ABSTRACT
Newcastle disease (ND) is an infectious viral poultry disease with great economic consequences. In developing countries, outbreaks of ND caused by virulent Newcastle disease virus (NDV) have been identified as a limiting factor to the growth of the poultry industry. Limited reports exist on the pathology of natural field infection caused by NDV genotype XVII in chickens. Here, we present clinical, pathological and molecular investigation of confirmed ND in a 24-week-old layer-type, semi-intensive poultry flock with recorded mortality of over 50%. During PM examination, tissues were harvested for virus isolation, histopathology and immunohistochemistry. Virus isolation was performed in 10-day-old embryonated chicken eggs, and a haemagglutinating agent thereof identified by one-step reverse transcription-polymerase chain reaction (RT–PCR). For the genotyping of the isolate, the full fusion gene was sequenced. Clinical signs observed included general body lethargy, inappetence and greenish diarrhoeic faeces from the cloaca before death with daily mortality exceeding 100 chickens. The pathology was characteristic of a viral haemorrhagic infection, with serosal haemorrhages, mucosal surface erosion and ulceration. In most of the carcasses, the main lesions seen included airsacculitis, meningeal congestion, haemorrhagic oophoritis, pancreatic necrosis, enteritis and faecal matting of the vent. Virus isolation and RT–PCR made a confirmatory diagnosis of ND. Based on the cleavage site motif sequence (112RRQKR/F117), the isolate was identified as a virulent strain with phylogenetic analysis showing clustering in genotype XVII viruses. To the best of the authors’ knowledge, this is the first report describing the pathological findings of a natural outbreak caused by NDV involving viruses of genotype XVII.
RESEARCH HIGHLIGHTS
First report of a natural outbreak of Newcastle disease in White Yarkon Leghorns.
The outbreak was caused by virulent NDV belonging to genotype XVII.
Pathology differed slightly from those in experimental studies using SPF and other unvaccinated chickens.
Introduction
Newcastle disease (ND) caused by virulent Newcastle disease virus (NDV) is an infectious poultry disease with great economic consequences. NDV, also referred to as Avian paramyxovirus 1, belongs to the species Avian orthoavulavirus 1 (AOAV-1), genus Orthoavulavirus, subfamily avulavirinae, and family Paramyxoviridae (Amarasinghe et al., Citation2019; ICTV, Citation2019). To date, two classes of AOAV-1 have been identified; class I and II. NDV belonging to class II are highly diverse with at least 21 genotypes (Dimitrov et al., Citation2016). Infection with the virus presents different clinical signs depending on several factors which include, but not limited to, the infecting virus strain, immune status of the birds, host species, age of the host, co-infection with other pathogens and environmental factors (Alexander, Citation2000). Based on clinical signs in unvaccinated chickens, five pathotypes have been identified: neurotropic velogenic (high mortality with respiratory and neurological disorders), viscerotropic velogenic (high mortality with haemorrhagic lesions), mesogenic (low mortality with respiratory and neurological signs), lentogenic (mild respiratory infections) and asymptomatic (inapparent infections) (Alexander, Citation2000; OIE, Citation2018).
The first confirmation of ND in Nigeria was in 1953 in Ibadan, southwestern Nigeria (Hills et al., 1953). Subsequently, evidence of the disease was reported across the entire country, and it has since become enzootic (Shittu et al., Citation2016). Over the years, ND has caused devastation of the Nigerian poultry industry. The effect of the devastation has resulted in many farmers abandoning poultry business which has greatly affected their source of livelihood (authors’ personal observation). In general, there are numerous papers describing ND outbreaks in Nigeria, but there are not many describing pathology in natural field infection in unvaccinated birds. In a 3-year prospective study, it was revealed that outbreaks occur regularly with the highest prevalence recorded in the dry harmattan season (November to February) which was attributed to stress and cold weather conditions (Okwor & Eze, Citation2010).
Of the 21 NDV genotypes circulating worldwide, seven genotypes (I, II, IV, VI, XIV, XVII and XVIII) have been identified in Nigerian poultry with genotype XVII being reported as the predominant genotype in Northern Nigeria (Shittu et al., Citation2016; Welch et al., Citation2019). Though molecular characterizations of the viruses have been well documented (Snoeck et al., Citation2013; Shittu et al., Citation2016; Welch et al., Citation2019), the clinical presentation and pathology of genotype XVII in the field still remain largely unexplored.
