ABSTRACT
Rooks (Corvus frugilegus) are considered migratory crows in Japan. Some rooks share a wintering site in the Izumi plain in Kagoshima Prefecture with hooded cranes (Grus monacha) and white-necked cranes (Grus vipio), which are designated as “endangered” in the International Union for Conservation of Nature (IUCN) Red List of Threatened Species. Highly pathogenic avian influenza (HPAI), caused by H5 subtype viruses, has recently been reported in these crane species in Japan, in conjunction with a massive decrease in their population. In the present study, the pathogenicity of HPAI virus was assessed in rooks to evaluate the likelihood that they are a source of infections in other bird species. One of four rooks intranasally inoculated with A/mandarin duck/Miyazaki/22M807-1/2011 (H5N1) died at 10 days post-inoculation (d.p.i.). The other three rooks exhibited seroconversion but no clinical signs. All the rooks had shed virus by the oral route at <103 50% egg infectious dose/ml until 7 d.p.i. Virus was also recovered from multiple tissues of the rook that succumbed to the infection. These results suggest that rooks are susceptible to infection with H5 HPAI viruses, leading to prolonged viral shedding. The rooks shed the virus at low titres however, indicating that they are likely to function as transmission vectors in wintering bird flocks. The rooks exhibited clear antibody responses against the H5 HPAI virus, and thus serological surveillance of them in the field should be helpful for assessing viral pervasion into the habitats of crane species.
Introduction
To date, H5 subtype highly pathogenic avian influenza (HPAI) viruses have spread to domestic poultry and wild birds in over 70 countries in Asia, Europe, the Middle East, and Africa since late 1996 when the virus, recognized as the precursor of present circulating viruses (Gs/Gd-like viruses) was isolated from a goose in Guangdong province, China (Xu et al., Citation1999). Phylogenetically, based on the HA genes, these progeny viruses have been organized into 10 clades, numbered clades 0–9 (WHO/OIE/FAO Evolution Working Group, Citation2008). Initial reports of highly pathogenic avian influenza were largely confined to poultry populations, and lethal Gs/Gd-like virus infection was not detected in wild birds. In 2002 however, outbreaks of Gs/Gd-like viruses in waterfowl and other captive/wild birds were reported for the first time at two waterfowl parks in Hong Kong (Ellis et al., Citation2004). Subsequently, more than a thousand migratory birds on Lake Qinghai in China died from HPAI infection in May and June of 2005 (Liu et al., Citation2005). Multiple HPAI outbreaks in wild birds have since been reported at numerous locations around the world.
In wild birds, HPAI outbreaks caused by Gs/Gd-like viruses have mostly been observed in migratory water birds such as ducks and swans, which are thought to be natural reservoirs of influenza A viruses (Alexander, Citation1982). A few cases of HPAI have been reported in crow species, mainly in Asian countries and the Middle East. House crows in Hong Kong were evidently infected with clade 2.3.4 H5N1 HPAI virus in 2006–2007 (Smith et al., Citation2009), and clade 2.3.2.1 H5N1 HPAI virus infections in crows were confirmed in India and Bangladesh in 2011 (Khan et al., Citation2014; Vijayakumar et al., Citation2015). Khan et al. (Citation2014) reported cannibalistic behaviour and pathological features in crows (Khan et al., Citation2014). In the Asian part of Russia, clade 2.3.2.1c H5N1 HPAI virus was isolated from rooks (Corvus frugilegus) in 2015 (Shipovalov et al., Citation2017). More recently, HPAI caused by clade 2.3.4.4b H5N8 virus was reported in hooded crows (Corvus cornix) in Iran in 2017 (Ghafouri et al., Citation2019). Outside of Asia, clade 2.2 H5N1 virus was isolated from rooks in the south of the European part of Russia in 2007 (Lvov et al., Citation2010). Clade 2.3.4.4b H5N8 HPAI virus was isolated from dead Corvus corone crows in Germany during the 2016–2017 winter, and cannibalistic behaviour was also observed in this outbreak (Globig et al., Citation2018). Furthermore, HPAI virus isolates from crows in Thailand, Nepal, Pakistan, and Egypt have been recorded as is evident via the National Center for Biotechnology Information and Global Initiative on Sharing All Influenza Data search engines (as at September 2019). Following such reports of HPAI in crows, studies investigating antibody prevalence in crow populations were conducted; in Egypt, H5, H7, and H9 haemagglutination inhibition antibodies were detected in house crows (Fadel & Afifi, Citation2017); avian influenza virus nucleoprotein antibody was also found in house crows in Bangladesh in 2012–2016, by competitive-ELISA (Hassan et al., Citation2017, Citation2018).
