ABSTRACT
A double construct vaccine of turkey herpesvirus (HVT) was prepared that contains the fusion (F) gene from Newcastle disease virus (NDV) and the viral protein 2 (VP2) gene from infectious bursal disease virus (IBDV). Safety of the vaccine (HVT-ND-IBD) was confirmed and efficacy was evaluated after subcutaneous (SC) vaccination at 1 day of age or the in ovo route of vaccination. Challenges were performed with velogenic NDV strains (Texas GB and Herts Weybridge 33/56), with different strains of IBDV (classical strain STC; very virulent strain CS89 and variant E strain) and with Marek’s disease virus (MDV) strain RB1B. Vaccination with HVT-ND-IBD induced a high level of protection against these challenges. Vaccination with HVT is often combined with Rispens CVI988 vaccine and live ND vaccines for higher and earlier, MD and ND protection, respectively. HVT-ND-IBD vaccination in combination with these vaccines showed MD protection as early as 4 days post vaccination and ND protection as early as 2 weeks post vaccination. The long protection as seen with HVT vaccination was confirmed by demonstrating protection against NDV up to 60 weeks. Finally, to evaluate the performance of the vaccine in commercial birds with maternally-derived antibodies, two field trials were performed, using in ovo vaccination in broilers and SC vaccination in combination with Rispens CVI988 vaccine in layer-type birds. The efficacy was confirmed for all components by challenges. These results demonstrate that HVT-ND-IBD is a safe and highly efficacious vaccine for simultaneous control of ND, IBD and MD.
RESEARCH HIGHLIGHTS
A double construct HVT vaccine with the NDV F and the IBDV VP2 genes was prepared.
The vaccine protects against three important diseases: MDV, NDV and IBDV.
In ovo and sub-cutaneous vaccination was evaluated in the field in commercial chickens.
Introduction
Turkey herpesvirus (HVT), also known as Meleagrid herpesvirus 1, belongs to the family Herpesviridae, subfamily Alphaherpesvirinae, genus Mardivirus and has been used since the 1970s as a vaccine to provide protection against Marek’s disease (MD) (Okazaki et al., Citation1970). HVT is a naturally apathogenic virus, very safe for use in birds, stimulates humoral and cellular immunity, induces life-long protection, overcomes maternally-derived antibodies and has the capacity to accept foreign gene insertions in multiple sites (Gergen et al., Citation2019). This has led to the use of HVT as a vector platform for the development of HVT construct vaccines for many different poultry diseases.
Currently, there are many commercially available HVT-vectored vaccines (Sondermeijer et al., Citation1993; Le Gros et al., Citation2009; Vagnozzy et al., Citation2012; Esaki et al., Citation2013; Kapczynski et al., Citation2015; Ingrao et al., Citation2017) that are used to protect chicken flocks against a number of important pathogens including Newcastle disease virus (NDV), infectious laryngotracheitis virus (ILTV), infectious bursal disease virus (IBDV), and avian influenza virus. However, each of these vaccines is monovalent in nature, i.e. they contain protective antigens from only one foreign pathogen and therefore protect chickens against one disease at a time. It is not possible to combine different monovalent HVT vaccines in one vaccination as this can lead to interference (Dunn et al., Citation2019). To provide protection against multiple diseases with a single HVT vaccination, we recently described a HVT construct vaccine, HVT-NDV-ILT, that expresses antigens from both NDV and ILTV, and demonstrated its utility in simultaneous protection against ILT, ND and MD (Gergen et al., Citation2019).
Infections with NDV and IBDV can both occur early in life. IBDV is a member of the Birnaviridae family, genus Avibirnavirus, and causes an acute and contagious viral infection of chickens affecting the bursa of Fabricius as the primary target organ (Eterradossi & Saif Citation2013). The economic impact of this disease is manifested as a result of a severe immune suppression in chickens infected at a young age leading to poor responses to vaccination and making birds more vulnerable to infections with other pathogens. In addition, some IBDV strains can cause high mortality in birds that are 3 weeks of age or older (Eterradossi & Saif Citation2013). Multiple HVT construct vaccines are available and have been demonstrated to provide protection against IBD (Gelb et al., Citation2016). Vaccination with these HVT vaccines is very safe and does not negatively impact the bursa of Fabricius (Lupini et al., Citation2020).
NDV is a member of the family Paramyxoviridae, genus Avulavirus, and causes disease of varying severity in poultry depending on virus virulence, age of chickens and immune status (Miller & Koch, Citation2013). Several pathotypes of the virus have been described and are used to refer to the virulence of NDV strains in descending order of virulence as velogenic, mesogenic and lentogenic strains. Velogenic strains produce up to 100% mortality in susceptible chickens of all ages and are further subdivided into viscerotropic velogenic strains (which cause lesions in the gastrointestinal tract) and neurotropic velogenic strains (which cause lesions in the nervous system and respiratory tract). Mesogenic NDV strains produce predominantly neurological disease with death only in young birds. Lentogenic NDV strains produce mild respiratory illness with little or no mortality, and some strains are commonly used as live vaccines (Morgan et al., Citation1992; Miller & Koch, Citation2013). Several HVT construct vaccines are available and have been demonstrated to provide high protection against ND (Sondermeijer et al., Citation1993; Rauw et al., Citation2010; Gergen et al., Citation2019).
In order to avoid the potential problems of interference among monovalent HVT-IBD and HVT-ND vaccines, and to additionally provide simultaneous protection against Marek’s disease from one HVT virus backbone, we constructed a HVT vaccine, HVT-ND-IBD, that concurrently expresses the F protein from NDV and the VP2 protein from IBDV. The present report describes the safety and efficacy of this vaccine. Protection was evaluated after vaccination at day-old or in ovo by challenge with different strains of virulent NDV, IBDV and MDV. For MD, protection was evaluated at 4–9 days post vaccination as early protection against MD is important in the field and immunity quickly builds after HVT vaccination. Protection against ND and IBD was evaluated from 2–4 weeks post vaccination to allow immunity to build. In the field, this is also the timing that immunity is needed, as levels of maternally-derived antibodies against NDV and IBDV have then declined. To study the duration of protection, challenges were performed with NDV as, for this challenge, birds are still sensitive at an older age. Protection was furthermore evaluated when the vaccine was used in the field in commercial birds.
