, the Hurdle part models the conditional probability for an inflammation depth >0 µm given the macroscopic categories. The Gamma part investigates the effect of the macro score on the expected depth of inflammation >0 µm. We chose the Gamma distribution to account for the skewed distribution of inflammation depth. All model parameters were estimated using the probabilistic programming language Stan (Carpenter et al., Citation2017) and the wrapper package brms (Bürkner, Citation2017) for the statistical programming language R (R Core Team, Citation2018).
For the evaluation of cut-off values and the classification of attributes, we used the performance measures sensitivity, specificity, positive predictive value, negative predictive value and accuracy according to the definitions presented in Supplementary Table 1.
Results
The PABAK values of the inter-observer reliability test for the visual assessment of hock burn with respect to the size of the alteration (scheme 1; ) varied from 0.75 to 0.87, depending on the compared observer pairs, with an average of 0.81 (Supplementary Table 2). Those for the assessment of hock burn with respect to the number of altered scales (scheme 2; ) were between 0.77 and 0.88, with an average of 0.82 (Supplementary Table 2). The PABAK values for the histological assessment varied from 0.80 to 0.93, with an average of 0.86 (Supplementary Table 2).
Macro score vs. micro score
Supplementary (a)–(c) shows examples of each possible combination of macro and micro scores. Macro score 0 was observed to be associated with micro score 0 (62.5%), 1 (35.0%) or 2 (2.5%), macro score 1 with micro score 0 (2.5%), 1 (7.5%), 2 (25.0%) or 3 (65.0%) and macro score 2 with micro score 3 (97.5%) or micro score 2 (2.5%). Macro scores 3 and 4 were always (100%) associated with micro score 3, i.e. an ulcer. shows the distribution of micro scores across the given macro scores with a four-staged ((a)) and with a simplified three-staged micro scoring ((b)), in which hocks were assigned to “no lesion”, “mild lesion” or “ulcer”. The aim was to identify if specific macro scores equalled specific micro scores. We found, in general, a similar tendency of macro and micro scores in that low or high scores in the macroscopic assessment corresponded to low or high scores, respectively, in the microscopic assessment. However, there was no exact one-to-one agreement between the scores. The categories of the macro scores corresponded to a mixture of micro scores in most cases. Only macro scores 3 and 4 always corresponded to micro score 3 (representing an ulcer).
Figure 2. Distribution (percentage) of micro scores at given macro scores of hock burn in broilers. (a) Four-staged micro scores at five-staged macro scores (see footnote for definition of macro scores). (b) Micro scores simplified to no lesion, mild lesion and ulcer at five-staged macro scores. (c) Micro scores simplified to no lesion, mild lesion and ulcer at macro scores simplified to visually identified healthy hock (no lesion), superficial lesion and deep lesion. Lesions were considered deep when a separation of the epidermal layers or a loss of epidermal substance was macroscopically visible; superficial lesions were discolourations and lesions in which the epidermis was not visually separated. Note: Macro score 0 = no lesion, macro score 1 = superficial, attached (single) lesion or several single superficial or deep lesions ≤0.5 cm in diameter, macro score 2 = deep lesion >0.5 cm to ≤1 cm or superficial lesion >0.5 cm in diameter, macro score 3 = deep lesion >1.0 cm in diameter, macro score 4 = whole hock extensively altered.
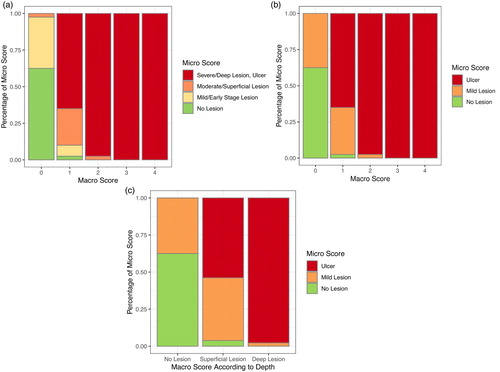
(a) presents the conditional probabilities of micro scores 0–3 given the macro score categories 0–4, and (b) shows the probabilities of the occurrence of no lesions, mild lesions or ulcers given the macro score categories 0–4. It was possible to identify the highest probability of certain micro scores for each macro score. In hock burns of macro score 1, micro score 3 (ulcer) was most likely with a probability of 65.0%. Taking this observation as basis for a forecast algorithm, the expected rules for assignments are presented in Supplementary a. Mild lesions did not have the highest probability of occurrence in any of the macro scores (32.5% macro score 1, 2.5% macro score 2, 0% macro score 3 and 0% macro score 4). Healthy hocks (no lesion) had the highest probability in macro score 0 (62.5%), and ulcers had the highest probability in macro scores 1 and above (65.0% macro score 1, 97.5% macro score 2, 100% macro scores 3 and 4).
