ABSTRACT
Infectious bronchitis virus (IBV) was first isolated in Australia in 1962. Ongoing surveillance and characterization of Australian IBVs have shown that they have evolved separately from strains found throughout the rest of the world, resulting in the evolution of a range of unique strains and changes in the dominant wild-type strains, affecting tissue tropism, pathogenicity, antigenicity, and gene arrangement. Between 1961 and 1976 highly nephropathogenic genotype GI-5 and GI-6 strains, causing mortalities of 40% to 100%, predominated, while strains causing mainly respiratory disease, with lower mortality rates, have predominated since then. Since 1988, viruses belonging to two distinct and novel genotypes, GIII and GV, have been detected. The genome organization of the GIII strains has not been seen in any other gammacoronavirus. Mutations that emerged soon after the introduction of vaccination, incursion of strains with a novel lineage from unknown sources, recombination between IBVs from different genetic lineages, and gene translocations and deletions have contributed to an increasingly complex IBV population. These processes and the consequences of this variation for the biology of these viruses provide an insight into the evolution of endemic coronaviruses during their control by vaccination and may provide a better understanding of the potential for evolution of other coronaviruses, including SARS-CoV-2. Furthermore, the continuing capacity of attenuated IBV vaccines developed over 40 years ago to provide protection against viruses in the same genetic lineage provides some assurance that coronavirus vaccines developed to control other coronaviruses may continue to be effective for an extended period.
Introduction
Infectious bronchitis (IB), caused by infectious bronchitis virus (IBV), is an economically important disease of commercial poultry worldwide and was first isolated in Australia in 1962 (Cumming, Citation1962). Australian IBVs share the features of IBVs from other countries and, as in other countries, vaccination with attenuated strains has been a major element of control programmes. However, vaccines in use in Australia are derived from isolates from Australian flocks, rather than from imported strains. Several reviews have described the characteristics of IBV strains that occur in other countries (de Wit, Cook et al., Citation2011; Jackwood, Citation2012; Promkuntod, Citation2016; Bande et al., Citation2017) with most sharing several common variants of IBV, including the Massachusetts 41 (M41)-, 4/91- and QX-like strains, with the emergence of some country-specific variants. In most reviews of IBV, Australian strains are only described briefly as a separate group of strains, distinct from IBVs of other countries. In a recent comprehensive phylogenetic comparison of the spike gene fragment of 1518 IBV strains from across the globe, Australian IBV were found to be the most genetically diverse group (Valastro et al., Citation2016), belonging to three distinct genetic groups (out of a total of six), with two of these groups unique to Australia. This review attempts to summarize the main features of Australian IBVs including their pathotypes, antigenic and genetic diversity, and differences in genetic organization. It also examines the phenotypic and genetic evolution that has occurred in these viruses in Australia since they were first identified.
History of IB and IBVs in Australia
A timeline with the date of isolation of each genotype found to date in Australia is shown in .
Figure 1. Timeline showing the emergence of the different infectious bronchitis virus lineages in Australia and the introduction of attenuated vaccines using selected strains belonging to those lineages.
