ABSTRACT
Salmonella Infantis is a major public health concern and has become established in the broiler sector in some European countries, as well as globally, and is frequently multidrug resistant (MDR). Three broiler farms in England and Wales, which had incursions of MDR S. Infantis between 2013 and 2017, were investigated longitudinally. The company feed mill and two associated hatcheries were intensively sampled. Following each visit, advice on cleaning, disinfection and other control measures for Salmonella was given to help eliminate S. Infantis from the premises. Four samples collected from inside the broiler houses after cleaning and disinfection were Salmonella-positive, indicating cleaning and disinfection within houses was generally effective. However, the exterior of persistently infected houses remained substantially contaminated and feeding systems could not be sampled. Clearance of S. Infantis from affected houses requires additional attention to decontamination of these aspects. Sixty S. Infantis isolates were tested for antimicrobial susceptibility by disk diffusion tests. All isolates were MDR, with resistance to at least nalidixic acid (Na), tetracycline (T), compound sulphonamide (Su), streptomycin (S) and furazolidone. This is a similar resistance pattern to the previously identified MDR (NaSSuT) clone in some European countries. The study shows that to remove S. Infantis from premises effectively, a combined approach to poultry houses and the surrounding farm environment is necessary. A revised cleaning and disinfection programme was developed that was associated with the clearance of MDR S. Infantis from persistently infected and newly infected broiler flocks, and UK livestock remains free of MDR S. Infantis.
RESEARCH HIGHLIGHTS
Standard cleaning and disinfection protocols did not completely eliminate infection.
A revised cleaning and disinfection programme was developed.
Disinfecting feeder lines and external areas was key to eliminating S. Infantis.
Identified similar antimicrobial resistance pattern to MDR epidemic S. Infantis.
Introduction
Salmonella is one of the commonest causes of food-borne outbreaks in people across Europe (EFSA and ECDC, Citation2019) There are over 2600 different Salmonella enterica serovars (Issenhuth-Jeanjean et al., Citation2014), only a small proportion of which have major impacts on human and animal health. Salmonella Infantis is non-host adapted and has been isolated from a wide range of animals including chickens, pigs, cattle and humans (Lindqvist & Pelkonen, Citation2007; Hauser et al., Citation2012).
In the European Union (EU), S. Infantis is one of the five regulated serovars in breeding flocks of chickens (Gallus gallus) (Regulation (EC) No 1003/2005) but is not a regulated serovar in commercial broiler flocks. In 2018, S. Infantis was the most commonly isolated serovar in the broiler sector, in both animal and meat samples from across the EU, with 36.5% and 56.7%, respectively, of all Salmonella serovars isolated from these sources being S. Infantis (EFSA and ECDC, Citation2019). However, the distribution of this serovar is very uneven, with a few Member States contributing to the majority of reports.
Salmonella Infantis frequently causes foodborne illness in people, consistently being the fourth most reported serovar in human cases of salmonellosis in the EU since 2011 (EFSA BIOHAZ, Citation2019). Compounding the public health concern is the emergence of multidrug-resistant (MDR) clones of S. Infantis around the world, including in Hungary, Austria, Slovenia, Poland, Israel and the United States of America (Nogrady et al., Citation2007; Gal-Mor et al., Citation2010; Nogrady et al., Citation2012; Tate et al., Citation2017; Pate et al., Citation2019). Multidrug resistance refers to isolates that are resistant to antimicrobials, in three or more different antimicrobial classes (Magiorakos et al., Citation2012). Multidrug-resistant Salmonella has been linked with more serious disease in humans, involving longer hospitalization and increased mortality rates compared to drug-susceptible strains (Helms et al., Citation2002; Su et al., Citation2004; Parisi et al., Citation2018).
Reported resistance patterns vary depending on the strain and the antimicrobials used in the test panel. A S. Infantis MDR clone isolated in some European countries, which had resistance to nalidixic acid (Na), streptomycin (S), sulphonamides (Su) and tetracycline (T), NaSSuT, has been reported (Hauser et al., Citation2012; Nogrady et al., Citation2012). Multidrug-resistant S. Infantis now contributes significantly to the proportion of Salmonella isolates, especially from broiler meat, in Europe that are MDR (EFSA and ECDC, Citation2018). Of particular concern is the recent emergence of an extended-spectrum-β-lactamase (ESBL)-producing MDR S. Infantis clone in Italy, Switzerland and the United States, with characteristic resistance to third-generation cephalosporins plus reduced susceptibility to fluoroquinolones (Franco et al., Citation2015; Hindermann et al., Citation2017; Tate et al., Citation2017; EFSA and ECDC, Citation2018). Transferable colistin resistance has also recently been demonstrated in some Italian S. Infantis strains (Carfora et al., Citation2018). Third-generation cephalosporins and fluoroquinolones are critically important human antimicrobials and are treatments of choice for invasive salmonellosis in humans (Collignon et al., Citation2009), so the global emergence of resistance in S. Infantis, involving a readily transferable mega-plasmid, is concerning (Bogomazova et al., Citation2019; Gymoese et al., Citation2019).
A combination of interventions is needed to prevent further spread and persistence of Salmonella on livestock premises. Effective terminal cleaning and disinfection of poultry houses, following depopulation of an infected flock, plays a vital role in the elimination of Salmonella from poultry farms (van de Giessen et al., Citation1994). The effectiveness of cleaning and disinfection is dependent on many factors, including the amount of residual organic matter after initial cleaning (Carrique-Mas et al., Citation2009), the efficacy of the disinfectant used (McDonnell & Russell, Citation1999), the disinfectant concentration, and the way that it is applied (Wales et al., Citation2006). Other control measures implemented on premises, such as biosecurity and pest control, help protect follow-on flocks and avoid re-contamination of poultry houses.
The present study describes recent incursions of S. Infantis into broiler flocks in England and Wales, thereby compromising the UK’s freedom from MDR S. Infantis in poultry, and the control measures implemented to eliminate infection effectively from affected premises.