In Japan, HPAI was reported in poultry farms in 2004 for the first time in 79 years (Mase et al., Citation2005). At that time, several crows that had evidently died from clade 2.5 H5N1 HPAI virus infection were found around the affected poultry farms, and characteristics of causal viruses and pathology in dead crows were assessed (Mase et al., Citation2005; Tanimura et al., Citation2006). In 2018, a massive HPAI outbreak caused by clade 2.3.4.4b H5N6 virus in jungle crows occurred around Koya Pond in Hyogo Prefecture, a known habitat of resident swans and crows (Mine et al., Citation2019). In that outbreak, at least 38 crows that ultimately succumbed to HPAI virus infection were continuously observed over 3 weeks beforehand, and virus dissemination via cannibalism was suggested.
To date, most HPAI cases in crows have been detected in resident bird species. Notably, however, some crow species in specific areas migrate for wintering. Rooks are distributed over a vast area of Eurasia, from Scandinavia and Western Europe to East Siberia (Madge, Citation2008). Although most of them are resident birds, some populations in the northernmost regions migrate in winter, as do some water birds in those regions. In the mid-twentieth century, the rook population declined with the use of organomercury and organochlorine compounds in the environment (Malmberg, Citation1973; Beyerbach et al., Citation1987). After subsequent improvement of the natural environment and agricultural innovations, the rook population was restored and their habitat range expanded (Marchant & Gregory, Citation1999; Takagi, Citation2010). In Japan, rooks share a wintering site with endangered crane species. Migration routes and wintering sites/periods are evidently closely related to the dynamics of HPAI epidemics (Soda, Ito, et al., Citation2013), and HPAI infections in endangered crane species have been reported in Japan (Ozawa et al., Citation2015, Citation2019). Therefore, elucidation of the pathogenicity of HPAI in rooks is important for the future conservation of cranes, which share habitat with them.
In the present study, rooks were experimentally inoculated with H5N1 HPAI virus and pathogenicity was assessed to evaluate the likelihood that they are a source of infection in other bird species, especially crane species. To the best of our knowledge, this is the first reported investigation of the pathogenicity of HPAI virus in rooks.
Materials and methods
Virus
Clade 2.3.2.1 HPAI virus A/mandarin duck/Miyazaki/22M807-1/2011 (H5N1) (M807) was applied for experimental infection of rooks. The strain was isolated from a mixture of tracheal and cloacal swabs of a mandarin duck that died in Miyazaki City located in the Kyusyu region of Japan (Soda, Ito, et al., Citation2013), and was already used in our previous study evaluating its pathogenicity in other duck species (Soda, Usui, et al., Citation2013). The accession numbers of the M807 gene sequences are AB677872–AB677879. Virus was propagated in 10-day-old chicken embryos (Aoki Breeder Farm, Tochigi, Japan) for 48 h at 35°C.
Birds
Four rooks (two adults and two juveniles, identified by feather growth and moult) were captured at Yamaguchi Prefecture, Japan, with the approval of the Ministry of the Environment of the Government of Japan (permission numbers 13101301-2 and 1401302-2) and Yamaguchi Prefecture (permission numbers 394-2 and 771-2). The birds were shipped to Tottori University, and confirmed to be influenza virus negative via an antigen detection kit (ESPLINE INFLUENZA A & B-N, Fujirebio Inc., Tokyo, Japan) and virus isolation study on eggs using their oral and cloacal swabs, and to be seronegative by haemagglutinin inhibition testing using the M807 strain (Sever, Citation1962). The birds were then immediately experimentally inoculated with the above-described influenza strain.
