ABSTRACT
Birds of prey, including endangered species, have been infected with H5 highly pathogenic avian influenza viruses (HPAIVs) in several countries. In this present study, we assessed the pathogenicity of the clade 2.3.2.1 H5N1 HPAIV in American kestrels (Falco sparverius) with a view to preventing future outbreaks in raptors. The kestrels were intranasally inoculated with the virus or fed the meat of chicks that had died from viral infection. Kestrels in both groups initially had reduced food intake, showed clinical signs such as depression and neurologic manifestations, and succumbed to the infection within 6 days. The kestrels primarily shed the virus orally from 1 day post-inoculation until death, with an average titre of 104.5–5.7 EID50/ml, which is comparable to the inoculum titre. The viruses replicated in almost all tested tissues; notably, the feather calamuses also contained infectious virions and/or viral genes. Pancreatic lesions were present in several infected birds, as shown in previous cases of HPAIV infection in raptors. These results indicate that kestrels are highly susceptible to infection by clade 2.3.2.1 H5 HPAIVs, which readily occurs through the consumption of infected bird carcasses. Early detection and removal of HPAIV infected carcasses in the field is essential for preventing outbreaks in raptors.
RESEARCH HIGHLIGHTS
Clade 2.3.2.1 H5 HPAIV caused lethal infection in American kestrels.
Kestrels with the HPAIV showed neurologic signs and eye disorders.
The HPAIV replicated in systemic tissues of kestrels, and was orally shed.
The HPAIV was recovered from feather calamus of kestrels.
Introduction
The H5 subtype highly pathogenic avian influenza viruses (HPAIVs) have spread to domestic poultry and wild birds in Asia, Europe, the Middle East, Africa, and North America since late 1996, when the precursor of the present circulating viruses (Gs/Gd-like viruses) was isolated from a goose in Guangdong province, China (Xu et al., Citation1999). Phylogenetically, these progeny viruses have been organized into 10 clades based on the HA genes, which include the numbered clades 0–9 (WHO/OIE/FAO-Evolution-Working-Group, Citation2008). At first, the reports of highly pathogenic avian influenza (HPAI) were largely confined to poultry populations; lethal Gs/Gd-like viral infections were not reported in wild birds. However, HPAI, caused by clade 1 viruses in waterfowl and other captive/wild birds, was reported for the first time at two parks in Hong Kong in 2002 (Ellis et al., Citation2004). Furthermore, clade 2.2 HPAIVs caused a massive outbreak among more than 1000 migratory birds on Lake Qinghai, China, in May and June 2005 (Liu et al., Citation2005). These incidents suggest the possibility of further spread of HPAIVs to endangered bird species.
Birds of prey provide critical ecosystem services and consist of 557 species altogether (McClure et al., Citation2018). Of these, 18% are threatened with extinction while 52% display declining global populations. To date, free-ranging or wild birds of prey, including endangered species, have been infected with H5 HPAIVs. In East Asia, a clade 1 H5N1 HPAIV was isolated from a sick condor in Southern China in 2003 (Jiao et al., Citation2012). In Hong Kong, HPAIVs belonging to various clades had been found in individual birds of different species: a clade 9 HPAIV from a peregrine falcon (Falco peregrinus) in 2004 (Li et al., Citation2004), and clade 2.3.2 or 2.3.4 HPAIVs from a common buzzard (Buteo buteo), a common kestrel (Falco tinnunculus), a crested goshawk (Accipiter trivirgatus), and peregrine falcons in 2006–2009 (Smith et al., Citation2009; Shichinohe et al., Citation2013). In Japan, an emaciated mountain hawk-eagle (Nisaetus nipalensis) infected with clade 2.2 HPAIV, similar to the causal viruses observed during the outbreak at lake Qinghai, was found in 2007 (Shivakoti et al., Citation2010). During 2010—2011 and 2016—2017, Japan experienced massive HPAI outbreaks caused by clade 2.3.2.1 H5N1 and clade 2.3.4.4 H5N6 HPAIVs in wild birds, including the Eastern buzzard (Buteo japonicas), grey-faced buzzard (Butastur indicus), Northern goshawk (Accipiter gentilis), peregrine falcon, snowy owl (Bubo scandiacus), and Ural owl (Strix uralensis) (Soda, Ito et al., Citation2013; Takemae et al., Citation2017; Ozawa et al., Citation2019; Usui et al., Citation2020). Similarly, clade 2.3.2.1 HPAIVs were isolated from the common kestrel and sparrow hawk in the neighbouring country, Korea, in 2011 (Choi et al., Citation2013).
Many cases of HPAI in raptors have been reported in Middle Eastern nations. This could be attributed to the fact that the art of falconry is an important cultural activity in the Arabian Peninsula (Monne et al., Citation2008). Marjuki et al. (Citation2009) characterized two clade 2.2 H5N1 HPAIV isolates from dead Saker falcons (Falco cherrug) in Saudi Arabia and Kuwait in 2005–2007. Saudi Arabia had experienced several HPAI outbreaks caused by clade 2.2.3 H5N1 HPAIVs in the falconry sectors since early 2007 (Monne et al., Citation2008; Khan et al., Citation2009). In 2011, a clade 2.2.1 H5N1 HPAIV was isolated from a harrier species in Israel (Accession numbers: EPI338186-7). Since 2014, two H5 HPAIVs have been found in falcons: clade 2.3.2.1 H5N1 HPAIV in United Arab Emirates in 2014 (Naguib et al., Citation2015) and clade 2.3.4.4 H5N8 HPAIV in Israel and Saudi Arabia (Al-Ghadeer et al., Citation2018) in 2016–2017.
In Europe, two smuggled crested hawk-eagles (Spizaetus cirrhatus) from Thailand presented with clade 1 H5N1 HPAIV infection in 2004, which was an epidemic in Southeast Asian countries at that time (Van Borm et al., Citation2005). In 2006—2010, clade 2.2 or 2.3.2.1 H5N1 HPAIVs were isolated from falcon and buzzard species in Bulgaria (Marinova-Petkova et al., Citation2012), Denmark (Bragstad et al., Citation2007), Germany (van den Brand et al., Citation2015), and Slovakia. Moreover, clade 2.3.4.4 H5N2, H5N5, and H5N8 HPAIVs have been prevalent in buzzards, kestrels, peregrine falcons, and white-tailed eagles (Haliaeetus albicilla) in Belgium, Denmark, Italy (Mulatti et al., Citation2018), Germany (Krone et al., Citation2018), Hungary, and the Netherlands (Kleyheeg et al., Citation2017) since 2016. Besides, H5 HPAIV infections in raptors were also reported in West Africa and North America: clade 2.2 H5N1 HPAIV from wild hooded vultures (Necrosyrtes monachus) in Burkina Faso in 2006 (Ducatez, Olinger et al., Citation2007; Ducatez, Tarnagda et al., Citation2007), and clade 2.3.4.4 H5N2/N8 HPAIVs from raptors in the United States of America (USA) in 2014–2015 (Shearn-Bochsler et al., Citation2019).