Figure 3. Probabilities and 95% uncertainty intervals of micro scores for given macro score categories of hock burn in broilers. (a) Four-staged micro scores at five-staged macro scores (see footnote for definition of macro scores). (b) Micro scores simplified to no lesion, mild lesion and ulcer at five-staged macro scores. (c) Micro scores simplified to no lesion, mild lesion and ulcer at macro scores simplified to visually identified healthy hock (no lesion), superficial lesion and deep lesion. Lesions were considered deep when a separation of the epidermal layers or a loss of epidermal substance was macroscopically visible; superficial lesions were discolourations and lesions in which the epidermis was not visually separated. Note: Macro score 0 = no lesion, macro score 1 = superficial, attached (single) lesion or several single superficial or deep lesions ≤0.5 cm in diameter, macro score 2 = deep lesion >0.5 cm to ≤1 cm or superficial lesion >0.5 cm in diameter, macro score 3 = deep lesion >1.0 cm in diameter, macro score 4 = whole hock extensively altered.
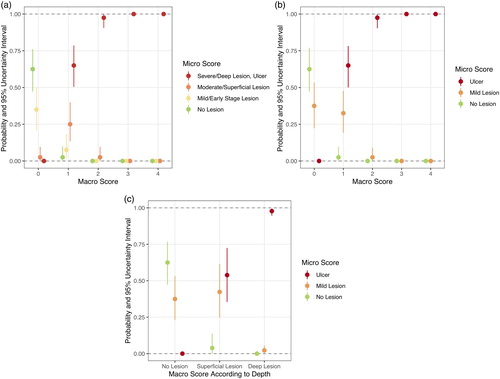
The evaluation and performance measures of cut-off values are presented in , given the prediction of macro score 0 corresponding to “no lesion”, macro score 1 corresponding to “mild lesion” and macro scores 2, 3 and 4 corresponding to “ulcer”. Ulcers were diagnosed with a sensitivity of 0.82, a specificity of 0.98 and an accuracy of 0.78. Thus, using these presented cut-off values, a hock with an ulcer can be diagnosed with macro score 2 or above with a probability of 0.82. However, using these cut-off values, mild lesions were diagnosed with a sensitivity of only 0.45 and a specificity of 0.84 at an accuracy of 0.78. Healthy hocks (no lesion), on the other hand, were diagnosed with a sensitivity of 0.96 and a specificity of 0.91. Another possibility of allocation of the macroscopic five-staged scoring system would be a simplified allocation to “healthy” (or “no lesion”, macro score 0) and “ulcer” (macro scores 1–4). Using this allocation, healthy hocks and ulcers were diagnosed with a sensitivity of 0.96 or 1.00, respectively, a specificity of 0.91 and 0.73, respectively, at an accuracy of 0.85 (). This scoring system led to a sensitivity for the diagnosis of mild lesions of 0.00 and a specificity of 1.00.
Table 2. Performance measures for predicted simplified micro scores at given macro scores for the evaluation of hock burn in broilers when the cut-off value was set at macro score 2.
Table 3. Performance measures for predicted simplified micro scores by using either a macro score system solely divided into “no lesion” and “deep lesion” or the five-staged macro scores (presented in one table due to similar results) for the evaluation of hock burn in broilers.
Furthermore, we simplified the macro scores for lesions into superficial or deep lesions without considering the size of the lesions and compared them with the histological micro scores “no lesion”, “mild lesion” and “ulcer” to evaluate whether it is possible to visually differentiate superficial and deep lesions. (c) presents the distribution of histological micro scores when the macro score exclusively considered if the visual lesion was superficial or deep. The respective probabilities for the micro scores using this redefined macro scoring are presented in (c). As shown, half (53.8%) of the visually classified superficial lesions represented histologically scored ulcers. Using this observation as basis for a forecast algorithm, Supplementary b shows that ulcers had the highest probability of occurrence in visually classified “superficial” (53.8%) or “deep” lesions (97.8%). Histological “mild” lesions did not have the highest probability (42.3%) in the visually classified “superficial” lesions. When visually classified “superficial” and “deep” lesions were classified as “ulcer”, the performance measures of the cut-off values allowed the histological diagnosis of ulcers with a sensitivity of 1.00 and a specificity of 0.73. Mild lesions were diagnosed with a sensitivity of 0.00 and a specificity of 1.00, and no lesions with a sensitivity of 0.96 and a specificity of 0.91, with an accuracy of 0.85 (). When the cut-off values were set in a way that visual superficial lesions represent histological mild lesions and visual deep lesions represent histological ulcers, mild lesions were diagnosed with a sensitivity of 0.38 and a specificity of 0.91. Ulcers were diagnosed with a sensitivity of 0.90 and a specificity of 0.95, with an accuracy of 0.84 ().
Table 4. Performance measures for predicted simplified micro scores by using a macro score system for the evaluation of hock burn in broilers divided into “no lesion”, “superficial lesion” and “deep lesion”.
