ABSTRACT
Salmonella Gallinarum (SG) is an avian-restricted pathogen that causes fowl typhoid in poultry. Although it has been reported frequently over many decades in poultry flocks worldwide, the microorganism is more commonly associated with poultry in developing countries, particularly those with high ambient temperatures, where the acute form of the disease results in considerable economic losses. A more detailed investigation of environmental factors that affect the course of disease may assist in identifying effective prevention and control measures. Heat stress is known to impair the immunological response to a variety of pathogens and clearly may be an important contributory factor in the prevalence of disease in countries with warm or hot climates. Thus, the objective of the present study was to evaluate the effects of heat stress on chickens infected with SG. For this, light and semi-heavy commercial laying hens were distributed randomly within four groups as follows: infected and non-infected groups in rooms held at ambient temperature, and infected and non-infected groups under heat stress. Clinical signs, egg production, and mortality were recorded daily. Bacteriological counts in liver and spleen samples were estimated at 2, 5, 7, and 14 days post-infection. The results showed that both SG infection and heat stress had similar effects on egg production and a synergistic effect of the two stressors was observed. The data show an interaction between disease and heat stress which could point towards environmental and biosecurity approaches to resolving the possible 30% fall in production observed in such countries.
Introduction
Acute and subclinical infections caused by Salmonella are responsible for a huge negative impact on public health and livestock economics (Shivaprasad, Citation2000; Wigley, Citation2016). Salmonella Gallinarum biovar Gallinarum (SG) is the causative agent of fowl typhoid (FT) in gallinaceous and other birds and is distributed worldwide (Freitas Neto et al., Citation2020). Under-reporting in some countries has hindered control of the pathogen (Celis-Estupiñan et al., Citation2017; OIE, Citation2020). A deeper and more extensive understanding of the factors associated with fowl typhoid pathogenesis and dissemination is an absolute requirement to avoid mortality, morbidity, poor production parameters, and the financial cost of control measures (Barrow & Freitas Neto, Citation2011; Garcia et al., Citation2013; Wigley, Citation2016).
The course and outcome of infection depend on the nature of the early interaction between the bacterium and the mononuclear phagocytic system of the host (Maskell et al., Citation1987; Kaiser et al., Citation2000; Zaninelli et al., Citation2019). The immune response to pathogens is directly affected by ambient temperature, and extreme heat stress can lead to increased disease severity (Welch, Citation1992; Padgett & Glaser, Citation2003; Hassani et al., Citation2009; Bhandare & Sheard, Citation2010; Liu et al. Citation2011; Lara & Rostagno, Citation2013).
In countries with tropical or sub-tropical climates, ambient temperatures can exceed 45°C (Accuweather, Citation2020). Under such conditions, a higher prevalence of FT has been reported (OIE, Citation2020). We hypothesized that heat stress could be an aggravating factor in SG infection. The objective of the present study was therefore to evaluate the impact of heat stress on the pathogenesis of FT in laying hen lines.
Materials and methods
Ethical Statement
The study was conducted at the Laboratory of Avian Pathology of the Department of Animal Pathology from the School of Agricultural and Veterinary Sciences (FCAV/UNESP), Jaboticabal, Brazil. It was carried out in accordance with the Ethical Principles on Animal Experimentation approved by the internal Ethical Committee on the Use of Animals (Process n° 017354/18).
Bacterial strains and preparation of inoculum
A spontaneous nalidixic acid-resistant of SG, strain SG 287/91, was stored at −80°C in Lysogeny Broth (LB – Sparks, MD, USA) supplemented with 30% glycerol. A 10 ml culture in LB was incubated at 37°C for 18 h at 150 rpm. From the overnight culture, an aliquot representing 0.1 ml of the volume of the inoculum was transferred to a 10 ml fresh LB and cultured for 3 h until it reached 108 CFU/ml. The inoculum administered to the brown semi-heavy hens was diluted to 105 CFU/ml prior to administering a dose of 1 ml per bird, due to the higher susceptibility of this line to FT.