The aim of this study was to describe the pathological findings of a natural outbreak caused by NDV genotype XVII under field conditions in an unvaccinated White Yarkon Leghorn chicken flock.
Materials and methods
Case history
A poultry farm in Vom (longitude 9.7269697/ 9°43′ 37″N and latitude 8.7890627/ 8°47′ 21″ E), Jos south local government area of Plateau state Nigeria, that supplies eggs from unvaccinated and antibiotic-free birds to academic and research institutions for research purposes, recorded high mortality (over 50%) in 24-week old unvaccinated White Leghorn layer and cockerel chickens. The initial flock size prior to the outbreak was 1229 chickens.
Post mortem examination and gross pathology
For post mortem examination, a total of 60 carcasses of layer hens and cockerels were submitted. Sections of liver, spleen, lung and trachea were removed for virus isolation. Gross pathological examination was performed in all the submitted carcasses. For histopathology and immunohistochemistry (IHC), the heart, proventriculus, duodenum, ileum, aecum, pancreas, airsac and brain were collected, in addition to tissues for virus isolation, and fixed in 10% buffered formalin for 48–72 h.
Histopathology and immunohistochemistry
The formalin fixed tissues were embedded in paraffin, sectioned at 5 µm, mounted on clean glass slides, and stained with haematoxylin and eosin for examination using low and high powered field of an Axio Imager A1 binocular microscope (Carl Zeiss®, Germany).
For IHC, sections were mounted on charged microscope slides (Menzel, Braunschweig, Germany), dewaxed and rehydrated. To detect Newcastle disease virus antigen, sections were incubated with an in-house rabbit anti-NDV serum in a dilution of 1:500 in Tris-buffered saline (TBS, 0.1 M Tris-base, 0.9% NaCl, pH 7.6). A biotinylated goat anti-rabbit IgG1 (Vector, Burlingame, CA, USA; diluted 1:200 in TBS) was used as a linker-antibody for the avidin–biotin-complex (ABC) method. As a negative control, the pre-immunization serum of the same rabbit was applied. Positive samples produced a bright red signal with an IHC kit (Vectastain Elite ABC Kit, Vector, Burlingame, CA, USA) and the substrate 3-amino-9-ethyl carbazole (DAKO AEC substrate-chromogen system; Dako, Carpinteria, CA, USA). The sections were counterstained with Mayer’s haematoxylin and sealed with an aqueous medium (Aquatex; Merck, Darmstadt, Germany). Positive and negative control tissues of chicken originating from animal experiments were included for each IHC procedure as described previously (Akanbi et al., Citation2017).
Virus isolation and identification
To isolate the pathogen(s) involved in the outbreak, tissue samples (liver, spleen, lung, trachea) from five dead birds were processed as a pool according to standard protocol (OIE, Citation2018). In brief, a 20% homogenate of the pooled tissues was prepared in phosphate-buffered saline (pH 7.2) containing penicillin (2000 units/ml), streptomycin (2 mg/ml), gentamycin (50 µg/ml) and amphotericin B (5 mg/ml). The homogenate was centrifuged at 1000×g for 10 min, and 0.2 ml of the supernatant was inoculated via the allantoic cavity into 10-day-old embryonated chicken eggs obtained from specific antibody-negative chickens. Inoculated eggs were incubated in a humidified chamber at 37°C with daily candling. Any dead embryos after 24 h post-inoculation were chilled at 4°C and allantoic fluid (ALF) harvested. To exclude bacterial contamination, the ALF was streaked on a blood agar plate. Bacteria-free ALF was subsequently tested in haemagglutination (HA) and haemagglutination-inhibition (HI) assays using NDV specific antiserum (Charles River Laboratory, South San Francisco, CA, USA).
