ABSTRACT
Fowl adenovirus (FAdV) infections in chickens have undergone substantial changes in recent decades, driven by host and pathogen factors. Based on the pathogenesis of inclusion body hepatitis (IBH) and hepatitis-hydropericardium syndrome (HHS), modern broilers are much more inclined to have difficulties keeping the metabolic homeostasis, whereas adenoviral gizzard erosion (AGE) is noticed equally in broilers and egg-layers. Defining the importance of certain serotypes for specific FAdV diseases is a major achievement of recent years but the isolation of viruses from clinically healthy birds remains unexplained, as virulence factors are hardly known and continue to be a “black box”. Together with further studies on pathogenesis of FAdV-induced diseases, such knowledge on virulence factors would help to improve protection strategies, which presently mainly concentrate on autogenous vaccines of breeders to prevent vertical transmission.
A brief history of fowl adenovirus (FAdV) diseases
Conforming with their historical role, fowl adenoviruses (FAdVs) were discovered by accident (van den Ende et al., Citation1949; Hess, Citation2020). Later on, various efforts to characterize FAdVs by neutralization assay revealed different serotypes, but also cross-reactions complicating clear distinction (McFerran, Citation1981; Hess, Citation2000). With immunosuppression noticed as a precondition to induce inclusion body hepatitis (IBH), the first FAdV disease, it was concluded that FAdVs alone are of low pathogenicity (McFerran, Citation1981). Simultaneous reports on the wide spread, or even ubiquity, of FAdVs in chicken flocks questioned the need for a targeted prophylaxis (McFerran & Smyth, Citation2000).
The emergence of hepatitis-hydropericardium syndrome (HHS) in 1987 in Pakistan changed the perception about the virulence of FAdVs, particularly certain FAdV-4 strains capable of inducing the disease in specific pathogen-free chickens first demonstrated in 1998 (Hess, Citation2017). The disease spread to other countries, with the most recent episode of outbreaks in China, besides the latest isolation from waterfowl (Shah et al., Citation2017).
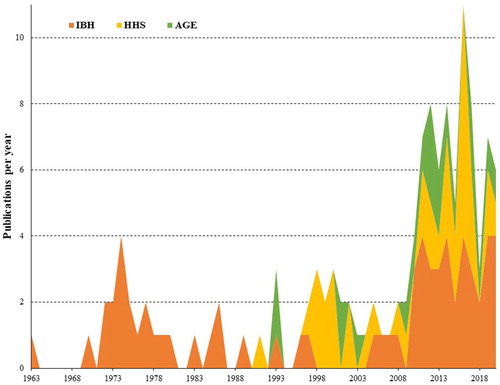
Additionally, the last two decades saw a worldwide increase of IBH outbreaks due to FAdV-2, −8a, −8b and −11, while gizzard erosions in chickens became linked with FAdV-1 (Schachner et al., Citation2018). The mounting systematic documentation of FAdV outbreaks () increasingly shifted perception of the virus from commensal towards primary pathogen, but the underlying mechanisms from the host side and the virus side still remain poorly understood.
The host perspective
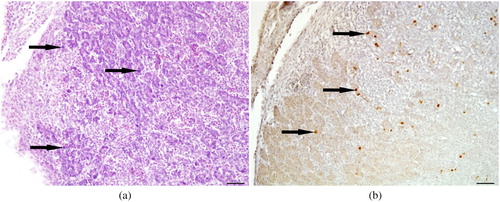
Over the past decades, specialization of the poultry sector has led to genetically highly distinctive hybrid birds, for either egg- or meat-production. Consequently, chickens differ in their physiological condition, which may enhance the intrinsic pathophysiological effects of IBH and HHS (Koenen et al., Citation2002; Julian, Citation2005). As metabolic disorders which, besides the well-known pathology of the liver, also include the often neglected pancreatic pathology (), the course of infection critically relies on bird type (Matos et al., Citation2016). In contrast, by nature of an unusual tissue tropism of FAdV-1 to gizzard epithelial cells, AGE was reported in young broilers as well as layers of various ages (Schachner et al., Citation2018).