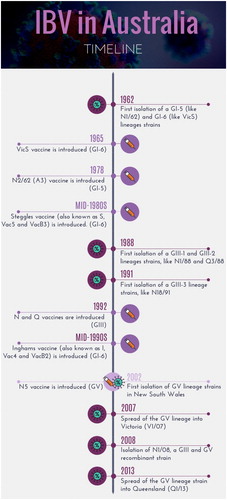
Up to the 1950s, the Australian poultry industry consisted mainly of egg-laying birds that also served as a source of chicken meat. The broiler industry began to develop in the early 1960s (Jackson, Citation2001). Quarantine was introduced for all live poultry imports in 1949 and has continued until the present day. Australian animal quarantine legislation identified infectious bronchitis (IB) of poultry as an exotic disease in 1958 and IB was not considered to be present in Australia up to 1962 (Anonymous, Citation1958; Peisley, Citation1961; Gilchrist, Citation1962). At the time, IB was considered to be predominantly a respiratory disease, similar to the disease prevalent in commercial poultry in the USA and the UK (Dawson et al., Citation1971; Jackwood et al., Citation2013). In 1946, Hart, the only veterinary poultry specialist in Australia between 1930 and the early 1950s, considered the possibility that outbreaks of respiratory disease seen in poultry in New South Wales (NSW) and Victoria characterized by mucoid tracheitis, sinusitis, and nasal discharge could be caused by IBV (Hart, Citation1946). In 1957, Hunt isolated an agent from an outbreak of “uraemia” in broilers that caused “nephritis” when re-inoculated into young chickens (Hunt, Citation1965), but the agent was not further characterized. The clinical syndrome of “uraemia” seen in young commercial broilers in the early 1960s differed from that seen in IB cases in the USA, from which the prototype and reference IBV strain, M41, was isolated (Cumming, Citation1962). The unusual clinical manifestation of IB in Australian poultry hindered recognition of the early, pioneering work of Rob Cumming, poultry disease specialist at the University of New England, Armidale, that demonstrated the link between IBV and nephritis/uraemia. This was further compounded by the lack of differential diagnostic methods for common avian pathogens then present in Australian poultry, in particular infectious laryngotracheitis, fowl pox, Mycoplasma species and members of the family Pasteurellaceae (Gilchrist, Citation1963).
An IBV designated “T” (N1/62) was isolated in 1962 by Cumming (Cumming, Citation1962, Citation1963) from a case of “uraemia” in commercial broilers near Tamworth, NSW. However, it took some years for “uraemia” to be accepted as a disease caused by IBV and the T (N1/62) strain virus to be recognized as a member of the same group of coronaviruses (CoV) as the M41 strain. Following isolation of T virus, other IBV strains, including Armidale [(A) (N2/62)], were isolated by Cumming from outbreaks of “uraemia” at other locations (Cumming, Citation1963). Several IBV strains were also isolated from outbreaks in Queensland (strains Q1/61, Q1/63, Q1/64, Q1/65, Q1/67, and Q1/69) (Newton et al., Citation1963). In addition to the “uraemia/nephritis” syndrome, respiratory disease, often described as chronic respiratory disease, was also seen in commercial poultry in NSW (Gilchrist, Citation1963; Hunt, Citation1965). Several IBVs were isolated from such cases (Gilchrist, Citation1963), but only G strain (N3/62) was characterized. By 1965, the infectious bronchitis-nephrosis (IBN) syndrome was considered to be the most serious disease problem in Australian poultry (Ranby, Citation1965). There was a suspicion that respiratory strains of IBV were also present, but less frequently, and that they were only partially related to nephropathogenic strains (Ranby, Citation1965). Vaccination with attenuated strains was introduced in Australia in 1966, but IBN continued to be a common disease in broilers for many years thereafter (Gilchrist, Citation1968; Watson, Citation1968; Jackson et al., Citation1972), with infection rates close to 100% seen in surveys of flocks (Watson, Citation1968; Jackson et al., Citation1972; Reid et al., Citation1984). In addition to the ease of spread between farms (Cumming, Citation1970), poor vaccine efficacy may have contributed to the persistence of disease, as IBVs were frequently isolated from vaccinated broilers (Chubb et al., Citation1976). Serological surveys in NSW (Cumming Citation1969a) and Queensland (Stephens et al., Citation1968) revealed that most flocks of adult birds had been exposed to IBV. Between 1966 and 1975, further isolates of IBV were obtained from broilers in Victoria, Queensland and NSW (), the majority from cases of IBN (Wadey & Faragher, Citation1981b). Since the early 1980s, poultry producers have suggested that incidence of IBN has decreased (P. Curtin, personal communication) and that respiratory disease has been seen more frequently in vaccinated broilers (Bagust, Citation1989). A number of isolates of IBV were obtained between 1981 and 1989 from cases of respiratory disease in NSW, Tasmania, and Northern Territory (Ignjatovic et al., Citation1997). All the strains (n = 52) isolated between 1962 and 1989, regardless of whether they were associated with IBN or respiratory disease, were classified as classical, or subgroup 1, strains (Sapats et al., Citation1996b; Ignjatovic et al., Citation1997), and have subsequently been classified as belonging to genotype GI-5 or GI-6 (Valastro et al., Citation2016) ().