Nucleic acid extraction, RT–PCR and sequencing
Total RNA was extracted from the ALF using QIAmp viral RNA mini kit (Qiagen, Hilden, Germany) following the manufacturer’s recommended procedure. For the detection of the isolate full fusion gene (F-gene), two pairs of primers (NDV-F1: TGCGGAGTGTGAAAGTCATCATT; NDV-R1: TGCTGAGGCAAACCCTTTGT; NDV-F2: ATTGGTAGCGGCTTGATCACTG; NDV-R2: CGTTCTACCCGTGTACTGCTCTTT) were designed based on the sequence of NDV isolate (2008_Mali_ML007_08; JF966385.1) using Primer3 (http://simgene.com/Primer3). To amplify the full F-gene, one-step RT–PCR (Qiagen) was performed in a 25 µl reaction mixture containing 5.0 µl of 5x PCR buffer, 1.0 µl dNTP mix (10 mM each), 1.0 µl of each primers (10 µM), 0.5 µl RNase Inhibitor (40 U/µl, Promega, Madison, WI, USA), 0.5 µl RT–PCR Enzyme Mix, 5.0 µl of RNA and nuclease-free water to make up to the final volume. The reaction was performed on a GeneAmp PCR system 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA) under the following cycling conditions: 50°C at 30 min, 94°C for 15 min; 40 cycles of 94°C for 30 s, 55°C for 1 min and 68°C for 2 min; and a final extension at 68°C for 10 min. The PCR amplicons were analysed by gel electrophoresis with 1.5% agarose and visualized using a Gel Documentation system (Biostep, Burkhardtsdorf, Germany). To exclude co-infection with other respiratory viruses, RNA extract from the positive ALF was tested for the matrix gene of avian influenza virus (Fouchier et al., Citation2000) and infectious bronchitis virus (Callison et al., Citation2006).
The amplicons were purified using the QIAquick PCR Purification kit (Qiagen, Germany). The purified amplicons were directly sequenced in both directions on an ABI3730XL sequencer (Applied Biosystems) by Macrogen Inc., Korea.
Nucleotide assembly, alignment and phylogenetic analysis
A consensus sequence was generated from both forward and reverse sequences using BioEdit 7.2.5 (Hall, Citation1999). The sequence of the isolates was aligned and BLAST conducted using ClustalW in MEGA 6.0 software (Tamura et al., Citation2013). To determine the genetic relatedness of the isolates, a phylogenetic tree was constructed using the Maximum Likelihood method and General Time Reversible model in MEGA 6.0 (Tamura et al., Citation2013) with other full F-gene sequences retrieved from GenBank representing major genotypes circulating in Nigeria.
GenBank accession number: The full F-gene sequence of the isolate was deposited to GenBank under the accession number MN043960.
Ethical approval statement
The sampling and post mortem examination of dead birds were approved by the Animal Use and Care Committee, and conducted in compliance with the Guidelines for Care and Use of Animals at the National Veterinary Research Institute, Vom, Plateau State, Nigeria.
Results
Clinical signs
These mixed-sex, unvaccinated White Leghorn chickens exhibited lethargy, inappetence and passed out greenish diarrhoeic faeces before death. Over a 5-day period, 626 birds died, from the initial flock size of 1229. The remaining 603 birds were depopulated on the fifth day. Daily carcass submission was above 20 ((a)). Also, a temporal increase in the severity of clinical signs and mortality was observed during the first 4 days ().
Figure 1. (a) Carcasses from the outbreaks showing high mortality. (b) Conjunctival and corneal congestion with comb and wattle congestion. (c) Greenish-yellow faecal pasting of the vent in carcasses. (d) Diffuse pancreatic necrosis. (e) Proventriculo-ventricular junction ecchymotic haemorrhages. (f) Testicular vascular congestion, severe. (g) Ovarian follicular congestion with ecchymotic haemorrhages and egg yolk peritonitis.
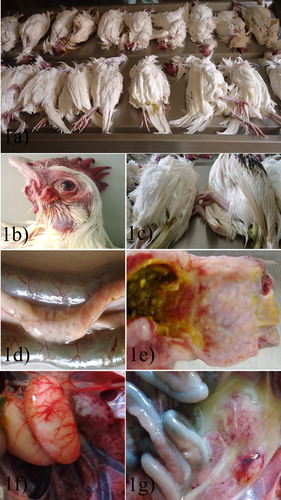
Table 1. Chronological events of the outbreak, clinical signs, and mortality.
Gross pathology
The carcasses appeared to be in good bodily condition but were dehydrated ((a)). The conjunctiva and cornea were congested ((b)) and the carcasses had greenish-yellow faecal matting of the vent ((c)). There was congestion of the comb and wattles while some were pale. Diffuse patchy necrosis was observed in the pancreas ((d)). There were ecchymotic haemorrhages on the proventriculo-ventricular junction ((e)). Testicular vascular congestion was observed in some carcasses ((f)). Egg yolks were broken leading to peritonitis ((g)) in most of the carcasses. Some of the carcasses had follicular congestion and ecchymotic haemorrhage. The gross lesion findings are presented in .
