ABSTRACT
Infection with a novel species of the genus Coxiella was first described in three Swainson’s blue mountain rainbow lorikeets from a zoological collection, and days later in a group of seven other psittacine birds and a toucan. We provide an update on coxiellosis in nine additional psittacines, and two non-psittacines. Psittacines originated in New England, the mid Atlantic, the Midwest, the South, and the Northwest. Psittacines most commonly had lesions in the brain, spleen, liver, and lungs, consisting of meningoencephalitis, hepatosplenomegaly, and interstitial pneumonia. Lesions contained histiocytic infiltrate, with intracytoplasmic, Gimenez-positive coccobacilli. Transmission electron microscopy revealed bacteria with trilaminar cell walls, electron dense cores, and spore-like forms. PCR revealed homology to the organism in index cases. In addition, one black-browed barbet and one paradise tanager were found with systemic coxiellosis; sequencing identified the same pathogen. These are the second piciforme and the first passerine affected by this pathogen, indicating expanded infectivity and pathogenicity.
RESEARCH HIGHLIGHTS
Report of coxiellosis in nine psittacines; lesions often in brain, spleen, liver, lung.
Second piciforme with coxiellosis, a black-browed barbet.
First case of avian coxiellosis described in a passerine, a paradise tanager.
Introduction
Coxiellosis has recently been described in psittacine birds. The first published report was of three Swainson’s blue mountain rainbow lorikeets raised in a zoological collection. All three birds were emaciated, with two developing progressive neurological signs (Woc-Colburn et al., Citation2008). Despite therapy, all three birds died. Gross lesions in two of these lorikeets were highlighted by hepatomegaly, while the third had splenomegaly. Microgranulomas were noted in the liver and spleen during histologic examination. In addition, microgranulomas were present in the brain, with lymphohistocytic perivascular encephalitis and vasculitis (Woc-Colburn et al., Citation2008). Some histiocytes within the brain parenchyma and the perivascular cuffs had displaced nuclei and smudged eosinophilic or amphophilic cytoplasmic inclusions that were azurophilic in Gimenez stains, and had a positive periodic acid-Schiff (PAS) reaction. Transmission electron microscopic (TEM) examination of these histiocytes revealed spherical to bacillary prokaryotes with trilaminar cell walls. Analysis by PCR revealed a novel species of Coxiella, most closely related to a symbiont bacterium of the brown dog tick (Rhipicephalus sanguineus) (Woc-Colburn et al., Citation2008). A similar, small, intracellular bacterium with a trilaminar membrane was implicated in disseminated inflammatory disease in seven unrelated psittacines and one toucan; the 16S rRNA gene of this organism was found to have 98% sequence identity homology to the same Rhipicephalus sanguineus symbiont as in the Swainson’s lorikeets (Shivaprasad et al., Citation2008). In the birds of this report, the most common lesion was necrotizing hepatitis with intralesional coxiella-like organisms, with histiocytic to lymphohistiocytic splenitis, interstitial pneumonia, nephritis, osteomyelitis, and adrenalitis also reported (Shivaprasad et al., Citation2008).
We present an update on avian coxiellosis via 11 novel cases.
Materials and methods
Ethical statement
All samples for this study were submitted by clinical veterinarians from birds that died naturally or were humanely euthanized due to severe, progressive clinical illness that was not responsive to treatment. All samples were therefore part of diagnostic case submissions and ethically obtained.
Selection of cases
All cases were submitted to the diagnostic service at Northwest ZooPath, Monroe, WA, USA. All diagnoses were confirmed by polymerase chain reaction as outlined below.
Gross pathology
Gross lesions noted by the submitting veterinarians were described in seven birds, all psittacines. No gross necropsies were performed at Northwest ZooPath, as all tissues were submitted fixed in formalin. Tissues submitted from psittacines included samples of the spleen (9), liver (9), kidney (9), brain (8), ventriculus (8), intestine (7), lung (7), pancreas (7), heart (6), adipose tissue (5), proventriculus (5), bone marrow (3), ganglia (3), trachea (3), oesophagus (2), skeletal muscle (2), testicle (2), adrenal (2), crop (2), thyroid (2), skin (1), epicardium (1), ovary (1), parathyroid (1), eyes (1), and cloaca (1).