Experimental design
The rooks were intranasally inoculated with 200 μl of allantoic fluid containing the M807 strain at 106 50% egg infectious dose (EID50), then observed for clinical signs at 24-h intervals for 10 days. Oral and cloacal swabs were also performed at 1, 2, 3, 5, 7 and 10 days post inoculation (d.p.i.) to assess viral shedding. The swabs were collected in 2 ml of nutrient broth medium (Nissui Pharmaceutical, Tokyo, Japan) with 10 mg of streptomycin sulfate (Meiji Seika Pharma, Tokyo, Japan) and 1 × 104 units of penicillin G (Meiji Seika Pharma). At the end of the 10-day period, the rooks were also checked for specific antibodies against M807 in serum via haemagglutinin inhibition testing. In addition to the rook that died of infection, the surviving birds were euthanized using isoflurane (FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan) and dissected after blood collection at 10 d.p.i., and their tissues (brain, trachea, breast muscle, lung, liver, pancreas, spleen, heart, kidney, and colon) were aseptically sampled. Portions of the tissue samples were homogenized using a Multi-Bead Shocker (Micro SmashTM MS-100R, Tomy Seiko, Tokyo, Japan, 3000 rpm, 30 s) to create a 10% (w/v) organ emulsion in nutrient broth medium with antibiotics. Samples serially ten-fold diluted in phosphate buffered saline with streptomycin sulfate and penicillin G were inoculated into 10-day-old chicken embryos. Eggs were incubated at 35°C for 48 h. HA testing was performed using allantoic fluid, and EID50 was calculated using the Reed and Müench method (Reed & Muench, Citation1938). The sampled tissues were also subjected to histopathological analysis. Tissues fixed in 10% neutral buffered formalin (FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan) were processed via routine methods then embedded in paraffin wax. Sections were stained with haematoxylin and eosin for histopathological examination. Immunohistochemical staining was also performed using antigen retrieval solution, 0.05% citraconic anhydride, pH 7.4 (Immunosaver; Nissin EM, Tokyo, Japan), mouse anti-influenza A virus matrix protein monoclonal antibody (clone GA2B; Serotec Ltd., Oxford, UK), and the Simple Stain MAX-PO (M) kit (Nichirei Bioscience Inc., Tokyo, Japan) in accordance with the manufacturers’ instructions.
Ethics statement
All bird experiments were conducted in self-contained isolator units (CLEA Japan, Tokyo) at a biosafety level 3 facility at the Avian Zoonosis Research Center, Tottori University, Japan. The experiments were approved by the Ethics Committee of the institute and performed in accordance with the guidelines of the institutional animal care and use committee of Tottori University (approval number 12-T-30). The predetermined study protocol stipulated that, throughout the study, any birds that became unable to eat or drink were to be euthanized, and recorded as dead at the following day’s observation time.
Results
Two of the four rooks in the study (#R01 and #R03) were adult birds and the other two (#R02 and #R04) were juveniles (). All the rooks were seronegative for H5N1 HPAI virus M807 strain. The two adults and one of the juveniles survived for the 10-day observation period after intranasal inoculation with 106 EID50 of M807 strain without showing any clinical signs. The remaining juvenile (#R02) exhibited mild lethargy after 8 d.p.i. without losing its appetite, and died at 10 d.p.i. (). Virus was recovered from numerous organs from #R02, with the highest titre detected in the pancreas (). Seroconversion was observed in the three rooks that did not succumb to the infection (#R01, #R03 and #R04). Interestingly, as well as #R02 which died of infection, virus was also isolated from the pancreases of #R01 and #R03.
Table 1. Antibody responses and viral titres/antigen-detection in the tissues of rooks intranasally inoculated with H5N1 HPAI virus (10 d.p.i).