Depth of the lesion
The depth of microscopically identified inflammation in hock burn lesions increased with increasing macro scores up to macro score 3, with an average of 1019 µm (). The deepest hock burn lesion was measured in macro score 3, with 2124 µm. shows the regression model for the estimation of the expected depth of the inflammation. For macro score 0, only a few observations with an inflammation depth >0 µm were noted, and therefore the uncertainty intervals were very wide. For the other macro scores, the precision of estimation was congruent and increased up to macro score 3, then decreased. To evaluate whether the estimation of inflammation depth differs between the macro scores, all possible combinations of macro scores were evaluated (). The denotation 0 vs. 1 implies that the difference between macro scores 0 and 1 is estimated. Positive estimated differences at the comparison of x vs. y indicate that the expected depth of inflammation in score x is deeper than in score y, whereas negative estimated differences indicate the opposite relationship. If the interval of estimation is completely below or above 0, the difference between considered macro scores is significant. The estimated depth (in µm) should also be considered besides the statistical differences. For hock burns of macro score 3, significantly deeper inflammations were expected than for hock burns of macro scores 1 and 2 (). However, the inflammation depth of hock burns of macro score 4 was expected to be significantly less than that of hock burns of macro score 3.
Figure 4. Estimation of the inflammation depth (µm) of hock burn in broilers with 95% uncertainty interval for macro scores 0–4 (see footnote for definition of macro scores). Note: Macro score 0 = no lesion, macro score 1 = superficial, attached (single) lesion or several single superficial or deep lesions ≤0.5 cm in diameter, macro score 2 = deep lesion >0.5 cm to ≤1 cm or superficial lesion >0.5 cm in diameter, macro score 3 = deep lesion >1.0 cm in diameter, macro score 4 = whole hock extensively altered.
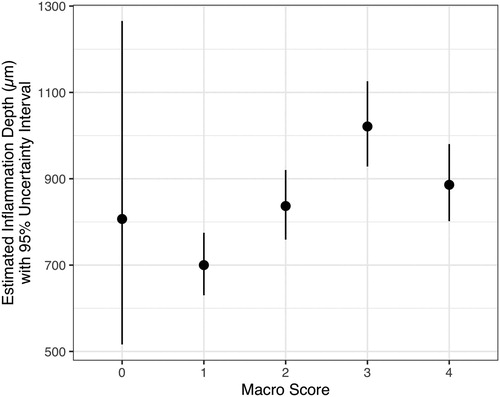
Figure 5. Differences of estimated inflammation depths of hock burn in broilers between the macro score categories (see footnote for definition of macro scores). The denotation 0 vs. 1 indicates that the difference between macro score 0 and macro score 1 is estimated. Positive estimated differences in the comparison of x vs. y indicate that the expected depth of inflammation is deeper in score x than in score y, and negative estimated differences indicate the opposite relationship. If the interval of estimation is completely below or above 0, the difference between considered macro score categories is significant. Note: Macro score 0 = no lesion, macro score 1 = superficial, attached (single) lesion or several single superficial or deep lesions ≤0.5 cm in diameter, macro score 2 = deep lesion >0.5 cm to ≤1 cm or superficial lesion >0.5 cm in diameter, macro score 3 = deep lesion >1.0 cm in diameter, macro score 4 = whole hock extensively altered.
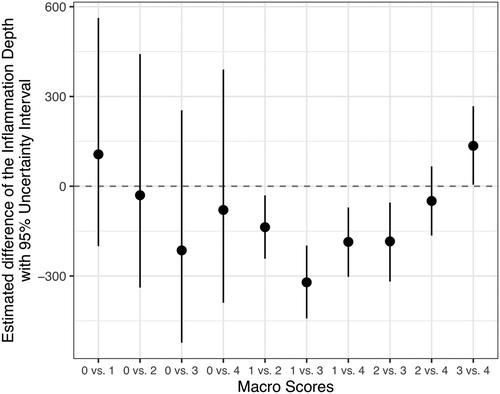
Table 5. Presentation of the mean inflammation depth (µm) of hock burn lesions in broilers for specific macro scores with standard deviation (SD), minimum and maximum depth of inflammation.
Affected tendon sheath
In hock burns identified with histological micro scores, 14.5% of the hocks assessed with micro score 3 (ulcer) and 17.6% of the hocks assessed with micro score 1 (mild hyperplasia and superficial inflammation) were accompanied by an inflammation of the tendon sheath underlying the skin (). In hock burns assessed with macro scores, the occurrence of inflammation of the tendon sheath was highest in macro scores 2 (22.5%; superficial lesions >0.5 cm or deep lesions ≤1 cm) and 4 (15.0%; ulcer).
Table 6. Numbers of hocks of broilers with tendon sheath affected by inflammation for micro scores 0–3 and macro scores 0–4, as percentage of examined hocks (40 for each macro score).