Materials and methods
Construction of the HVT vector vaccine virus
A double HVT vector vaccine designated HVT-ND-IBD (also known as strain HVP360, marketed as Innovax®-ND-IBD), which contains both the NDV-F gene and the IBDV VP-2 gene with regulatory sequences, was constructed using overlapping clones as described before (Gergen et al., Citation2019). First, the insertion plasmid was constructed. The NDV F gene from NDV Genotype II Clone 30 vaccine with an upstream immediate-early (IE1) promoter from human cytomegalovirus (HCMV) and a downstream CMV terminator, and the IBDV VP2 gene from the classical IBDV strain Faragher 52/70 with an upstream IE1 promoter from murine cytomegalovirus (MCMV) and a downstream SV40 poly-adenylation site were cloned in the US region (US2 gene) of HVT FC126. Subsequently, chicken embryo fibroblast (CEF) cells were transfected with the set of cloned, overlapping cosmid/plasmid-derived DNA fragments from the HVT vaccine strain FC126, plus the insertion plasmid, as described before by Gergen et al. (Citation2019), and the cells were allowed to recombine the fragments at the overlap regions. The viral stock obtained following transfection of CEF was plaque-purified on CEF cells to assure purity.
Other vaccines
The live ND vaccine used was Nobilis® ND C2 (Batch A153AJ01). The Rispens CVI988 vaccine used in the duration of immunity study was Rismavac (Batch 02760105); in the laboratory RB1B challenge study Nobilis® Rismavac batch 23I16 was used, and in the field trial Nobilis® Rismavac batch A963C was used.
Fertile eggs and chickens
For the laboratory trials specific pathogen-free (SPF) White Leghorn chickens were used and chickens were housed in isolators or in isolation rooms with floor pens. Food and water were provided ad libitum. All experimental procedures were performed in compliance with the relevant animal use guidelines. For the field trials white commercial (future) layer and broiler chickens were used.
Vaccination
Eggs with viable embryos, at 18 days of embryonation, were inoculated by the in ovo route with 0.05–0.1 ml of the respective vaccine or Marek’s vaccine diluent (placebo). The inoculum was administered into the amniotic sac using a 1 mL syringe fitted with a 22G × 1¼″ needle. One-day-old chicks were vaccinated by the sub-cutaneous route with 0.2 ml in the back of the neck using a 22G × 1″ needle to administer the respective vaccine or Marek’s vaccine diluent (placebo).
Vaccine titration
The HVT construct vaccine was titrated in 6-well plates or dishes on confluent monolayers of CEF cells. Serial dilutions of the vaccine sample were prepared and plates inoculated. Plates were incubated at 38.0 ± 1°C with 4-5% CO2 for 5 days. On day 5, the CEF monolayers were fixed and stained with the appropriate primary antibody and corresponding fluorescein-labelled secondary antibody. Plates were observed under a fluorescent microscope and titres were calculated by multiplying the average number of plaques by the dilution factor. Titres are reported as plaque forming units (PFU).
Immunofluorescence antibody assay
CEF monolayers were infected with HVT-ND-IBD and, after 5 days incubation, medium was decanted and the monolayer was fixed with 100% methanol for 5–10 min at room temperature. Methanol was decanted and monolayers allowed to air dry followed by staining with mouse anti-NDV-F (Mab #57) or mouse anti-VP2 (Mab #R63). Antibody was incubated at room temperature for 3 h. After antibody incubation, plates were washed three times with PBS and subsequently incubated with fluorescein isothiocyanate-conjugated secondary anti-mouse IgG-FITC (Sigma catalog no. F9137, diluted 1:100). After 1 h room temperature incubation, plates were washed with PBS and evaluated under a fluorescent microscope.
Challenge procedures and viruses
NDV challenge was conducted by the intramuscular (IM) route using the velogenic Texas GB strain with 104 embryo lethal dose (ELD50) or with 105 ELD50 of Herts 33/56 in 0.2 ml. IBDV challenge was conducted by the intraocular route with classical IBDV STC strain using 103 EID50 per dose, with variant E IBDV strain using 102.2 EID50 per dose or with very virulent IBDV strain CS89 using 15–150 chicken infective dose (CID50). MDV challenge was carried out using the RB1B strain by the IM route in the leg (0.2 ml/bird with 3–8 CID50/bird for the birds vaccinated with HVT-ND-IBD only, and 26 CID50/bird for the birds vaccinated with HVT-ND-IBD in combination with the Rispens CVI988 vaccine strain).
Experimental design of the laboratory efficacy studies
Overdose safety studies in chicken
The safety of the HVT construct vaccine virus was evaluated following vaccination by in ovo and sub-cutaneous (SC) routes using a vaccine dose representing at least 10 times as much viable virus as would normally be used for vaccination. At 18 days of embryonation, 60 eggs were vaccinated with 93,780 PFU of HVT-ND-IBD per dose in 0.1 ml and 60 eggs were inoculated with 0.1 ml of a Marek’s diluent as placebo. Fifty 1-day-old chicks were vaccinated with 108,720 PFU of HVT-ND-IBD per dose in 0.2 ml by the sub-cutaneous route. At 5 days of age, 50 non-vaccinated chicks were challenged with the very virulent Marek’s disease virus (vvMDV) RB1B to determine if the birds used in the study were susceptible to Marek’s disease. The chickens challenged with RB1B virus were monitored for 7 weeks and any surviving chickens were necropsied at 7 weeks of age and observed for gross lesions indicative of Marek’s disease. Chickens in the other groups were kept and monitored for adverse events or clinical signs of MDV. At 120 days of age these chickens were necropsied and evaluated for any observable lesions.
Efficacy against NDV
Protection against challenge with NDV was evaluated with different NDV challenge strains. Challenges were performed with Genotype II strain Texas GB and Genotype IV strain Herts Weybridge 33/56. HVT-ND-IBD was given either by the in ovo route using eggs at 18 d of embryonation or by the sub-cutaneous (SC) route in day-old chickens. After challenge, protection was evaluated over a 14-day period and birds were scored positive if observed clinical signs included typical signs of NDV infection such as lack of coordination, paralysis and/or death.
Two studies were performed with the Texas GB challenge. The first study evaluated protection after SC or in ovo vaccination using different vaccine doses, and the second study evaluated the duration of protection after HVT-ND-IBD vaccination up to 60 weeks. In the first study, three groups of eggs at 18 d of embryonation were inoculated in ovo with 1199, 1658, or 2235 PFU/dose of HVT-ND-IBD vaccine and one group of eggs was inoculated with a diluent placebo as a challenge control. In addition, three groups of day-old chicks were inoculated SC with 1662, 2400 or 2880 PFU/dose of HVT-ND-IBD vaccine and one group was inoculated SC with a diluent placebo to serve as challenge control. Challenge with NDV Texas GB by the IM route route was carried out 4 weeks after vaccination.