In the barbet, the great vessels, heart, spleen, liver, brain, skeletal muscle, adrenal gland, testicle, ventriculus, proventriculus, kidney, adipose tissue, oesophagus, and trachea were submitted.
In the tanager, the adrenal gland, spinal cord, gastrointestinal tract, oesophagus, kidney, feathered skin, liver, lung, bone, heart, skeletal muscle, adipose tissue, vitelline diverticulum, ovary, oviduct, spleen, brain, eyes, nasal passages, lacrimal glands, vertebrae, and cloaca were submitted.
Histopathology
Selected tissues from each bird were fixed in 10% neutral buffered formalin for 3–5 days, processed routinely, sectioned at 5 µm, mounted on frosted glass slides, and stained with haematoxylin and eosin (H&E). Some sections also were stained routinely with PAS, Fite’s acid fast, and Gimenez techniques.
Transmission electron microscopy
Tissues were removed from paraffin-embedded blocks and deparaffinized. Samples were then fixed in a mixture of 2.5% glutaraldehyde + 2.5% paraformaldehyde in 0.1 M cacodylate buffer at 4°C for 24 h, postfixed in 1% osmium tetroxide and dehydrated in a graded acetone series. Samples were infiltrated and embedded in Poly/Bed 812 resin (Polysciences, Warrington, PA). Thin sections (70 nm) were obtained with a PTXL ultramicrotome (RMC, Boeckeler Instruments, Tucson, AZ, USA) on 200 mesh copper grids stained with uranyl acetate and lead citrate. Sections were imaged using a JEOL 100CX Transmission Electron Microscope (Japan) at a 100 kV accelerating voltage.
PCR technique
Genomic DNA was isolated from formalin-fixed paraffin-embedded samples using a commercial DNA extraction kit (QIAamp DNA FFPE Tissue Kit, Qiagen, Valencia, CA, USA). A PCR specific for avian Coxiella species was performed using previously described primers and methods (Shivaprasad et al., Citation2008). DNA from a previously sequenced lorikeet coxiellosis case from the Washington Animal Disease Diagnostic Laboratory was used as a positive control. Extracted sterile diethyl pyrocarbonate water was used as a negative control, and water was used as a no-template control for all PCR reactions. Amplification products were purified using the Freeze ‘N Squeeze™ DNA Gel Extraction Spin Column (BioRad, Hercules, CA USA) and were directly sequenced in both directions by an outside vendor (Genewiz, South Plainfield, NJ, USA). Sequences were compared with all sequences in the GenBank database using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) (Altschul et al., Citation1990). Multiple sequence alignments of the amplified 16S regions were constructed using ClustalW (http://www.ebi.ac.uk/clustalw/index.html) (Higgins et al., Citation1996). BLAST was used to analyse the consensus sequences.
Results
Signalment and clinical history
The signalment, clinical signs, and clinicopathological data of each case are shown in . The affected psittacines included three species of lorikeets, two species of macaws, two cockatiels, and two budgerigars. These birds lived in Ohio, New Jersey, New Hampshire, Rhode Island, Iowa, Tennessee, Washington, and North Carolina; the barbet was from Florida, and the tanager was from the same Ohio institution as the budgerigar in case 5. Clinical signs in psittacines included weakness or ataxia (5/9), weight loss or thin body condition (2/9), and feather loss or feather destructive behaviour (2/9). The barbet had progressive ataxia, while the paradise tanager had acute onset dyspnoea and died 3 h after the presentation. All four birds that had a complete blood count (CBC) performed were psittacines, and had moderate leukocytosis, characterized by moderate heterophilia (2/4), moderate lymphocytosis (2/4) or moderate monocytosis (1/4). Hypergammaglobulinaemia was noted in 2/4 birds. Ancillary laboratory work included negative PCR for Chlamydia sp. (4/9), and negative tests for polyomavirus (1/9), circovirus (1/9), paramyxovirus (1/9) and bornavirus (1/9). Medications administered to the birds included amphotericin B, enrofloxacin, tetracycline, clindamycin, terbutaline and meloxicam. Two psittacines died during intracoelomic biopsy while the other seven were euthanized. The barbet was euthanized because of progressive intractable ataxia, and the tanager died naturally soon after presentation.