Discussion
We observed a tendency of higher micro scores with increasing macro scores, but an exact allocation of macro to micro scores was not possible. Ulcers showed the highest probability already at macro score 1, whereas mild lesions were not found to have an estimated highest probability at any macro score. The depth of inflammation of hock burn lesions increased with an increasing macro score, and visually more severe and deep lesions were also histologically rated with higher scores. Our hypothesis that lesions of a higher visual score (“macro score”) also have a higher histological score (“micro score”) and deeper inflammation than lesions of a lower macro score was confirmed to some extent. Considering limitations, the validated macroscopic assessment scheme for hock burn allows an estimation of histological alterations in hocks of broilers.
The average of the inter-observer reliability for both assessment methods (visual and histological) was in the range of the reliability scores (0.81–1.00) defined as “almost perfect” by Landis & Koch (Citation1977). Fleiss et al. (Citation2003) described a value for kappa of above 0.75 as “excellent” agreement; values of 0.40–0.75 represent a “fair” to “good” agreement. Thus, the kappa values of our visual and histological observers were “good” to “excellent”. Because both assessment methods seemed to result in similar inter-observer reliability, we chose to use the five-staged assessment method based on the size of the alteration. This method has a higher congruence with the assessment by camera systems at slaughter, which assesses the size of alterations (e.g. used for the assessment of FPD by Vanderhasselt et al. Citation2013, Louton et al., presumably Citation2020, under review). Additionally, it can be compared more easily with assessment methods suggested by other authors (Allain et al., Citation2009; Hepworth et al., Citation2011).
Hock burn is an indicator of flock health and can be linked to bird welfare (Allain et al., Citation2009; Hepworth et al., Citation2011). The assessment of this important bird welfare indicator can be performed at slaughter even though this assessment method has a few disadvantages (de Jong et al., Citation2016). However, the currently used visual scoring systems for hock burn in broilers have not been validated histologically. The microscopic evaluation of the macroscopically scored hocks in our study revealed a similar tendency in both scoring systems. High visually determined macro scores corresponded to high histologically determined micro scores in the examined hocks. Hocks scored with macro scores 3 and 4 (deep lesions >1 cm and whole hock extensively altered, respectively) were always assessed with micro score 3 (ulcer). Michel et al. (Citation2012), who performed histological evaluation of foot pad lesions, also observed that macroscopic lesions of type III, defined as severe deep lesions, were typically ulcerative. Heitmann et al. (Citation2018) observed a tendency of increasing severity with increasing size of foot pad lesions. Mayne et al. (Citation2006) observed infiltration of heterophils into the stratum germinativum in mild lesions and into the dermis, dermo-epidermal junction and epidermis in severe lesions of FPD in turkeys. The authors described that keratin in the centre of severe lesions was often completely destroyed and replaced by inflammatory cells (Mayne et al., Citation2006). McKeegan (Citation2010) found a clear correlation of external lesion scoring with the severity of ulcers in hock burns of broilers and concluded that the histological results corresponded to visual scores.
Evaluating the highest probability of micro scores for given macro scores in the present study, we found that micro score 0 (no lesion) had the highest probability in macro score 0 (no lesion). Micro score 3 (ulcer) had the highest probability in all macro scores above 0. Micro scores 1 and 2 (mild lesions) did not have a definite probability in any macro score. However, they were present at a high ratio in macro scores 0 and 1. Mayne et al. (Citation2006) also observed microscopic cellular changes in turkeys with no external signs of FPD. Pressure ulcers in humans have been reported as painful (Günes, Citation2008). Gentle (Citation1992) stated that no major differences were measured with respect to anatomical, physiological and behavioural parameters when comparing pain in birds and mammals. The author concluded that ethical considerations as applied to mammals should also be considered for birds. In addition, Machin (Citation2005) proposed a generally similar perception of pain in birds compared with mammals. Similarly, Douglas et al. (Citation2018) proposed that birds have the neurologic components to perceive pain similarly to mammals. Gentle et al. (Citation2001) provided evidence of nociceptors in the scaly skin of the chicken leg. The authors furthermore proposed that birds experience pain rather than just showing simple nociceptive responses because they found afferent mechanothermal A-delta fibres in the nociceptors (Gentle et al., Citation2001). Based on our observations that even small macroscopic lesions might correspond to ulcers, and thus may be associated with pain for the birds, we propose to re-define the macro scoring and reduce it to a three- or even two-staged scoring system. Especially when a scoring system is used by authorities to classify hock burn lesions at slaughter concerning erosions and ulcers, it could be simplified to “healthy” (or “no lesion”, macro score 0), “mild lesion” (macro score 1) and “ulcer” (macro scores 2 and above) or even to “healthy” (or “no lesion”, macro score 0) and “ulcer” (macro scores 1 and above) with the respective sensitivity of diagnosis. This simplified visual scoring system would also be in line with the suggestion made by Heitmann et al. (Citation2018) that visual assessment systems should be simple to use.