As indicated above, birds of prey have been infected with H5 HPAIVs belonging to various clades. Based on these reports, several research groups experimentally assessed the pathogenicity of clade 2.2 HPAIVs in Gyr-saker hybrid falcons (Falco rusticolus × F. cherrug) (Lierz et al., Citation2007; Bertran et al., Citation2012) and American kestrels (Falco sparverius) (Hall et al., Citation2009). In these studies, most falcons and kestrels died within 3–7 days following intranasal and/or intrachoanal inoculation of the viruses; in addition, some infected birds exhibited neurological signs. The infected raptors shed the viruses orally earlier than cloacally. In addition, Bertran et al. (Citation2012) elucidated that HPAIV also caused lethal infection in hybrid falcons via natural feeding routes. However, clade 2.3.2.1 and 2.3.4.4 HPAIVs, but not clade 2.2, have recently caused natural infection cases in raptors (Marinova-Petkova et al., Citation2012; Choi et al., Citation2013; Soda, Ito et al., Citation2013; Naguib et al., Citation2015; Kleyheeg et al., Citation2017; Takemae et al., Citation2017; Al-Ghadeer et al., Citation2018; Krone et al., Citation2018; Mulatti et al., Citation2018; Ozawa et al., Citation2019; Shearn-Bochsler et al., Citation2019; Usui et al., Citation2020), and their pathogenicity remains unclear.
In the present study, we assessed the pathogenicity of a clade 2.3.2.1 HPAIV in the American kestrel belonging to the Genus Falco by an experimental study on the infection. The virus used in this study was isolated during the HPAI outbreak with many fatal cases in Japan in 2010–2011 (Soda, Usui et al., Citation2013). American kestrels are small compared with other birds of prey, measuring 21–31 cm from head to tail (White, Citation1994a). Additionally, various H5 HPAIVs have been found in kestrels in the field: clade 2.3.4 HPAIV in Hong Kong (Smith et al., Citation2009), clade 2.3.2.1 in Korea (Choi et al., Citation2013), clade 2.3.4.4 in Italy (Mulatti et al., Citation2018), and an unknown clade in Egypt (ElBakrey et al., Citation2016). The novelty of the present study is displayed by the use of the clade 2.3.2.1 HPAIV strain as the challenge virus, as well as by determining infectious virus titre of the tissues and swabs of the subjects, instead of viral gene quantification as shown in previous studies (Hall et al., Citation2009; Bertran et al., Citation2012), to further validate the possibility that the American kestrels function as a source of infection for other birds in the field. In addition, we focused on ophthalmic findings from experimental infections to explain the previous HPAI case regarding a raptor with an eye disorder (ElBakrey et al., Citation2016). To the best of our knowledge, this is the first report experimentally regarding the pathogenicity of clade 2.3.2.1 HPAIV in raptors.
Materials and methods
Virus
A/mandarin duck/Miyazaki/22M807-1/2011 (H5N1) (M807) – clade 2.3.2.1 HPAIV was used for experimental infection of American kestrels. The strain was isolated from a mixture of tracheal and cloacal swabs of a mandarin duck that had died in Miyazaki city, located in the Kyusyu region of Japan (Soda, Usui et al., Citation2013), and had previously been used in a study evaluating its pathogenicity in other crow and duck species (Soda, Usui et al., Citation2013, Citation2020). The virus was propagated in 10-day-old embryonated chicken eggs (Aoki breeder farm, Tochigi, Japan) for 48 h at 35°C (Hirst, Citation1942).
Birds
Six American kestrels (three females and three males of unknown age, body weights: 161–223 g) were obtained from a breeder (Moukin House Co. Ltd., Ibaraki, Japan). Laryngopharyngeal and cloacal swabs were collected in 2 ml nutrient broth medium (Nissui Pharmaceutical, Tokyo, Japan) containing 10 mg streptomycin (Meiji Seika Pharma, Tokyo, Japan) and 10,000 units of penicillin G (Meiji Seika Pharma), and confirmed as influenza virus-negative by inoculation in to embryonated chicken eggs and the hemagglutination assay (Sever, Citation1962). The birds were housed in individual self-contained isolator units (CLEA Japan, Tokyo, Japan). Throughout the study, they were fed a quarter body of edible dead quail, with the head, wings, legs, and intestine removed by the manufacturer in advance (Animal planning, Osaka, Japan), and water every day. Quarter bodies of the edible quails were cut into several small pieces (bite-size) prior to feeding the kestrels. Before experimental infection, the birds were allowed to adapt to the new environment for 5 days. Two days before virus challenge, their body weights were 161–230 g. The day before virus challenge, a blood sample was collected from each kestrel. The blood samples were incubated at 37°C for 2 h, allowed to stand at 4°C for 12 h, and then centrifuged to collect the supernatant (serum). Using inactivated antigens of the M807, the haemagglutinin inhibition test (Sever, Citation1962) was carried out to confirm that serum antibodies to the challenge virus strain were negative (within the limits of detectability).
Experimental design
The kestrels were distributed into groups A [two females (#N1 and #N3) and one male (#N2)] and B [one female (#O2) and two males (#O1 and #O3)]. The kestrels in group A were intranasally inoculated with 100 μl allantoic fluid containing M807 at 106.0 50% egg infections dose (EID50). They were fed edible quails on challenge day as usual. Each kestrel in group B was challenged via the natural feeding route with one entire chick, which had previously died from infection with M807, prepared as indicated below, and was not fed the edible quail on the virus challenge day. The air sacs of six 3-day-old chicks (GHEN Corporation Co., Ltd., Gifu, Japan) were inoculated with 100 μl allantoic fluid, containing M807 at 106.5 EID50. One day post-inoculation (d.p.i), all chicks died and laryngopharyngeal swabs were confirmed positive for the nucleoprotein antigen of the influenza A virus using a rapid diagnosis kit, ESPLINE Influenza A&B-N (FUJIREBIO INC., Tokyo, Japan). Three dead chicks were dissected and tissue viral titres were determined to estimate the inoculum titre for the kestrels. The viral titres of liver, lung, kidney and brain homogenate ranged from 105.25 to 107.75 EID50/g, thought to be comparable to the inoculum titre for group A. The remaining three chicks were applied for inoculum to the kestrels. Eventually, all kestrels in group B ate the chicks, with nothing left over.