Table 1. Signalment, clinical signs and clinicopathological findings in coxiellosis in nine psittacine birds, one black-browed barber, and one paradise tanager.
Necropsy findings
Gross necropsy information was available for seven cases, and is summarized in . Emaciation was noted in two of seven cases. Gross lesions were seen in the liver in 4/7 cases, and findings included rounded edges, mottling, and congestion. Splenomegaly was also noted in 3/7 cases, and was accompanied by renomegaly in one case. The lungs were grossly affected in one case, as were the pericardium and air sacs. One bird was noted to have a haemorrhagic duodenum. Gross evidence of meningitis was noted in one case ((1)).
Figure 1. (1) Coxiellosis in a 12-month-old, male budgerigar (case 5). Gross image of the fresh brain tissue, ventral view, showing alternating meningeal hyperaemia (h) and clouding (i; interpreted as inflammation). There is malacia (softening to liquefaction) of the cerebellum, resulting in an irregular surface (*). Photo courtesy of Lauren V Powers, DVM, DABVP. Service Head, Avian and Exotic Pet Service, Carolina Veterinary Specialists,12117 Statesville Road, Huntersville, North Carolina 28078. (2) Coxiellosis in a 12-month-old, male budgerigar (case 5). Haematoxylin and eosin stain, brain, 40×. Meningoencephalitis characterized by increased cellularity of the meninges (black arrow) and the white and grey matter of the brain itself (arrowheads). Bar = 65 µm. Inset – higher magnification showing an infected cell. 400×, bar = 15 µm. (3) Coxiellosis in a male Swainson’s lorikeet (case 6). Haematoxylin and eosin stain, lung, 400×. There is increased cellularity of the interstitium. There are multiple infected cells denoted by the white arrows. Bar = 30 µm. (4) Coxiellosis in a male Swainson’s lorikeet. Gimenez histochemical stain, 400×. The infectious organisms are azurophilic intracytoplasmic inclusions in the infiltrating macrophages. Bar = 30 µm.
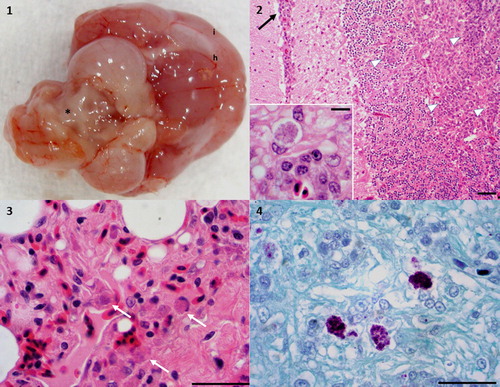
Table 2. Coxiellosis in nine psittacine birds, one black-browed barbet, and one paradise tanager.
Histological findings attributed to coxiellosis were those with either discrete granulomas, or those with dense histiocytic, lymphohistiocytic, or lymphoplasmacytic and histiocytic infiltrates. The granulomas consisted of well-delineated nodular aggregates of epithelioid macrophages, and these lesions were seemingly randomly distributed within the spleen, liver, and lungs. Similar granulomas were noted in the brain tissue, often adjacent to vasculature that was surrounded and infiltrated by activated macrophages. There was often lymphoplasmacytic perivascular infiltrate adjacent to areas of granulomatous infiltrate. Though the presence of intralesional, intracellular, eosinophilic cytoplasmic inclusions on histopathology was helpful, it was not a consistent finding. Lesions attributed to coxiellosis in psittacines included histiocytic to granulomatous meningoencephalitis (7/9) ((2)), splenitis (6/9), hepatitis (5/9), pneumonia (5/9) ((3)), proventriculitis/ventriculitis (3/9), aortitis (2/9), and single occurrences of myocarditis, visceral steatitis, enteritis, and dermatitis. In the barbet, there was coxiella-associated inflammation in the great vessels, heart, spleen, liver, and meninges. In the tanager, coxiella-induced adrenalitis, myelitis, and alimentary ganglioneuritis were noted.
Gimenez staining was positive in 4/8 cases in which it was run, negative in 1/8, and equivocal in 3/8 cases. Positive Gimenez staining identified variably sized azurophilic granules within histiocytic inclusions in brain tissue in two psittacines ((4)), the liver and spleen in one psittacine, the lung in one psittacine, and in the great vessels in the barbet. A Gimenez stain was not performed on tissues from the cockatiel in case 8, or the tanager.