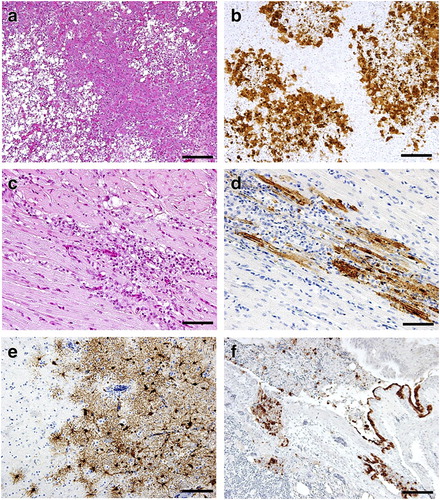
No gross lesions were detected in any of the rooks in the study at 10 d.p.i., except for an incidental finding of Sarcocystis spp. in skeletal and cardiac muscles of #R01, without inflammation. Histopathological changes were mainly observed in the rooks’ pancreases, wherein multifocal to coalescing necrotic lesions and inflammatory infiltration were detected in #R01, #R02 and #R03 ((a), representative lesion in #R02). In these three rooks, virus antigens were detected in acinar cells and macrophages, and a larger number of positive cells was detected in #R02 than in the other rooks ((b) and ). In #R02, the heart exhibited mild multifocal degeneration with nonsuppurative infiltration ((c)), and viral antigens were detected in affected myocardial cells ((d) and ). In the brains of #R02 and #R03, neuronal degeneration, mild perivascular cuffing and gliosis were observed and virus antigens were distributed in neurons, astrocytes, microglia and meningeal cells ((e)). #R02 was histologically diagnosed with mild nonsuppurative bronchopneumonia, necrotizing bronchitis, and myositis of skeletal muscle. Multifocal necrotizing bronchitis with inflammatory infiltration and sloughed epithelial cells was evident, accompanied by viral antigens in necrotizing and degenerated secondary bronchial epithelium, macrophages and bronchial smooth muscle cells ((f) and ). Irrespective of virus recovery results, virus antigen was detected in all of the tissues tested in #R02 ().
Figure 1. Representative histopathological findings of the pancreas (a) and heart (c), and immunohistochemical demonstration of type A influenza virus antigens in the pancreas (b), heart (d), brain (e), and bronchi and lung (f) of #R02, which died at 10 d.p.i. Bars indicate 100 µm (a, b, e, f) and 50 µm (c, d).
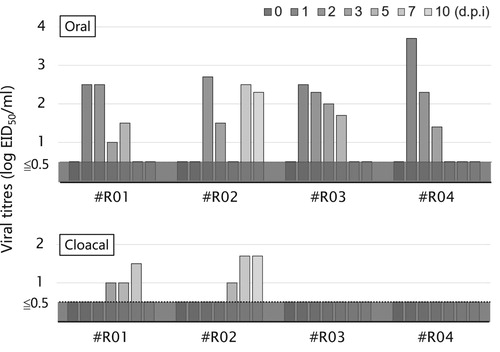
Virus shedding was also assessed (). The three rooks that survived for 10 days (#R01, #R03 and #R04) shed virus by the oral route during 1–5 d.p.i., in the acute phase of infection, with titres of approximately 102 EID50/ml. A bimodal pattern of viral shedding was observed in #R02, with virus also isolated from oral swabs at 7 and 10 d.p.i. Although viral titres were lower, delayed viral shedding was detected in cloacal swabs from #R01 and #R02 at 3–10 d.p.i.
Figure 2. Viral titres of oral (upper) and cloacal (lower) swabs collected from the rooks. Viral titres were calculated by the method of Reed and Muench (Citation1938) and are expressed as EID50/ml of the samples.
Discussion
The present study has identified the pathology and occasional occurrence of lethal H5 HPAI virus infection in rooks (). Rooks infected with H5 HPAI virus mainly shed the pathogen orally (), and the virus may remain in the pancreas of infected rooks in the late phase of infection, 10 d.p.i. ().