After virus inoculation, the kestrels were monitored and scored for the development of clinical signs every 8 h based on the Intravenous Pathogenicity Test Method (OIE, Citation2019); “sick” birds would show one of the following signs and “severely sick” more than one of following signs: respiratory involvement, depression, diarrhoea, cyanosis of the exposed skin, oedema of the face and/or head, nervous signs. Moreover, their body weight, food uptake, and rectal temperature were measured daily. Laryngopharyngeal and cloacal swabs were collected on 1, 2, 3, and 5 d.p.i. The birds which died of infection were immediately dissected, and their tissues (brain, trachea, lung, liver, pancreas, spleen, kidney, heart, colon, glandular stomach, eyeballs, and feather calamus) were aseptically collected. These samples, except spleen, glandular stomach, eyeballs, and feather calamus, were homogenized to make 20% (w/v) suspensions in nutrient broth medium with antibiotics. Spleen and feather calamus were homogenized to make 10% and 2% suspensions, respectively. Infectious viral titres in the collected samples were determined using embryonated eggs and the method described by Reed and Muench (Citation1938). The viral titre differences between groups A and B were assessed using the Student’s t-test.
Ethics statement
All bird experiments were carried out at a biosafety level 3 facility at the Avian Zoonosis Research Center, Tottori University, Japan. The experiments were performed according to the guidelines of the institutional animal care and use committee of Tottori University (approval number: 12-T-30). Humane endpoints were set as follows: any bird that lost more than 20% of body weight was euthanized using isoflurane (FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan), and recorded as dead on the following day’s observation time; any birds showing neurological signs were observed at a maximum interval of 24 h to confirm their pathology and then immediately euthanized.
Histopathology and immunostaining of viral antigens
Tissues (brain, trachea, lung, liver, pancreas, spleen, kidney, heart, colon, glandular stomach, and eyeballs) of the kestrels that had died of infection were fixed in 10% neutral buffered formalin (FUJIFILM Wako Pure Chemical Corporation), routinely processed, and embedded in paraffin wax. Sections were stained with haematoxylin and eosin (HE) for histopathological examination. Immunohistochemistry for viral antigen was performed using the Simple Stain MAX-PO (M) kit (Nichirei Bioscience Inc., Tokyo, Japan) according to the manufacturer’s instructions. Briefly, the rehydrated sections were incubated with 0.1% H2O2 in methanol for endogenous peroxidase blocking and were subjected to heat-induced antigen retrieval with 0.05% citraconic anhydride, pH 7.4 (Immunosaver; Nissin EM, Tokyo, Japan). Subsequently, the sections were treated with a blocking reagent (Protein Block Serum-Free; Dako, CA, USA), followed by overnight incubation with the primary antibody (1:5000 dilution, mouse anti-influenza A virus matrix protein monoclonal antibody, clone: GA2B; Serotec Ltd., Oxford, UK), at 4°C. Thereafter, sections were treated with the immunohistochemical detection system mentioned above, followed by detection with 3,3′-diaminobenzidine tetrahydrochloride solution (ImmPACT DAB Substrate; Vector Laboratories, CA, USA).
Virus detection from feather calamus using RT–PCR
If HPAIV genes can be rapidly detected from dropped feathers of infected birds in the field, we will be able to recognize HPAIV invasion in the environment and take prompt measures to prevent further viral spread. We obtained five feather calamus samples from each kestrel that had died from experimental infection for verification using RT–PCR. The samples were homogenized and suspended in 140 μl PBS. Viral RNA was extracted from these suspensions using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. RT–PCR was performed using the SuperScript III One-Step RT–PCR System with Platinum Taq High Fidelity (Invitrogen, Carlsbad, CA, USA) using a viral matrix gene-specific primer pair (forward: 5′-AAG ACC AAT CCT GTC ACC TCT GA-3′ and reverse: 5′-CAA AGC GTC TAC GCT GCA GTC C-3′) (Karlsson et al., Citation2007). The RT–PCR mixture contained 2 μl sample RNA, 0.5 μl forward and reverse primers (10 μM each), 12.5 μl 2× reaction mix, 0.5 μl SuperScript III RT/Platinum Taq High Fidelity Enzyme Mix, and 9 μl nuclease-free water. The PCR conditions were as follows: one cycle at 55°C for 30 min and 94°C for 2 min, 40 cycles at 94°C for 15 s, 60°C for 30 s, 68°C for 30 s, and one cycle of final extension at 68°C for 5 min. Amplified products were examined using 1.2% agarose gel electrophoresis.
Results
Clinical signs and mortality
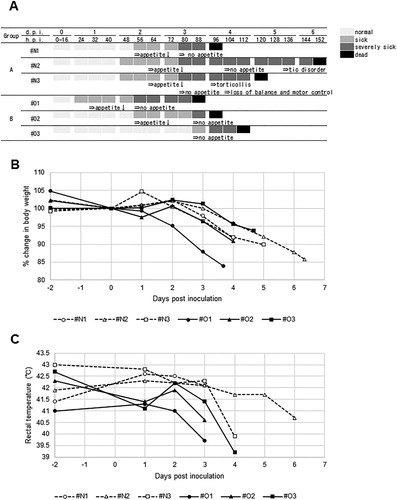
A/mandarin duck/Miyazaki/22M807-1/2011 (H5N1) produced 100% mortality in American kestrels via both intranasal and natural feeding routes ((A)); all birds died 88–152 h post-inoculation (h.p.i). The mean death times of the birds in groups A and B were 5.1 and 4.1 d, respectively. Disease onset in all birds, other than #O3, was first recognized by decreased activity and loss of appetite at 1–2 d.p.i. The clinical conditions of these birds subsequently worsened with weakness, depression, and no feeding behaviour. #O3 suddenly showed complete loss of appetite at 88 h.p.i and died the next day. Two birds in group A (#N2 and #N3) showed additional neurological signs: tic disorder, torticollis, and loss of balance/motor control, frequently with a half-closed or fully closed eyelid. Throughout the study, neither typical respiratory signs, such as nasal discharge, nor diarrhoea, were observed. The body weight of each bird had apparently decreased since the day after complete loss of appetite ((A,B)). Rectal temperatures of group A were comparatively higher than those of group B at 1 d.p.i ((C)). In most birds, rectal temperatures suddenly fell prior to death.