Additional histological lesions are noted in , with the most prevalent in psittacines being extramedullary haematopoiesis (6/9), splenic or hepatic erythrophagocytosis (5/9), lymphoplasmacytic to plasmacytic periportal hepatitis (5/9), proliferative glomerulopathy (4/9), and plasmacytic splenitis (3/9). The barbet had adrenal inter-renal cell hypertrophy and vacuolation, and no additional lesions were noted in the tanager.
Concurrent infectious diseases detected in the psittacines included skeletal muscle sarcocystosis (1/9), cryptosporidiosis in the proventricular epithelial cells (1/9), and adenoviral nephritis (1/9). Chronic enteritis of undetermined cause was noted in all sections of all segments of the small intestine in four of nine cases (cases 1, 2, 3, and 6). The tanager had ureteral trematodiasis.
Transmission electron microscopy and PCR
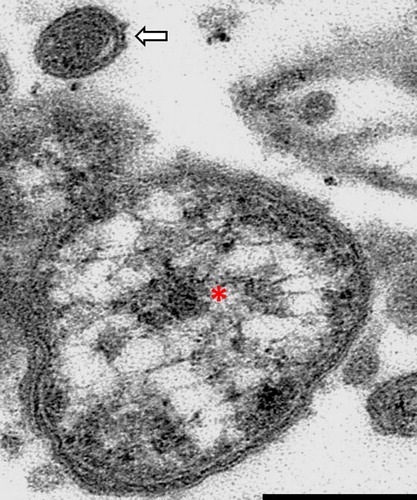
Transmission electron microscopy identified small coccobacilli (0.5–1.1 µm long, 0.2–0.5 µm wide), consistent with members of the genus Coxiella, within cytoplasmic parasitophorous vacuoles (). Two major hallmarks of members of the genus Coxiella are trilaminar cell membranes, and electron dense cores (). Occasionally a small cell variant was identified as an almost homogeneous, small, electron dense bacterial particle within the characteristic cell membrane (). Organisms were not found by TEM in three of the birds with characteristic lesions and a positive PCR result.
Figure 2. Coxiellosis in hyacinth macaw (case 2). Transmission electron micrograph, 14,000×. A macrophage in the spleen contains myriad intracellular bacteria, with peripheralization of the nucleus (N). The membrane of the parasitophorous vacuole (arrowhead) can be seen associated with bacteria (arrow), some of which have a characteristic electron dense core (asterisk). N – host cell nucleus. Bar = 0.5 µm.
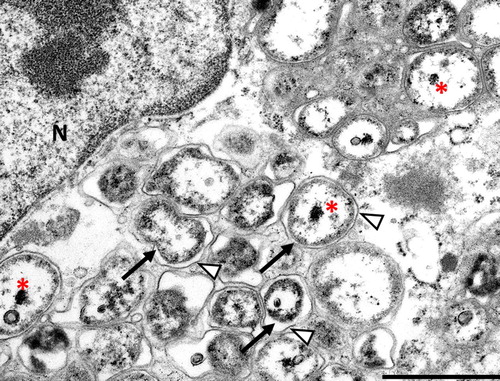
Figure 3. Coxiellosis in hyacinth macaw (case 2). Transmission electron micrograph, 19,000×. An aggregate of bacteria, showing a single large cell variant with electron lucent vacuoles and an electron dense core (asterisk), and a single small cell variant above it (arrow). The small cell variant is almost uniformly electron dense. Note the trilaminar wall of the bacteria. Bar = 0.15 µm.
All birds, including the barbet and tanager, had positive PCR results that were specific for the same novel Coxiella species identified in three Swainson’s blue mountain rainbow lorikeets (Woc-Colburn et al., Citation2008). PCR analysis resulted in the amplification of 232-nucleotide-long amplicons that were 100% identical to the 16S rRNA sequence of the Coxiella-like organism previously detected in hepatic tissue of a rosella (GenBank accession no EU143670). The avian Coxiella isolated from a parrot that is not part of our set, GenBank accession EU143669, is almost identical to that of the rosella isolate, but differs by a 6 base pair insertion. This insertion was not found in any of the birds in this study. No amplicons were obtained from samples from the negative control tissues or the water negative template control.