Taking these suggested cut-off values and using the three-staged visual scoring system as suggested above, healthy hocks would be diagnosed with a sensitivity of 0.96 and a specificity of 0.91 and hocks with an ulcer with a sensitivity of 0.82 and a specificity of 0.98, at an accuracy of 0.78. However, in this case, mild lesions would be diagnosed with a sensitivity of only 0.45 and a specificity of 0.84, at an accuracy of 0.78. Thus, a hock with a mild lesion would have a probability of only 45% of being identified as such. Because of the importance of the diagnosis of ulcers, it might make sense to use a two-staged visual scoring system defining macro scores 1–4 as “ulcer”. Applying these adapted cut-off values, healthy hocks and ulcers would be diagnosed with a sensitivity of 0.96 or 1.00, respectively, and a specificity of 0.91 and 0.73, respectively, at an accuracy of 0.85. To evaluate if the visual differentiation of superficial and deep lesions is possible, we used another visual scoring system and re-defined the macro scores to visually classified “no lesions”, “superficial lesions” and “deep lesions”. However, this additional analysis showed if lesions are exclusively classified as superficial or deep lesions without regarding the size of the lesions, more than half of the visually classified “superficial” lesions are histologically defined as ulcers. Thus, the extrapolation to histological alterations or the classification of mild lesions and ulcers by visual assessment is only possible with limitations, because the majority of the visual lesions are ulcers. Other authors suggested that for the diagnosis of ulcers in FPD an incision should be performed to improve the accuracy of assessment (Lund et al., Citation2017; Riber et al., Citation2020). An incision might improve the diagnosis of ulcers in hock burn of broilers after slaughter as well. However, for assessments at the slaughterhouse, making incisions on the hocks may not be practicable because the broilers are hung on the shackles by the legs and hocks.
Furthermore, we measured the inflammation depth of lesions to evaluate and discuss if the depth (in µm) could be used as an additional indicator of severity. However, to our knowledge, no studies have been performed with an exact measurement of the depth of hock burn lesions. Thus, a threshold depth indicating a painful ulcer does not exist. McKeegan (Citation2010) stated that the depths of ulcers were shallow without giving exact measurements. Heitmann et al. (Citation2018) measured the depth of foot pad lesions (defined as an increase in the thickness of the skin layer) and measured depths of more than 2500 µm. In the present study, the depth increased with an increasing macro score, up to macro score 3, with an average of 1019 µm. Thus, a revised scoring system could incorporate depth as an indicator of severity. Before implementing this scoring system, we suggest that more research should be done with respect to pain caused by ulcers of different depths. Compared with lesions of the foot pad, hock burn lesions seem to be less deep, which was also observed by Greene et al. (Citation1985). Similarly, Piller et al. (presumably Citation2020, under review) observed in a comparable examination of foot pads of broilers an increasing depth from 938 µm in macro score 1 up to 1443 µm in macro score 3, with a slight decrease to 1422 µm in macro score 4. The average inflammation depth of 1443 µm in macro score 3 of the foot pad lesions examined by Piller et al. (presumably Citation2020, under review) was greater than in the hock burn lesions of our study, which had an average depth of 1019 µm in macro score 3. We also observed that ulceration, and thus deep lesions, occurred in relatively small macroscopic hock burn lesions (65% probability in macro score 1), contrasting with the findings in the foot pad lesions similarly examined by Piller et al. (presumably Citation2020, under review) (38% probability in macro score 1, 58% probability in macro score 2). This could be because the subcutaneous layer of the hock region, in contrast to that of the foot pads, has little fat and connective tissue.
Another possibility to differentiate the levels of severity of hock burn could incorporate different stages of ulceration and severity of granulation tissue. This option should be studied further and could provide indices of the duration or chronicity of potential lesions. Because our study focused on determining ulceration and differentiating ulcers from mild lesions, different stages of ulceration and the presence or absence of granulation tissue were not evaluated separately. This evaluation should be included in future research.
The assessment of hock burn as a bird welfare indicator can be performed at slaughter even though this method has a few disadvantages (de Jong et al., Citation2016). In one third of the member states of the European Union, FPD is assessed at the slaughter of broilers (European Union, Citation2016). The governmental regulation for FPD currently considers lesions involving ulcers as animal-welfare-relevant (References for the Implementation of the German Order on the Protection of Animals and the Keeping of Production Animals, Citation2011). A survey among slaughterhouses for broilers in the German-speaking territories (Austria, Switzerland, Germany) revealed that FPD and hock burn are commonly assessed (Louton, Erhard & Wöhr Citation2018). Because hock burn is an indicator of flock health and can be linked to bird welfare (Allain et al., Citation2009; Hepworth et al., Citation2011), it should be assessed additionally to FPD. Furthermore, in contrast to the risk factors for FPD, the main risk factor for hock burn is the weight of the broilers rather than the litter characteristics (McKeegan, Citation2010; Louton, Bergmann et al., Citation2018). From our point of view, until the pain caused by ulcers of different depths or severity can be identified, all ulcers should be considered in the assessment. Thus, a two-staged visual scoring system as presented herein, incorporating all ulcers and all visible lesions, or a three-staged scoring system (no lesion, mild lesion, ulcer) should be used until then.