Figure 2. Pancreas at 7 days post-infection from an SPF chicken infected orally with FAdV-D (FAdV-11) 106.7 TCID50. (a) Basophilic intranuclear inclusion bodies (arrows) are present in the acinar cells of the pancreas. (b) Specific detection of FAdV (arrows) by immunohistochemistry in the same organ. Scale bars = 50 µm.
In recent years, the host environment has undergone similar significant changes (McKay, Citation2009). Although no data exist on the actual infection status of breeders, it is conceivable that birds are less exposed to FAdVs due to increased biosecurity intended primarily against Avian Influenza, Mycoplasma spp. and Salmonella spp. Antibodies in breeders have an undisputed role to prevent vertical FAdV transmission and provide protection early in life (Toro et al., Citation2001; Grafl et al., Citation2012), while their absence puts progeny birds at an increased risk of infection. Additionally, the susceptibility of young birds may be enhanced by immunosuppressive diseases (CAV, IBDV) but also FAdVs per se compromise the immune function, and the infection might even result in an overreaction of the immune response (Hess, Citation2017; Zhao et al., Citation2020). With antibodies as an important indicator of protection, the finding that injected antigens outperform oral live virus-infection holds implications for vaccination strategies (Grgić et al., Citation2013).
While mammalian adenoviruses are relatively host-specific (Benkő & Harrach, Citation2003), FAdVs have been recovered from a number of phylogenetically disparate hosts (McFerran & Smyth, Citation2000; Harrach et al., Citation2019; Hess, Citation2020). Particularly concerning are data about the spill-over of highly virulent FAdV-4 to other domestic birds indicating possible trans-species transmission, although HHS in ducks could not be reproduced following a natural route of infection (Wang & Zhao, Citation2019).
The virus perspective
Following the implementation of hexon-based molecular typing and first whole-genome sequencing efforts (Chiocca et al., Citation1996; Raue & Hess, Citation1998; Meulemans et al., Citation2004), FAdV sequence data started rapidly expanding from the early 2000s on ward. While interest for complete genomes has focused mainly on FAdV prototypes dating back to the middle of the last century and sequenced ex-post, those historical strains are separated by a decade-long gap from the almost inflationary abundant, contemporary hexon sequences – a development driven by the acutely changing situation of FAdVs in the field.
Contrary to actual prevalence trends, it is likely that more recent sequence information from historical types without clinical background are increasingly missed. Conversely, it cannot be outruled that external factors have contributed to a limited viral spectrum, through competitive disadvantages, such as shorter host lifespans, to strains with slower replication/transmission kinetics. Although such FAdVs are underrepresented in existing datasets, they might still be circulating and serving as a source of natural recombination, demonstrated recently in the FAdV species associated with IBH (Schachner et al., Citation2019). Despite components from non-IBH types, certain recombinants obviously maintain individual fitness, and thus overall diversity of FAdVs, through other, yet undefined, genomic traits.
Another development is the large representation of FAdV-4 in datasets, the majority being contributions from HHS-outbreaks in China during the past 5 years. Distinct from previous FAdV-4 genomes, all these strains share the same deletion first described by Zhao et al. (Citation2015), pinpointing a regional variant. Recently, strain variants have also gained support by a documentation in the so-far underrepresented species FAdV-B (Kaján et al., Citation2019).