Figure 1. Time course of clinical conditions of American kestrels inoculated with the clade 2.3.2.1 H5 HPAIV. (A) (Upper) Clinical scores. Each kestrel was observed for disease manifestation at 8 h intervals. The judgement of sick and severely sick was based on the Intravenous Pathogenicity Test method (OIE, Citation2019). (A) (Lower) The onset of major clinical signs: changes in body weight (B) and rectal temperature (C) before/after virus inoculation.
Viral shedding and replication in tissues
All tested birds in groups A and B had shed the virus orally from 1 d.p.i until death at average titres of 104.5—5.7 EID50/ml and 104.5—5.4 EID50/ml, respectively ((A)). Virus shedding to the cloaca was comparably delayed and limited; 101.6—3.5 EID50 of the virus on average had been recovered since 2 d.p.i ((B)). Interestingly, the viral titres in cloacal swabs of group B at 2 d.p.i were significantly higher than those observed in group A (P < 0.05).
Figure 2. Viral shedding and replication in tissues of American kestrels inoculated with the clade 2.3.2.1 H5 HPAIV. Average viral titres of laryngopharyngeal (A) and cloacal (B) swabs, and tissues (C) were calculated from those of virus-positive samples. The numerator and denominator of fractions above bars indicate the number of virus-positive and surviving individuals, respectively, at that time; the descriptions “3/3” and “0/3” were omitted. Asterisks denote significant differences between groups (Student’s t-test, P < 0.05).
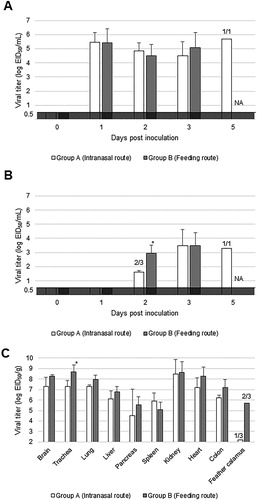
The virus was recovered from almost all tested tissues (brain, trachea, lung, liver, pancreas, spleen, kidney, heart, and colon) from kestrels which had died of HPAIV infection, at an average titre of over 104.5 EID50/g ((C)). Only the virus titres in the tracheas of group B were significantly higher than those of group A (P < 0.05). Viruses were also isolated from feather calamuses of #N3, #O2, and #O3 ((C)). The viral matrix gene was detected in feather calamuses of all birds (data not shown).
Necropsy and histopathology
All American kestrels, which had died of HPAIV infection, were grossly and histologically examined (). The gross and histological pathology were similar between groups A and B. Distinctive severe necrotizing pancreatitis was confirmed in five birds ( and (A)). The virus antigen predominantly disseminated to exocrine cells, macrophages, and surfaces of pancreatic duct cells ((B)). Minute foci of neuronal degeneration and necrosis with minimal inflammatory reactions and viral antigens were occasionally observed in the cerebrum of all birds ( and (C)). The virus antigen was multifocally and extensively detected in the nuclear/cytoplasm regions of neurons, glial cells, meningeal cells, and ependymal cells in all kestrel brains (). The antigen was also found in the nuclear/cytoplasmic regions of the bronchial mucosa and alveolar epithelial cells ((D)). Mild degeneration and necrosis of bronchial and tracheal epithelium with mild submucosal inflammatory infiltrations were observed in all kestrels (). Mild multifocal degeneration, obscure striations, and mild pyknotic nuclei were observed in kestrel hearts, and viral antigens were detected in those myocardial cells ( and (E)). In addition, all kestrels exhibited viral antigen in cells at the conjunctiva ((F)) and Schlemm’s canal ((H)). Mild degeneration of conjunctival cells with oedema and a few infiltrations of nonsuppurative inflammatory cells were occasionally observed. Further, corneal epithelium ((I)) and endothelium ((J)), optic nerves ((K)), and orbicularis oculi muscles contained virus-positive cells. Multifocal, small necrotic foci with viral antigens were additionally found in the livers of #O1 and #O2 (). #O3 developed mild mucosal erosion and ulceration in the colon. Histopathological changes were relatively mild, except in the pancreas. Other tissues did not show apparent histological lesions despite viral antigen distribution in parenchymal cells in most tissues ().
Figure 3. Histological analysis of tissues from American kestrels inoculated with the clade 2.3.2.1 H5 HPAIV. Representative gross (inset) and histological lesions of pancreas sections (A). Histological structure around Schlemm’s canal with HE staining (G). Representative immunohistochemical staining of pancreas (B), brain (C), lung (D), heart (E), conjunctiva (F), Schlemm’s canal (H), corneal epithelium and endothelium (I and J), and optic nerve (K). Arrowheads in (C) indicate multifocal cerebral necrosis. The asterisk in (D) indicates bronchial lumen. In (G,H), arrowheads and asterisk indicate Schlemm’s canal and ciliary body, respectively. Scale bars: 100 µm (A–E, G, H); 50 µm (F, I–K).
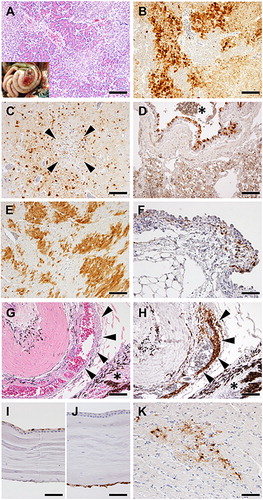
Table 1. Distribution of histological lesions and viral antigen in the tissues of American kestrels inoculated with H5N1 HPAIV.