Materials and methods
Premises and control measures
Three broiler farms in England and Wales that initially had S. Infantis detected in broiler flocks between October 2013 and October 2017 were included in further investigations. The initial positive boot swab samples were collected as part of the Salmonella National Control Plan (Defra, Citation2008). All three farms were contracted to the same poultry company.
All broiler houses across all premises were single-storey conventional buildings with wood shaving-based bedding, pan feeder system, nipple drinkers with spillage cups and wall-mounted exhaust fans and supplementary fans. Farm 1 (F1) consisted of three broiler houses with approximately 43,000 broilers in each. Farm 2 (F2) had four broiler houses with around 32,000 broilers in each, and Farm 3 (F3) consisted of nine houses with approximately 40,000 broilers in each house. The turnaround time between flocks varied between premises and consecutive flocks, but was usually between 5 and 10 days. Poultry houses were always dry cleaned, power washed and disinfected between flocks. All three premises had rodent control programmes in place and rodents were not deemed to be a problem on any of the farms.
Following initial visits, advice was given regarding cleaning and disinfection, as well as other control measures to help facilitate the elimination of the S. Infantis from the premises. During subsequent visits, any changes made on-site were noted and appropriate advice given.
A feed mill and two company hatcheries, from the same integrated poultry company that supplied the broiler premises with feed and chicks, were also intensively sampled.
Sampling
Each broiler farm was visited at least twice, with farm sampling visits taking place between January 2014 and May 2018. Sampling visits and postal sample submissions were continued until S. Infantis was no longer detected on the premises. Sampling after cleaning and disinfection was focused on the broiler houses where S. Infantis had been detected, as well as the surrounding farm environment. During the five post-cleaning and disinfection visits, the inside of each broiler house was intensively sampled. The inside of each house was split into quadrants and samples were collected evenly throughout the house. A sampling protocol was followed which always included collecting samples from floors, walls, air vents, feeders, drinkers, cracks in walls and floors, the anteroom floor and any fixed or moveable fixtures and fittings. The number of samples collected from the different sampling locations varied for each visit depending on the number of houses that were being sampled and the availability of staff, as there was a maximum number of samples that could be collected and processed per visit. Environmental sampling varied between premises and visits, depending on circumstances at the time of the visit and structure of the farm and broiler houses. It usually included the area immediately adjacent to the affected broiler houses such as the concrete apron, drains and drain channels as well as other areas of the poultry farm, encompassing an array of different sample types such as farm driveways, old litter, puddles, feed bins, wild bird faeces, rodent faeces, farm footwear and vehicles on site. Seven of the visits were conducted post re-stocking. Initially, in 2016, flocks at farm 1 (F1) were sampled by a combination of boot, litter and dust swabs. Latterly, flocks at F1 and the other two premises were sampled using 20 litter and 20 dust swabs, and environmental samples were also collected. The number of samples collected at each visit ranged between 140 and 320.
Samples were principally collected using clean disposable gloves and sterile hand-held gauze swabs which were used to swab 0.5 m2 of the area of interest, before being placed into plastic jars containing 225 ml of buffered peptone water (BPW) (Merck: Merck Millipore, Watford, UK). On visits conducted post re-stocking, litter and dust samples were collected using sterile hand-held gauze swabs, which were also placed into plastic jars containing 225 ml BPW. Boot swabs were taken using at least 100 shuffling steps per pair of boot swabs, covering a representative area of the broiler house. Boot swabs were then placed into plastic jars containing 225 ml of BPW and processed in the same way as the hand-held gauze swabs.
The two hatcheries were each visited once. The hatcheries were intensively sampled throughout, including egg storage areas, setters, hatchers and chick processing areas. Hand-held gauze swabs were used to swab 0.5 m2 of the area of interest, before being placed into plastic jars containing 225 ml BPW.
The feed mill was visited once. Sampling focused on dust, any feed spillages and aggregated material collected from all stages of production. The material was placed into new plastic jars, using clean disposable gloves. Dry samples collected at the feed mill were processed the following day. Each sample was spilt into four sub-samples (25 g) before being added to 225 ml BPW and processed as for the hand-held gauze swabs. Additionally, some hand-held swabs were used to collect samples from pooled water outside of the feed mill.
Postal submissions of 376 samples collected by the farm manager or company field manager were received from Farm F1 at the Animal and Plant Health Agency (APHA) Weybridge intermittently from December 2013 through to March 2018. The majority (227/376) of samples were collected using boot swabs. Faecal samples were frequently (119/376) also collected and occasionally litter (6/376) and/or dust (24/376) were additionally collected. On the day of receipt, samples were added to 225 ml BPW and processed using the same method as samples collected during visits.
Salmonella isolation
After collection, all samples were processed at the APHA Weybridge laboratory. Samples were pre-enriched by incubation in 225 ml BPW at 37 ± 1°C for 16–20 h, with 0.1 ml of the broth then used to inoculate modified semi-solid Rappaport-Vassiliadis agar (MSRV; Mast: Mast Group Ltd, Bootle, UK) containing 1 mg/ml novobiocin (Sigma; Sigma-Aldrich, Dorset, UK). Following incubation at 41.5 ± 1°C for 21–27 h, a 1 µl loop was used to transfer spreading growth from the MSRV onto Rambach agar (Merck), which was then incubated at 37 ± 1°C for 21–27 h. Any suspect Salmonella colonies were confirmed using slide agglutination tests, with all positive isolates being confirmed and serotyped according to the White-Kaufmann-Le Minor Scheme (Grimont & Weill, Citation2007).
Statistical analysis
Data from postal submissions were split according to the age of the birds when the sampling took place (13–21 days old versus 29–35 days old). A binomial test was used to compare the proportion of samples that were S. Infantis-positive in each age group using the in-built prop.test function in R (v3.4.4).
Considering samples collected at Farm 1 (F1) only, a binomial test was used to compare the proportion of samples that were S. Infantis-positive collected inside the broiler houses versus outside the houses using the in-built prop.test function in R (v3.4.4).