Kierończyk et al. (Citation2017) describe tenosynovitis as an infectious skeletal system disorder of meat-type fowl. Bradshaw et al. (Citation2002) summarize that arthritis and tenosynovitis cause lameness and swollen joints in affected birds and are caused by bacterial and viral infections. This may lead to an inflammation of the hock or associated ligaments and tendons. In our study, a relationship between inflammation of tendon sheaths and the macro or micro score of hock burn was not observed; inflammation of tendon sheaths was observed both in low and high scores of hock burn. Thus, the aetiological factor for the observed inflammations of tendon sheaths seems to be unrelated to the observed hock burn.
The presented study is novel and provides detailed information on the histological assessment of hock burn lesions and the depth of these lesions in broilers. Limitations of the study are that the used scoring system considers visual deep lesions (≤0.5 cm in diameter) already at macro score 1, and thus ulcers may be included in macro score 1. However, even when lesions were exclusively classified as superficial or deep, without regarding their size, more than half of the visually classified “superficial” lesions were histologically defined as ulcers. Another limitation is that, based on the results of our study, an assessment of pain associated with the different scores is not possible. Thus, welfare-relevant alterations can only be classified in terms of ulcerations. Further research should focus on the actual pain perceived at different scores of hock burn and the different stages of ulceration, which might give evidence considering the duration of the lesion.
In this study, the histological validation of a visual scoring system for hock burn in broilers revealed a tendency of higher micro scores with increasing macro scores. Considering limitations, the validated macroscopic assessment scheme for hock burn allows an estimation of histological alterations in hocks of broilers. The results could be used to classify hock burn lesions concerning mild lesions and ulcers. Scoring systems could be simplified to “healthy” (or “no lesion”, macro score 0), “mild lesion” (macro score 1) and “ulcer” (macro scores 2 and above) or even to “healthy” (or “no lesion”, macro score 0) and “ulcer” (macro scores 1 and above) with the respective sensitivity of diagnosis presented in our study. Our hypothesis that lesions of a higher visual score (“macro score”) also have a higher histological score (“micro score”) and deeper inflammation than lesions of a lower macro score was confirmed to some extent. The inflammation depth of hock burn lesions in broilers increased with increasing macro score up to macro score 3. More research is needed to identify the welfare relevance of hock burn assessments. To conclude, our study only provides threshold values for the assessment of ulcers. For future governmental regulations, it could be discussed whether the presence of ulcers in general, the depth of inflammation or the perception of pain at different ulcer stages should be used for defining the welfare relevance.
Supplemental Material
Download Zip (284.4 KB)Acknowledgements
The authors thank Astrid Nagel and Birgit Isele-Rüegg for the excellent technical assistance. We thank Dr. Verena Lietze for the scientific language editing. We thank Stefan and Yeliz Wiesbeck and PD Dr. Sven Reese for their valuable and dedicated support during data collection.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
H. Louton http://orcid.org/0000-0001-9658-823X
S. Bergmann http://orcid.org/0000-0001-9888-653X
J. Stracke http://orcid.org/0000-0002-9986-9720
N. Kemper http://orcid.org/0000-0003-0092-4302
Additional information
Funding
References
- Allain, V., Mirabito, L., Arnould, C., Colas, M., Le Bouquin, S., Lupo, C. & Michel, V. (2009). Skin lesions in broiler chickens measured at the slaughterhouse: relationships between lesions and between their prevalence and rearing factors. British Poultry Science, 50, 407–417. doi: 10.1080/00071660903110901
- Bergmann, S., Louton, H., Westermaier, C., Wilutzky, K., Bender, A., Bachmeier, J., Erhard, M. & Rauch, E. (2016). Field trial on animal-based measures for animal welfare in slow growing broilers reared under an alternative concept suitable for the German market. Berliner und Münchener Tierärztliche Wochenschrift, 129, 453–461.
- Bessei, W. (2006). Welfare of broilers: a review. World’s Poultry Science Journal, 62, 455–466. doi: 10.1079/WPS2005108
- Bradshaw, R.H., Kirkden, R.D. & Broom, D.M. (2002). A review of the aetiology and pathology of leg weakness in broilers in relation to welfare. Avian and Poultry Biology Reviews, 13, 45–103. doi: 10.3184/147020602783698421
- Broom, D.M. & Reefmann, N. (2005). Chicken welfare as indicated by lesions on carcases in supermarkets. British Poultry Science, 46, 407–414. doi: 10.1080/00071660500181149
- Bürkner, P.-C. (2017). Brms: an R package for Bayesian multilevel models using Stan. Journal of Statistical Software, 80, 1–28. doi: 10.18637/jss.v080.i01
- Byrt, T., Bishop, J. & Carlin, J.B. (1993). Bias, prevalence and kappa. Journal of Clinical Epidemiology, 46, 423–429. doi: 10.1016/0895-4356(93)90018-V
- Carpenter, B., Gelman, A., Hoffman, M.D., Lee, D., Goodrich, B., Betancourt, M., Brubaker, M., Guo, J., Li, P. & Riddell, A. (2017). Stan: a probabilistic programming language. Journal of Statistical Software, 76, 1–32. doi: 10.18637/jss.v076.i01
- Douglas, J.M., Guzman, D. & Paul-Murphy, J.R. (2018). Pain in birds: the anatomical and physiological basis. Veterinary Clinics of North America: Exotic Animal Practice, 21, 17–31.