Discussion
The present study revealed that clade 2.3.2.1 HPAIVs, as well as clade 2.2 HPAIVs (Hall et al., Citation2009), cause lethal infection in American kestrels via respiratory or oral infections ((A)). Several kestrels displayed neurologic manifestations, such as tremors and torticollis, at disease end-stage ((A)), as similarly observed in previous studies (Hall et al., Citation2009; Bertran et al., Citation2012). In the present study, we could not prepare non-inoculated controls, and hence cannot completely exclude the possibility that stress under the rearing conditions was involved in disease development. However, the virus replicated in almost all tested tissues (), and the histopathological lesions were certainly accompanied by viral antigens ( and ); these results support the notion that American kestrels are highly vulnerable to the clade 2.3.2.1 HPAIV.
Normally, American kestrels largely feed on insects and small vertebrates, mainly small rodents up to 89 g in weight (White, Citation1994a), which differs from peregrine falcons that chiefly feed on birds (White, Citation1994b). However, certain populations of American kestrels in North America feed on small birds, such as passerines (White, Citation1994a; Doyle & Smith, Citation2001). Schulwitz et al. (Citation2019) reported a rare feeding strategy in American kestrels, which involves scavenging the carcass of a domesticated turkey. In the present study, the subjected kestrels actively fed on edible quails or chicks that were infected with the HPAIV. In addition, kleptoparasitism (stealing of food from another bird) was observed in kestrel species (White et al., Citation1994). These observations support the hypothesis that small kestrels, as well as larger falcon species, can be infected with HPAIVs by scavenging bird carcasses infected with HPAIVs in the field. The kestrels studied in the previous case reports (Smith et al., Citation2009; Choi et al., Citation2013; ElBakrey et al., Citation2016; Mulatti et al., Citation2018) might be infected with HPAIVs because of such behaviour.
In the present study, five of the six American kestrels lost appetite (leaving edible quail uneaten) at the early phase of infection, 1–2 d.p.i ((A)), corresponding to the preceding study (Lierz et al., Citation2007). In contrast, their body weights and rectal temperatures had not changed distinctly until 2–3 d.p.i ((B,C)). Monitoring food intake should be essential to recognize HPAIV infection in captive raptors, especially when an HPAI outbreak occurs in the immediate area. The subjected kestrels had shed the virus to oral cavities at high titres since 1 d.p.i ((A)); detection of viral antigen using rapid diagnosis kits should also be available. #N2 and #N3 showed neurological signs at the terminal stage of infection ((A)), as did the raptors in the previous reports (Ducatez, Olinger et al., Citation2007; Hall et al., Citation2009; Bertran et al., Citation2012; ElBakrey et al., Citation2016; Krone et al., Citation2018). #N2 and #N3 incompletely or completely closed their eyes. Virus antigens were distributed across brains and ophthalmic tissues of all subjected kestrels, this was consistent with observed histopathological lesions, such as neuronal necrosis and conjunctivitis ((C,F), and ). Virus replication in these tissues may negatively impact the ability to fly and hunt, leading to debility or falling to the ground. Actually, the fallen falcon in the field showed ophthalmic signs and conjunctivitis with crust formation on eyelids (ElBakrey et al., Citation2016).
As most falconids live alone or in solitary pairs with strong territoriality (White et al., Citation1994), the risk of massive infection among raptors should be lower than that in water birds and crows. To date, HPAI cases in raptors in the field have been sporadic. Conversely, American kestrels are highly susceptible to HPAIV infection. Hall et al. (Citation2009) reported that 101 EID50 of the virus was sufficient to cause lethal infection. The subjected kestrels in the present study shed 104—6 EID50 of the virus, comparable to the inoculum titre, into oral cavities ((A)). HPAIVs may disseminate among densely reared captive raptors in hatcheries, breeding farms, sectors, and zoos; zoonotic infections to humans (caretakers, falconers, etc.) are an additional cause for concern. Indeed, mass infections in falcons and owls occurred in zoos in Kuwait (Marjuki et al., Citation2009) and Japan (Usui et al., Citation2020), respectively. Separation of raptors from poultry and water birds, major hosts of influenza viruses, should be taken into consideration. Besides, virus found in the cloaca was unremarkable compared with that in the oral cavity ((B)). The kestrels in group B shed more viruses at 2 d.p.i, and viral antigens spread widely in the colon of #O3 ((B) and ); the viruses might readily reach the intestines via a natural feeding route and replicate in epithelial cells. Faeces containing infectious virions may function as a source of infection to other birds, while the kestrels shed low viral titres to the cloaca. Members of the genus falco at high latitudes are principally diurnal migrants (White et al., Citation1994). A previous study (Bertran et al., Citation2012) showed that subclinical infection also occurred in the kestrels inoculated with HPAIVs, although all birds died from infection under the present experimental condition ((A)). Therefore, we propose that falcons with inapparent infection may be involved in the dissemination of HPAIVs through their movement, as well as migratory water birds.
The HPAIV systemically replicated in the subjected kestrels ((C)) and antigens were found in all tested tissues ( and ). We previously isolated HPAIVs from systemic organs of falcon and goshawk carcasses in the field (Soda, Ito et al., Citation2013); such bodies could become a source of infection to other birds. To prevent dissemination of HPAIVs to scavengers, raptors that are suspected to be infected with viruses should be immediately incinerated or transported to appropriate institutes with biosecurity for definitive diagnosis. Oral swabs or tracheas can be readily sampled, and should be well-suited for virus detection/isolation, according to the present results: high viral titres in these samples ((A,C)) would aid accurate diagnoses. Histological lesions were observed in the pancreas of kestrels ((A) and ), while viral titres were lower in the pancreas compared to other tissues ((C)). Other research groups also reported remarkable pancreatic lesions observed in raptor carcasses with HPAIVs (Bertran et al., Citation2012; Naguib et al., Citation2015; van den Brand et al., Citation2015). It remains unclear as to why the HPAIVs showed high tissue tropism for the pancreas in raptors. It can be assumed that pancreatic enzyme activity contributes to effective cleavage activation of viral haemagglutinin, indispensable for multiple replications of the influenza virus. Intriguingly, feather calamuses of the subjected kestrels contained virions and/or viral genes ((C)). Yamamoto et al. (Citation2008) showed that domestic ducks retained HPAIVs in feathers longer than in oral and cloacal mucosa. They also reported on the time period that HPAIV would remain infective in feather tissues (Yamamoto et al., Citation2017). Hence, feathers cast from raptors with HPAIVs may contaminate environments. Conversely, we can use the feathers with HPAIVs to recognize the invasion of specific HPAIVs.