Antimicrobial susceptibility testing
To determine antimicrobial resistance, a selection of S. Infantis isolates was subjected to disk diffusion tests using ISO-Sensitest agar (Oxoid; Oxoid Ltd, Basingstoke, UK) and antimicrobial-containing discs (Oxoid). The discs contained the following antimicrobials: amikacin (30 µg), apramycin (15 µg), gentamicin (10 µg), neomycin (10 µg), streptomycin (10 µg), tetracycline (10 µg), nalidixic acid (30 µg), ciprofloxacin (1 µg), ampicillin (10 µg), amoxycillin/clavulanic acid (30 µg), ceftazidime (30 µg), cefotaxime (30 µg), furazolidone (15 µg), sulphamethoxazole trimethoprim (25 µg), compound sulphonamide (300 µg) and chloramphenicol (30 µg). Where available, British Society for Antimicrobial Chemotherapy (BSAC) breakpoints were used to determine whether cultures were resistant or sensitive (APHA, Citation2019). For apramycin, neomycin and tetracycline, where BSAC breakpoints were not available, previously derived breakpoints were used (UK-VARSS, Citation2018).
Results
Farm 1 (F1)
F1, the index case, was visited on seven occasions (visits A–G) between 2014 and 2018; visits were either carried out after cleaning and disinfection or post-restocking, and findings are summarized in . Salmonella Infantis was initially isolated from the premises in October 2013, and the first sampling visit (visit A) in January 2014 followed intensive cleaning and disinfection of the affected houses. No S. Infantis was isolated from inside the main part of the affected houses indicating that the cleaning and disinfection programme was effective. However, contamination was found inside the anteroom, including in a slave feed hopper that would have been replaced inside the house before the replacement chicks were introduced. There was extensive contamination of the area outside the houses, including on farm boots for use inside the broiler house. Salmonella Montevideo was also found in a puddle during the first visit.
Table 1. Findings from sampling visits to broiler farm F1.
Visit B was conducted post re-stocking in July 2016, following positive postal samples in the previous flock. Salmonella Infantis was not isolated from samples collected within the houses when birds were 5 days old, but there were multiple positive environmental samples from outside the houses. Further positive postal submissions preceded visit C which was made after cleaning and disinfection. Salmonella Infantis was isolated inside the sampled house from the floor, walls and pan feeders. Areas outside the house were contaminated, especially the farm track and also a pair of farm boots.
Visit D (September 2016) was conducted after cleaning and disinfection just before placement of birds. No Salmonella was isolated from inside the house sampled, but again there was extensive contamination of the external environment with S. Infantis. Additionally, Salmonella 13,23:i:- was isolated from a sample collected from the concrete surfaces immediately outside the house.
A further post re-stocking visit (visit E) in October 2016 had no Salmonella isolated from the premises, when birds were 10 days old. Postal submissions continued to be intermittently S. Infantis-positive until May 2017, when there was then a period of 4 months with Salmonella-negative submissions. However, postal submissions from flocks, following electricians replacing light fittings within houses after cleaning and disinfection, were again positive.
Post re-stocking visits F and G in 2018, were both negative and were carried out after consecutive negative postal submissions.
The proportion of samples that were positive for S. Infantis inside the broiler houses at F1 (0.005) was lower than the proportion of samples positive for S. Infantis collected from outside the broiler houses at F1 (0.16). This difference was highly significant (χ2 = 88.8, df = 1, P < 0.01), and the difference between the two proportions had a 95% confidence interval of 0.11–0.20.
Postal submissions
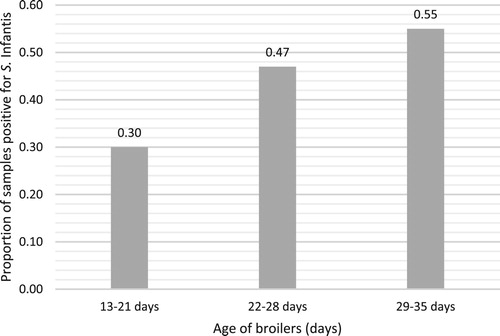
Postal submissions received from farm F1 were collected when broilers’ ages ranged from 13–35 days old. The highest proportion of Salmonella-positive samples were collected from the oldest groups of broilers, aged between 29 and 35 days, see . Samples collected from birds aged 29–35 days old (0.55 proportion of samples were positive) were significantly more likely to be positive than samples collected when birds were 13–21 days old (0.30 proportion of samples were positive) (χ2 = 16.7, df = 1, P < 0.01).
Farm 2 (F2)
F2 was visited three times, in April, July and October 2017; findings are summarized in . Visit A was conducted after cleaning and disinfection. Salmonella Infantis was isolated from a single sample taken from the fan housing inside one of the houses and from a sample of old litter that remained outside another house. Salmonella Infantis-positive postal submissions were received from this premises until May 2017.
Table 2. Findings from sampling visits to broiler farm F2.
During the first post re-stocking visit (visit B), S. Infantis was only isolated from a trailer outside the houses. No Salmonella was found in samples collected during the final post re-stocking visit.
Farm 3 (F3)
F3 was visited twice, and findings are summarized in . The first visit in September 2017 had no Salmonella isolated from any of the samples collected after cleaning and disinfection. The second visit, which was post re-stocking in January 2018, only found S. Montevideo in one house, as well as in a litter and a dust sample.
Table 3. Findings from sampling visits to broiler farm F3.
Feed mill (M1)
M1, a company feed mill that supplies feed to the affected broiler premises, was visited once, in June 2016. Three hundred and fourteen subsamples were cultured and results are summarized in . Salmonella Durham was isolated from a sample collected from the ingredient storage, S. Mbandaka from an intake pit and Salmonella 4,12:d:- from the fat coater and cooler. No S. Infantis was isolated from the feed mill and none of the serovars found in the mill were found in farm samples.
Table 4. Findings from sampling visit to company feed mill associated with Salmonella Infantis affected broiler farms, June 2016.