- European Union. (2016). Use of slaughterhouse data to monitor welfare of broilers on farm. Overview report of a series of audits of the Directorate-General for Health and Food Safety from 2013 to 2015 to evaluate the official controls on the welfare of chickens kept for meat production using slaughterhouse data to establish farm checks. European Commission.
- Fleiss, J.L., Levin, B. & Cho Paik, M. (2003). Statistical Methods for Rates and Proportions 3rd edn ( p. 604). Hoboken, NJ: John Wiley & Sons.
- Gentle, M.J. (1992). Pain in birds. Animal Welfare, 1, 235–247.
- Gentle, M.J., Tilston, V. & McKeegan, D.E.F. (2001). Mechanothermal nociceptors in the scaly skin of the chicken leg. Neuroscience, 106, 643–652. doi: 10.1016/S0306-4522(01)00318-9
- Greene, J.A., McCracken, R.M. & Evans, R.T. (1985). A contact dermatitis of broilers – clinical and pathological findings. Avian Pathology, 14, 23–38. doi: 10.1080/03079458508436205
- de Gruyter, W. (2002). Pschyrembel. Klinisches Wörterbuch 259th edn ( p. 480). Berlin: Walter de Gruyter GmbH. ISBN 3-11-016522-8.
- Gunnarsson, S., Algers, B. & Svedberg, J. (2000). Description and evaluation of a scoring system of clinical health in laying hens. In S. Gunnarsson Laying Hens in Loose Housing Systems. Doctoral thesis, Swedish University of Agricultural Sciences, Uppsala.
- Günes, U.Y. (2008). A descriptive study of pressure ulcer pain. Ostomy Wound Management & Prevention, 54, 56–61.
- Haslam, S.M., Knowles, T.G., Brown, S.N., Wilkins, L.J., Kestin, S.C., Warriss, P.D. & Nicol, C.J. (2007). Factors affecting the prevalence of foot pad dermatitis, hock burn and breast burn in broiler chicken. British Poultry Science, 48, 264–275. doi: 10.1080/00071660701371341
- Heitmann, S., Stracke, J., Petersen, H., Spindler, B. & Kemper, N. (2018). First approach validating a scoring system for foot-pad dermatitis in broiler chickens developed for application in practice. Preventive Veterinary Medicine, 154, 63–70. doi: 10.1016/j.prevetmed.2018.03.013
- Hepworth, P.J., Nefedov, A.V., Muchnik, I.B. & Morgan, K.L. (2010). Early warning indicators for hock burn in broiler flocks. Avian Pathology, 39, 405–409. doi: 10.1080/03079457.2010.510500
- Hepworth, P.J., Nefedov, A.V., Muchnik, I.B. & Morgan, K.L. (2011). Hock burn: an indicator of broiler flock health. Veterinary Record, 168, 303. doi: 10.1136/vr.c6897
- Jacob, F.G., Baracho, M.S., Nääs, I.A., Lima, N.S.D., Salgado, D.D. & Souza, R. (2016). Risk of incidence of hock burn and pododermatitis in broilers reared under commercial conditions. Revista Brasileira de Ciência Avícola, 18, 357–362. doi: 10.1590/1806-9061-2015-0183
- de Jong, I.C., Gunnink, H. & van Harn, J. (2014). Wet litter not only induces footpad dermatitis but also reduces overall welfare, technical performance, and carcass yield in broiler chickens. Journal of Applied Poultry Research, 23, 51–58. doi: 10.3382/japr.2013-00803
- de Jong, I.C., Hindle, V.A., Butterworth, A., Engel, B., Ferrari, P., Gunnink, H., Perez Moya, T., Tuyttens, F.A.M. & van Reenen, C.G. (2016). Simplifying the Welfare Quality® assessment protocol for broiler chicken welfare. Animal, 10, 117–127. doi: 10.1017/S1751731115001706
- Kierończyk, B., Rawski, M., Józefiak, D. & Świątkiewicz, S. (2017). Infectious and non-infectious factors associated with leg disorders in poultry – a review. Annals of Animal Science, 17, 645–669. doi: 10.1515/aoas-2016-0098
- Kjaer, J.B., Su, G., Nielsen, B.L. & Sørensen, P. (2006). Foot pad dermatitis and hock burn in broiler chickens and degree of inheritance. Poultry Science, 85, 1342–1348. doi: 10.1093/ps/85.8.1342
- Landis, J.R. & Koch, G.G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33, 159–174. doi: 10.2307/2529310
- Louton, H., Bergmann, S., Reese, S., Erhard, M., Bachmeier, J., Rösler, B. & Rauch, E. (2018). Animal- and management-based welfare indicators for a conventional broiler strain in two barn types (Louisiana barn and closed barn). Poultry Science, 97, 2754–2767. doi: 10.3382/ps/pey111
- Louton, H., Erhard, M. & Wöhr, A.C. (2018). Acquisition of animal-based welfare measures at slaughter of poultry. Fleischwirtschaft, 11, 94–98.