Again, raptors have recently been infected with clade 2.3.2.1 and 2.3.4.4 HPAIVs. Bertran et al. (Citation2012) revealed that alpha 2,3 receptors are predominantly distributed in respiratory tissues and rectum of falcons. Gs/Gd-like viruses bind the alpha 2,3 N-linked sialic acids (Gao et al., Citation2018), thus predicting HPAIV infection cases in raptors hereafter. Raptor species, which had died as a result of HPAIV infection in the field, can be regarded as sentinels for HPAI occurrence not only in their prey but in other wild birds and poultry in the surrounding area. Although the number of examined birds was limited in the present study, we expect that the findings will contribute to minimizing the casualties of wild birds and poultry exposed to future HPAI outbreaks, and also to alert falcon-holders to recognize the risk of possible interspecies (bird to human) transmission of HPAIVs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Al-Ghadeer, H., Chu, D.K.W., Rihan, E.M.A., Abd-Allah, E.A., Gu, H., Chin, A.W.H., Qasim, I.A., Aldoweriej, A., Alharbi, S.S., Al-Aqil, M.A., Al-Sahaf, A., Abdel Rahman, S.S., Aljassem, A.H., Abdul-Al, A., Aljasir, M.R., Alhammad, Y.M.O., Kasem, S., Peiris, M., Zaki, A. & Poon, L.L.M. (2018). Circulation of influenza A(H5N8) virus, Saudi Arabia. Emerging Infectious Diseases, 24, 1961–1964. doi: 10.3201/eid2410.180846
- Bertran, K., Busquets, N., Abad, F.X., Garcia de la Fuente, J., Solanes, D., Cordon, I., Costa, T., Dolz, R. & Majo, N. (2012). Highly (H5N1) and low (H7N2) pathogenic avian influenza virus infection in falcons via nasochoanal route and ingestion of experimentally infected prey. PLoS One, 7, e32107. doi: 10.1371/journal.pone.0032107
- Bragstad, K., Jorgensen, P.H., Handberg, K., Hammer, A.S., Kabell, S. & Fomsgaard, A. (2007). First introduction of highly pathogenic H5N1 avian influenza A viruses in wild and domestic birds in Denmark, Northern Europe. Virology Journal, 4, 43. doi: 10.1186/1743-422X-4-43
- Choi, J.G., Kang, H.M., Jeon, W.J., Choi, K.S., Kim, K.I., Song, B.M., Lee, H.S., Kim, J.H. & Lee, Y.J. (2013). Characterization of clade 2.3.2.1 H5N1 highly pathogenic avian influenza viruses isolated from wild birds (mandarin duck and Eurasian eagle owl) in 2010 in Korea. Viruses, 5, 1153–1174. doi: 10.3390/v5041153
- Doyle, H. & Smith, J. (2001). Chapter 16 Raptors and Scavengers. In C.J. Krebs, S.A. Boutin, & R. Boonstra (Eds.), Ecosystem Dynamics of the Boreal Forest: The Kluane Project (pp. 377–404). New York: Oxford University Press.
- Ducatez, M.F., Olinger, C.M., Owoade, A.A., Tarnagda, Z., Tahita, M.C., Sow, A., De Landtsheer, S., Ammerlaan, W., Ouedraogo, J.B., Osterhaus, A.D., Fouchier, R.A. & Muller, C.P. (2007). Molecular and antigenic evolution and geographical spread of H5N1 highly pathogenic avian influenza viruses in western Africa. Journal of General Virology, 88, 2297–2306. doi: 10.1099/vir.0.82939-0
- Ducatez, M.F., Tarnagda, Z., Tahita, M.C., Sow, A., de Landtsheer, S., Londt, B.Z., Brown, I.H., Osterhaus, D.M., Fouchier, R.A., Ouedraogo, J.B. & Muller, C.P. (2007). Genetic characterization of HPAI (H5N1) viruses from poultry and wild vultures, Burkina Faso. Emerging Infectious Diseases, 13, 611–613. doi: 10.3201/eid1304.061356
- ElBakrey, R., Mansour, S., Ali, H., Knudsen, D. & Eid, A. (2016). First detection of highly pathogenic avian influenza virus H5N1 in common kestrel falcon (Falco tinnunculus) in Egypt. Japanese Journal of Veterinary Research, 64, S9–S14.
- Ellis, T.M., Bousfield, R.B., Bissett, L.A., Dyrting, K.C., Luk, G.S., Tsim, S.T., Sturm-Ramirez, K., Webster, R.G., Guan, Y. & Malik Peiris, J.S. (2004). Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathology, 33, 492–505. doi: 10.1080/03079450400003601
- Gao, R., Gu, M., Liu, K., Li, Q., Li, J., Shi, L., Li, X., Wang, X., Hu, J., Liu, X., Hu, S., Chen, S., Peng, D., Jiao, X. & Liu, X. (2018). T160a mutation-induced deglycosylation at site 158 in hemagglutinin is a critical determinant of the dual receptor binding properties of clade 2.3.4.4 H5NX subtype avian influenza viruses. Veterinary Microbiology, 217, 158–166. doi: 10.1016/j.vetmic.2018.03.018
- Hall, J.S., Ip, H.S., Franson, J.C., Meteyer, C., Nashold, S., TeSlaa, J.L., French, J., Redig, P. & Brand, C. (2009). Experimental infection of a North American raptor, American kestrel (Falco sparverius), with highly pathogenic avian influenza virus (H5N1). PLoS One, 4, e7555. doi: 10.1371/journal.pone.0007555
- Hirst, G.K. (1942). Adsorption of influenza hemagglutinins and virus by red blood cells. The Journal of Experimental Medicine, 76, 195–209. doi: 10.1084/jem.76.2.195
- Jiao, P., Yuan, R., Song, Y., Wei, L., Ren, T., Liao, M. & Luo, K. (2012). Full genome sequence of a recombinant H5N1 influenza virus from a condor in Southern China. Journal of Virology, 86, 7722–7723. doi: 10.1128/JVI.01043-12
- Karlsson, M., Wallensten, A., Lundkvist, A., Olsen, B. & Brytting, M. (2007). A real-time PCR assay for the monitoring of influenza A virus in wild birds. Journal of Virological Methods, 144, 27–31. doi: 10.1016/j.jviromet.2007.03.013