Hatchery (H1)
H1 was visited in August 2016, when S. Livingstone was isolated from multiple samples collected from the setter, egg transfer and hatcher areas, see .
Table 5. Findings from sampling visits to two hatcheries associated with Salmonella Infantis affected broiler farms.
Hatchery (H2)
H2 was visited later on in the investigations, in September 2017; results are summarized in . Only one sample yielded Salmonella, which was S. Typhimurium isolated from a swab from the floor of a workshop, collected outside the hatchery.
Antimicrobial resistance
All 60 S. Infantis isolates that were antimicrobial sensitivity tested had resistance to nalidixic acid, tetracycline, furazolidone, streptomycin and compound sulphonamide. The majority of isolates (43/55; 78%) collected during visits to farms F1 and F2, had an additional resistance to sulphamethoxazole and trimethoprim combination. None of the original operator samples (5), collected from all three premises, had resistance to sulphamethoxazole/trimethoprim.
Discussion
The three broiler farms investigated during the present study experienced varying lengths of time to clear S. Infantis from their sites. Elimination of S. Infantis in poultry houses is known to be very difficult (Drauch et al., Citation2020) and it became evident that standard cleaning and disinfection protocols, using orchard sprayers to apply disinfectant within the poultry houses, were not completely eliminating infection from within affected poultry houses. Very few samples collected from inside the poultry houses after cleaning and disinfection had Salmonella isolated, indicating that, generally, cleaning and disinfection was effective inside the houses. Nogardy et al. (Citation2008) also found that on most premises cleaning and disinfection inside of the houses appeared to be effective against S. Infantis, based on bacteriological sampling, although this does not always seem to be the case (Pate et al., Citation2019). However, the interior of pan feeding systems could not be sampled routinely and S. Infantis-positive samples collected during the third visit to farm F3, after cleaning and disinfection, included residual feed from inside a pan feeder. Furthermore, at the first visit to farm F1, a clean slave feed hopper had been placed within the anteroom during cleaning and disinfection of the house. When sampled, S. Infantis was isolated, highlighting the risk posed by open slave feed hoppers, which can be contaminated when dust originating from the faeces and integument of infected birds settles within them. Contaminated feed is then able to disseminate through the feeder lines.
Salmonella has the ability to form protective biofilms, which can act as a continuous source of contamination (Joseph et al., Citation2001). Recent studies have suggested that S. Infantis is a relatively poor biofilm producer (Schonewille et al., Citation2012; Pate et al., Citation2019). Moraes et al. (Citation2018) recently found that S. Infantis ranged in its biofilm forming ability between isolates from strong to weak. Interestingly, some of the S. Infantis strains isolated from these premises have been shown to be good biofilm producers under laboratory conditions (M. Chambers, personal communication). To what extent these laboratory experiments replicate conditions in the natural environment is the subject of an on-going study. Biofilm formation likely contributes to Salmonella survival in certain areas such as feed or drinker lines: areas which are both difficult to clean and disinfect, and in which to detect infection (Maes et al., Citation2019).
Although, no longer an option following the ban on formaldehyde use in feed within the European Union from February 2018 (Regulation (EU) 2018/183), a commercially available product containing formaldehyde was initially used to treat feed at farm F1 to help eliminate Salmonella from biofilms within the feeding system. Latterly, dismantling feeders and soaking in formalin, although time consuming, seemed to be an effective way of preventing residual contamination. Alternatively, disinfecting feeder lines and feeder pans at high pressure using a glutaraldehyde and formaldehyde combination product, plus formalin by pressure washing, along with sufficient drainage of feeder pans, was also found to be effective. This is in agreement with previous farm studies which found formalin application to be the most effective disinfection methodology against Salmonella (Davies & Wray, Citation1995; Carrique-Mas et al., Citation2009). Combination products containing formaldehyde and glutaraldehyde have also been found to be effective against Salmonella on farms (Carrique-Mas et al., Citation2009; Mueller-Doblies et al., Citation2010) and results of a recent in vitro disinfectant study specifically focused on S. Infantis concurred (Drauch et al., Citation2020).
Multiple visits to farm F1 found widespread external environmental contamination. The ability of Salmonella to survive well in the environment, from months to years (Sandvang et al., Citation2000; Baloda et al., Citation2001), means that any attempt to eradicate Salmonella from a premises requires a combined approach to both the poultry houses and the surrounding farm environment. Salmonella Infantis was repeatedly isolated from mud, puddles and tracks around farm F1, until these reservoirs of infection were addressed. Reducing the contamination of these high risk areas, by a combination of approaches, was essential in elimination of S. Infantis from the premises. Different strategies included disinfection with formalin, and lime application. Formaldehyde is an effective disinfectant in the presence of organic matter (Davies & Wray, Citation1995; McDonnell & Russell, Citation1999; Davies & Breslin, Citation2003), helping reduce contamination in some external areas, especially concreted areas. Lime, which increases the pH and helps to reduce viable numbers of Salmonella (Bennett et al., Citation2003; Nyberg et al., Citation2011), was also applied in some areas.
In the current study, S. Infantis was isolated from various samples collected from anterooms including an electrical control panel, floor and a slave feed hopper. Particular attention needs to be paid to the cleaning and disinfection of anterooms, as they act as a buffer zone between the broiler houses and the external environment. These areas, with non-waterproof electrical fixtures and fittings, can be more difficult to clean and disinfect effectively. Other ancillary areas found contaminated included a toilet and a tea room. The tea room was a particular concern because of the zoonotic implications of S. Infantis.
On the same premises, farm boots were found to be contaminated, thus personnel were likely to be spreading contamination on footwear. Contaminated vehicles, personnel and equipment that may be used within poultry houses to lay litter, feed and place or thin birds can act as vectors for re-contaminating poultry houses, and consideration also needs to be given to rodents and wild birds (Davies & Breslin, Citation2003). Another potential route of re-contamination into the houses was new litter that had spilled during delivery onto the apron being swept into the houses.