In vivo experiment and sampling
Two hundred and thirty day-old white line (light variety) and brown line (semi-heavy variety) female hens were obtained from a commercial hatchery where they were vaccinated against Marek’s disease. They were housed within metallic pens in temperature-controlled rooms, receiving water and feed as recommended commercially until the start of the experimental period. During the rearing phase, birds were vaccinated against viral diseases (avian infectious bronchitis, Newcastle disease, infectious bursal disease and infectious coryza) and microbiological culture was performed monthly to guarantee the absence of Salmonella spp.
Prior to the onset of lay (16 weeks), chickens were distributed in experimental batteries into four experimental groups (). Chickens were inoculated with 1 ml of inoculum orally directly into the crop, with non-infected birds receiving sterile LB instead of SG broth culture. Infection and heat stress were only initiated after egg production had become stable (22 weeks old). Up to that time, room temperatures were adjusted according to the commercial manual. Groups W2, W4, B2, and B4 were submitted to 6 h of heat stress immediately before and after inoculation; from the following day, these groups experienced 8 h of heat stress per day for 7 days.
Table 1. Experimental design of White (W) and Brown (B) lines infected or not with S. Gallinarum at room temperature recommended for the lineages or heat stress conditions.
Clinical signs, mortality, and egg production were recorded daily for 14 days post-inoculation (dpi). At 2, 5, 7, and 14 dpi, three chickens per group were euthanized to evaluate gross lesions and to harvest samples of liver, spleen and caecal content for bacterial enumeration. This was performed with decimal dilutions of tissue homogenates with selenite enrichment plating on Brilliant Green agar containing sodium nalidixate, as done previously (Rodrigues-Alves et al., Citation2018).
Statistical analysis
Data were analysed using GraphPad Prism version 8.0.1 for Windows (GraphPad Software, La Jolla, CA, USA). Mortality values were compared by the chi-square test. ANOVA followed by Tukey’s and Dunnett’s tests were used for bacterial counts and daily egg production, respectively. The results were considered statistically significant when the P-value was lower than 0.05 (P < 0.05).
Results
shows the effects of SG infection and heat stress on egg production in the white and brown egg-layers. In white egg-layers both infection and heat stress produced marked and significant reductions in production, with the effects of heat stress immediately apparent and that following infection only becoming clear 5 days after infection. The effect of heat stress appeared to begin to return to normal in the later stages of the experiment. The effect of combined heat stress and infection were reduced a little more than either infection or heat stress alone but this was not statistically significant compared with infection alone. Nevertheless, it was statistically the greatest reduction from the control group.
Figure 1. Effects of heat stress on daily egg production of commercial laying hens challenged by S. Gallinarum. Different symbols represent significant differences (P < 0.05) using Dunnet’s test.
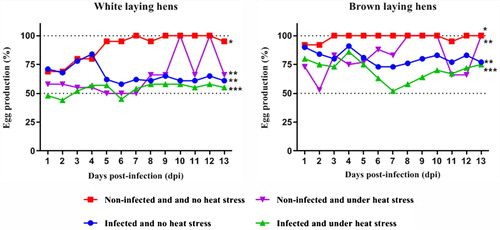
In brown egg-layers, the effect of heat stress was less than in white egg-layers but still statistically significant, and also appeared to return intermittently towards normal as the experiment progressed. Despite brown egg-layers being more susceptible to SG infection than white egg-layers, the effect on egg production was less but still significant. The effect of the combined stresses was greater than in white egg-layers and also highly statistically significant.
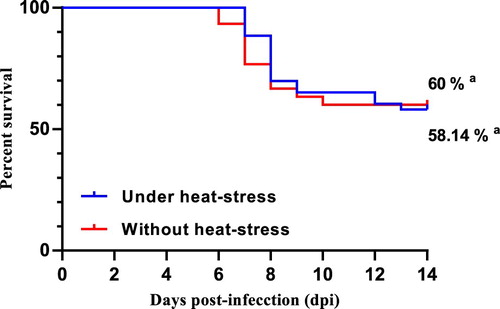
Although white egg-layers showed mild clinical signs with some diarrhoea, no birds died. On the other hand, brown egg-layers had severe clinical signs including ruffled feathers, apathy, somnolence, and diffuse greenish diarrhoea. In addition, 41.9% of the chickens under heat stress succumbed to the disease, while 40% reared in ambient conditions died ().
Figure 2. Effects of heat stress on mortality of commercial brown laying hens challenged by S. Gallinarum. aNon-significant statistical differences (P > 0.05) using Dunnet’s test.
