ABSTRACT
Cestodes belonging to the genus Raillietina are a major veterinary health problem affecting the poultry industry, particularly chickens (Gallus gallus domesticus) and ducks (Anas playtrhynchos domesticus). The traditional method for accurately detecting this cestode based on their morphological characteristics is rather difficult due to the large number of morphological similarities. Consequently, this study aimed to develop specific primers for R. echinobothrida, R. tetragona, and R. cesticillus detection that could be used to indicate epidemic areas for protection and infection control. Specific primers were manually designed based on the internal transcribed spacer 2 region and validated, establishing the optimal temperature, final concentration in PCR mixture, specificity, and sensitivity of each primer set. The results showed that the primers amplify specific species without cross-amplifying other parasites and hosts. The PCR products were about 473, 352, and 397 bp long for R. echinobothrida, R. tetragona, and R. cesticillus, respectively. The sensitivity test demonstrated that R. echinobothrida and R. cesticillus-specific primers detect a minimum of 5×10−2 ng DNA, while R. tetragona-specific primers detect a minimum of 0.5 ng genomic DNA. The specific primers successfully developed in this study might be useful for detecting cysticercoids in intermediate hosts or adult stages in poultry for epidemiological surveys, management and control of infection.
RESEARCH HIGHLIGHTS
This study established specific primers for Raillietina species detection.
The ITS2 region is an effective molecular marker for Raillietina identification.
GRAPHICAL ABSTRACT
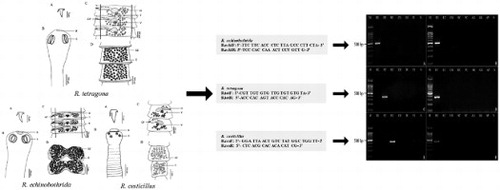
Introduction
In Thailand, poultry production is an important part of the economy, which is indicated by the constantly increasing poultry production during the decade. Moreover, eggs and meat products are affordable sources of high protein. Cestodes belonging to the genus Raillietina are a major veterinary health problem affecting the poultry industry, particularly chickens (Gallus gallus domesticus) and ducks (Anas playtrhynchos domesticus). Intestinal cestodes in the genus Raillietina have been reported in several countries worldwide. Three worldwide species of Raillietina (R. echinobothrida, R. tetragona, and R. cesticillus) have been reported to infect chickens and ducks in Tanzania, Nigeria, Ethiopia, Zimbabwe, and Bangladesh (Permin et al., Citation1997; Catelli et al., Citation1999; Eshetu et al., Citation2001; Mukaratirwa & Hove, Citation2009; Butboonchoo et al., Citation2016; Begum et al., Citation2019). Several reports have indicated that Thailand has a high Raillietina infection prevalence (8.40–66.80%) (Sangvaranond, Citation1994; Butboonchoo & Wongsawad, Citation2017). These cestode species are highly pathogenic parasites that can cause severe clinical signs, such as epithelial cell inflammation and destruction, nutritional loss, weight loss, slow growth rate, and reduced egg production (Nadakal et al., Citation1973; Samad et al., Citation1986; McDougald, Citation2020; Chen & Li, Citation2014; Bashini et al., Citation2017). This results in increased production cost and feed conversion ratio, and the sub-standard quality of meat and eggs leads to the need for rapid and accurate diagnosis for control, prevention, and timely treatment.
The main morphological characteristics for Raillietina identification include rostellar hook shape and arrangement, genital pore opening position, and number of eggs per egg capsule (Yamaguti, Citation1959; Sawada, Citation1964; Butboonchoo et al., Citation2016). However, reliance on morphological identification can lead to misidentification due to shared morphological similarities. Hence, molecular identification has been used as an alternative to the existing morphological diagnostic methods, as molecular techniques are more reliable, inexpensive, accurate, and sensitive than morphological methods. Several molecular procedures have been applied to identify Raillietina, including reverse transcription polymerase chain reaction (RT-PCR) (Chen & Li, Citation2014), random amplified polymorphic DNA polymerase chain reaction (RAPD-PCR) (Ghobashy & Taeleb, Citation2015), phylogenetic analysis (Butboonchoo et al., Citation2016), and high annealing temperature random amplified polymorphic DNA (HAT-RAPD) (Butboonchoo & Wongsawad, Citation2017). However, the methods available to date have not been used for species detection.
Therefore, this study aimed to develop a simple PCR to detect and distinguish each Raillietina species using specific primers based on the internal transcribed spacer 2 (ITS2) region. The specific primers successfully developed in this study could be applied to indicate the epidemic veterinary areas for preventing, managing, and controlling infection.