Postal samples received over a 3-year period from farm F1, were intermittently positive for S. Infantis. Samples collected later in a flock’s life were significantly more likely to be positive, suggesting a slow spread of infection across the house and suggesting that the recommended sampling time of around 3 weeks of age may not always be suitable for this serovar (Gradel et al., Citation2002). It is unknown whether S. Infantis remained within affected houses between positive flocks, possibly below the limit of detection, or whether it was intermittently being re-introduced into the house from the external environment. This is similar to a study in Hungary, with flocks placed in two previously S. Infantis-contaminated broiler houses; S. Infantis was only detected at very low levels in one flock when the birds were a week old and the other at 2 weeks old and prevalence continued to increase during the rearing period (Nogrady et al., Citation2008).
Over time and following consecutive visits to farm F1, cleaning, disinfection and other interventions were progressively carried out, culminating in no S. Infantis being isolated during the final three post-restocking visits. Disinfecting at high pressure using a glutaraldehyde and formaldehyde combination product, plus formalin power washing, was associated with no further carry over of infection and rapid clearance of new infection. Increased attention to feeder lines and feeder pans, as well as the external environment, was key. Additional important contributing steps taken included increased site security and biosecurity, such as introducing a hygiene lock, limiting vehicle entry to the site, more effective use of hygiene barriers at house entrances and increased disinfectant boot dip availability and use. These steps, coupled with decreasing contamination of areas adjacent to houses, by a combination of concreting tracks, disinfection and lime application, were likely to have resulted in limited re-contamination of houses.
Incursions of MDR S. Infantis onto both broiler farms F2 and F3 were more quickly addressed. Control measures, refined through previous experience on farm F1, were successfully implemented by highly motivated farm managers. Intensive cleaning and disinfection protocols were followed on the inside of affected houses and potential external environmental sources of contamination were immediately addressed. These rapid interventions reduced the spread of S. Infantis across the site, preventing persistence of infection on the premises.
All S. Infantis isolates that were susceptibility tested in this study were MDR. The BSAC breakpoints used in this study were fully harmonized with the clinical breakpoints developed by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (APHA, Citation2019) although, unlike EUCAST and Clinical and Laboratory Standard Institute (CLSI), BSAC does not have an intermediate classification. A similar resistance pattern was observed to the previously identified MDR (NaSSuT) clone in some European countries, such as Austria, Hungary, Romania and Poland (Nogrady et al., Citation2012), suggesting a possible common European origin. A recent study into the molecular epidemiology of S. Infantis in Europe confirmed that the UK isolates, which included isolates from these UK premises, were closely related to other European strains (Alba et al., Citation2020).
MDR S. Infantis with the same antimicrobial resistance profile as the isolates from the broiler premises had previously been found within the company meat processing plant in products that included meat imported from Europe. Whole genome sequencing (WGS) of a selection of isolates from the three broiler premises and the meat processing plant found that all the isolates had the IncFIB(pN55391) plasmid (Publication in progress, APHA) using Staramr (https://github.com/phac-nml/staramr) searches against the PlasmidFinder database (Carattoli et al., Citation2014).
All three premises were broiler farms from within the same integrated company. Feed and day-old chicks are known to be potential common sources of Salmonella within integrated poultry companies (Davies et al., Citation1997, Citation2001; Kim et al., Citation2007; Crabb et al., Citation2018). Therefore, the company hatcheries and feed mill were visited and comprehensively sampled for the presence of Salmonella to explore potential common sources. No S. Infantis was isolated from either of the hatcheries or the feed mill, making them an unlikely common source. Similarly, Karacan Sever & Akan (Citation2019) found that S. Infantis was predominantly being spread to broilers by environmental contamination, rather than through the breeding pyramid. Selected isolates from the three broiler premises that were sequenced were closely related, with some isolates from different premises having identical single nucleotide polymorphism (SNP) addresses and others were within a 5-SNP single linkage cluster and considered highly likely to be epidemiologically linked (Chattaway et al., Citation2019; Tang et al., Citation2019). Additionally, several isolates from the company meat processing plant and two of the broiler premises were within the 5-SNP cluster and within eight SNP differences with the third broiler premises (Petrovska et al., Citation2016; Dallman et al., Citation2018). No direct epidemiological links between the processing plant and broiler farms were identified in this investigation, but it seems likely that the strains involved originated from outside the UK and these isolates have recently been confirmed by WGS as similar to the European epidemic strain (Alba et al., Citation2020).
As confirmed by WGS results, incursions onto the different premises were highly likely to be epidemiologically linked. The affected premises shared the same company cleaning and catching teams for thinning and depopulation, as well as electricians, so it is possible that S. Infantis could have been spread by the movement of equipment or personnel between premises. Processing plant transportation, vehicle and personnel movements have also been highlighted as potential sources of Salmonella within other integrated poultry chains (Davies et al., Citation1997; Crabb et al., Citation2018).
Consideration of other routes of infection onto poultry units is through the movement of animal by-products (ABP). Many units do not have on-site incinerators and rely on the collection of ABP disposal bins from farm. Disposal bins are recycled, travelling to and from high risk areas, so should be regarded as a potential risk pathway. For S. Infantis in particular, the risk is increased if ABP disposal bins have contact with imported broiler meat from certain high risk European countries whilst being emptied and held at ABP plants.
Once introduced within a country, the speed at which S. Infantis spreads and establishes itself within the production chain of that country gives rise to great concern (Nogrady et al., Citation2008; Gal-Mor et al., Citation2010; Hauser et al., Citation2012). Any future incursions of S. Infantis into the poultry sector will require timely, coordinated effort from all involved stakeholders. The revised cleaning and disinfection programme that was developed through the present study was associated with the clearance of MDR S. Infantis from persistently infected and newly infected broiler flocks and should be utilized to ensure that UK livestock remains free of major European epidemic S. Infantis.