Table 2. Distribution and intensity of gross and histological lesions with immunohistochemical (IHC) staining for NDV nucleoprotein.
Histopathology and immunohistochemistry
The meninges were expanded by infiltration of inflammatory mononuclear cells and vascular congestion ((a)). Also, cerebral neurons showed central chromatolysis, and pyknosis, and the grey matter was infiltrated by lymphocytes. Some of the neurons had small, 8 µm in diameter, 1–3 eosinophilic intranuclear inclusions, while some of the neurons were necrotic. Formation of perivascular lymphocytic cuffs is frequently observed ((b)). Pancreatic acinar cells were necrotic ((c)). Splenic lymphoid follicles were diffusely necrotic and often destroyed ((d)). There were severe intestinal laminar propria and cryptic necrosis with mononuclear inflammatory cell infiltration ((e, f)). There was ganglioneuritis in the intestinal wall ((e) inset) with lymphoplasmacytic infiltration. The mucosal-associated lymphoid tissues (MALT) in the ileum showed necrosis and depletion. Other histopathological lesions are represented in .
Figure 2. (a) Meningoencephalitis with severe lymphocytic cellular infiltration. (b) Encephalitis with formation of perivascular lymphocytic cuffs. (c) Pancreatic acinar cells are diffusely necrotic with obliteration of islands of Langerhans. (d) Splenic lymphoid follicular necrosis. (e) Enteritis, with severe lymphocytic cellular infiltration. (f) Ganglioneuritis with lymphoplasmacytic infiltration in the intestinal wall, H &E.
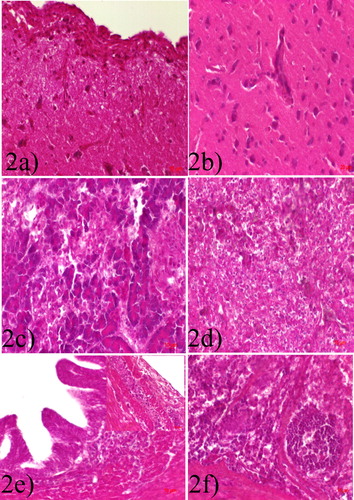
Figure 3. (a) Airsac, high intranuclear and intracytoplasmic staining in cells. (b) Meningeal epithelial lining cells intranuclear and intracytoplasmic staining. (c) Cerebral and meningeal epithelial lining cells intranuclear and intracytoplasmic staining, moderate. (d) Pancreas, severe intranuclear and intracytoplasmic staining in acinar cells X100, IHC.
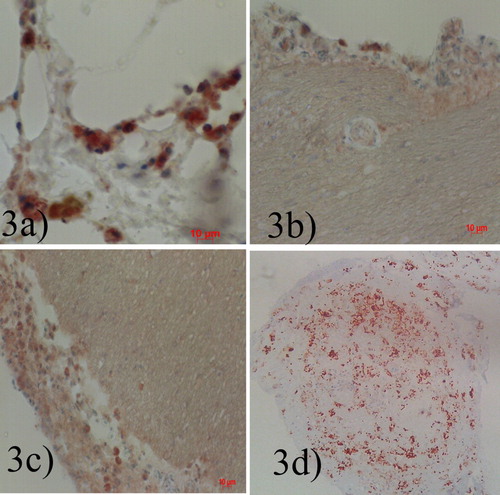
Immunohistochemical stain against Newcastle disease virus nucleoprotein showed brick-red or brown staining in several tissues including meningeal epithelial cells and hepatocytes. Detection of other cell types by IHC is represented in as represented in (a–d).
Virus isolation and identification
All embryonated eggs inoculated with the homogenate died within 48 h of inoculation. The HA titre of the ALF harvested from the dead embryos was log28. The haemagglutinating agent was inhibited by NDV specific antiserum in the HA/HI test identifying the isolate as NDV which was designated, chicken/Nigeria/VRD14.12/2014 (VRD14.12). No growth was observed on blood agar streaked with the ALF.