Materials and methods
Specimen preparation
The parasite specimens including Raillietina spp. and other closely related species were collected from chickens and ducks from Bangkok, Suphan Buri, and Maha Sarakham provinces, Thailand, from June to September 2019, examined under a stereo microscope (Nikon SMZ445), and fixed in 4% formaldehyde for morphological studies, or preserved in absolute ethanol for DNA extraction.
Species confirmation
For morphological identification, the fixed parasites were washed with distilled water and stained with Mayer’s haematoxylin (Bio-Optica; Milan, Italy), dehydrated using an ethyl alcohol dilution series for 45 min in each step (10, 20, 30, 50, and 70%), de-stained with 2% destain reagent (2% HCl in 70% ethyl alcohol), and washed in 70% ethyl alcohol. Subsequently, they were dehydrated using the ethanol series (80 and 95%) and butanol, cleared with xylene before being mounted with Permount (Fisher scientific; Pennsylvania, United States of America), and the species were identified based on taxonomic keys (Schultz, Citation1940; Yamaguti, Citation1958, Citation1959; Sawada, Citation1964, Citation1965; Heneberg et al., Citation2015; Butboonchoo et al., Citation2016).
Total genomic DNA of Raillietina spp. and related species was extracted using a GF-1 Tissue DNA Extraction Kit (Vivantis; Selangor, Malaysia) according to the manufacturer’s instructions. Then, genomic DNA concentration was measured by the NanoDrop Lite spectrophotometer (Thermo Scientific; California, USA) that yielded approximately 3.3–23.2 ng per gravid proglottid, and the final concentration was adjusted to 5 ng/µl with ultrapure water. The ITS2 regions were amplified using the primers ITS3 (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (Barber et al., Citation2000). The final volume of PCR was 25 µl, containing 1 mM MgCl2, 0.2 µM each primer, 0.1 mM each dNTP, 1.5 unit Taq DNA polymerase (Vivantis), 1× PCR buffer, and 1 µl (5 ng) extracted DNA as the template. PCR amplification used a FlexCycler2 (Analytik Jena AG; Jena, Germany). The DNA samples were initially denatured at 94°C for 5 min, followed by 35 cycles (denaturation at 94°C for 1 min, primer annealing at 59°C for 1 min, and extension at 72°C for 1 min), and final extension at 72°C for 10 min. The amplicons were separated using 1.5% agarose gel electrophoresis in TBE buffer (Tris base, boric acid, EDTA, pH 8, 0.5 M) and stained with ViSafe Green (Vivantis). The size of the PCR products was estimated by comparison with a 100 bp ladder marker and sequenced (1st BASE DNA Sequencing Division; Selangor, Malaysia) for species confirmation. The DNA sequences were analysed and submitted to the GenBank database [accession numbers: R. echinobothrida (MN902341, MN902342, MN902343); R. tetragona (MN902344, MN902345, MN902346); R. cesticillus (MN902346)].
Species-specific primer design
The ITS2 sequence data were aligned and R. echinobothrida-, R. tetragona-, and R. cesticillus-specific primers were manually designed based on the complete and/or partial sequence, together with sequences from the GenBank database including those for R. echinobothrida (MN902341, MN902342, MN902343), R. tetragona (MH421967, MH421970, MH421995, MN902344, MN902345, MN902346), R. cesticillus (KP893422, MN902346), R. beveridgei (AY382318), R. australis (AY382317), R. chiltoni (AY382319), R. dromaius (AY382320), and those of other related species and hosts including Hymenolepis nana (AF461124), H. diminuta (AF461125), Taenia saginata (AY825542, AY825541), Prosthogonimus cuneatus (KP192736, KP192725), P. pellucidus (KP192732, KP192734), P. ovatus (KP192733, KP192722), Echinostoma miyagawai (MH796365), E. revolutum (GQ463129, AF067850), Hypoderaeum conoideum (KJ944311, KJ944314, KJ944315), Echinoparyphium recurvatum (AY168931), Ascaridia galli (KY789471, AJ007452), and G. gallus (DQ018755). The details of each Raillietina species-specific primer are shown in .
Table 1. The specific primers used in this study.