In the second study two groups of SPF chickens were used, and were vaccinated at day-old by the SC route with 0.2 ml of HVT-ND-IBD at 2000 PFU/dose mixed with 1000 PFU/dose of Rispens CVI988 vaccine (group 1) or left non-vaccinated as controls (group 2). Challenges with NDV Texas GB by the IM route were performed at 9, 50 and 60 weeks post vaccination.
Two studies were performed with the NDV Herts Weybridge 33/56 challenge. In the first study two groups of SPF chickens were used, which were vaccinated at day-old by the SC route with 0.2 ml of HVT-ND-IBD at 2000 pfu/dose or left unvaccinated as controls. At 4 weeks post vaccination, half of the chickens of each group were challenged and at 8 weeks post vaccination the other half of vaccinated and control chickens were challenged by the IM route with Herts Weybridge 33/56.
In the second study the efficacy of HVT-ND-IBD in combination with a live attenuated ND vaccine (Nobilis® ND C2) was evaluated to study earlier onset of protection. Two groups of SPF chickens were used; the first group was vaccinated at day-old with 7924 pfu/dose of HVT-ND-IBD by the SC route and with Nobilis® ND C2 by the oculo-nasal route, and the second group was left unvaccinated as controls. At 2 weeks post vaccination the chickens were challenged by the IM route with Herts Weybridge 33/56.
Efficacy against IBDV
Protection against challenge with IBDV was evaluated with different IBDV challenge strains. Challenges were performed with a classical IBDV strain (STC), a very virulent IBDV strain (CS89) and a variant E IBDV strain. HVT-ND-IBD was either given by the in ovo route using eggs at 18–19 d of embryonation or by the sub-cutaneous (SC) route in day-old chickens.
Classical IBDV challenge: three groups of eggs at 18 d of embryonation were inoculated in ovo with three different doses of the HVT-ND-IBD vaccine (1566, 2228 and 2860 PFU/dose) and one group of eggs was inoculated with only the diluent (placebo) and served as a challenge control. In addition, three groups of day-old chicks were inoculated SC with three different doses of the HVT-ND-IBD vaccine (1752, 2224, and 2910 PFU/ dose) and one group was inoculated SC with the diluent only and served as the challenge control. Challenge with classical IBDV was carried out 4 weeks after vaccination using USDA IBDV STC challenge strain. Three days postchallenge, birds were necropsied and examined for typical gross lesions of IBDV, which include peri-bursal oedema and/or oedema and haemorrhage in the bursal tissue.
Variant IBDV challenge: A group of eggs at 18 d of embryonation was inoculated in ovo with the HVT-ND-IBD vaccine (2096 PFU/dose), and one group of eggs was inoculated with only the diluent and served as a challenge control. A negative control group was included, and these birds did not receive a challenge. In addition, a group of day-old chicks was inoculated SC with the HVT-ND-IBD vaccine (2184 PFU/dose) and one group was inoculated SC with only the diluent and served as the challenge control. A negative control group which was not challenged was included. Challenge with variant IBDV was carried out 4 weeks after inoculation using variant E challenge strain. Ten days following challenge, birds of the challenged (vaccinated and control) and the non-challenged control group were necropsied, the sex was recorded, and bursa/body weight ratio was calculated for each bird. B/BW ratios were calculated according to the following formula: B/BW ratio = [bursa weight (g)/body weight (g)] × 1000. An average ratio was calculated, and the standard deviation was computed for the negative (non-challenged) control group. A chicken was scored positive if its bursa/body weight ratio was >2 standard deviations below the average bursa/body weight ratio of the negative (non-challenged) control group.
Very virulent IBDV challenge: two studies were performed. In the first study, three groups of SPF chicken eggs were used. Two groups were inoculated with two different doses of HVT-ND-IBD (0.05 ml): 1996 and 3157 pfu/dose. The controls were inoculated with diluent only. At 2 and 3 weeks post vaccination a challenge was performed using 30 CID50 IBDV CS89 challenge virus, and at 8 weeks post vaccination a challenge was performed using 150 CID50 IBDV CS89 challenge virus. After challenge, the birds were monitored for 10 days for clinical signs or deaths due to IBDV. At the end of the observation period the surviving chickens were euthanized and histological examination for lesions of the bursa of Fabricius was performed. If more than 50% of the follicles showed signs of lymphoid depletion the bird was scored positive. In the second study four groups of chickens were included. Three groups were vaccinated by the SC route with different doses of HVT-ND-IBD (1993, 2515 and 3170 pfu/dose) and one group was not vaccinated (control). All groups were challenged two weeks post vaccination with 15 CID50 IBDV CS89 challenge virus and unvaccinated controls were included. Post challenge birds were observed for 10 days followed by histological examination of the bursa of the surviving chickens as described above.
Efficacy against MDV
In the first study, two groups of eggs were inoculated in ovo with 1996, or 3157 pfu/dose of HVT-ND-IBD and one group of eggs was inoculated with a diluent as a challenge control. In the second study, one group of day-old chicks was inoculated SC with 1000 pfu/dose of HVT-ND-IBD and one group was not inoculated and served as a challenge control. Challenge with MDV was carried out 9 days after vaccination in study 1 and 2 using very virulent MDV RB1B strain. Protection was evaluated over a 70 and 75 d-period for study 1 and 2, respectively, following challenge and chickens were observed for clinical signs such as lack of coordination, paralysis, depression, ruffled feathers and/or death, or gross lesions of Marek’s disease such as mortality, neurological signs, paralysis and prostration. At the end of the observation period the chickens were examined for macroscopic lesions of MD, such as atrophic bursa and thymus, visceral tumours and nerve lesions.
In the third study, chickens were vaccinated by the SC route with 2000 pfu/dose of HVT-ND-IBD and 1000 pfu/dose of Rispens CVI988 vaccine. Challenge with MDV strain RB1B was carried out 4 days after vaccination. An unvaccinated control group was included in the challenge. Birds were observed for 77 days following challenge for clinical signs of MD, such as mortality, neurological signs, paralysis and prostration. At the end of the 77 d observation period the chickens were examined for macroscopic lesions of MD, such as atrophic bursa and thymus, visceral tumours and nerve lesions.