So far, virulence differences between FAdVs remain a major conundrum as there is no particular genomic trait which could be unanimously identified as a stand-alone virulence factor. Although functional studies have attributed virulence to the type determinants (Pallister et al., Citation1996; Zhang et al., Citation2018), this is contrasted by cohort sequence data showing no consistent distinction between hexon and fibre genes of differentially pathogenic serotype members. Investigations of genomic differences between pathogenic and non-pathogenic FAdVs (Slaine et al., Citation2016; Vera-Hernández et al., Citation2016; Absalón et al., Citation2017) increasingly support the idea of virulence modulation outside the type determinants, mainly in the genome ends for which we still have major sequence deficiencies. This, once again, provides a context for external factors acting on virus genetics, as terminally encoded products are tentative interplayers of host immune components. Yet another example could be viral structures prone to inter-passage mutation, such as tandem repeats in the FAdV termini, which may be particularly affected by large-scale, high turnover production settings.
Outlook and future challenges
FAdV infections have undergone substantial changes in recent years leading to the appearance of new diseases, an increased number of disease outbreaks reported for IBH, HHS and AGE on various continents, and an occasional spread to other poultry and bird species.
Considering changes in the birds’ environment, more epidemiological studies are needed covering the whole production chain. Such studies should combine virus isolation with serology to obtain a more complete picture of the infection dynamics. Determining the presence of antibodies against specific serotypes prior to the onset of lay could be useful to guide a protection strategy for broilers by breeder vaccination. Furthermore, studies are needed to determine possible correlations between antibody titres and protection, and the contribution of different epitopes to neutralizing activity independent of the antigen (whole virus (live or killed) or recombinant protein). Applied consistently in experimental studies, such investigations should help to elucidate cross-protection between vaccine and challenge viruses and to define a protection spectrum against heterologous strains. Cellular components of the host defence should be determined, especially in the case of live virus vaccination, although their contribution to protection should be addressed for inactivated antigens as well.
Studies on the pathogenesis of FAdV-induced diseases should include the whole range of target organs compromising metabolic and mechanical functions, mainly pancreas (IB + HHS) and heart (HHS), but also gizzard (AGE). For IBH and HHS the spectrum should be extended towards immunological organs, to determine the underlying mechanisms of immunosuppression on a cellular level. Furthermore, experimental confirmation would be needed for atypical manifestations and occurrence in hosts other than chickens.
Virus neutralization test (VNT) remains a certain gold standard for FAdV characterization, in a taxonomic as well as functional context. However, insufficient demarcation of various strains due to one- or two-way cross-reactions pose an obstacle, which may be overcome with genetic data, having largely replaced traditional serology. Although hexon-based typing correlates reasonably well with VNT, the recent demonstration of FAdVs with natural recombinations or deletions favours complete genomic approaches. Such genomic information would support another highly requested category of studies, the targeted modification of FAdV genomes and the study of viral genes in isolation.
Overall, the FAdV˗host interaction bears numerous challenges driven by the variety of viruses, and also the complexity of the host reaction, altogether complicating the situation. However, it can be predicted that the (re-)emergence in the field, combined with unique characteristics of the FAdV-host interplay, will foster future studies and gain substantial new knowledge.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Absalón, A.E., Morales-Garzón, A., Vera-Hernández, P.F., Cortés-Espinosa, D.V., Uribe-Ochoa, S.M., García, L.J. & Lucio-Decanini, E. (2017). Complete genome sequence of a non-pathogenic strain of fowl adenovirus serotype 11: minimal genomic differences between pathogenic and nonpathogenic viruses. Virology, 501, 63–69. doi: 10.1016/j.virol.2016.11.006
- Benkő, M. & Harrach, B. (2003). Molecular evolution of adenoviruses. In W. Doerfler & P. Böhm (Eds.), Adenoviruses: Models and vectors in virus–host interactions. Current Topics in Microbiology and Immunology (Vol. 272, pp. 3–35). Berlin, Heidelberg, Germany: Springer.