Primer validation
The primers were validated with PCR optimization, testing for specificity and sensitivity. First, each primer set was tested to determine the optimal annealing temperature (57–64°C) and final concentration of magnesium chloride (0.5–1.0 mM) and primers (0.1–0.5 µM) in PCR mixture using gradient PCR. The primer specificity was tested with parasites that commonly co-infect poultry with Raillietina, namely Cotugnia sp., Diorchis sp., Fimbriaria sp., Echinostoma sp., E. miyagawai, H. conoideum, P. cuneatus, and A. galli. Moreover, the primer specificity was tested on the genomic DNA of definitive hosts G. g. domesticus and A. p. domesticus. To verify the target product of specific primers, the PCR products were directly sequenced by 1st BASE DNA Sequencing Division and analysed using the BLASTn algorithm tool in the GenBank database to confirm species identity. For testing primer sensitivity, 5 ng Raillietina DNA was ten-fold serially diluted based on three independent replicates per species to identify the minimum detectable concentration.
Field detection
Adult cestode specimens were randomly collected from infected poultry. The genomic DNA was extracted from a gravid proglottid and detected using Raillietina-specific primers. Subsequently, these specimens were confirmed morphologically.
Results
Species confirmation
Our study found three species of cestode belonging to the genus Raillietina, namely R. echinobothrida, R. tetragona, and R. cesticillus, which were identified by their rostellum shape, sucker shape, genital pore opening position, and number of eggs per egg capsule.
PCR optimization and primer validation
The conditions for PCR using each primer set were optimized by testing the best annealing temperature and final concentration of magnesium chloride and primers in PCR volume (). Primer specificity was tested using DNA from 11 parasitic and two host species. The results indicated that each primer set amplifies the target region without cross-amplifying DNA from other parasites, including R. echinobothrida, R. tetragona, R. cesticillus, Cotugnia sp., Diorchis sp., Fimbriaria sp., Echinostoma sp., E. miyagawai, H. conoideum, P. cuneatus, A. galli, and definitive hosts (G. g. domesticus and A. p. domesticus). The PCR products were 473, 352, and 397 bp long for R. echinobothrida, R. tetragona, and R. cesticillus, respectively (). These products were sequenced and analysed by the BLASTn algorithm tool that identified Raillietina with a similarity of 100% to the sequence in the GenBank database. The results of the sensitivity test showed that R. echinobothrida- and R. cesticillus-specific primer sets detected a minimum of 5×10−2 ng DNA, while R. tetragona primers detected 0.5 ng genomic DNA (; Supplementary Figure 1).
Figure 1. Specificity test for R. echinobothrida (A,B), R. tetragona (C,D) and R. cesticillus (E,F) primer sets. M: DNA marker 100 bp; RE: R. echinobothrida; RT: R. tetragona; RC: R. cesticillus; CO: Cotugnia sp.; DI: Diorchis sp.; FI: Fimbriaria sp.; EC: Echinostoma sp.; EM: E. miyagawai; HC: H. conoideum; PC: P. cuneatus; AG: A. galli; GG: G. g. domesticus; AP: A. p. domesticus and N: negative control.
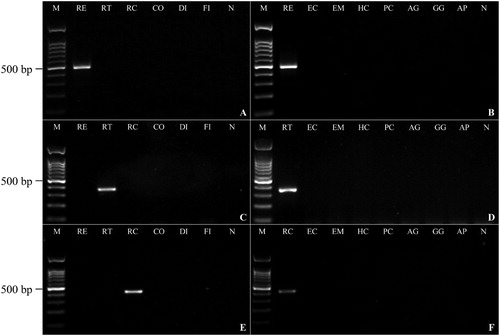
Figure 2. Results of the sensitivity test of primer sets. The initial DNA concentration was 5 ng and then ten-fold serial dilutions were prepared. A-C show the minimum DNA concentration that can be amplified by R. echinobothrida, R. tetragona and R. cesticillus primer sets, respectively.
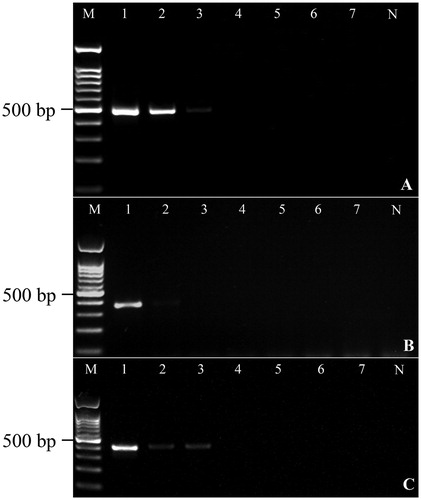
Table 2. The optimal PCR conditions for specific primers.