Efficacy in the field
The efficacy of the vaccine was also evaluated in the field in broiler chickens (in ovo vaccination) and in layer chickens (SC vaccination in combination with Rispens CVI988 vaccine). In the first trial 11,902 18-day-old embryonated eggs were in ovo vaccinated with HVT-ND-IBD (test group) and placed in the test house. On the same day 12,869 18-day-old embryonated eggs were injected in ovo with the vaccine solvent only (control group) and placed in the control house. After hatching, chickens were taken from the field and challenged with NDV Herts, IBD CS89 and MD RB1B as described above. In the second trial, 210,009 d-old white commercial (future) layer chickens were SC vaccinated with HVT-ND-IBD mixed with Rispens CVI988 vaccine (Nobilis® Rismavac). Vaccinated chickens and non-vaccinated chickens were taken from the field and challenged with NDV Herts, IBD CS89 and MD RB1B as described above. MDV challenges were performed at 8 days of age (in ovo vaccination) or 7 days of age (SC) vaccination. NDV and IBDV challenges were performed when the levels of maternally-derived antibodies had sufficiently declined.
Ethical approval
The studies presented were carried out in an ethical and responsible manner, in full compliance with all relevant codes of experimentation and legislation of the countries where the studies were performed.
Statistical evaluation
To evaluate the level of protection, the vaccinated groups were compared to the non-vaccinated controls using Fisher's exact test when comparing % protection at the end of challenge or using log-rank (Mantel–Cox) test when evaluating survival curves.
Results
Vaccine construction and verification
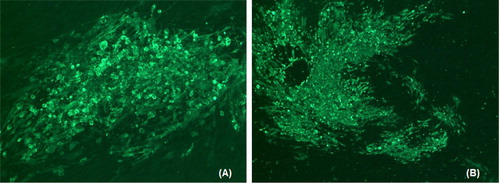
The correct assembly of the HVT-ND-IBD virus was confirmed and the sequences of the inserted expression cassette with the NDV F and IBDV VP2 genes were confirmed following sequencing (data not shown). In addition, strong expression of the proteins encoded by the inserted genes was confirmed by immunofluorescence ().
Figure 1. Photographs of HVT-ND-IBD plaques stained with antibodies specific to the two inserted gene products. (A) HVT-ND-IBD plaque stained with anti-NDV F monoclonal antibody, 57NDV, followed by a FITC-labelled goat anti-mouse IgG. (B) HVT-ND-IBD plaque stained with anti-IBD VP2 monoclonal antibody, R63, followed by a FITC-labelled goat anti-mouse IgG.
Overdose safety
The safety of the HVT-ND-IBD vaccine, in terms of its effects on hatchability and potential to cause disease in susceptible chickens, was evaluated using SPF birds injected in ovo or SC with more than 10 times as much viable virus as would be contained in one dose of vaccine. The hatchability of eggs vaccinated with the HVT-ND-IBD was 93.3% while the hatchability of eggs inoculated with a Marek’s vaccine diluent was 95.0% (); there was no significant difference in hatchability as evaluated by Fisher’s exact test (P = 1), demonstrating the vaccine had no negative impact on the hatchability. During the 120-day observation period of the two groups vaccinated with HVT-ND-IBD (in ovo or SC) and the control group vaccinated in ovo with diluent, in each group one chicken died due to reasons not related to the vaccination. All birds in the HVT-ND-IBD vaccinated groups and the diluent vaccinated group were free of clinical signs of Marek’s disease. At the time of necropsy there were no birds in the HVT-ND-IBD vaccine groups with signs of MD lesions. In the group of chickens challenged with vvMDV RB1B strain 98% (49 out of 50) of birds showed clinical signs and/or lesions typical of Marek’s disease confirming that the birds used in this study were susceptible to MDV. These results demonstrate that HVT-ND-IBD vaccine is safe for use in chickens.
Table 1. Marek’s disease lesions and hatchability following administration of an overdose of HVT-ND-IBD vaccine.
Efficacy against NDV
The efficacy of HVT-ND-IBD vaccine used as a single vaccination or in combination with live ND vaccine was evaluated by challenge with the different NDV strains.
NDV Texas GB challenge
SPF chickens were vaccinated using different doses of HVT-ND-IBD using both in ovo and SC vaccination. At 4 weeks post inoculation the vaccinated chickens (31–33 chickens/group) and a control group inoculated with diluent only (13 chickens/group) were challenged with the NDV Texas GB challenge strain (genotype II). As shown in (A) (in ovo vaccination) and (B) (SC vaccination), all non-vaccinated birds died or were euthanized within 4 days following NDV challenge. The birds vaccinated in ovo or SC with the different doses of HVT-ND-IBD all showed a high level of protection (in ovo: 97%, 97% and 100% after vaccination with 1199, 1658 and 2235 pfu/dose, respectively; and SC: 100%, 100% and 97% after vaccination with 1662, 2400, and 2880 pfu/dose, respectively), demonstrating a significant reduction in mortality (P < 0.0001, log-rank test).
Figure 2. Survival curves after NDV challenge. (A) Texas GB challenge 4 weeks post in ovo vaccination with HVT-ND-IBD at three different doses (1: 1199 PFU/dose, 2: 1658 PFU/dose, 3: 2235 PFU/dose), (B) Texas GB challenge 4 weeks post SC vaccination with HVT-ND-IBD at three different doses (1: 1662 PFU/dose, 2: 2400 PFU/dose, 3: 2880 PFU/dose), (C) Texas GB challenge 9 weeks (graph C1), 50 weeks (graph C2) and 60 weeks (graph C3) post SC vaccination with HVT-ND-IBD (2000 PFU/dose) mixed with Rispens CVI988 vaccine (1000 PFU/dose); (D) Herts Weybridge 33/56 challenge 4 weeks (graph D1) and 8 weeks (graph D2) post SC vaccination with HVT-ND-IBD (2000 PFU/dose), (E) Herts Weybridge 33/56 challenge 2 weeks post SC vaccination with HVT-ND-IBD (7924 PFU/dose) and live ND C2 vaccine.