Table 1. Infectious bronchitis virus strains isolated in Australia.
In the late 1980s, clinical disease characterized by hard coughing with increased mortalities was detected in broilers after 35 days of age at two large poultry complexes, Appin in NSW and Redland Bay in Queensland (Ignjatovic et al., Citation1997). The IBVs isolated from these outbreaks, represented by strains N1/88 and Q3/88 (Ignjatovic et al., Citation1991; Sapats et al., Citation1996b), were antigenically and genetically distinct from all previously characterized classical strains and were classified as novel, or subgroup 2, strains, and have subsequently been classified as constituting genotype GIII (GIII-1 and GIII-2 lineages, respectively) (Valastro et al., Citation2016). The disease caused by these novel strains persisted for about two years on both sites, causing late, pre-slaughter mortalities, and many antigenically identical strains were isolated in the years that followed () until new vaccines, strains N and Q, were developed in 1992 (more details are discussed in the vaccine section below).
In the mid-1990s, disease characterized by sudden coughing was reported in boilers, usually older than 38 days, on several farms in the Somerville area in Victoria. Between November 1991 and June 1993, three antigenically different groups of strains, represented by V18/91 and V19/91, V6/92 and V9/92, and V1/93, V2/93 and V3/93, were isolated from seven Victorian farms () (Ignjatovic et al., Citation1997). These strains were also classified as novel, or subgroup 2, strains, and were subsequently found to belong to the different lineage within the GIII genotype (GIII-3 lineage) (Valastro et al., Citation2016). However, unlike the situation in NSW and Queensland, these novel Victorian variants did not persist on the farms from which they were first isolated and were not isolated thereafter from any other farms in Victoria.
Another respiratory variant of IBV, represented by strain N1/03, was first detected between December 2002 and February 2003 in vaccinated broilers from four different poultry producers, at several distinct locations around Sydney, NSW. The strains isolated from 10 farms (N1/02, N3/02, N4/02, N1/03, N2/03, N3/03, N4/03, N5/03, N6/03, N7/03) were typed and found to represent a novel, genetically unique strain, distinct from the subgroup 2 strains, and hence were classified as novel subgroup 3 strains. Subsequently, these stains were classified as representatives of the genotype GV (Valastro et al., Citation2016). Cross-protection studies indicated that existing vaccines were not protective and, as a result, a new variant IB vaccine, N5, was developed. More details are discussed in the vaccine section below.
In 2008, strain N1/08 was isolated from a flock with respiratory disease that had been vaccinated twice with serotype B vaccines (Hewson et al., Citation2010). Subsequent genomic sequencing revealed that this strain was derived from recombination between two highly divergent strains, belonging to the novel subgroups 2 and 3 (or GIII and GV) (Hewson et al., Citation2014). In 2007 and 2013, strains V1/07 and Q1/13, which are similar genetically to N1/03, were isolated from broilers with respiratory signs in Victoria and Queensland, respectively, indicating further geographical spread of genotype GV strains (Hewson et al., Citation2009; Quinteros et al., Citation2016).