Sample detection
The gravid proglottid of nine adult cestode specimens was verified with Raillietina-specific primers; the results showed that six out of the nine specimens were identified as R. echinobothrida, R. tetragona, and R. cesticillus, with two specimens for each species (Supplementary Figure 2), which is consistent with the morphological characteristics (accuracy 100%) and the other three samples were identified as cestodes belonging to the genera Cotugnia, Fimbriaria, and Diorchis by morphological characteristics. Thus, it was shown that the primers have high specificity and precision for detecting the three Raillietina species.
Discussion
Raillietina species were identified based on taxonomic keys given by Yamaguti (Citation1959), Sawada (Citation1964), and Sawada (Citation1965). In this study, R. echinobothrida and R. cesticillus have double rows of rostellar hooks, whereas R. tetragona has only one row. The suckers of R. cesticillus were rounded and unarmed, but R. echinobothrida and R. tetragona were rounded armed and oval armed, respectively. The patterns of unilateral genital pore opening of R. echinobothrida and R. tetragona can be discriminated, however, from the anterior or posterior proglottid opening, while R. cesticillus has an irregularly alternating genital pore. In addition, R. cesticillus has one egg per egg capsule, but R. echinobothrida and R. tetragona have several eggs. Consequently, these morphological characteristics can be used to identify R. echinobothrida, R. tetragona, and R. cesticillus, which is similar to that reported in previous studies (Butboonchoo et al., Citation2016; McDougald, Citation2020).
To validate the primers, PCR was performed with each primer set; the results indicated that Raillietina species DNA was amplified with a high level of specificity and the DNA from other parasites and definitive hosts was not cross-amplified. The results of the sensitivity test demonstrated that the lowest DNA concentration detected by the primers was 5 × 10−2 ng for R. echinobothrida and R. cesticillus and 0.5 ng for R. tetragona, whereas the genomic DNA concentration in gravid proglottid was approximately 3.3–23.2 ng. Thus, these primers have high sensitivity and might be used to detect adult parasites in the definitive host because the three species release gravid proglottids from a definitive host. R. cesticillus releases nearly 10–12 gravid proglottids per day (McDougald, Citation2020). Previous studies have described several PCR-based methods for the identification of Raillietina species, such as HAT-RAPD, using six arbitrary primers to show the specific pattern of each species, but it is not clearly visible, allowing a possibility of misidentification (Butboonchoo & Wongsawad, Citation2017), Alternatively, loop-mediated isothermal amplification coupled with a lateral flow dipstick (LAMP-LFD) is used for the simultaneous detection of these three Raillietina species in one reaction, but it cannot differentiate each species (Panich et al., Citation2021). Consequently, the primers developed in this study may be used as an alternative for detecting and identifying worldwide Raillietina species in poultry.
This study confirms that the ITS2 region can be classified and identified in the Raillietina group distinctively from other related species. In addition, to the best of our knowledge, this study is the first to successfully develop specific primers for identifying R. echinobothrida, R. tetragona, and R. cesticillus in poultry. Moreover, these primers are high-performance new diagnostic tools that might be useful for detecting other stages of Raillietina, such as eggs in the environment or cysticercoids in the intermediate host, for epidemiological investigation and veterinary research in the future due to the short time required for diagnosis, improving accuracy and speed over morphological methods.
Conclusion
In conclusion, the specific primers developed in this study are highly effective for identifying the three cosmopolitan Raillietina species (R. echinobothrida, R. tetragona, and R. cesticillus). They can specifically amplify the DNA from each Raillietina species without cross-amplification from other parasitic species and definitive hosts. Furthermore, the lowest detectable concentration was sufficient for parasite detection in the definitive host. This could be applied to detect epidemic outbreaks and manage infection prevention and control.
Ethics approval statement
All experimental methods related to poultry were proceeded by the National Research Council of Thailand and approved by the Committee for Animal Care and Use Committee Srinakharinwirot University (License NO. SWU-A-025-2562).
Supplemental Material
Download MS Word (28.5 MB)Acknowledgements
We considerably acknowledge Srinakharinwirot University (grant number 663/2563), Thailand for granting us the use of many of their facilities and the Science Achievement Scholarship of Thailand (SAST) for providing research funding.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Barber, K.E., Mkoji, G.M. & Loker, E.S. (2000). PCR-RFLP analysis of the ITS2 region to identify Schistosoma haematobium and S. bovis from Kenya. The American Journal of Tropical Medicine and Hygiene, 62, 434–440.
- Bashini, J.M., Pandiammal, S. & Senthilkumaar, P. (2017). Isolation of genomic DNA and restriction endonuclease analysis in cestode parasite. Raillietina echinobothrida infecting the intestine of the country fowl (Gallus domesticus). World Journal of Pharmaceutical Research, 6, 1761–1772.