As of 2019, only a few studies have reported HPAI virus infection in species of crows, which implies that HPAI virus infection in crows is usually subclinical. The present results also support this notion; three of the four rooks inoculated with the HPAI virus survived without manifesting any clinical signs (). Viral shedding from the oral cavity and cloaca was <103 EID50/ml (). We previously showed that mandarin ducks (Aix galericulata) intranasally inoculated with M807 orally shed the virus at a titre of <103.5 TCID50/ml (Soda, Usui, et al., Citation2013), comparable to the rooks in the present study. Hiono et al. (Citation2016) also reported that experimental inoculation of HPAI virus in jungle crows induced viral shedding with a titre of <103.6 PFU/ml. These results indicate that crows, including rooks, could function as the host reservoirs for HPAI virus as well as wild aquatic birds, for transmission to other birds with shared habitats.
In the present study, obvious seroconversions were observed in the surviving rooks (). Similarly, a rise in serum antibody titres was observed in jungle crows inoculated with clade 1, 2.3.2.1, or 2.3.4.4 HPAI virus (Hiono et al., Citation2016). Antibodies against avian influenza viruses were also detected in the serum of crows in Bangladesh and Egypt (Fadel & Afifi, Citation2017; Hassan et al., Citation2017, Citation2018). Hence, crow species are susceptible to avian influenza viruses, including various H5 HPAI viruses, despite low morbidity and mortality. The presence of antibodies against HPAI virus in crows should be effective to identify the HPAI virus invasion in migratory bird flocks.
Cannibalism was observed during the past HPAI outbreaks in crows in Bangladesh, Germany, and Japan (Khan et al., Citation2014; Globig et al., Citation2018; Mine et al., Citation2019), accompanied by consecutive cases of mortality in crows and other birds with virus infection. In the present study, viral antigens were detected in the systemic tissues, including the muscles of dead rooks ( and ). The discordance between the results of virus isolation and distribution of viral antigen in some tissues () could be explained by induced antibody already neutralizing viruses in tissues, or interfering viral proliferation on isolation studies using eggs. Infectious virions were confirmed in systemic tissues in the dead rook, #R02, supporting the notion that HPAI virus could be transmitted via consumption of the carcasses of infected crows. Bertran et al. (Citation2012) successfully reproduced natural infection in raptors by feeding on infected prey with H5N1 HPAI virus or H7N2 low pathogenic avian influenza virus; the fed raptors orally shed the virus as well as nasochoanally inoculated birds. Susceptibility of crow species, including rooks, to HPAI viruses via digestive route should be of interest, and examined further. To prevent possible dissemination of HPAI virus to scavengers, crows suspected of HPAI virus infection should be immediately incinerated or transported to appropriate institutes with biosafety measures for definitive diagnosis. For an accurate diagnosis, not only swabs but also tissue samples should be used because of limited virus shedding by the oral and cloacal routes in crows () (Hiono et al., Citation2016). In addition, protecting poultry farms from the invasion of crows, both under ordinary and emergency circumstances, should be essential to prevent the dissemination of HPAI virus.
In the present study, necrosis in acinar cells in the pancreas and neuronal degeneration in the brain were found in the rooks inoculated with the HPAI virus, which is in line with the previous case reports in the field (Tanimura et al., Citation2006; Khan et al., Citation2014). Remaining viruses in the pancreas might cause viral shedding to the cloaca in the late phase of the infection (). The reason why the HPAI viruses showed high tissue tropism for the pancreas in crows remains unclear. It can be assumed that pancreatic trypsin activity contributes to effective cleavage activation of viral haemagglutinin, leading to high viral growth (Klenk et al., Citation1975). Neuronal damage in the brains of crows may cause more severe clinical signs in the field (Tanimura et al., Citation2006; Khan et al., Citation2014) than in experimental infection.