Discussion
We describe nine additional cases of coxiellosis in psittacine birds, as well as one piciforme and one passerine, all tentatively diagnosed via histopathology and histochemistry, and confirmed by molecular testing and TEM. This study highlights the diversity of affected psittacine species, widespread geographic distribution, the most common clinical signs and prominent lesions in psittacines, as well as indicating infectivity and pathogenicity outside of psittacines, in the orders Piciformes and Passeriformes.
Within the psittacines presented in our study, affected birds ranged from amongst the smallest (budgerigar) to amongst the largest (macaw) of the family. These cases suggest that coxiellosis must be considered in a differential diagnosis for any psittacine bird with weight loss, ataxia, leukocytosis, chronic enteritis, and other evidence of disseminated or multi-organ nonsuppurative inflammatory disease. Two of the submitted birds died during diagnostic procedures, highlighting the fact that these birds can be more compromised than they appear clinically. Medical treatment protocols included enrofloxacin, tetracycline, and clindamycin, none of which resulted in cure. During necropsy or exploratory coeliotomy, coxiellosis should be amongst the differential diagnoses for psittacine birds with gross lesions in either the liver or the spleen, though other infectious agents must also be considered. In birds with a histopathological diagnosis of lymphoplasmacytic, histiocytic encephalitis, splenitis, hepatitis, pneumonia, or proventriculitis/ventriculitis, Gimenez staining, followed by either PCR or transmission electron microscopy, may be helpful in identifying Coxiella infection. As suggested by equivocal results on Gimenez staining in three of eight cases where this stain was performed, the value of this stain as a diagnostic tool may be limited, with other stains such as Giemsa being potentially more appropriate. The data that we have generated indicate that PCR may be more sensitive (and less expensive) than electron microscopy for identification of Coxiella sp. in infected psittacine tissue, though nonrepresentative sample site selection, or the random distribution of organisms, may have biased these results.
It is interesting to note that there appear to be two genetically distinct populations of Coxiella sp. circulating within psittacine birds in the USA. As noted earlier, there is a recorded Coxiella sp. in a parrot for which the amplified segment of 16S ribosomal nucleic acid is identical to that reported in our cases and those noted in the index cases, but has a 6 base pair insertion. To our knowledge, this other case is the only one where the strain with the 6 base pair insertion has been identified. It is tempting to speculate as to the reasons for the existence of these two divergent populations, i.e. does it relate to emergence of altered pathogenesis or host specificity, or is it simply due to distinct origins and coincidental emergence (or detection) as a pathogen, with infection of each individual bird simply randomly due to exposure to one strain or the other?
Within our psittacines, the commonly identified lesions not consisting of granulomatous or histiocytic infiltrate were highlighted by lymphoplasmacytic to plasmacytic hepatitis or splenitis, a proliferative glomerulopathy, extramedullary haematopoieisis (hepatic, splenic, and renal), and hepatic or splenic erythrophagocytosis. These lesions were interpreted as representing organ-specific responses to systemically disseminated coxiellosis. The hepatitis and splenitis often involved plasma cells, and the observed proliferative glomerulopathy may have been a result of antigen–antibody complex deposition within the glomeruli. This constellation of lesions indicates that if there is evidence of non-specific lymphocytic or lymphoplasmacytic infiltrate in the liver or spleen, evidence of tubulointerstitial nephritis or glomerulopathy, or hepatic of splenic erythrophagocytosis in a psittacine bird, coxiellosis should be considered as a potential aetiology.