Acknowledgements
The authors would like to thank all farm, feed mill and hatchery personnel that were involved in this investigation. Also laboratory staff at APHA for processing and serotyping isolates, Liljana Petrovska-Holmes and Jaromir Guzinski for whole genome sequence analysis and to Arthur Barker for his help in collating the data. This work was funded by the Department for Environment, Food and Rural Affairs (Defra) under Project CR2000A.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alba, P., Leekitcharoenphon, P., Carfora, V., Amoruso, R., Cordaro, G., Di Matteo, P., Ianzano, A., Iurescia, M., Diaconu, E.L., Study Group, E.N., Pedersen, S.K., Guerra, B., Hendriksen, R.S., Franco, A. & Battisti, A. (2020). Molecular epidemiology of Salmonella Infantis in Europe: insights into the success of the bacterial host and its parasitic pESI-like megaplasmid. Microbial Genomics, 6. doi: 10.1099/mgen.0.000365
- APHA, Animal and Plant Health Agency. (2019). Salmonella in Livestock Production in GB, 2018.
- Baloda, S.B., Christensen, L. & Trajcevska, S. (2001). Persistence of a Salmonella enterica serovar typhimurium DT12 clone in a piggery and in agricultural soil amended with Salmonella-contaminated slurry. Applied Environmental Microbiology, 67, 2859–2862. doi: 10.1128/AEM.67.6.2859-2862.2001
- Bennett, D.D., Higgins, S.E., Moore, R.W., Beltran, R., Caldwell, D.J., Byrd, J.A. & Hargis, B.M. (2003). Effects of lime on Salmonella enteritidis survival in vitro. Journal of Applied Poultry Research, 12, 65–68. doi: 10.1093/japr/12.1.65
- Bogomazova, A.N., Gordeeva, V.D., Krylova, E.V., Soltynskaya, I.V., Davydova, E.E., Ivanova, O.E. & Komarov, A.A. (2019). Mega-plasmid found worldwide confers multiple antimicrobial resistance in Salmonella Infantis of broiler origin in Russia. International Journal of Food Microbiology, 319, 108497. doi: 10.1016/j.ijfoodmicro.2019.108497
- Carattoli, A., Zankari, E., Garcia-Fernandez, A., Voldby Larsen, M., Lund, O., Villa, L., Moller Aarestrup, F. & Hasman, H. (2014). In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrobial Agents and Chemotherapy, 58, 3895–3903. doi: 10.1128/AAC.02412-14
- Carfora, V., Alba, P., Leekitcharoenphon, P., Ballaro, D., Cordaro, G., Di Matteo, P., Donati, V., Ianzano, A., Iurescia, M., Stravino, F., Tagliaferri, T., Battisti, A. & Franco, A. (2018). Colistin resistance mediated by mcr-1 in ESBL-producing, multidrug resistant Salmonella Infantis in broiler chicken industry, Italy (2016–2017). Frontiers in Microbiology, 9, 1880. doi: 10.3389/fmicb.2018.01880
- Carrique-Mas, J.J., Marin, C., Breslin, M., McLaren, I. & Davies, R. (2009). A comparison of the efficacy of cleaning and disinfection methods in eliminating Salmonella spp. from commercial egg laying houses. Avian Pathology, 38, 419–424. doi: 10.1080/03079450903193768
- Chattaway, M.A., Dallman, T.J., Larkin, L., Nair, S., McCormick, J., Mikhail, A., Hartman, H., Godbole, G., Powell, D., Day, M., Smith, R. & Grant, K. (2019). The transformation of reference microbiology methods and surveillance for Salmonella with the use of whole genome sequencing in England and Wales. Frontiers in Public Health, 7, 317. doi: 10.3389/fpubh.2019.00317
- Collignon, P., Powers, J.H., Chiller, T.M., Aidara-Kane, A. & Aarestrup, F.M. (2009). World Health Organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies for the use of antimicrobials in food production animals. Clinical Infectious Diseases, 49, 132–141. doi: 10.1086/599374
- Crabb, H.K., Allen, J.L., Devlin, J.M., Firestone, S.M., Wilks, C.R. & Gilkerson, J.R. (2018). Salmonella spp. transmission in a vertically integrated poultry operation: clustering and diversity analysis using phenotyping (serotyping, phage typing) and genotyping (MLVA). PloS One, 13, e0201031. doi: 10.1371/journal.pone.0201031
- Dallman, T., Ashton, P., Schafer, U., Jironkin, A., Painset, A., Shaaban, S., Hartman, H., Myers, R., Underwood, A., Jenkins, C. & Grant, K. (2018). SnapperDB: a database solution for routine sequencing analysis of bacterial isolates. Bioinformatics (Oxford, England), 34, 3028–3029. doi: 10.1093/bioinformatics/bty212
- Davies, R. & Breslin, M. (2003). Observations on Salmonella contamination of commercial laying farms before and after cleaning and disinfection. Veterinary Record, 152, 283–287. doi: 10.1136/vr.152.10.283
- Davies, R., Breslin, M., Corry, J.E., Hudson, W. & Allen, V.M. (2001). Observations on the distribution and control of Salmonella species in two integrated broiler companies. Veterinary Record, 149, 227–232. doi: 10.1136/vr.149.8.227
- Davies, R.H., Nicholas, R.A., McLaren, I.M., Corkish, J.D., Lanning, D.G. & Wray, C. (1997). Bacteriological and serological investigation of persistent Salmonella enteritidis infection in an integrated poultry organisation. Veterinary Microbiology, 58, 277–293. doi: 10.1016/S0378-1135(97)00157-0
- Davies, R.H. & Wray, C. (1995). Observations on disinfection regimens used on Salmonella enteritidis infected poultry units. Poultry Science, 74, 638–647. doi: 10.3382/ps.0740638
- Defra, Department for Environment, Food and Rural Affairs. (2008). UK National Control Programme for Salmonella in Chickens (Gallus gallus) Reared for Meat (Broilers ).
- Drauch, V., Ibesich, C., Vogl, C., Hess, M. & Hess, C. (2020). In-vitro testing of bacteriostatic and bactericidal efficacy of commercial disinfectants against Salmonella Infantis reveals substantial differences between products and bacterial strains. International Journal of Food Microbiology, 328, 108660. doi: 10.1016/j.ijfoodmicro.2020.108660
- EFSA and ECDC, European Food Safety Authority and European Centre for Disease Prevention and Control. (2018). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA Journal, 270.