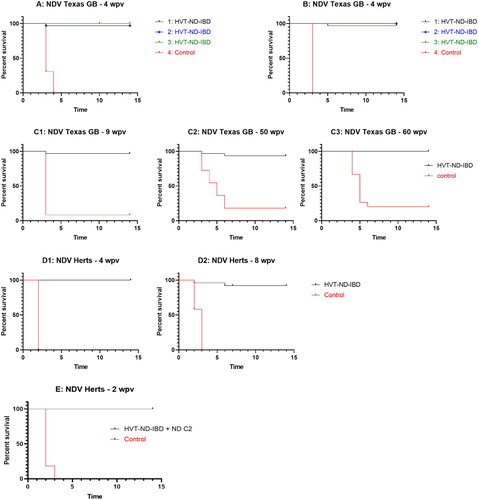
In the field, long-lived birds are often vaccinated with a combination of a HVT vaccine and a second MDV vaccine strain, such as Rispens CVI988 or SB-1 vaccine strain to obtain a higher level of protection against MD. Therefore, in a second study, birds were vaccinated by the SC route with HVT-ND-IBD combined with Rispens CVI988 vaccine and the duration of protection was evaluated by performing NDV challenges at 9, 50 and 60 weeks post vaccination (32–35 vaccinated birds and 11-15 control birds for each challenge timepoint). As indicated in (C) most non-vaccinated birds died or showed clinical ND signs (92%, 82% and 80% for the 9, 50 and 60 week post vaccination challenges, respectively) and were euthanized following NDV challenge with Texas GB, whereas HVT-ND-IBD vaccinated birds showed a high level of protection (97%, 94% and 100% for the 9, 50 and 60 week post vaccination challenges, respectively). These results demonstrate that the HVT-ND-IBD vaccine is highly efficacious at each timepoint (P < 0.0001, log-rank test) in protection against NDV, and at 60 weeks post vaccination birds are still fully protected.
NDV Herts Weybridge 33/56 challenge
To evaluate the protection against NDV genotype IV challenge, birds were vaccinated with HVT-ND-IBD (22–24 birds/group) and challenged at both 4 and 8 weeks post SC vaccination with the Herts Weybridge 33/56 NDV strain. At both challenge timepoints 100% of the control chickens (12 chickens/group) died or showed severe clinical signs of Newcastle disease within 2–3 days post challenge ((D)). At 4 weeks post vaccination, 100% protection was obtained after ND challenge of the group of vaccinated chickens. At 8 weeks post vaccination, 91% protection was obtained after ND challenge of the vaccinated chickens. In the second study, the effect of the addition of a live ND vaccination at day-old was evaluated and challenge was performed at 2 weeks post vaccination (24 vaccinated chickens and 11 unvaccinated controls were used). As shown in (E), 100% of the control chickens died or showed severe clinical signs of Newcastle disease within 2 days post challenge, whereas 100% protection was obtained after NDV challenge in the group of chickens vaccinated with HVT-ND-IBD and Nobilis® ND C2 (P < 0.0001, log-rank test).
Efficacy against IBD
Protection against challenge with IBDV was evaluated with different IBDV challenge strains. Challenges were performed with a classical IBDV strain (STC), a variant E IBDV strain and a very virulent IBDV strain (CS89).
Classical IBDV challenge
The protection afforded by HVT-ND-IBD after vaccination of chickens with different doses was assessed following challenge 4 weeks post vaccination with a classical IBDV strain (USDA IBDV STC challenge strain). At 3 days postchallenge, all birds were euthanized, necropsied, and examined for gross lesions, which included peri-bursal oedema, oedema, and haemorrhage in the bursal tissue. Vaccination was done either by the in ovo route in 18 d old embryonated chicken eggs or by the SC route in day-old chickens. In the groups vaccinated in ovo with the HVT-ND-IBD vaccine, 97% of birds were protected from challenge with classical STC IBDV strain, whereas 90% of placebo vaccinated birds showed lesions typical of IBDV such as bursal oedema and haemorrhage at 3 days post challenge (). In the groups vaccinated with HVT-ND-IBD by the SC route, 100% of birds were protected from challenge, whereas 100% of placebo vaccinated birds showed bursal lesions typical of IBDV ().
Table 2. Protection against IBDV (challenge with classical STC IBDV).
Variant IBDV challenge
Protection at 4 weeks after vaccination for Variant E IBDV challenge was evaluated at 10 days post challenge by calculation of the bursa/body weight ratio. When birds were vaccinated by the in ovo route and challenged 4 weeks post vaccination with variant E IBDV, 91% of the birds were protected, whereas 100% of birds inoculated in ovo with a diluent were positive for IBDV. After SC vaccination 97% of birds were protected and 100% of the placebo inoculated birds were positive for IBD ().
Table 3. Protection against IBDV (challenge with variant E IBDV).
Very virulent IBDV challenge
Two studies were performed where birds were challenged with very virulent IBDV challenge strain CS89. In the first study, birds (20–22 birds/group) were vaccinated in ovo with different doses of HVT-ND-IBD and challenged at 2, 3 and 8 weeks post vaccination. All HVT-ND-IBD vaccinated birds were 100% protected against CS89 challenge. None of the birds showed clinical signs or lesions of the bursa when evaluated after the 10-day observation period. At the 2 and 3 week challenge timepoints all control birds (10–12 birds/group) succumbed to IBD or were euthanized due to severe clinical signs of IBD by day 4 (all bursas had more than 75% of the follicles with lymphoid depletion). At 8 weeks post vaccination eight birds succumbed to IBD or were euthanized due to severe clinical signs of IBD by day 6; the two other birds showed clinical signs and both had 100% lymphoid depletion of the bursa when evaluated after the 10-day observation period ((A)).
Figure 3: Survival curves after IBDV CS89 challenge. (A) Very virulent IBDV CS89 challenge 2 weeks (graph A1), 3 weeks (graph A2) and 8 weeks (graph A3) post in ovo vaccination with HVT-ND-IBD at two different doses (1: 1996 PFU/dose, 2: 3157 PFU/dose); (B) Very virulent IBDV CS89 challenge 2 weeks post SC vaccination with HVT-ND-IBD at three different doses (1: 1993 PFU/dose, 2: 2515 PFU/dose 3: 3170 PFU/dose). At the end of the observation period (day 10) the bursas of surviving birds were histologically evaluated for lesions specific for IBD and scored positive if present.
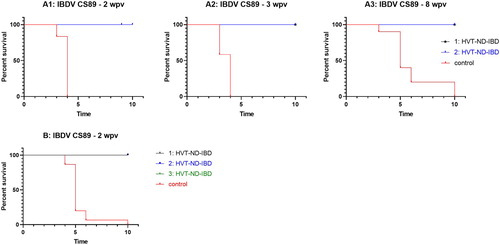
In the second study, chickens were vaccinated by the SC route with three different doses of HVT-ND-IBD (25 chickens/group) and challenged 2 weeks later with very virulent CS89. All HVT-ND-IBD vaccinated birds were protected against CS89 challenge. None of the birds showed clinical signs or lesions of the bursa when evaluated after the 10-day observation period. Fourteen of the control birds succumbed to IBD or were euthanized due to severe clinical signs of IBD by day 6. The remaining bird showed clinical signs and had 100% lymphoid depletion of the bursa when evaluated after the 10-day observation period ((B)).