SG-infected birds had congestive hepatosplenomegaly with the presence of whitish multifocal lesions on both organs. Gross lesions were more severe in brown chickens, as described in . Nevertheless, no statistical significance (P > 0.05) was observed either in bacterial counts () or in mortality rates between groups subjected or not subjected to heat stress.
Figure 3. Viable cells of S. Gallinarum in liver, spleen, and caecal content of commercial laying hens. ns: no statistically significant difference (P > 0.05).
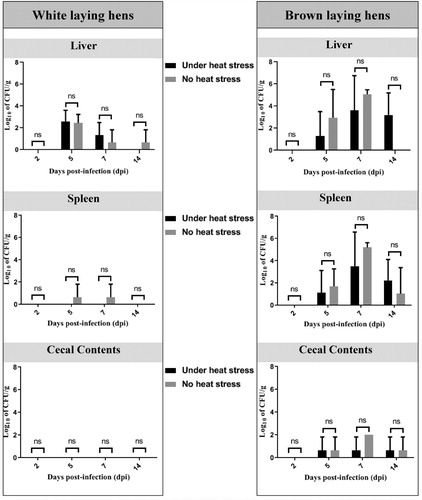
Table 2. Effects of heat stress on gross lesion scores of commercial laying hens challenged by S. Gallinarum.
Discussion
Heat stress alters the secretion of hormones, such as gonadotropin, luteinizing hormone, prolactin and/or thyroid hormone, that affect ovulation impacting on egg production (Novero et al., Citation1991; Visser et al., Citation2009), and causes a deterioration in egg quality due to the imbalance of available calcium as a consequence of respiratory alkalosis (Franco-Jimenez et al., Citation2007). Moreover, it reduces the capacity of the immune system to respond effectively to pathogens, increasing disease severity and mortality (Bhandare & Sheard, Citation2010; Lara & Rostagno, Citation2013). Fowl typhoid is a systemic disease that can lead to high mortality rates, mainly observed in mature birds. The pathogenicity depends on the virulence of the bacterial strain involved, the infectious dose, the presence of any co-infection, and the age, lineage, and nutritional status of the bird (Freitas Neto et al., Citation2020; Shivaprasad, Citation2000; Wigley, Citation2016). Generating a greater understanding of the effect of heat stress on SG-infected chickens could assist poultry technicians and producers in those countries with warm climates to increase their knowledge of one factor contributing to production losses.
The clinical signs and gross lesions in the organs of birds of both lines infected with SG were similar to those reported in the literature. The mild clinical signs and less severe gross lesions of white chickens in comparison to brown hens are likely due, in part at least, to the more efficient phagocytic mononuclear system of the white chickens on the infection control which is likely to be genetically determined by the SAL1 locus (Wigley et al., Citation2002). Light birds usually show limited foci of inflammation in the liver although these are more extensive in the spleen, while semi-heavy lines typically develop extensive areas of necrosis in both organs with higher influx of inflammatory cells (Garcia et al., Citation2013; Psifidi et al., Citation2018). The severity of the effects following infection in chickens under heat stress could be the result of the inability to dissipate heat, which can lead to the collapse of several organs, including the nervous system, cardiovascular, gastrointestinal, hepatic, renal and muscle systems (Lara & Rostagno, Citation2013), that provokes mainly necrosis and haemorrhages, as observed in canines (Marcondes Santos et al., Citation2003), humans (Tarini et al., Citation2006), baboons (Bouchama et al., Citation2005), pigs (Pearce et al., Citation2013) and chickens (Wang et al., Citation2018).
We showed higher mortality rates of infected brown line birds in comparison to the white egg-layers. Nevertheless, the white egg-layers become asymptomatic carriers, which favours dissemination and maintenance of SG in poultry farms (Shivaprasad, Citation1997; Kaiser et al., Citation2000; Barrow & Freitas Neto, Citation2011; Wigley, Citation2016). On the other hand, SG-infected brown hens showed a pronounced decrease in egg production at 6–10 dpi associated with the peak of mortality. Semi-heavy hens are susceptible to fowl typhoid, and death has been reported to occur between 5 and 14 dpi (Berchieri Junior et al., Citation2001; Oliveira et al., Citation2005; Barrow & Freitas Neto, Citation2011).