- Khan, O.A., Shuaib, M.A., Rhman, S.S., Ismail, M.M., Hammad, Y.A., Baky, M.H., Fusaro, A., Salviato, A. & Cattoli, G. (2009). Isolation and identification of highly pathogenic avian influenza H5N1 virus from Houbara bustards (Chlamydotis undulata macqueenii) and contact falcons. Avian Pathology, 38, 35–39. doi: 10.1080/03079450802609815
- Kleyheeg, E., Slaterus, R., Bodewes, R., Rijks, J.M., Spierenburg, M.A.H., Beerens, N., Kelder, L., Poen, M.J., Stegeman, J.A., Fouchier, R.A.M., Kuiken, T. & van der Jeugd, H.P. (2017). Deaths among wild birds during highly pathogenic avian influenza A(H5N8) virus outbreak, the Netherlands. Emerging Infectious Diseases, 23, 2050–2054. doi: 10.3201/eid2312.171086
- Krone, O., Globig, A., Ulrich, R., Harder, T., Schinkothe, J., Herrmann, C., Gerst, S., Conraths, F.J. & Beer, M. (2018). White-tailed sea eagle (Haliaeetus albicilla) die-off due to infection with highly pathogenic avian influenza virus, subtype H5N8, in Germany. Viruses, 10, 478. doi: 10.3390/v10090478
- Li, K.S., Guan, Y., Wang, J., Smith, G.J., Xu, K.M., Duan, L., Rahardjo, A.P., Puthavathana, P., Buranathai, C., Nguyen, T.D., Estoepangestie, A.T., Chaisingh, A., Auewarakul, P., Long, H.T., Hanh, N.T., Webby, R.J., Poon, L.L., Chen, H., Shortridge, K.F., Yuen, K.Y., Webster, R.G. & Peiris, J.S. (2004). Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in Eastern Asia. Nature, 430, 209–213. doi: 10.1038/nature02746
- Lierz, M., Hafez, H.M., Klopfleisch, R., Luschow, D., Prusas, C., Teifke, J.P., Rudolf, M., Grund, C., Kalthoff, D., Mettenleiter, T., Beer, M. & Hardert, T. (2007). Protection and virus shedding of falcons vaccinated against highly pathogenic avian influenza A virus (H5N1). Emerging Infectious Diseases, 13, 1667–1674. doi: 10.3201/eid1311.070705
- Liu, J., Xiao, H., Lei, F., Zhu, Q., Qin, K., Zhang, X.W., Zhang, X.L., Zhao, D., Wang, G., Feng, Y., Ma, J., Liu, W., Wang, J. & Gao, G.F. (2005). Highly pathogenic H5N1 influenza virus infection in migratory birds. Science, 309, 1206. doi: 10.1126/science.1115273
- Marinova-Petkova, A., Georgiev, G., Seiler, P., Darnell, D., Franks, J., Krauss, S., Webby, R.J. & Webster, R.G. (2012). Spread of influenza virus A (H5N1) clade 2.3.2.1 to Bulgaria in common buzzards. Emerging Infectious Diseases, 18, 1596–1602. doi: 10.3201/eid1810.120357
- Marjuki, H., Wernery, U., Yen, H.L., Franks, J., Seiler, P., Walker, D., Krauss, S. & Webster, R.G. (2009). Isolation of highly pathogenic avian influenza H5N1 virus from saker falcons (Falco cherrug) in the Middle East. Advances in Virology, 2009, 1. doi: 10.1155/2009/294520
- McClure, C.J.W., Westrip, J.R.S., Johnson, J.A., Schulwitz, S.E., Virani, M.Z., Davies, R., Symes, A., Wheatley, H., Thorstrom, R., Amar, A., Buij, R., Jones, V.R., Williams, N.P., Buechley, E.R. & Butchart, S.H.M. (2018). State of the world’s raptors: distributions, threats, and conservation recommendations. Biological Conservation, 227, 390–402. doi: 10.1016/j.biocon.2018.08.012
- Monne, I., Fusaro, A., Al-Blowi, M.H., Ismail, M.M., Khan, O.A., Dauphin, G., Tripodi, A., Salviato, A., Marangon, S., Capua, I. & Cattoli, G. (2008). Co-circulation of two sublineages of HPAI H5N1 virus in the Kingdom of Saudi Arabia with unique molecular signatures suggesting separate introductions into the commercial poultry and falconry sectors. Journal of General Virology, 89, 2691–2697. doi: 10.1099/vir.0.2008/004259-0
- Mulatti, P., Fusaro, A., Scolamacchia, F., Zecchin, B., Azzolini, A., Zamperin, G., Terregino, C., Cunial, G., Monne, I. & Marangon, S. (2018). Integration of genetic and epidemiological data to infer H5N8 HPAI virus transmission dynamics during the 2016-2017 epidemic in Italy. Scientific Reports, 8, 18037. doi: 10.1038/s41598-018-36892-1
- Naguib, M.M., Kinne, J., Chen, H., Chan, K.H., Joseph, S., Wong, P.C., Woo, P.C., Wernery, R., Beer, M., Wernery, U. & Harder, T.C. (2015). Outbreaks of highly pathogenic avian influenza H5N1 clade 2.3.2.1c in hunting falcons and kept wild birds in Dubai implicate intercontinental virus spread. Journal of General Virology, 96, 3212–3222. doi: 10.1099/jgv.0.000274
- OIE. (2019). Section 3.3. Aves, Chapter 3.3.4. avian influenza (infection with avian influenza viruses). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 2019, 821–843.
- Ozawa, M., Matsuu, A., Khalil, A.M., Nishi, N., Tokorozaki, K., Masatani, T., Horie, M., Okuya, K., Ueno, K., Kuwahara, M. & Toda, S. (2019). Phylogenetic variations of highly pathogenic H5N6 avian influenza viruses isolated from wild birds in the Izumi plain, Japan, during the 2016-17 winter season. Transboundary and Emerging Diseases, 66, 797–806. doi: 10.1111/tbed.13087
- Reed, L.J. & Muench, H. (1938). A simple method of estimating fifty percent endpoints. American Journal of Epidemiology, 27, 493–497. doi: 10.1093/oxfordjournals.aje.a118408
- Schulwitz, S.E., Griffith, M.C. & McClure, C.J.W. (2019). American kestrel (Falco sparverius) scavenging on domestic turkey (Meleagris gallopavo) carcass. The Wilson Journal of Ornithology, 131, 410–413. doi: 10.1676/18-101
- Sever, J.L. (1962). Application of a microtechnique to viral serological investigations. Journal of Immunology, 88, 320–329.