- Chiocca, S., Kurzbauer, R., Schaffner, G., Baker, A., Mautner, V. & Cotten, M. (1996). The complete DNA sequence and genomic organization of the avian adenovirus CELO. Journal of Virology, 70, 2939–2949. doi: 10.1128/JVI.70.5.2939-2949.1996
- Grafl, B., Aigner, F., Liebhart, D., Marek, A., Prokofieva, I., Bachmeier, J. & Hess, M. (2012). Vertical transmission and clinical signs in broiler breeders and broilers experiencing adenoviral gizzard erosion. Avian Pathology, 41, 599–604. doi: 10.1080/03079457.2012.740614
- Grgić, H., Poljak, Z., Sharif, S. & Nagy, E. (2013). Pathogenicity and cytokine gene expression pattern of a serotype 4 fowl adenovirus isolate. PLoS One, 8, e77601. doi: 10.1371/journal.pone.0077601
- Harrach, B., Tarján, Z.L. & Benkő, M. (2019). Adenoviruses across the animal kingdom: a walk in the zoo. FEBS Letters, 593, 3660–3673. doi: 10.1002/1873-3468.13687
- Hess, M. (2000). Detection and differentiation of avian adenoviruses: a review. Avian Pathology, 29, 195–206. doi: 10.1080/03079450050045440
- Hess, M. (2017). Commensal or pathogen – a challenge to fulfil Koch’s Postulates. British Poultry Science, 58, 1–12. doi: 10.1080/00071668.2016.1245849
- Hess, M. (2020). Aviadenovirus infections. In D.E. Swayne, M. Bouliann, C.M. Logue, L.R. McDougald, V. Nair, D.L. Suarez, S. de Wit, T. Grimes, D. Johnson, M. Kromm, T.Y. Prajitno, I. Rubinoff & G. Zavala (Eds.), Diseases of poultry (14th ed., pp. 322–332). Hoboken, NJ: Wiley-Blackwell.
- Julian, R.J. (2005). Production and growth related disorders and other metabolic diseases of poultry – a review. The Veterinary Journal, 169, 350–369. doi: 10.1016/j.tvjl.2004.04.015
- Kaján, G.L., Affranio, I., Tóthné Bistyák, A., Kecskeméti, S. & Benkő, M. (2019). An emerging new fowl adenovirus genotype. Heliyon, 5, e01732. doi: 10.1016/j.heliyon.2019.e01732
- Koenen, M.E., Boonstra-Blom, A.G. & Jeurissen, S.H.M. (2002). Immunological differences between layer- and broiler-type chickens. Veterinary Immunology and Immunopathology, 89, 47–56. doi: 10.1016/S0165-2427(02)00169-1
- Matos, M., Grafl, B., Liebhart, D. & Hess, M. (2016). The outcome of experimentally induced inclusion body hepatitis (IBH) by fowl aviadenoviruses (FAdVs) is crucially influenced by the genetic background of the host. Veterinary Research, 47, 69. doi: 10.1186/s13567-016-0350-0
- McFerran, J.B. (1981). Adenoviruses of vertebrate animals. In E. Kurstak & C. Kurstak (Eds.), Comparative diagnosis of viral diseases III (pp. 102–165). New York: Academic Press.
- McFerran, J.B. & Smyth, J.A. (2000). Avian adenoviruses. Revue Scientifique et Technique de L’Office International des Epizooties, 19, 589–601. doi: 10.20506/rst.19.2.1238
- McKay, J.C. (2009). The genetics of modern commercial poultry. In P. Hocking (Ed.), Biology of breeding poultry (pp. 3–9). Wallingford, United Kingdom: CABI.