Once infected, the host directs nutrients and energy generation from production to control the pathogen (Humphrey & Klasing, Citation2004), and fowl typhoid is known to decrease daily egg production (Celis-estupiñan et al., Citation2017) as we observed here. Furthermore, stress conditions alter physiological parameters, such as reproductive hormone secretion, bird behaviour, and the energy used to adapt to heat stress (Novero et al., Citation1991; Bhandare & Sheard, Citation2010; Al-Fataftah & Abdelqader, Citation2014; Alhenaky et al., Citation2017). These alterations lead to reduced production (Line et al., Citation1997; Mashaly et al., Citation2004), to poorer egg quality and, consequently, to economic losses (Novero et al., Citation1991; Pereira et al., Citation2008; Pereira et al., Citation2010; Garcia et al., Citation2012). Although the high temperature did not influence the mortality of infected chickens, the association of fowl typhoid and heat stress favoured a substantial decrease (P < 0.05) of about 10% on egg production in both lines in comparison to birds solely infected or submitted to heat stress alone. Furthermore, the losses were even higher in both lineages when comparing with unchallenged chickens reared at thermoneutral conditions, which were 24% and 33% for semi-heavy and light lineages, respectively. It is noteworthy that the economic losses in infected brown birds did not reside only in a fall in egg-laying production, but also in increased mortality. Thus, we can state firmly that heat stress decreases egg production of birds with fowl typhoid.
It must be remembered that the heat stress was intermittent (8 h per day) and the impact on mortality in semi-heavy lineages challenged with SG was due to the acute infectious course of fowl typhoid, and not to the heat stress (Shivaprasad, Citation1997; Celis-estupiñan et al., Citation2017). Thus, we presume that chronic heat stress observed in warm-climate countries could have a more negative influence in long-term infections, although this clearly remains to be determined and the possibility of physiological adaptation must be taken into account. The disease is present in all continents, being frequently reported in Africa, Asia, and Central and South Americas (OIE, Citation2020) where temperatures throughout the year are higher than in temperate regions and the association with SG leads to more economic losses.
Conclusion
Heat stress by itself can increase production losses and the severity of anatomopathological changes in chickens infected by S. Gallinarum, but not the mortality rates. Furthermore, the association of both infection and temperature stresses resulted in egg production losses that varied from 24% to 33%. Thus, our results reinforce the importance of investment in biosecurity measures to avoid the introduction of S. Gallinarum in poultry flocks and also in climate control in poultry houses to offer thermoneutral conditions essential to bird welfare and to reach production goals.
Acknowledgements
Thanks to Post Graduate Program in Veterinary Medicine, Agricultural and Livestock Microbiology and Pro-rectory of Teaching and Research (PROPe), School of Agricultural and Veterinary Sciences Sao Paulo State University (FCAV/UNESP), Jaboticabal, SP, Brazil.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdelqader, A. & Al-Fataftah, A. (2014). Thermal aclimation of broiler birds by intermittent heat exposure. Journal of Thermal Biology, 39, 1–5.
- Alhenaky, A., Abdelqader, A., Abuajamieh, M. & Al-fataftah, A.R. (2017). The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. Journal of Thermal Biology, 70, 9–14.
- Accuweather. (2020). NMHSs - National Meteorological & Hydrological Services.
- Barrow, P.A. & Freitas Neto, O.C. (2011). Pullorum disease and fowl typhoid - new thoughts on old diseases: a review. Avian Pathology, 40, 1–13.
- Berchieri, J.A., Murphy, C.K., Marston, K. & Barrow, P.A. (2001). Observations on the persistence and vertical transmission of Salmonella enterica serovars Pullorum and Gallinarum in chickens: effect of bacterial and host genetic background. Avian Pathology, 30, 221–231.
- Bhandare, S. & Sheard, P. (2010). Cuidado em el transporte de las aves. World Poultry, 2, 56–57.
- Bouchama, A., Roberts, G., Al Mohanna, F., El-Sayed, R., Lach, B., Chollet-Martin, S., Ollivier, V., Al Baradei, R., Loualich, A., Nakeeb, S., Eldali, A. & De Prost, D. (2005). Inflammatory, hemostatic, and clinical changes in a baboon experimental model for heatstroke. Journal of Applied Physiology, 98, 697–705.