Pathotyping and genotyping
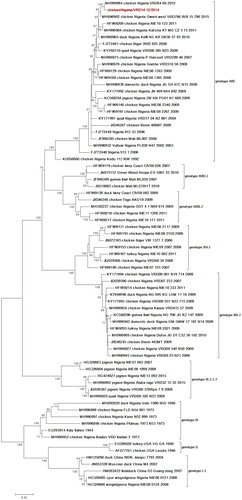
The BLAST hit result of the nucleotide sequence revealed closest identity (99.8%) of the isolate with NDV previously isolated in Nigeria (chicken/Nigeria/VRD64/66/2012; MH996964.1) belonging to genotype XVII. Multiple basic amino acids (112RRQKR/F117) were observed at the cleavage site of the isolate which is a characteristic of virulent ND viruses. A phylogenetic tree based on the full F-gene sequence of the isolate with other sequences retrieved from GenBank showed clustering with contemporary class II genotype XVII viruses (). Using the Maximum Composite Likelihood model, the nucleotide sequence similarities of VRD14.12 with those of NDV genotype XVII viruses used in the phylogenetic tree reconstruction ranged from 99.8 (closest to chicken/Nigeria/VRD64/66/2012) to 94.2% (distant to duck/Nigeria/KUDU 113/N56/1992).
Figure 4. Phylogenetic tree of Newcastle disease virus based on the complete fusion gene. The tree was constructed using Maximum Likelihood in MEGA 6 with 500 boostrap replicates to assign confidence to the groupings. The tree with the highest log likelihood (−9569.8326) is shown. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The isolate in this study is highlighted in red. Colour online.
Discussion
The present study investigated and isolated virulent NDV, now known as Avian orthoavulavirus 1 (Amarasinghe et al., Citation2019; Butt et al., Citation2019), from an outbreak in an unvaccinated White Leghorn layer-type chicken flock. The isolate exhibited virulence in the White Leghorn typical for viscerotropic velogenic pathotype, with high mortality and haemorrhagic lesions as described previously (Alexander, Citation2000; OIE, Citation2018). This pathotype of NDV has been found to be the most common and enzootic in Africa, Middle and the Far-East, but also in countries in Central and South America (Echeonwu et al.,Citation1993; Liu et al., 2003; Ponman et al., Citation2012). In Africa, ND is enzootic and is responsible for large economic losses. According to reports, an estimated 78,526 outbreaks of ND were reported across Nigeria in 2008 alone, with an estimated financial burden of $24.72 million (8.9 billion naira) for local chickens alone (Fadiga et al., Citation2013; Shittu et al., Citation2016).
In this study, the hallmark of the chronological events of the outbreak leading up to death was severe lethargy exhibited by these birds in addition to anorexia and greenish diarrhoeic faeces. Over a 5-day period, 626 birds died with a mortality rate of 50.94%. The lack of characteristic clinical signs in many bird species, infected with AOAV-1 isolates is known to pose a serious challenge for the rapid identification and diagnosis of the infection by this virus (OIE, Citation2008). There was a temporal increase in the severity of clinical signs and mortality during the first 4 days (). Although neurotropic lesions were seen during the pathological examination, the birds did not develop clinical signs of a neurological disorder as would have been seen in a velogenic neurotropic strain (Susta et al., Citation2015; Butt et al., Citation2019). An investigation by Susta et al. (Citation2015) on the clinical signs and pathology of genotype XVII in specific pathogen-free (SPF) birds reported severe clinical signs and death within 4 days post-infection with NDV genotype XVII, a finding consistent with the present study. However, in this field outbreak, more than 50% mortality was recorded within a 4-day period. This therefore suggests that mortality is not influenced by age of the chickens at infection and that the severity of the infection may largely be attributed to the unvaccinated status of these birds. It is known that the wide array of clinical signs in Newcastle disease depends on both strain virulence and host-related factors, like host species infected, immunologic status, and the presence of coinfections in the host (Cattoli et al., Citation2011).