In addition to psittacines, our study includes the first report of a passerine bird affected by coxiella, and the second member of the order Piciformes. The previous piciform was a toucan that had coxiella-induced lesions in the liver and spleen (Shivaprasad et al., Citation2008). In addition, the toucan had lymphoplasmacytic endocarditis involving the left atrioventricular valve. There are few reports of significant disease in the barbet and tanager, or other birds in their genera; most of what is described relates to ectoparasites and endoparasites (Adkesson et al., Citation2005; Rotstein et al., Citation2005; Constantinescu et al., Citation2014; Delaski et al., Citation2015). As in the psittacines, the barbet also had lesions in the liver and spleen, with additional lesions in the great vessels, heart, and meninges. By comparison, in what appears a distinct lesion due to coxiellosis, the tanager had lesions restricted to ganglioneuritis and myelitis. The broader significance of this neurotropic phenotype in this single passerine bird is not clear. What is perhaps clearer is the ability of this Coxiella species to infect non-psittacine birds. While there is no case record of avian coxiellosis where the black-browed barbet came from, the paradise tanager came from the same facility as the budgerigar of case 5. This tanager was born roughly two years after the budgerigar died. This implies both the possibility of interspecies transmission, and the potential of subclinical or undiagnosed persistence in institutional birds or in the native wild bird population. Further, it may indicate the potential for spread of the agent from a zoo bird with outdoor access to native passerine birds. It is possible that, in all cases in our series, the agent originated from wild birds that came into contact with captive birds that had environmental exposure.
Though unlikely based on the disparate geographic origin of the 11 birds we report, there does exist the potential for contamination of a nationally distributed, common food or environmental object. Based on the obligate intracellular lifecycle of Coxiella bacteria, and the taxonomic proximity to other vector-borne bacteria, it is possible, but unlikely, that this novel bacterium is transmitted via ticks, or other biting insect vectors. As an emerging pathogen affecting a long-lived bird that is often housed in groups prior to purchase or adoption, and then lives within the house in close contact with people and other birds, this agent is of substantial epidemiologic concern. The increased host ranges make ongoing research more important. Further studies should include isolation of the agent in culture, experiments to fulfil Koch’s postulates, and further epidemiological and molecular characterization of the two distinct genetic populations of Coxiella sp. in psittacines. In addition, investigations into transmission between psittacines, passerines, piciformes, and domesticated fowl are warranted, as are studies investigating prevalence in wild birds, and the potential of infection in non-avian hosts.
Acknowledgement
The authors would like to acknowledge Ralph Common, Center for Advanced Microscopy, Michigan State University, for assistance with TEM sample preparation and image acquisition.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
David B. Needle http://orcid.org/0000-0002-7215-5306
References
- Adkesson, M.J., Zdziarski, J.M. & Little, S.E. (2005). Atoxoplasmosis in Tanagers. Journal of Zoo and Wildlife Medicine, 36, 265–272. doi: 10.1638/03-091.1
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410. doi: 10.1016/S0022-2836(05)80360-2
- Constantinescu, I.C., Chişamera, G., Mukhim, D.K.B. & Adam, C. (2014). Two new species of feather mites (Acarina: Psoroptidia) from the Great Barbet, Psilopogon virens (Piciformes: Megalaimidae). Zootaxa, 3893, 127–142. doi: 10.11646/zootaxa.3893.1.6
- Delaski, K.M., Nelson, S., Dronen, N.O., Craig, T.M., Pond, J. & Gamble, K.C. (2015). Detection and management of air sac trematodes (Szidatitrema species) in captive multispecies avian exhibits. Journal of Avian Medicine and Surgery, 29, 345–353. doi: 10.1647/2015-085
- Higgins, D.G., Thompson, J.D. & Gibson, T.J. (1996). Using CLUSTAL for multiple sequence alignments. Methods in Enzymology, 266, 383–402. doi: 10.1016/S0076-6879(96)66024-8
- Rotstein, D.S., Flowers, J.R., Wolfe, B.A. & Loomis, M. (2005). Renal trematodiasis in captive double-toothed barbets (Lybius bidentatus). Journal of Zoo and Wildlife Medicine, 36, 124–126. doi: 10.1638/03-119
- Shivaprasad, H.L., Cadenas, M.B., Diab, S.S., Nordhausen, R., Bradway, D., Crespo, R. & Breitschwerdt, E.B. (2008). Coxiella-like infection in psittacines and a toucan. Avian Diseases, 52, 426–432. doi: 10.1637/8192-120707-Reg
- Woc-Colburn, A.M., Garner, M.M., Bradway, D., West, G., D’Agostino, J., Trupkiewicz, J., Barr, B., Anderson, S.E., Rurangirwa, F.R. & Nordhausen, R.W. (2008). Fatal coxiellosis in Swainson’s Blue Mountain Rainbow Lorikeets (Trichoglossus haematodus moluccanus). Veterinary Pathology, 45, 247–254. doi: 10.1354/vp.45-2-247