- Celis-estupinan, A.L.D.P., Batista, D.F.A., Cardozo, M.V., Souza, A.I.S., Rodrigues Alves, L.B., Almeida, A.M., Barrow, P.A., Berchieri Junior, A. & Freitas Neto, O.C. (2017). Further investigations on the epidemiology of fowl typhoid in Brazil. Avian Pathology, 3, 1–10.
- Franco-Jimenez, D.J., Scheideler, S.E., Kittok, R.J., Brown-Brandl, T.M., Robeson, L.R., Taira, H. & Beck, M.M. (2007). Differential effects of heat stress in three strains of laying hens. Journal of Applied Poultry Science, 4, 628–634.
- Freitas Neto, O.C., Penha Filho, R.A. & Berchieri Junior, A. (2020). Salmoneloses aviárias. In R.L. Andreatti Filho, A. Berchieri Junior, E.N. Silva, A. Back, J.D. Fabio & M.A.F. Zuanaze (Eds.), Doenças das Aves (Vol. 4, pp. 495–517). Campinas, Brazi: Ed FACTA.
- Garcia, R.G., Almeida Paz, I.C.L., Caldara, F.R., Nääs, I.A., Pereira, D.F. & Ferreira, V.M.O.S. (2012). Selecting the most adequate bedding material for broiler production in Brazil. International Journal of Poultry Science, 14, 71–158.
- Garcia, K.O., Berchieri Junior, A., Santana, A.M., Alarcon, M.F.F., Freitas Neto, O.C. & Fagliari, J.J. (2013). Experimental infection of commercial layers with wild or attenuated Salmonella Gallinarum mutant strains: anatomic pathology, total blood cell count and serum protein levels. Brazilian Journal of Poultry Science, 15, 91–104.
- Hassani, A.S., Amirmozafari, N. & Ghaemi, A. (2009). Virulence increasing of Salmonella typhimurium in Balb/c mice after heat-stress induction of phage shock protein A. Current Microbiology, 59, 446–450.
- Humphrey, B.D. & Klasing, K.C. (2004). Modulation of nutrient metabolism and homeostasis by the immune system. World’s Poultry Science Journal, 60, 90–100.
- Kaiser, P., Rothwell, L., Galyov, E.E., Barrow, P.A., Burnside, J. & Wigley, P. (2000). Differential cytokine expression in avian cells in response to invasion by Salmonella enteritidis and Salmonella gallinarum. Microbiology, 146, 3217–3226.
- Lara, L.J. & Rostagno, M.H. (2013). Impact of heat stress on poultry production. Animals, 3, 356–369.
- Line, J.E., Bailey, J.S., Cox, N.A. & Stern, N.J. (1997). Yeast treatment to reduce Salmonella and Campylobacter populations associated with broiler chickens subjected to transport stress. Poultry Science, 76, 1227–1231.
- Liu, Z., Sun, X., Tang, J., Tang, Y., Tong, H., Wen, Q., Liu, Y. & Su, L. (2011). Intestinal inflammation and tissue injury in response to heat stress and cooling treatment in mice. Molecular Medicine Reports, 4, 437–443.
- Marcondes, S.M., Fragata, F.S., Merlo, A. & Sakai, S.P. (2003). Hipertermia não pirogênica. Relato de casos. Brazilian Jounal Veterinary Research Animal Science, 40, 194.
- Mashaly, M.M., Hendricks, G.L., Kalama, M.A., Gehad, A.E., Abbas, A.O. & Patterson, P.H. (2004). Effect of heat stress on production parameters and immune responses of commercial laying hens. Poultry Science, 83, 889–894.
- Maskell, D.J., Hormaeche, C.E., Harrington, K.A., Joysey, H.S. & Liew, F.Y. (1987). The initial suppression of bacterial growth in a salmonella infection is mediated by a localized rather than a systemic response. Microbial Pathogenesis, 2, 295–305.
- Nazir, S., Kamil, S.A., Riyaz, A., Mir, M.S., Darzi, M.M., Yasine, A. & Goudar, K.S. (2014). Pathology and colonization of internal organs after experimental infection of broiler chickens with Salmonella Gallinarum through oral or intraperitoneal routes. Pathologie Infectieuse, 67, 53–60.