The habitat range of rooks has expanded as a result of environmental changes such as global warming (Takagi, Citation2010). The present study revealed that the pathogenicity of the HPAI virus in rooks is not very high. Furthermore, as of August 2019, few HPAI viruses were isolated from rooks in the field (Lvov et al., Citation2010; Shipovalov et al., Citation2017). However, it cannot be denied that rooks will be involved in future HPAI outbreaks because of their characteristic migration behaviour. Although the number of examined rooks was limited in the present study, we expect that the findings will provide a basis for further pathological studies in rooks, and contribute to protect the endangered bird species, which are sharing their habitat with rooks with HPAI virus infection.
Acknowledgements
Capture and transportation of the rooks were facilitated by Yamashina Institute for Ornithology and Rhino LLC. We thank Dr H. Takeuchi (Rhino LLC) for giving technical suggestions about aviculture.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alexander, D.J. (1982). Ecological aspects of influenza A viruses in animals and their relationship to human influenza: a review. Journal of the Royal Society of Medicine, 75, 799–811.
- Bertran, K., Busquets, N., Abad, F.X., García de la Fuente, J., Solanes, D., Cordón, I., Costa, T., Dolz, R., Majó, N. & Davis, T. (2012). Highly (H5N1) and low (H7N2) pathogenic avian influenza virus infection in falcons via nasochoanal route and ingestion of experimentally infected prey. PLoS One, 7, e32107. doi: 10.1371/journal.pone.0032107
- Beyerbach, M., Büthe, A., Heidmann, W., Dettmer, R. & Knüwer, H. (1987). Chlorierte Kohlenwasserstoffe in Eiern und Lebern von Saatkrähen (Corvus frugilegus) aus niedersächsischen Brutkolonien. Journal für Ornithologie, 128, 277–290. doi: 10.1007/BF01640298
- Ellis, T.M., Barry Bousfield, R., Bissett, L.A., Dyrting, K.C., Luk, G.S.M., Tsim, S.T., Sturm-ramirez, K., Webster, R.G., Guan, Y. & Peiris, J.S.M. (2004). Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathology, 33, 492–505. doi: 10.1080/03079450400003601
- Fadel, H.M. & Afifi, R. (2017). Investigation of avian influenza infection in wild birds in Ismailia and Damietta cities, Egypt. Veterinary World, 10, 695–701. doi: 10.14202/vetworld.2017.695-701
- Ghafouri, S.A., Fallah Mehrabadi, M.H., Talakesh, S.F., Hosseini, H., Ziafati, Z., Malekan, M., Aghaeean, L. & Ghalyanchilangeroudi, A. (2019). Full genome characterization of Iranian H5N8 highly pathogenic avian influenza virus from hooded crow (Corvus cornix), 2017: the first report. Comparative Immunology, Microbiology and Infectious Diseases, 64, 73–80. doi: 10.1016/j.cimid.2019.03.005
- Globig, A., Staubach, C., Sauter-Louis, C., Dietze, K., Homeier-Bachmann, T., Probst, C., Gethmann, J., Depner, K.R., Grund, C., Harder, T.C., Starick, E., Pohlmann, A., Höper, D., Beer, M., Mettenleiter, T.C. & Conraths, F.J. (2018). Highly pathogenic avian influenza H5N8 clade 2.3.4.4b in Germany in 2016/2017. Frontiers in Veterinary Science, 4, 240. doi: 10.3389/fvets.2017.00240
- Hassan, M.M., Hoque, M.A., Debnath, N.C., Yamage, M. & Klaassen, M. (2017). Are poultry or wild birds the main reservoirs for avian influenza in Bangladesh? Ecohealth, 14, 490–500. doi: 10.1007/s10393-017-1257-6
- Hassan, M.M., Hoque, M.A., Ujvari, B. & Klaassen, M. (2018). Live bird markets in Bangladesh as a potentially important source for avian influenza virus transmission. Preventive Veterinary Medicine, 156, 22–27. doi: 10.1016/j.prevetmed.2018.05.003