- Begum, A., Mukutmoni, M. & Akter, F. (2019). Parasite diversity in mallard: a cross sectional study on Anas platyrhynchos from Munshiganj, Dhaka. Bangladesh Journal of Zoology, 47, 121–128.
- Butboonchoo, P. & Wongsawad, C. (2017). Occurrence and HAT-RAPD analysis of gastrointestinal helminths in domestic chickens (Gallus gallus domesticus) in Phayao province, northern Thailand. Saudi Journal of Biological Sciences, 24, 30–35.
- Butboonchoo, P., Wongsawad, C., Rojanapaibul, A. & Chai, J.Y. (2016). Morphology and molecular phylogeny of Raillietina spp. (Cestoda: Cyclophyllidea: Davaineidae) from domestic chickens in Thailand. The Korean Journal of Parasitology, 54, 777.
- Catelli, C.T.E., Poglayen, G. & Gadale, A.T.O. (1999). Preliminary study of the helminths of the chicken digestive tract in Somalia. Pathologie Infectieuse, 52, 107–112.
- Chen, L. & Li, H. (2014). Biochemical and molecular characterization of the tegument protein RT10 from Raillietina tetragona. Parasitology Research, 113, 1239–1245.
- Eshetu, Y., Mulualem, E., Ibrahim, H., Berhanu, A. & Aberra, K. (2001). Study of gastro-intestinal helminths of scavenging chickens in four rural districts of Amhara region, Ethiopia. Revue Scientifique Et Technique-Office International Des Epizooties, 20, 791–793.
- Ghobashy, M.A. & Taeleb, A.A. (2015). Molecular characterization of Raillietina (R.) spp. Ortlepp, 1938 (Cestoda: Cyclophyllidea: Davaineidae) infecting domestic and wild birds (Columba livia and Columba livia domestica). Journal of Zoology, 10, 136–141.
- Heneberg, P., Sitko, J. & Bizos, J. (2015). Integrative taxonomy of central European parasitic flatworms of the family Prosthogonimidae Lühe, 1909 (Trematoda: Plagiorchiida). Parasitology International, 64, 264–273.
- McDougald, L. R. (2020). Internal parasites. Diseases of Poultry, 1157–1191.
- Mukaratirwa, S. & Hove, T. (2009). A survey of ectoparasites, cestodes and management of free-range indigenous chickens in rural Zimbabwe. Journal of The South African Veterinary Association, 80, 188–191.
- Nadakal, A.M., Mohandas, A., John, K.O. & Muraleedharan, K. (1973). Contribution to the biology of the fowl cestode Raillietina echinobothrida with a note on its pathogenicity. Transactions of the American Microscopical Society, 92, 273–276.
- Panich, W., Tejangkura, T. & Chontananarth, T. (2021). Novel high-performance detection of Raillietina echinobothrida, Raillietina tetragona, and Raillietina cesticillus using loop-mediated isothermal amplification coupled with a lateral flow dipstick (LAMP-LFD). Veterinary Parasitology, 292, 109396.
- Permin, A., Magwisha, H., Kassuku, A.A., Nansen, P., Bisgaard, M., Frandsen, F. & Gibbons, L. (1997). A cross-sectional study of helminths in rural scavenging poultry in Tanzania in relation to season and climate. Journal of Helminthology, 71, 233–240.
- Samad, M.A., Alam, M.M. & Bari, A.S.M. (1986). Effect of Raillietina echinobothrida infection on blood values and intestinal tissues of domestic fowls of Bangladesh. Veterinary Parasitology, 21, 279–284.
- Sangvaranond, A. (1994). Parasitic helminths of native chickens in the central part of Thailand. Kasetsart Journal (Natural Science), 28, 402–412.
- Sawada, L. (1964). On the genus Raillietina Fuhrmann 1920 (I). Journal of the Nara Gakugei University, 12, 19–36.
- Sawada, L. (1965). On the genus Raillietina Fuhrmann 1920 (II). Journal of the Nara Gakugel University, 13, 5–38.
- Schultz, R.L. (1940). The genus Diorchis Clerc 1903. The American Midland Naturalist, 23, 382–389.
- Yamaguti, S. (1958). Systema Helminthum. Vol. I. The Digenetic Trematodes of Vertebrates-Part II. New York: Interscience Publishers Inc.
- Yamaguti, S. (1959). Systema Helminthum. Vol. II. The Cestodes of Vertebrates. New York: Interscience Publishers Inc.