- Louton, H., Erhard, M., Wirsch, K., Bergmann, S., Piller, A., Schmidt, P. & Rauch, E. (presumably 2020). Four assessment methods of foot pad dermatitis and hock burn of broilers at slaughter if compared to findings in living animals. Berliner und Münchener Tierärztliche Wochenschrift, under review after revision.
- Lund, V.P., Nielsen, L.R., Oliveira, A.R.S. & Christensen, J.P. (2017). Evaluation of the Danish footpad lesion surveillance in conventional and organic broilers: misclassification of scoring. Poultry Science, 96, 2018–2028. doi: 10.3382/ps/pex024
- Machin, K.L. (2005). Avian analgesia. Journal of Exotic Pet Medicine, 14, 236–242.
- Mayne, R.K., Hocking, P.M. & Else, R.W. (2006). Foot pad dermatitis develops at an early age in commercial turkeys. British Poultry Science, 47, 36–42. doi: 10.1080/00071660500475392
- McKeegan, D. (2010). Foot pad dermatitis and hock burn in broilers: risk factors, aetiology and welfare consequences. Research project final report, Faculty of Veterinary Medicine, University of Glasgow, Scotland, UK.
- Michel, V., Prampart, E., Mirabito, L., Allain, V., Arnould, C., Huonnic, D., Le Bouquin, S. & Albaric, O. (2012). Histologically-validated footpad dermatitis scoring system for use in chicken processing plants. British Poultry Science, 53, 275–281. doi: 10.1080/00071668.2012.695336
- Ministry of Food, Agriculture and Consumer Protection of the German State Lower Saxony. (2014). Decree by the Ministry of Food, Agriculture and Consumer Protection of the German State Lower Saxony. Reference Number 204.1-42503/2-828, Date of Decree 11th Dec 2014, Version 31st Jul 2015, Rule § 20 Abs. 4 und 5 TierSchNutztV, § 11 TierSchG, § 17 TierSchG, § 20 TierSchNutztV.
- Pass, D.A. (1989). The pathology of the avian integument: a review. Avian Pathology, 18, 1–72. doi: 10.1080/03079458908418580
- Piller, A., Bergmann, S., Schwarzer, A., Erhard, M., Stracke, J., Spindler, B., Kemper, N., Schmidt, P., Bachmeier, J., Schade, B., Boehm, B., Kappe, E. & Louton H. (presumably 2020). Validation of histological and visual scoring systems for foot pad dermatitis in broiler chicken. Animal Welfare, accepted for publication.
- R Core Team. (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
- References for the Implementation of the German Order on the Protection of Animals and the Keeping of Production Animals. (2011). Ausführungshinweise Masthühner. Beschlossen von der AG Tierschutz der LAV am 29. November 2011. Tierschutz-Nutztierhaltungsverordnung (TierSchNutztV) i. d. F. der Bekanntmachung vom 22. August 2006 (BGBl. I S. 2043), die durch die Verordnung vom 1. Oktober 2009 (BGBl. I S. 3223) geändert worden ist, Abschnitt 4, Anforderungen an das Halten von Masthühnern.
- Riber, A.B., Rangstrup-Christensen, L., Hansen, M.S., Hinrichsen, L.K. & Herskin, M.S. (2020). Characterisation of footpad lesions in organic and conventional broilers. Animal, 14, 119–128. doi: 10.1017/S1751731119001551
- Saraiva, S., Saraiva, C. & Stilwell, G. (2016). Feather conditions and clinical scores as indicators of broilers welfare at the slaughterhouse. Research in Veterinary Science, 107, 75–79. doi: 10.1016/j.rvsc.2016.05.005
- Sørensen, P., Su, G. & Kestin, S.C. (2000). Effects of age and stocking density on leg weakness in broiler chickens. Poultry Science, 79, 864–870. doi: 10.1093/ps/79.6.864
- Vanderhasselt, R.F., Sprenger, M., Duchateau, L. & Tuyttens, F.A.M. (2013). Automated assessment of footpad dermatitis in broiler chickens at the slaughter-line: evaluation and correspondence with human expert scores. Poultry Science, 92, 12–18. doi: 10.3382/ps.2012-02153
- Welfare Quality®. (2009). Welfare Quality® Assessment Protocol for Poultry (Broilers, Laying Hens). Lelystad: Welfare Quality® Consortium.