- Meulemans, G., Couvreur, B., Decaesstecker, M., Boschmans, M. & van den Berg, T.P. (2004). Phylogenetic analysis of fowl adenoviruses. Avian Pathology, 33, 164–170. doi: 10.1080/03079450310001652086
- Pallister, J., Wright, P.J. & Sheppard, M. (1996). A single gene encoding the fiber is responsible for variations in virulence in the fowl adenoviruses. Journal of Virology, 70, 5115–5122. doi: 10.1128/JVI.70.8.5115-5122.1996
- Raue, R. & Hess, M. (1998). Hexon based PCRs combined with restriction enzyme analysis for rapid detection and differentiation of fowl adenoviruses and egg drop syndrome virus. Journal of Virological Methods, 73, 211–217. doi: 10.1016/S0166-0934(98)00065-2
- Schachner, A., Gonzalez, G., Endler, L., Ito, K. & Hess, M. (2019). Fowl adenovirus (FAdV) recombination with intertypic crossovers in genomes of FAdV-D and FAdV-E, displaying hybrid serological phenotypes. Viruses, 11, 1094. doi: 10.3390/v11121094
- Schachner, A., Matos, M., Grafl, B. & Hess, M. (2018). Fowl aviadenovirus (FAdV) induced diseases and strategies for their control – a review on the current global situation. Avian Pathology, 47, 111–126. doi: 10.1080/03079457.2017.1385724
- Shah, M.S., Ashraf, A., Khan, M.I., Rahman, M., Habib, M., Chughtai, M.I. & Qureshi, J.A. (2017). Fowl adenovirus: history, emergence, biology and development of a vaccine against hydropericardium syndrome. Archives of Virology, 162, 1833–1843. doi: 10.1007/s00705-017-3313-5
- Slaine, P.D., Ackford, J.G., Kropinski, A.M., Kozak, R.A., Krell, P.J. & Nagy, E. (2016). Molecular characterization of pathogenic and nonpathogenic fowl aviadenovirus serotype 11 isolates. Canadian Journal of Microbiology, 62, 993–1002. doi: 10.1139/cjm-2016-0297
- Toro, H., Gonzalez, O., Escobar, C., Cerda, L., Morales, M.A. & Gonzalez, C. (2001). Vertical induction of the inclusion body hepatitis/hydropericardium syndrome with fowl adenovirus and chicken anemia virus. Avian Diseases, 45, 215–222. doi: 10.2307/1593031
- Van den Ende, M., Don, P.A. & Kipps, A. (1949). The isolation in eggs of a new filtrable agent which may be the cause of bovine lumpy skin disease. Journal of General Microbiology, 3, 174–183. doi: 10.1099/00221287-3-2-174
- Vera-Hernández, P.F., Morales-Garzón, A., Cortés-Espinosa, D.V., Galiote-Flores, A., García-Barrera, L.J., Rodríguez-Galindo, E.T., Toscano-Contreras, A., Lucio-Decanini, E. & Absalón, A.E. (2016). Clinicopathological characterization and genomic sequence differences observed in a highly virulent fowl aviadenovirus serotype 4. Avian Pathology, 45, 73–81. doi: 10.1080/03079457.2015.1125443
- Wang, Z. & Zhao, J. (2019). Pathogenesis of hypervirulent fowl adenovirus serotype 4: the contributions of viral and host factors. Viruses, 11, 741. doi: 10.3390/v11080741
- Zhang, Y., Liu, R., Tian, K., Wang, Z., Yang, X., Gao, D., Zhang, Y., Fu, J., Wang, H. & Zhao, J. (2018). Fiber2 and hexon genes are closely associated with the virulence of the emerging and highly pathogenic fowl adenovirus 4. Emerging Microbes & Infections, 7, 199.
- Zhao, W., Li, X., Li, H., Han, Z., Wang, F., Liu, C., Shao, Y. & Ma, D. (2020). Fowl adenoviruse-4 infection induces strong innate immune responses in chicken. Comparative Immunology, Microbiology and Infectious Diseases, 68, 101404. doi: 10.1016/j.cimid.2019.101404
- Zhao, J., Zhong, Q., Zhao, Y., Hu, Y.X. & Zhang, G.Z. (2015). Pathogenicity and complete genome characterization of fowl adenoviruses isolated from chickens associated with inclusion body hepatitis and hydropericardium syndrome in China. PLoS One, 10, e0133073. doi: 10.1371/journal.pone.0133073