- EFSA and ECDC, European Food Safety Authority and European Centre for Disease Prevention and Control. (2019). The European Union One Health 2018 Zoonoses Report. EFSA Journal, 17, 296.
- EFSA BIOHAZ, European Food Safety Authority Panel on Biological Hazards. (2019). Salmonella control in poultry flocks and its public health impact. EFSA Journal, 17, 94.
- Franco, A., Leekitcharoenphon, P., Feltrin, F., Alba, P., Cordaro, G., Iurescia, M., Tolli, R., D'Incau, M., Staffolani, M., Di Giannatale, E., Hendriksen, R.S. & Battisti, A. (2015). Emergence of a clonal lineage of multidrug-resistant ESBL-producing Salmonella Infantis transmitted from broilers and broiler meat to humans in Italy between 2011 and 2014. PloS One, 10, e0144802. doi: 10.1371/journal.pone.0144802
- Gal-Mor, O., Valinsky, L., Weinberger, M., Guy, S., Jaffe, J., Schorr, Y.I., Raisfeld, A., Agmon, V. & Nissan, I. (2010). Multidrug-resistant Salmonella enterica serovar Infantis, Israel. Emerging Infectious Diseases, 16, 1754–1757. doi: 10.3201/eid1611.100100
- Gradel, K.O., Andersen, J. & Madsen, M. (2002). Comparisons of sampling procedures and time of sampling for the detection of Salmonella in Danish infected chicken flocks raised in floor systems. Acta Veterinaria Scandinavica, 43, 21–30. doi: 10.1186/1751-0147-43-21
- Grimont, P. & Weill, F. (2007). Antigenic Formulae of the Salmonella SErovars 9th Edn. Paris: WHO Collaborating Centre for Reference and Research on Salmonella.
- Gymoese, P., Kiil, K., Torpdahl, M., Osterlund, M.T., Sorensen, G., Olsen, J.E., Nielsen, E.M. & Litrup, E. (2019). WGS based study of the population structure of Salmonella enterica serovar Infantis. BMC Genomics, 20, 870. doi: 10.1186/s12864-019-6260-6
- Hauser, E., Tietze, E., Helmuth, R., Junker, E., Prager, R., Schroeter, A., Rabsch, W., Fruth, A., Toboldt, A. & Malorny, B. (2012). Clonal dissemination of Salmonella enterica serovar Infantis in Germany. Foodborne Pathogens and Disease, 9, 352–360. doi: 10.1089/fpd.2011.1038
- Helms, M., Vastrup, P., Gerner-Smidt, P. & Molbak, K. (2002). Excess mortality associated with antimicrobial drug-resistant Salmonella Typhimurium. Emerging Infectious Diseases, 8, 490–495. doi: 10.3201/eid0805.010267
- Hindermann, D., Gopinath, G., Chase, H., Negrete, F., Althaus, D., Zurfluh, K., Tall, B.D., Stephan, R. & Nuesch-Inderbinen, M. (2017). Salmonella enterica serovar Infantis from food and human infections, Switzerland, 2010–2015: poultry-related multidrug resistant clones and an emerging ESBL producing clonal lineage. Frontiers in Microbiology, 8, 1322. doi: 10.3389/fmicb.2017.01322
- Issenhuth-Jeanjean, S., Roggentin, P., Mikoleit, M., Guibourdenche, M., de Pinna, E., Nair, S., Fields, P.I. & Weill, F.X. (2014). Supplement 2008-2010 (no. 48) to the White-Kauffmann-Le Minor scheme. Research in Microbiology, 165, 526–530. doi: 10.1016/j.resmic.2014.07.004
- Joseph, B., Otta, S.K., Karunasagar, I. & Karunasagar, I. (2001). Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. International Journal of Food Microbiology, 64, 367–372. doi: 10.1016/S0168-1605(00)00466-9
- Karacan Sever, N. & Akan, M. (2019). Molecular analysis of virulence genes of Salmonella Infantis isolated from chickens and turkeys. Microbial Pathogenesis, 126, 199–204. doi: 10.1016/j.micpath.2018.11.006
- Kim, A., Lee, Y.J., Kang, M.S., Kwag, S.I. & Cho, J.K. (2007). Dissemination and tracking of Salmonella spp. in integrated broiler operation. Journal of Veterinary Science, 8, 155–161. doi: 10.4142/jvs.2007.8.2.155
- Lindqvist, N. & Pelkonen, S. (2007). Genetic surveillance of endemic bovine Salmonella Infantis infection. Acta Veterinaria Scandinavica, 49, 15. doi: 10.1186/1751-0147-49-15
- Maes, S., Vackier, T., Nguyen Huu, S., Heyndrickx, M., Steenackers, H., Sampers, I., Raes, K., Verplaetse, A. & De Reu, K. (2019). Occurrence and characterisation of biofilms in drinking water systems of broiler houses. BMC Microbiology, 19, 77. doi: 10.1186/s12866-019-1451-5
- Magiorakos, A.P., Srinivasan, A., Carey, R.B., Carmeli, Y., Falagas, M.E., Giske, C.G., Harbarth, S., Hindler, J.F., Kahlmeter, G., Olsson-Liljequist, B., Paterson, D.L., Rice, L.B., Stelling, J., Struelens, M.J., Vatopoulos, A., Weber, J.T. & Monnet, D.L. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection, 18, 268–281. doi: 10.1111/j.1469-0691.2011.03570.x
- McDonnell, G. & Russell, A.D. (1999). Antiseptics and disinfectants: activity, action, and resistance. Clinical Microbiology Reviews, 12, 147–179. doi: 10.1128/CMR.12.1.147
- Moraes, J.O., Cruz, E.A., Souza, E.G.F., Oliveira, T.C.M., Alvarenga, V.O., Pena, W.E.L., Sant'Ana, A.S. & Magnani, M. (2018). Predicting adhesion and biofilm formation boundaries on stainless steel surfaces by five Salmonella enterica strains belonging to different serovars as a function of pH, temperature and NaCl concentration. International Journal of Food Microbiology, 281, 90–100. doi: 10.1016/j.ijfoodmicro.2018.05.011
- Mueller-Doblies, D., Carrique-Mas, J.J., Sayers, A.R. & Davies, R.H. (2010). A comparison of the efficacy of different disinfection methods in eliminating Salmonella contamination from turkey houses. Journal of Applied Microbiology, 109, 471–479.