- Hiono, T., Okamatsu, M., Yamamoto, N., Ogasawara, K., Endo, M., Kuribayashi, S., Shichinohe, S., Motohashi, Y., Chu, D.-H., Suzuki, M., Ichikawa, T., Nishi, T., Abe, Y., Matsuno, K., Tanaka, K., Tanigawa, T., Kida, H. & Sakoda, Y. (2016). Experimental infection of highly and low pathogenic avian influenza viruses to chickens, ducks, tree sparrows, jungle crows, and black rats for the evaluation of their roles in virus transmission. Veterinary Microbiology, 182, 108–115. doi: 10.1016/j.vetmic.2015.11.009
- Khan, S.U., Berman, L., Haider, N., Gerloff, N., Rahman, M.Z., Shu, B., Rahman, M., Dey, T.K., Davis, T.C., Das, B.C., Balish, A., Islam, A., Teifke, J.P., Zeidner, N., Lindstrom, S., Klimov, A., Donis, R.O., Luby, S.P., Shivaprasad, H.L. & Mikolon, A.B. (2014). Investigating a crow die-off in January–February 2011 during the introduction of a new clade of highly pathogenic avian influenza virus H5N1 into Bangladesh. Archives of Virology, 159, 509–518. doi: 10.1007/s00705-013-1842-0
- Klenk, H.D., Rott, R., Orlich, M. & Blodorn, J. (1975). Activation of influenza A viruses by trypsin treatment. Virology, 68, 426–439. doi: 10.1016/0042-6822(75)90284-6
- Liu, J., Xiao, H., Lei, F., Zhu, Q., Qin, K., Zhang, X.W., Zhang, X.L., Zhao, D., Wang, G., Feng, Y., Ma, J., Liu, W., Wang, J. & Gao, G.F. (2005). Highly pathogenic H5N1 influenza virus infection in migratory birds. Science, 309, 1206. doi: 10.1126/science.1115273
- Lvov, D.K., Shchelkanov, M.Y., Prilipov, A.G., Vlasov, N.A., Fedyakina, I.T., Deryabin, P.G., Alkhovsky, S.V., Grebennikova, T.V., Zaberezhny, A.D. & Suarez, D.L. (2010). Evolution of highly pathogenic avian influenza H5N1 virus in natural ecosystems of northern Eurasia (2005–08). Avian Diseases, 54, 483–495. doi: 10.1637/8893-042509-Review.1
- Madge, C.S. (2008). Handbook of the birds of the world. PLATE 37 family CORVIDAE (CROWS) 96. Rook. Handbook of the Birds of the World, 14, 625–626.
- Malmberg, T. (1973). Pesticides and the rook Corvus frugilegus in Scania, Sweden between 1955 and 1970. Oikos, 24, 377–387. doi: 10.2307/3543814
- Marchant, J.H. & Gregory, R.D. (1999). Numbers of nesting rooks Corvus frugilegus in the United Kingdom in 1996. Bird Study, 46, 258–273. doi: 10.1080/00063659909461138
- Mase, M., Tsukamoto, K., Imada, T., Imai, K., Tanimura, N., Nakamura, K., Yamamoto, Y., Hitomi, T., Kira, T., Nakai, T., Kiso, M., Horimoto, T., Kawaoka, Y. & Yamaguchi, S. (2005). Characterization of H5N1 influenza A viruses isolated during the 2003–2004 influenza outbreaks in Japan. Virology, 332, 167–176. doi: 10.1016/j.virol.2004.11.016
- Mine, J., Uchida, Y., Nakayama, M., Tanikawa, T., Tsunekuni, R., Sharshov, K., Takemae, N., Sobolev, I., Shestpalov, A. & Saito, T. (2019). Genetics and pathogenicity of H5N6 highly pathogenic avian influenza viruses isolated from wild birds and a chicken in Japan during winter 2017–2018. Virology, 533, 1–11. doi: 10.1016/j.virol.2019.04.011
- Ozawa, M., Matsuu, A., Khalil, A.M., Nishi, N., Tokorozaki, K., Masatani, T., Horie, M., Okuya, K., Ueno, K., Kuwahara, M. & Toda, S. (2019). Phylogenetic variations of highly pathogenic H5N6 avian influenza viruses isolated from wild birds in the Izumi plain, Japan, during the 2016–17 winter season. Transboundary and Emerging Diseases, 66, 797–806. doi: 10.1111/tbed.13087
- Ozawa, M., Matsuu, A., Tokorozaki, K., Horie, M., Masatani, T., Nakagawa, H., Okuya, K., Kawabata, T. & Toda, S. (2015). Genetic diversity of highly pathogenic H5N8 avian influenza viruses at a single overwintering site of migratory birds in Japan, 2014/15. Euro Surveillance, 20.