Efficacy against MDV challenge
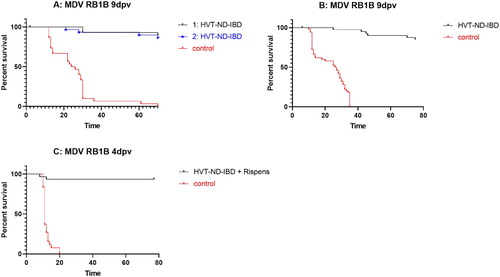
Embryonated eggs were vaccinated in ovo with different doses of HVT-ND-IBD (1996 pfu/dose or 3157 pfu/dose). Controls were inoculated with diluent. Thirty birds per group were challenged with the RB1B strain. As can be seen in (A), a high level of protection was obtained. In the groups vaccinated with HVT-ND-IBD: 90 and 87% of birds vaccinated with 1996 and 3157 pfu/dose, respectively, had no signs of MD and did not show macroscopic lesions of MD at the end of the observation period, whereas 100% of placebo vaccinated birds showed clinical signs and lesions typical of MD. Protection was also assessed when birds were vaccinated by the SC route and 50 birds/group were included. Eighty-eight percent of the SC vaccinated birds were free of clinical signs or lesions typical of MD, whereas 100% of the control birds had clinical signs and lesions typical of MD ((B)). These results demonstrate that the insertion of multiple genes from two different pathogens did not alter the ability of the HVT backbone to provide a high level of protection against challenge with a virulent MDV (P < 0.0001, log-rank test for all vaccinated groups, compared to the relevant unvaccinated control group).
In a third study, birds were vaccinated with HVT-ND-IBD mixed with Rispens CVI988 vaccine as this combination is often used in layer and breeder birds in the field to increase the level of protection against MD. Thirty birds were vaccinated with 2000 pfu/dose of HVT-ND-IBD and 1000 pfu/dose of Rispens CVI988 vaccine, and 30 unvaccinated birds were used as controls. When the birds were challenged with very virulent MDV strain RB1B as early as 4 days after vaccination, 100% of the unvaccinated control birds died or were euthanized when showing severe clinical signs of MD by 20 days post vaccination, demonstrating the high challenge pressure applied. From the vaccinated birds 93% were protected, demonstrating a high level of protection (P < 0.0001, log-rank test) ((C)).
Figure 4: Survival curves after MDV challenge. (A) RB1B challenge at 9 days post vaccination in birds vaccinated in ovo with HVT-ND-IBD (1: 1996 PFU/dose, 2: 3157 PFU/dose); (B) RB1B challenge at 9 days post vaccination in birds vaccinated SC with HVT-ND-IBD (1000 PFU/dose); (C) RB1B challenge at 4 days post vaccination of birds vaccinated SC with HVT-ND-IBD (2000 PFU/dose) + Rispens CVI988 vaccine (1000 PFU/dose). At the end of the observation period (75, 70 and 77 days post challenge for A, B, and C, respectively), surviving birds were necropsied and birds were examined for macroscopic lesions of MD and scored positive if present.
Efficacy in the field
The efficacy of the vaccine was also evaluated under field conditions. Two field trials were performed: one trial in broiler chickens, which were in ovo vaccinated with HVT-ND-IBD (test group) or with the vaccine diluent (control group). The second trial was in layer chickens which were SC vaccinated with HVT-ND-IBD mixed with Rispens CVI988 vaccine. After vaccination, chickens from both trial locations were transported to the laboratory for evaluation of vaccine efficacy against MD, ND and IBD. Challenges for NDV and IBDV were performed when the levels of maternally-derived antibodies for these components had sufficiently declined as monitored in the control birds. The results of the trials are summarized in . All challenges were successful as controls showed a high level of disease prevalence: 100% of the control birds were positive for disease after the NDV and IBDV challenges and 85 and 93% were positive for MD after the RB1B challenge in the broilers and layers, respectively. The level of protection against all challenges was 90% or higher demonstrating excellent efficacy of the HVT-ND-IBD vaccine when used under commercial conditions.
Table 4. Protection of commercial birds against ND, MD and IBD (field efficacy).
Discussion
Vaccination is used world-wide to effectively control infectious agents causing severe economic losses in poultry flocks (Sharma, Citation1999; Marangon & Busani, Citation2006). With the emphasis on avoiding the use of antibiotics in poultry production systems, vaccines will have an even larger role in health management programmes for protection of poultry flocks across the industry. Furthermore, additional trends in the industry place more demands on vaccination as a method of disease control as a result of the desire for safe convenient live vaccines that are delivered by mass application, preferably in the hatchery, unaffected by maternally-derived antibodies, which provide long lasting immunity after one dose. On the other hand, a large number of live vaccines are required to be given at a young age to chickens sometimes causing difficulties with compatibility, vaccine reactions and potentially reversion to virulence (Guy et al., Citation1991; Gelb et al., Citation2007; Müller et al., Citation2012; Landman et al., Citation2017). Live vaccines based on HVT as a vector have played an important role in meeting many of the aforementioned demands and have significantly improved some of these aspects, thus improving the welfare of chickens and user convenience.
Although several HVT-based vaccines have been used successfully to protect poultry flocks against a number of important avian pathogens (Sondermeijer et al., Citation1993; Le Gros et al., Citation2009; Vagnozzi et al., Citation2012; Esaki et al., Citation2013; Kapczynski et al., Citation2015), these vaccines are monovalent in nature; i.e. protect against one disease in addition to MD. Moreover, it has proved difficult to achieve robust polyvalent protection by simply combining these monovalent vaccines due to the interference seen when combining different HVT construct vaccines or conventional HVT vaccines with HVT construct vaccines (Dunn et al., Citation2019). In order to address the interference problem, we recently developed an HVT-based double insert vaccine and demonstrated its utility in protection of chickens against NDV and ILTV as well as MDV from one virus backbone (Gergen et al., Citation2019). In order to broaden the versatility of HVT as a polyvalent vaccine vector platform and extend the application of our approach to overcoming potential interference among HVT construct vaccines, we prepared a double HVT construct vaccine (HVT-ND-IBD) and assessed its safety and potential as a polyvalent vaccine for protection against three key avian pathogens; MDV, NDV and IBDV.