Gross pathological findings from the study of Susta et al. (Citation2015) on the genotype XVII in SPF birds with the presence of conjunctiva inflammation, splenomegaly and pancreatic necrosis were consistent with our observations. These findings are typical for viscerotropic velogenic pathotype (Alexander, Citation2000; OIE, Citation2018), although we found lesions typical of a neurotropic velogenic virulent strain of AOAV-1 in the brain of these chickens. In addition, pneumonia and airsacculitis seen in these chickens may be due to opportunistic copathogens (Escherichia coli and Klebsiella spp.) which were isolated from the lungs and peritoneum on media (data not shown). The histopathologic lesions of meningoencephalitis with perivascular cuffs of lymphoplasmacytic cellular infiltrates in the cerebrum and neuronal necrosis, including lymphoid tissue necrosis, were in agreement with our findings. Other lesions seen in our study that are not consistent with previous studies include follicular and testicular haemorrhages which may be attributed to the fact that these chickens are of laying age as compared to what was observed in young chickens which do not have developed reproductive organs.
Also, viscerotropic lesions were more severe in these adult birds, as seen in the proventriculus () and intestine (), while neurotropic lesions were more prominent in these birds as observed in the meninges and cerebrum ((a, b)). In an experimental study using a Nigerian strain of the velogenic viscerotropic NDV known as duck/Nigeria/903/KUDU–113/1992 (Echeonwu et al., Citation1993) belonging to NDV class II, genotype XVII (Shittu et al., Citation2016) to challenge NDV antibody negative 10-week-old broilers and pullets, severe clinical signs which included depression, greenish diarrhoea and inappetence were observed in the birds 2 and 3 days post-infection (Onyema et al., Citation2019) which were consistent with our findings. However, higher (75%) mortality was recorded in the experimental study (Onyema et al., Citation2019). Elsewhere, infection of 2-week-old chickens with NDV isolates from West Africa resulted in 100% mortality within 4 days (Samuel et al., Citation2013), whereas the mortality rate was 50.94% in this current study, corroborating the effect of age on the pathogenicity of NDV (Alexander, Citation2000). This invariably implies that severity of Avian orthoavulavirus 1 infection increases with decreasing age. A temporal comparison with the study of Samuel et al. (Citation2013) is in disagreement with our finding of signs of weakness, depression and diarrhoea which led to a high number of deaths as the infection progressed in these 24-week-old layers, whereas in 2-week-old chicks, no clinical signs were seen 3–4 dpi before death. Pathological lesions of proventricular haemorrhage, catarrhal, and haemorrhagic enteritis seen by Onyema et al. (Citation2019) were also consistent with our study. However, other than the similar lymphoid lesions, no detailed histopathology was given in their report.
Virus localization in tissue as seen by IHC detection was similar to previous study with genotype XVII under experimental condition (Susta et al., Citation2015), especially with the meninges, airsacs, enterocytes and other parenchymatous tissues. Although the pneumonia seen in our study was not seen in the young SPF chickens of Susta et al. (Citation2015), IHC detected NDV genotype XVII in respiratory tissues such as nasal turbinates, airsac, trachea, larynx and also the lung which was attributed to systemic viral distribution.
The farm under investigation rears unvaccinated layer-type chickens, i.e. a no vaccination and no drug policy is practiced because the farm supplies academic and research institutions with antibody-free eggs for research purposes. This no vaccination policy was responsible for the severity of the infection in these chickens.
It is obvious from the result of the pathological lesions confirmed by virus isolation and sequencing of the full F-gene that the outbreak was caused by Avian orthoavulavirus 1 belonging to genotype XVII. Though the possibility of concurrent infections with other avian viruses (infectious bronchitis virus [IBV], avian influenza virus [AIV]) is plausible, further testing with specific primers for AIV and IBV yielded negative results (data not shown). Genotype XVII has been identified as the predominantly circulating genotype (Shittu et al., Citation2016; Welch et al., Citation2019) along with the other previously described genotypes (Ponman et al., Citation2012; Snoeck et al., Citation2013) in Nigeria. Also, this genotype XVII has been circulating in Nigeria since 2006, Ivory Coast 2007, in Niger and Burkina Faso since 2008 (Susta et al., Citation2015), Cameroon, Mali and Central African Republic, 2008 and Benin, 2009 (Snoeck et al., Citation2013); all of these countries share international borders with each other. It is possible that this genotype might have spread from Nigeria to the other West African countries through international movement and trade.
This study, therefore, has elucidated the clinical, pathological, and molecular characterization of Newcastle disease from a natural outbreak in an unvaccinated layer-type farm with White Yarkon Leghorn chickens in Plateau State, Nigeria. To the best of the authors’ knowledge, this is the first report describing a natural outbreak of ND involving viruses of genotype XVII in White Yarkon Leghorns.