- Novero, R.P., Beck, M.M., Gleaves, E.W., Johnson, A.L. & Deshazer, J.A. (1991). Plasma progesterone, luteinizing hormone concentrations, and granulosa cell responsiveness in heat-stressed hens. Poultry Science, 70, 2335–2339.
- OIE. World Organisation for Animal Health. (2020). Wahis interface: disease information. 2020. Retrieved August 3, 2020.
- Oliveira, G.H., Berchieri Junior, A. & Fernandes, A.C. (2005). Experimental infection of laying hens with Salmonella enterica serovar Gallinarum. Brazilian Journal of Microbiology, 36, 51–56.
- Padgett, D.A. & Glaser, R. (2003). How stress influences the immune response. Trends in Immunology, 24, 444–448.
- Pearce, S.C., Mani, V., Boddicker, R.L., Johnson, J.S., Weber, T.E., Ross, J.W., Rhoads, R.P., Baumgard, L.H. & Gabler, N.K. (2013). Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLOS ONE, 8, 1–9.
- Pereira, D.F., Vitorasso, G., Oliveira, S.C., Kakimoto, S.K., Togashi, C.K. & Soares, N.M. (2008). Correlations between thermal environment and egg quality of two layer commercial strains. Brazilian Journal of Poultry Science, 10, 81–88.
- Pereira, D.F., Vale, M.M., Zevolli, B.R. & Salgado, D.D. (2010). Estimating mortality in laying hens as the environmental temperature increases. Brazilian Journal of Poultry Science, 12, 265–271.
- Psifidi, A., Russell, K.M., Matika, O., Sánchez-Molano, E., Wigley, P., Fulton, J.E., Stevens, M.P. & Fife, M.S. (2018). The genomic architecture of fowl typhoid resistance in commercial layers. Frontiers in Genetics, 9, 1–11.
- Rodrigues-Alves, L.B., Freitas Neto, O.C., Batista, D.F.A., Barbosa, F.O., Rubio, M.S., Souza, A.I.S., Almeida, A.M., Barrow, P.A. & Berchieri Junior, A. (2018). Inactivation of phoPQ genes attenuates Salmonella Gallinarum biovar Gallinarum to susceptible chickens. Brazilian Journal of Microbiology, 49, 601–606.
- Shivaprasad, H.L. (1997). Pullorum disease and fowl typhoid. In B.W. Calnek, H.J. Barnes, C.W. Beard, L.R.L. Mcdougald & Y.M. Saif (Eds.), Diseases of Poultry 10th ed. (pp. 82–96). Ames: Iowa State University.
- Shivaprasad, H.L. (2000). Fowl typhoid and pullorum disease. Revue Scientifique et Technique, 19, 405–424.
- Tarini, V.A.F, Vilas, L., Zanuto, R., Silva, H.C.A. & Oliveira, A.S.B. (2006). Heat, physical exercise, and hyperthermia: epidemiology, etiopatogeny, complications, risk factors, interventions, and preventions. Revista Neurociências, 14, 144–152.
- Visser, M.E., Holleman, L.J.M. & Caro, S.P. (2009). Temperature has a causal effect on avian timing of reproduction. Proceedings of the Royal Society B, 276, 2323–2331.
- Wang, X.J., Feng, J.H., Zhang, M.H., Li, X.M., Ma, D.D. & Chang, S.S. (2018). Effects of high ambient temperature on the community structure and composition of ileal microbiome of broilers. Poultry Science, 97, 2153–2158.
- Welch, W.J. (1992). Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiological Reviews, 72, 1063–1081.
- Wigley, P., Hulme, S.D., Bumstead, N. & Barrow, P.A. (2002). In vivo and in vitro studies of genetic resistance to systemic salmonellosis in the chicken encoded by the SAL1 locus. Microbes and Infection, 4, 1111–1120.
- Wigley, P. (2016). Salmonella enterica serovar Gallinarum: addressing fundamental questions in bacteriology sixty years on from the 9R vaccine. Avian Pathology, 46, 1–20.
- Zaninelli, R.L., Gobetti, S.T.C., Oliveira, K.M.B. & Campanha, J.E.T. (2019). Salmoneloses na produção avícola – revisão bibliográfica. Ciência Veterinária UniFil, 1, 154–163.