The safety of the HVT-ND-IBD vaccine described here was confirmed by overdose studies with susceptible SPF embryos at 18 days of age or 1-day-old chicks inoculated in ovo or SC, respectively, with a high virus dose. These birds did not show any adverse reactions or MD lesions. In addition, in ovo vaccination with this overdose did not affect hatchability. These results indicate that insertion of foreign genes did not alter the safety profile of the HVT vector backbone.
Of critical importance for the generation of HVT construct vaccines with multiple gene inserts is the genetic stability of the construct. The sequence of the inserted expression cassettes (promoters and inserted genes) and the location of the insert in the HVT genome all play a role. However, it cannot be predicted which sequences will lead to a stable construct (Willemsen & Zwart, Citation2019). To generate the HVT-ND-IBD different promoters and different insertion sites were tested (data not shown) leading to the selection of the current HVT-ND-IBD construct which uses the IE1 gene promoter from HCMV for the NDV F gene expression and the IE1 gene promoter from MCMV for the IBDV VP2 gene expression, and the insertion of both heterologous genes in one locus in the US2 region of the HVT genome. The efficacy studies performed with this construct demonstrated protection levels in line with single HVT constructs, which indicated the suitability of the promoters used to obtain early and sustained protection against ND and IBD.
In vitro construct stability is essential for commercial production of a HVT construct vaccine, and in vivo stability is essential to obtain the long duration of protection which is one of the key advantages for HVT vectors, as HVT causes a persistent infection in chickens and the virus remains present lifelong (Nair et al., Citation2020). If the construct is not fully stable, the inserted sequences could be lost, which would result in decreased protection at a later age of the birds. To evaluate the duration of protection birds were kept up to 60 weeks post vaccination in containment. These birds were not exposed to any live ND vaccines or field viruses. When challenged with NDV Texas GB at 60 weeks post vaccination, the birds were still fully protected (100%).
The inserted NDV gene was taken from the NDV Clone 30 vaccine strain which is a cloned La Sota NDV strain of genotype II. To evaluate NDV protection two different challenge strains were used: the Texas GB which is also a genotype II NDV (96% amino acid identity between the F-protein of Nobilis® ND Clone 30 and the Texas GB strain) and the Herts Weybridge 33/56 NDV challenge strain which is a genotype IV strain (92% amino acid identity). A high level of protection was obtained (over 94% protection) against both genotypes.
The inserted VP2 gene was taken from the classical IBDV strain Faragher 52/70 and protection was demonstrated against different IBDV challenge strains: classical IBDV strain (STC), a very virulent IBDV strain (CS89) and a variant E IBD strain. The challenge evaluation was done in different ways and at different timepoints post challenge for the challenge viruses used. Protection against STC was evaluated very early at 3 days post challenge by evaluation of gross lesions in the bursa; such early evaluation gives insight into the acute effects (and protection) of IBDV challenge. Protection was also evaluated 10 days post challenge for the CS89 (clinical signs and bursa lesions by histology) and variant E (B/BW evaluation); in all cases a high level of protection was observed demonstrating vaccine protection. The homology of the VP2 sequence inserted in the HVT-ND-IBD vaccine to the different challenge strains is very high (98–99% amino acid identity). As demonstrated earlier for HVT vaccines with a single VP2 insert, protection can be obtained against homologous and heterologous challenges (Bublot et al., Citation2007; Gelb et al., Citation2016).
To demonstrate protection against MD, challenges were performed where HVT-ND-IBD was used by itself or mixed with the Rispens CVI988 vaccine strain (Nobilis® Rismavac). The combination of the Rispens CVI988 vaccine strain with a HVT vaccine is often used in layer birds. It was demonstrated that 93% protection against the vvMDV challenge RB1B was obtained at 4 days post vaccination when Rispens CVI988 vaccine was combined with HVT-ND-IBD.
The combination of HVT-ND-IBD with a live ND vaccine was also evaluated as the combination of a HVT construct vaccine with a live NDV vaccine can increase the overall protection level (Rauw et al., Citation2010) and live ND vaccines induce early protection. The combination of HVT-ND-IBD with a live ND vaccine was evaluated as early as 2 weeks post vaccination and a 100% protection against Herts challenge virus was obtained. In regions where NDV is prevalent and causing disease issues, the combined use of a rHVT with another live ND vaccine could increase the overall protection with early onset and a long duration (Kapczynski et al., Citation2013).
The presence of maternally-derived antibody is known to negatively influence vaccination, but HVT and some HVT construct vaccines have demonstrated to be effective in the presence of maternally-derived antibody (Morgan et al., Citation1993; Bublot et al., Citation2007; Le Gros et al., Citation2009; Rauw et al., Citation2010). The HVT-ND-IBD was also tested in the field in birds with maternally-derived antibodies. Efficacy against MD, ND and IBD was evaluated after both in ovo vaccination in broilers and SC vaccination in commercial layer birds and it was confirmed that HVT-ND-IBD was able to provide protection against all these diseases.
In summary, the double HVT construct vaccine described in this report, HVT-ND-IBD, provides the poultry industry with a new tool that expands the range of HVT as a vector platform for a new generation of safe and well-characterized HVT-based vaccines, which provide excellent protection against three key poultry pathogens from a single HVT backbone with a single dose given by mass application.
Acknowledgements
The authors thank the technical staff and Animal Care Staff at Elkhorn, Nebraska and Millsboro, Delaware facilities and at Boxmeer, the Netherlands for their excellent support in the conduct of these studies.
Disclosure statement
The authors of the presented studies are employees of Intervet Inc. USA, Intervet UK Ltd and Intervet International BV.
References
- Bublot, M., Pritchard, N., LeGros, F.X. & Goutebroze, S. (2007). Use of a vectored vaccine against infectious bursal disease of chickens in the face of maternally derived antibody. Journal of Comparative Pathology, 137, S81–S84.
- Dunn, J.R., Dimitrov, K.M., Miller, P.J., Garcia, M., Turner-Alston, K., Brown, A. & Hartman, A. (2019). Evaluation of protective efficacy when combining turkey herpesvirus–vector vaccines. Avian Diseases, 63, 75–83.
- Esaki, M., Noland, L., Eddins, T., Godboy, A., Saeki, S., Saitoh, S., Yasuda, A. & Dorsey, K.M. (2013). Safety and efficacy of a turkey herpes virus vector laryngotracheitis vaccine for chickens. Avian Diseases, 57, 192–198.