Acknowledgement
We the authors will like to acknowledge the excellent technical assistance of Gyang Dung and Gabriele Czerwinski.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Akanbi, B.O., Fereidouni, S., Taiwo, V.O., Starick, E., Okewole, P.A., Binder, A., Heenemann, K. & Teifke, J.P. (2017). Formalin-fixed and paraffin-embedded tissues of chickens are useful for retrospective studies on pathology of H5N1 highly pathogenic avian influenza virus outbreaks in Nigeria. Nigerian Veterinary Journal, 38, 1–10.
- Alexander, D.J. (2000). Newcastle disease. British Poultry Science, 42, 5–22. doi: 10.1080/713655022
- Amarasinghe, G.K., Ayllón, M.A., Bào, Y., Basler, C.F., Bavari, S., Blasdell, K.R., Briese, T., Brown, P.A., Bukreyev, A., Balkema-Buschmann, A., Buchholz, U.J., Chabi-Jesus, C., Chandran, K., Chiapponi, C., Crozier, I., de Swart, R.L., Dietzgen, R.G., Dolnik, O., Drexler, J.F., Dürrwald, R., Dundon, W.G., Duprex, W.P., Dye, J.M., Easton, A.J., Fooks, A.R., Formenty, P.B.H., Fouchier, R.A.M., Freitas-Astúa, J., Griffiths, A., Hewson, R., Horie, M., Hyndman, T.H., Jiāng, D., Kitajima, E.W., Kobinger, G.P., Kondō, H., Kurath, G., Kuzmin, I.V., Lamb, R.A., Lavazza, A., Lee, B., Lelli, D., Leroy, E.M., Lǐ, J., Maes, P., Marzano, S.-Y.L., Moreno, A., Mühlberger, E., Netesov, S.V., Nowotny, N., Nylund, A., Økland, A.L., Palacios, G., Pályi, B., Pawęska, J.T., Payne, S.L., Prosperi, A., Ramos-González, P.L., Rima, B.K., Rota, P., Rubbenstroth, D., Shī, M., Simmonds, P., Smither, S.J., Sozzi, E., Spann, K., Stenglein, M.D., Stone, D.M., Takada, A., Tesh, R.B., Tomonaga, K., Tordo, N., Towner, J.S., van den Hoogen, B., Vasilakis, N., Wahl, V., Walker, P.J., Wang, L.-F., Whitfield, A.E., Williams, J.V., Zerbini, F.M., Zhāng, T., Zhang, Y.-Z. & Kuhn, J.H. (2019). Taxonomy of the order Mononegavirales: update 2019. Archives of Virology, 164, 1967–1980. doi: 10.1007/s00705-019-04247-4
- Butt, S.L., Moura, V.M.B.D., Susta, L., Miller, P.J., Hutcheson, J.M., Cardenas-Garcia, S., Brown, C.C., West, F.D., Afonso, C.L. & Stanton, J.B. (2019). Tropism of Newcastle disease virus strains for chicken neurons, astrocytes, oligodendrocytes, and microglia. British Medical Council Veterinary Research, 15, 317–327.
- Callison, S.A., Hilt, D.A., Boynton, T.O., Sample, B.F., Robison, R., Swayne, D.E. & Jackwood, M.W. (2006). Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. Journal of Virological Methods, 138, 60–65. doi: 10.1016/j.jviromet.2006.07.018
- Cattoli, G., Susta, L., Terregino, C. & Brown, C. (2011). Newcastle disease: a review of field recognition and current methods of laboratory detection. Journal of Veterinary Diagnostic Investigation, 23, 637–656. doi: 10.1177/1040638711407887
- Dimitrov, K.M., Ramey, A.M., Qiu, X., Bahl, J. & Afonso, C.L. (2016). Temporal, geographic, and host distribution of avian paramyxovirus 1 (Newcastle disease virus). Infection, Genetics and Evolution, 39, 22–34. doi: 10.1016/j.meegid.2016.01.008
- Echeonwu, G.O.N., Ireogbu, C.I. & Emeruwa, A.C. (1993). Recovery of velogenic Newcastle disease virus from dead and healthy free-roaming birds in Nigeria. Avian Pathology, 22, 383–387. doi: 10.1080/03079459308418928
- Fadiga, M., Jost, C. & Ihedioha, J. (2013). Financial costs of disease burden, morbidity and mortality from priority livestock diseases in Nigeria. Disease burden and cost-benefit analysis of targeted interventions. ILRI Research Rep. Nairobi, Kenya, pp. 1–84.