Australian IBV Reference Collection
The Australian IBV Reference Collection was established by Trevor Faragher in 1970 at the National Biological Standard Laboratories (NBSL), Melbourne, where the collection was maintained until 1987, when it was transferred to the CSIRO Australian Animal Health Laboratory, Geelong. The collection consists of strains that have been isolated since 1961 and characterized to a variable degree by several authors (Cumming, Citation1963; Gilchrist, Citation1963; Newton et al., Citation1963; Chubb et al., Citation1974; Wadey & Faragher, Citation1981a, Citation1981b; Ignjatovic et al., Citation1997, Citation2006). Part of the collection has been also maintained since 2004 at the Melbourne Veterinary School, in the Faculty of Veterinary and Agricultural Sciences at the University of Melbourne, where strains isolated since 2006 have been deposited. The aims in establishing the collection were to ensure classification, maintenance and distribution, on request, of samples of IBV strains (Geering et al., Citation1970). The strain designation given to an isolate is based on the model SI/YY, where S indicates the state where the strain was isolated from (N, New South Wales; NT, Northern Territory; Q, Queensland; T, Tasmania or V, Victoria), I indicates the number of the isolate, and YY indicates the year of isolation. For example, the Australian IBV strain originally designated “T” was renamed N1/62, indicating that it was the first isolate collected in NSW (N1) in 1962. The strains originally described in the literature with names that do not follow this convention that have been renamed in the reference collection include N9/74 (Appin), Q1/73 (H104), N2/62 (Armidale), T1/82 (2032) and N1/63 (Gilvax) (Klieve & Cumming, Citation1988a, Citation1988b). The reference collection nomenclature is similar to the international nomenclature initially proposed by Cavanagh (IBV/bird type/country of origin/ strain designation/year of isolation, Cavanagh (Citation2001)). Using this nomenclature, all Australian IBV strains would be preceded by the prefixes Ck (chicken) and Aus (for example, the revised name for the N1/62 (T) strain is IBV/Ck/Aus/N1/62). However, in this review, the designation used in the Australian reference collection (e.g. N1/62) has been used for simplicity, as all strains share the prefix IBV/Ck/Aus/.
The accession numbers in the GenBank database for sequence data for Australian IBV strains are listed in .
Antigenic relationships
Virus neutralization tests were initially used to detect antigenic differences between IBV isolates as the virus serotype was considered likely to be important in determining the protective efficacy of vaccines (Wadey & Faragher, Citation1981a, Citation1981b). Serotyping indicated that the most frequently used vaccines, VicS, I and S, were the same serotype B (genotype GI-6), whereas the A3 vaccine was serotype C (genotype GI-6). By 1993, a total of 20 serotypes (A–T) had been defined (Ignjatovic et al., Citation1991; Ignjatovic et al., Citation1997), and were shown to be distinct from those seen elsewhere (Lohr, Citation1976; Wu et al., Citation1998). However, it had become apparent that serotyping had limited value in identifying variants that could escape protective vaccinal immunity. The haemagglutination inhibition test, also a standard test at the time, was not feasible for use in strain differentiation, as only a small number of serotype B strains could haemagglutinate (Faragher, Citation1987).
Sera from IBV-infected birds contain cross-reactive antibodies that are detectable by immunofluorescence, western blotting and ELISA (Endo-Munoz & Faragher, Citation1989b; Ignjatovic & Galli, Citation1994a; Ignjatovic Citation1995; Ignjatovic et al., Citation1996). All the structural proteins of IBV, the glycoproteins (gp) S1, S2 and M, and the N protein, induce cross-reactive antibodies (Ignjatovic & Galli, Citation1994b; Ignjatovic et al., Citation1995). The N protein is the most immunogenic, eliciting the highest antibody titres in both infected and vaccinated birds. The least immunogenic is the M protein, and M protein-specific antibodies are often not detected by western blotting or ELISA (Ignjatovic et al., Citation1997). The S1, S2, M and N proteins of all the classical subgroup 1 strains are antigenically similar, and sera from infected or vaccinated birds infected with one strain cross-react with and recognize the S1, S2, M, and N proteins of all other subgroup 1 strains in both western blotting and ELISA. However, sera against subgroup 1 strains do not react with the S1, S2 and M proteins of novel subgroup 2 strains, and sera against subgroup 2 strains do not react with the S1, S2 and M proteins of subgroup 1 strains, with only minor cross-reactions seen between their N proteins. Because of these antigenic differences between IBV strains, the choice of test antigen for diagnostic ELISAs for serological surveillance of flocks will be critical. An ELISA based on classical, subgroup 1, strains such as N1/62 (as detecting antigen), will detect exposure to all classical strains, but will not detect exposure to novel strains such as N1/88, Q3/88 and V18/91.