- Eterradossi, N. & Saif, M.Y. (2013). Infectious bursal disease. In D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez, & V. Nair (Eds.), Diseases of Poultry 13th edn (pp. 219–246). Hoboken: Wiley-Blackwell.
- Gelb, J.J., Jackwood, D.J., Brannick, E.M. & Ladman, B.S. (2016). Efficacy of recombinant HVT-IBD vaccines administered to broiler chicks from a single breeder flock at 30 and 60 weeks of age. Avian Diseases, 60, 603–612.
- Gelb, J.J., Ladman, B.S., Lacata, M.J., Shapiro, M.H. & Campion, L.R. (2007). Evaluating viral interference between infectious bronchitis virus and Newcastle disease virus vaccine strains using quantitative reverse transcription-polymerase chain reaction. Avian Diseases, 51, 924–934.
- Gergen, L., Cook, S., Ledesma, B., Cress, W., Higuchi, D., Counts, D., Cruz-Coy, J., Crouch, C., Davis, P., Tarpey, I. & Morsey, M. (2019). A double recombinant herpes virus of turkeys for the protection of chickens against Newcastle, infectious laryngotracheitis and Marek’s diseases. Avian Pathology, 48, 45–56.
- Guy, J.S., Barnes, H.J. & Smith, L.G. (1991). Increased virulence of modified-live infectious laryngotracheitis vaccine virus following bird-to-bird passage. Avian Diseases, 35, 348–355.
- Ingrao, F., Rauw, F., van den Berg, T. & Lambrecht, B. (2017). Characterization of two recombinant HVT-IBD vaccines by VP2 insert detection and cell-mediated immunity after vaccination of specific-pathogen free chickens. Avian Pathology, 46, 289–299.
- Kapczynski, D.R., Alfonso, C.L. & Miller, P.J. (2013). Immune responses of poultry to Newcastle disease virus. Developmental and Comparative Immunology, 41, 447–453.
- Kapczynski, D.R., Esaki, M., Dorsey, K.M., Jiang, H., Jackwood, M., Moraes, M. & Gardin, Y. (2015). Vaccine protection of chickens against antigenically diverse H5 highly pathogenic avian influenza isolates with a live HVT vector vaccine expressing the influenza hemagglutinin gene derived from a clade 2.2 avian influenza. Vaccine, 33, 1197–1205.
- Landman, W.J.M., Vervaet, C., Remon, J.P., Huyge, K. & van Eck, J.H.H. (2017). Primary Newcastle disease vaccination of broilers: comparison of the antibody seroresponse and adverse vaccinal reaction after eye–nose drop or coarse spray application, and implication of the results for a previously developed coarse dry powder vaccine. Avian Pathology, 46, 451–461.
- Le Gros, F.X., Dancer, A., Giacomini, C., Pizzoni, L., Bublot, M., Graziani, M. & Prandini, F. (2009). Field efficacy trial of a novel HVT-IBD vector vaccine for 1-day-old broilers. Vaccine, 27, 592–596.
- Lupini, C., Quaglia, G., Mescolini, G., Russo, E., Salaroli, R., Forni, M., Boldini, S. & Catelli, E. (2020). Alteration of immunological parameters in infectious bronchitis vaccinated–specific pathogen-free broilers after the use of different infectious bursal disease vaccines. Poultry Science, 99, 4351–4359.
- Marangon, S. & Busani, L. (2006). The use of vaccination in poultry production. Scientific and Technical Review of the Office International des Epizooties (Paris), 26, 265–274.
- Miller, P.J. & Koch, G. (2013). Newcastle disease, other avian paramyxoviruses, and avian metapneumovirus infections. In D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez, & V. Nair (Eds.), Diseases of Poultry 13th edn (pp. 89–138). Hoboken: Wiley-Blackwell.
- Müller, H., Mundt, E., Eterradossi, N. & Islam, M.R. (2012). Current status of vaccines against infectious bursal disease. Avian Pathology, 41, 133–139.
- Morgan, R.W., Gelb, J.C., Schreurs, S., Lutticken, D., Rosenberger, J.K. & Sondermeijer, J.P. (1992). Protection of chicken from Newcastle and Marek’s diseases with a recombinant herpes virus of turkeys vaccine expressing the Newcastle disease virus fusion protein. Avian Diseases, 36, 858–872.
- Morgan, R.W., Gelb, J.J., Pope, C.R. & Sondermeijer, P.J. (1993). Efficacy in chickens of a herpesvirus of turkeys recombinant vaccine containing the fusion gene of Newcastle disease virus: onset of protection and effect of maternal antibodies. Avian Diseases, 37, 1032–1040.
- Nair, V., Gimeno, I. & Dunn, J. (2020). Marek’s disease. In D.E. Swayne, M. Boulianne, C.M. Logue, L.R. McDougald, V. Nair & D.L. Suarez (Eds.), Diseases of Poultry 14th edn (pp. 550–701). Hoboken: Wiley-Blackwell.
- Okazaki, W., Purchase, H.G. & Burmester, B.R. (1970). Protection against Marek’s disease by vaccination with a herpesvirus of turkeys. Avian Diseases, 14, 413–429.
- Rauw, F., Gardin, Y., Palyac, V., Anbari, S., Lemaire, S., Boschmans, M., Van den Berg, T. & Lambrecht, B. (2010). Improved vaccination against Newcastle disease by an in ovo recombinant HVT-ND combined with an adjuvanted live vaccine at day-old. Vaccine, 28, 823–833.
- Sharma, J.M. (1999). Introduction to poultry vaccines and immunity. Advances in Veterinary Medicine, 41, 481–494.
- Sondermeijer, P.J., Claessens, J.A., Jenniskens, P.E., Mockett, A.P., Thijssen, R.A., Willemse, M.J. & Morgan, R.W. (1993). Avian herpesvirus as a live viral vector for the expression of heterologous antigens. Vaccine, 11, 349–358.
- Vagnozzi, A., Zavala, G., Riblet, S.M., Mundt, A. & Garcia, M. (2012). Protection induced by commercially available live-attenuated and recombinant viral vector vaccines against infectious laryngotracheitis virus in broiler chickens. Avian Pathology, 41, 21–31.
- Willemsen, A. & Zwart, M.P. (2019). On the stability of sequences inserted into viral genomes. Virus Evolution, 5, vez045.