- Fouchier, R.A., Bestebroer, T.M., Herfst, S., Van Der Kemp, L., Rimmelzwaan, G.F. & Osterhaus, A.D.M.E. (2000). Detection of influenza A viruses from different species by PCR amplification of conserved sequences in the matrix gene. Journal of Clinical Microbiology, 38, 4096–4101. doi: 10.1128/JCM.38.11.4096-4101.2000
- Hall, T. (1999). Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows during 1885–2001. Archives of Virology, 148, 1387–1403.
- OIE, World Organization for Animal Health. (2008). Newcastle disease. In OIE Biological Standards Commission, Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 6th edn (Chapter 2.3.14, pp. 576–589). Paris: OIE.
- OIE, World Organization for Animal Health. (2018). Newcastle disease. In OIE Biological Standards Commission, Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (pp. 969–971). Paris: OIE.
- Okwor, E.C. & Eze, D.C. (2010). The annual prevalence of Newcastle disease in commercial chickens reared in Southeastern savannah zone of Nigeria. Research Journal of Poultry Science, 3, 23–26. doi: 10.3923/rjpscience.2010.23.26
- Onyema, I., Eze, D.C., Abba, Y., Emennaa, P.E., Shoyinka, S.V.O., Okwor, E.C., Ezema, W.S., Ihedioha, J.I. & Okoye, J.O.A. (2019). Lesions of velogenic viscerotropic Newcastle disease virus infection were more severe in broilers than pullets. Journal of Applied Animal Research, 47, 189–194. doi: 10.1080/09712119.2019.1598420
- Ponman, S., Abolnik, C., Joannis, T.M. & Bisschop, S. (2012). Virulent Newcastle disease virus in Nigeria: identification of a new clade of sub-lineage 5f from live bird markets. Virus Genes, 44, 98–103. doi: 10.1007/s11262-011-0678-5
- Samuel, A., Nayak, B., Paldurai, A., Xiao, S., Aplogan, G.L., Awoume, K.A., Webby, R.J., Ducatez, M.F., Collins, P.L. & Samal, S.K. (2013). Phylogenetic and pathotypic characterization of Newcastle disease viruses circulating in West Africa and efficacy of a current vaccine. Journal of Clinical Microbiology, 51, 771–781. doi: 10.1128/JCM.02750-12
- Shittu, I., Joannis, T.M., Odaibo, G.N. & Olaleye, O.D. (2016). Newcastle disease in Nigeria: epizootiology and current knowledge of circulating genotypes. Virus Disease, 27, 329–339. doi: 10.1007/s13337-016-0344-6
- Snoeck, C.J., Owoade, A.A., Couacy-Hymann, E., Alkali, B.R., Okwen, M.P., Adeyanju, A.T., Komoyo, G.F., Nakouné, E., Le Faou, A. & Muller, C.P. (2013). High genetic diversity of Newcastle disease virus in poultry in West and Central Africa: cocirculation of genotype XIV and newly defined genotypes XVII and XVIII. Journal of Clinical Microbiology, 51, 2250–2260. doi: 10.1128/JCM.00684-13
- Susta, L., Jones, M.E.B., Cattoli, G., Cardenas-Garcia, S., Miller, P.J., Brown, C.C. & Alfonso, C.L. (2015). Pathologic characterization of genotypes XIV and XVII Newcastle disease viruses and efficacy of classical vaccination on specific pathogen-free birds. Veterinary Pathology, 52, 120–131. doi: 10.1177/0300985814521247
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729. doi: 10.1093/molbev/mst197
- Welch, C.N., Shittu, I., Abolnik, C., Solomon, P., Dimitrov, K.M., Taylor, T.L., Williams-Coplin, D., Goraichuk, I.V., Meseko, C.A., Ibu, J.O., Gado, D.A., Joannis, T.M. & Afonso, C.L. (2019). Genomic comparison of Newcastle disease viruses isolated in Nigeria between 2002 and 2015 reveals circulation of highly diverse genotypes and spillover into wild birds. Archives of Virology, 164, 2031–2047. doi: 10.1007/s00705-019-04288-9