- Shearn-Bochsler, V.I., Knowles, S. & Ip, H. (2019). Lethal infection of wild raptors with highly pathogenic avian influenza H5N8 and H5N2 viruses in the USA, 2014-15. Journal of Wildlife Diseases, 55, 164–168. doi: 10.7589/2017-11-289
- Shichinohe, S., Okamatsu, M., Yamamoto, N., Noda, Y., Nomoto, Y., Honda, T., Takikawa, N., Sakoda, Y. & Kida, H. (2013). Potency of an inactivated influenza vaccine prepared from a non-pathogenic H5N1 virus against a challenge with antigenically drifted highly pathogenic avian influenza viruses in chickens. Veterinary Microbiology, 164, 39–45. doi: 10.1016/j.vetmic.2013.01.041
- Shivakoti, S., Ito, H., Otsuki, K. & Ito, T. (2010). Characterization of H5N1 highly pathogenic avian influenza virus isolated from a mountain hawk eagle in Japan. Journal of Veterinary Medical Science, 72, 459–463. doi: 10.1292/jvms.09-0478
- Smith, G.J., Vijaykrishna, D., Ellis, T.M., Dyrting, K.C., Leung, Y.H., Bahl, J., Wong, C.W., Kai, H., Chow, M.K., Duan, L., Chan, A.S., Zhang, L.J., Chen, H., Luk, G.S., Peiris, J.S. & Guan, Y. (2009). Characterization of avian influenza viruses A (H5N1) from wild birds, Hong Kong, 2004-2008. Emerging Infectious Diseases, 15, 402–407. doi: 10.3201/eid1503.081190
- Soda, K., Ito, H., Usui, T., Nagai, Y., Ozaki, H., Yamaguchi, T. & Ito, T. (2013). Incursion and spread of H5N1 highly pathogenic avian influenza viruses among wild birds in 2010-11 winter in Japan. Journal of Veterinary Medical Science, 75, 605–612. doi: 10.1292/jvms.12-0512
- Soda, K., Tomioka, Y., Usui, T., Ozaki, H., Yamaguchi, T. & Ito, T. (2020). Pathogenicity of H5 highly pathogenic avian influenza virus in rooks (Corvus frugilegus). Avian Pathology, 49, 261–267. doi: 10.1080/03079457.2020.1724876
- Soda, K., Usui, T., Uno, Y., Yoneda, K., Yamaguchi, T. & Ito, T. (2013). Pathogenicity of an H5N1 highly pathogenic avian influenza virus isolated in the 2010-2011 winter in Japan to mandarin ducks. Journal of Veterinary Medical Science, 75, 619–624. doi: 10.1292/jvms.12-0487
- Takemae, N., Tsunekuni, R., Sharshov, K., Tanikawa, T., Uchida, Y., Ito, H., Soda, K., Usui, T., Sobolev, I., Shestopalov, A., Yamaguchi, T., Mine, J., Ito, T. & Saito, T. (2017). Five distinct reassortants of H5N6 highly pathogenic avian influenza A viruses affected Japan during the winter of 2016-2017. Virology, 512, 8–20. doi: 10.1016/j.virol.2017.08.035
- Usui, T., Soda, K., Sumi, K., Ozaki, H., Tomioka, Y., Ito, H., Murase, T., Kawamoto, T., Miura, M., Komatsu, M., Imanishi, T., Kurobe, M., Ito, T. & Yamaguchi, T. (2020). Outbreaks of highly pathogenic avian influenza in zoo birds caused by HA clade 2.3.4.4 H5N6 subtype viruses in Japan in winter 2016. Transboundary and Emerging Diseases, 67, 686–697.
- Van Borm, S., Thomas, I., Hanquet, G., Lambrecht, B., Boschmans, M., Dupont, G., Decaestecker, M., Snacken, R. & van den Berg, T. (2005). Highly pathogenic H5N1 influenza virus in smuggled Thai eagles, Belgium. Emerging Infectious Diseases, 11, 702–705. doi: 10.3201/eid1105.050211
- van den Brand, J.M., Krone, O., Wolf, P.U., van de Bildt, M.W., van Amerongen, G., Osterhaus, A.D. & Kuiken, T. (2015). Host-specific exposure and fatal neurologic disease in wild raptors from highly pathogenic avian influenza virus H5N1 during the 2006 outbreak in Germany. Veterinary Research, 46, 24. doi: 10.1186/s13567-015-0148-5
- White, M. (1994a). Plate 26 FALCONIDAE III (Falco), 32. American Kestrel. In D. Hoyo, J. Cabot, & J. Sargatal (Eds.), Handbook of the Birds of the World Volume 2: New World Vultures to Guineafowl (pp. 261). Barcelona: Lynx Edicions.
- White, M. (1994b). Plate 28 FALCONIDAE V (Falco), 60. Peregrine Falcon. In D. Hoyo, J. Cabot, & J. Sargatal (Eds.), Handbook of the Birds of the World Volume 2: New World Vultures to Guineafowl (pp. 274–275). Barcelona: Lynx Edicions.
- White, M., Olsen, D. & Kiff, F. (1994). Family FALCONIDAE (FALCONS AND CARACARAS). In D. Hoyo, J. Cabot, & J. Sargatal (Eds.), Handbook of the Birds of the World Volume 2: New World Vultures to Guineafowl (pp. 216–247). Barcelona: Lynx Edicions.
- WHO/OIE/FAO-Evolution-Working-Group. (2008). Toward a unified nomenclature system for highly pathogenic avian influenza virus (H5N1). Emerging Infectious Diseases, 14, e1.
- Xu, X., Subbarao, K., Cox, N.J. & Guo, Y. (1999). Genetic characterization of the pathogenic influenza A/goose/guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology, 261, 15–19. doi: 10.1006/viro.1999.9820
- Yamamoto, Y., Nakamura, K. & Mase, M. (2017). Survival of highly pathogenic avian influenza H5N1 virus in tissues derived from experimentally infected chickens. Applied and Environmental Microbiology, 83, e00604–17. doi: 10.1128/AEM.00604-17
- Yamamoto, Y., Nakamura, K., Okamatsu, M., Miyazaki, A., Yamada, M. & Mase, M. (2008). Detecting avian influenza virus (H5N1) in domestic duck feathers. Emerging Infectious Diseases, 14, 1671–1672. doi: 10.3201/eid1410.080415