- Reed, L.J. & Muench, H. (1938). A simple method of estimating fifty per cent endpoints. American Journal of Epidemiology, 27, 493–497. doi: 10.1093/oxfordjournals.aje.a118408
- Sever, J.L. (1962). Application of a microtechnique to viral serological investigations. Journal of Immunology, 88, 320–329.
- Shipovalov, A.V., Durymanov, A.G., Petrova, O.V., Ivanova, E.V., Epanchintseva, A.V., Svyatchenko, S.V., Maltsev, S.V., Marchenko, V.Y., Mikheev, V.N., Ryzhikov, A.B. & Ilicheva, T.N. (2017). [Analysis of population immunity against influenza prior to 2014 and 2015 epidemic seasons]. Zhurnal Mikrobiologii Epidemiologii i Immunobiologii, 53–60.
- Smith, G.J.D., Vijaykrishna, D., Ellis, T.M., Dyrting, K.C., Leung, Y.H.C., Bahl, J., Wong, C.W., Kai, H., Chow, M.K.W., Duan, L., Chan, A.S.L., Zhang, L.J., Chen, H., Luk, G.S.M., Peiris, J.S.M. & Guan, Y. (2009). Characterization of avian influenza viruses A (H5N1) from wild birds, Hong Kong, 2004–2008. Emerging Infectious Diseases, 15, 402–407. doi: 10.3201/eid1503.081190
- Soda, K., Ito, H., Usui, T., Nagai, Y., Ozaki, H., Yamaguchi, T. & Ito, T. (2013). Incursion and spread of H5N1 highly pathogenic avian influenza viruses among wild birds in 2010–11 winter in Japan. Journal of Veterinary Medical Science, 75, 605–612. doi: 10.1292/jvms.12-0512
- Soda, K., Usui, T., Uno, Y., Yoneda, K., Yamaguchi, T. & Ito, T. (2013). Pathogenicity of an H5N1 highly pathogenic avian influenza virus isolated in the 2010–2011 winter in Japan to mandarin ducks. Journal of Veterinary Medical Science, 75, 619–624. doi: 10.1292/jvms.12-0487
- Takagi, K. (2010). Expanding wintering distributions of rooks in Japan. Bird Research, 6, A13–A28.
- Tanimura, N., Tsukamoto, K., Okamatsu, M., Mase, M., Imada, T., Nakamura, K., Kubo, M., Yamaguchi, S., Irishio, W., Hayashi, M., Nakai, T., Yamauchi, A., Nishimura, M. & Imai, K. (2006). Pathology of fatal highly pathogenic H5N1 avian influenza virus infection in large-billed crows (Corvus macrorhynchos) during the 2004 outbreak in Japan. Veterinary Pathology, 43, 500–509. doi: 10.1354/vp.43-4-500
- Vijayakumar, P., Mishra, A., Ranaware, P.B., Kolte, A.P., Kulkarni, D.D., Burt, D.W. & Raut, A.A. (2015). Analysis of the crow lung transcriptome in response to infection with highly pathogenic H5N1 avian influenza virus. Gene, 559, 77–85. doi: 10.1016/j.gene.2015.01.016
- WHO/OIE/FAO Evolution Working Group. (2008). Toward a unified nomenclature system for highly pathogenic avian influenza virus (H5N1). Emerging Infectious Diseases, 14, e1.
- Xu, X., Subbarao, K., Cox, N.J. & Guo, Y. (1999). Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology, 261, 15–19. doi: 10.1006/viro.1999.9820