- Nogrady, N., Kardos, G., Bistyak, A., Turcsanyi, I., Meszaros, J., Galantai, Z., Juhasz, A., Samu, P., Kaszanyitzky, J.E., Paszti, J. & Kiss, I. (2008). Prevalence and characterization of Salmonella Infantis isolates originating from different points of the broiler chicken-human food chain in Hungary. International Journal of Food Microbiology, 127, 162–167. doi: 10.1016/j.ijfoodmicro.2008.07.005
- Nogrady, N., Kiraly, M., Davies, R. & Nagy, B. (2012). Multidrug resistant clones of Salmonella Infantis of broiler origin in Europe. International Journal of Food Microbiology, 157, 108–112. doi: 10.1016/j.ijfoodmicro.2012.04.007
- Nogrady, N., Toth, A., Kostyak, A., Paszti, J. & Nagy, B. (2007). Emergence of multidrug-resistant clones of Salmonella Infantis in broiler chickens and humans in Hungary. Journal of Antimicrobial Chemotherapy, 60, 645–648. doi: 10.1093/jac/dkm249
- Nyberg, K.A., Vinneras, B., Lewerin, S.S., Kjellberg, E. & Albihn, A. (2011). Treatment with Ca(OH)2 for inactivation of Salmonella Typhimurium and Enterococcus faecalis in soil contaminated with infected horse manure. Journal of Applied Microbiology, 110, 1515–1523. doi: 10.1111/j.1365-2672.2011.05006.x
- Parisi, A., Crump, J.A., Glass, K., Howden, B.P., Furuya-Kanamori, L., Vilkins, S., Gray, D.J. & Kirk, M.D. (2018). Health outcomes from multidrug-resistant Salmonella infections in high-income countries: a systematic review and meta-analysis. Foodborne Pathogens and Disease, 15, 428–436. doi: 10.1089/fpd.2017.2403
- Pate, M., Micunovic, J., Golob, M., Vestby, L.K. & Ocepek, M. (2019). Salmonella Infantis in broiler flocks in Slovenia: the prevalence of multidrug resistant strains with high genetic homogeneity and low biofilm-forming ability. Biomed Research International, 2019, 4981463. doi: 10.1155/2019/4981463
- Petrovska, L., Mather, A.E., AbuOun, M., Branchu, P., Harris, S.R., Connor, T., Hopkins, K.L., Underwood, A., Lettini, A.A., Page, A., Bagnall, M., Wain, J., Parkhill, J., Dougan, G., Davies, R. & Kingsley, R.A. (2016). Microevolution of monophasic Salmonella Typhimurium during epidemic, United Kingdom, 2005-2010. Emerging Infectious Diseases, 22, 617–624. doi: 10.3201/eid2204.150531
- Sandvang, D., Jensen, L.B., Baggesen, D.L. & Baloda, S.B. (2000). Persistence of a Salmonella enterica serotype typhimurium clone in Danish pig production units and farmhouse environment studied by pulsed field gel electrophoresis (PFGE). FEMS Microbiology Letters, 187, 21–25. doi: 10.1111/j.1574-6968.2000.tb09130.x
- Schonewille, E., Nesse, L.L., Hauck, R., Windhorst, D., Hafez, H.M. & Vestby, L.K. (2012). Biofilm building capacity of Salmonella enterica strains from the poultry farm environment. FEMS Immunology and Medical Microbiology, 65, 360–365. doi: 10.1111/j.1574-695X.2012.00966.x
- Su, L.H., Chiu, C.H., Chu, C. & Ou, J.T. (2004). Antimicrobial resistance in nontyphoid Salmonella serotypes: a global challenge. Clinical Infectious Diseases, 39, 546–551. doi: 10.1086/422726
- Tang, Y., Davies, R. & Petrovska, L. (2019). Identification of genetic features for attenuation of two Salmonella enteritidis vaccine strains and differentiation of these from wildtype isolates using whole genome sequencing. Frontiers in Veterinary Science, 6, 447. doi: 10.3389/fvets.2019.00447
- Tate, H., Folster, J.P., Hsu, C.H., Chen, J., Hoffmann, M., Li, C., Morales, C., Tyson, G.H., Mukherjee, S., Brown, A.C., Green, A., Wilson, W., Dessai, U., Abbott, J., Joseph, L., Haro, J., Ayers, S., McDermott, P.F. & Zhao, S. (2017). Comparative analysis of extended-spectrum-beta-lactamase CTX-M-65-producing Salmonella enterica serovar Infantis isolates from humans, food animals, and retail chickens in the United States. Antimicrobial Agents and Chemotherapy, 61. doi: 10.1128/AAC.00488-17
- UK-VARSS, Veterinary Medicines Directorate. (2018). UK Veterinary Antibiotic Resistance and Sales Surveillance, UK-VARSS, Supplementary Material.
- van de Giessen, A.W., Ament, A.J. & Notermans, S.H. (1994). Intervention strategies for Salmonella enteritidis in poultry flocks: a basic approach. International Journal of Food Microbiology, 21, 145–154. doi: 10.1016/0168-1605(94)90207-0
- Wales, A., Breslin, M. & Davies, R. (2006). Assessment of cleaning and disinfection in Salmonella-contaminated poultry layer houses using qualitative and semi-quantitative culture techniques. Veterinary Microbiology, 116, 283–293. doi: 10.1016/j.vetmic.2006.04.026