Antigenic typing using monoclonal antibodies (Mabs) has been useful for differentiation of Australian IBVs (Ignjatovic et al., Citation1991). Patterns of reactivity with Mabs against the N and M structural proteins also showed that these proteins, which had been considered to be highly conserved, also vary antigenically between strains. The N protein has at least seven distinct antigenic epitopes that are recognized by different Mabs, but none of these is conserved, making it possible to differentiate 57 Australian IBV strains into five antigenic types. Four epitopes, recognized by Mab1, Mab16, Mab24 and Mab27, are present in most classical IBV strains isolated between 1962 and 1987. Two epitopes, recognized by Mab7 and Mab9, are found only in serotypes B and L, respectively, enabling differentiation of the serotype B vaccines (VicS, I and S) and serotype L strains (the N1/88-like novel subgroup 2 strains) from all other IBVs (Ignjatovic et al., Citation1996). The Q3/88-like and V18/92-like novel subgroup 2 strains are antigenically the most different and have none of the N protein epitopes detected by the Mabs against the classical strains.
The Australian classical strains N1/62 and VicS are serologically distinct from New Zealand strains, which fall into four different serotypes and also differ from all the major North American serotypes, Massachusetts, Connecticut, Arkansas, Iowa, JMK, Florida 18288, Se17 and Maryland (Lohr, Citation1976) (the serotypes JMK, Florida 18288, Se17 and Maryland, prevalent at the time, are no longer detected in North America). The Australian classical IBV strains have also been shown to differ antigenically from the M41, Connecticut, and Arkansas99 strains based on their reactivity with a panel of monoclonal antibodies (Wu et al., Citation1998).
As a result of the antigenic variation between Australian strains and those found in other countries, an ELISA based on an IBV strain, or antigen, exotic to Australia cannot be expected to reliably detect the serum antibody status of birds vaccinated with local IB vaccines.
Pathogenicity
Respiratory disease
Since 1931, IB has been described as a respiratory disease of both young and older chickens (Schalk, Citation1931; Avellaneda et al., Citation1994; Fabricant, Citation1998) and this continues to be the predominant clinical manifestation of the disease in all countries around the world (Promkuntod, Citation2016; de Wit et al., Citation2018). Respiratory IBV strains show a predilection for and replicate predominantly in the trachea, causing histopathological changes without mortality unless there is concurrent bacterial infection, when flock mortality can reach 20% (Avellaneda et al., Citation1994). It is uncertain whether some of these strains can also replicate in the kidneys without causing pathological changes (Benyeda et al., Citation2010; de Wit et al., Citation2018). Respiratory IBV strains have undergone significant antigenic evolution in all countries, but their tissue tropism has remained largely unchanged.
Nephritis
Nephropathogenic IBV strains have been detected sporadically since the early 1960s in a number of countries, including the USA, Belgium and Israel, but their occurrence in these countries is considered transient and not significant epidemiologically (de Wit, Cook et al., Citation2011), with the exception of the nephropathogenic 793B strain (also known as 4/91 or CR88), which emerged at the beginning of the 1990s (Gough et al., Citation1992; Awad et al., Citation2014). Subsequently, QX-strain also emerged in 1996 (Promkuntod, Citation2016).
Classical subgroup 1 strains
The disease described in Australia as “uraemia”, and later as IB nephritis, was first described in 1962, with mortalities of 30% to 40% seen in unvaccinated commercial flocks (Cumming, Citation1962, Citation1969c). The proposition by Cumming that the causative agent was an IBV, similar to the respiratory IBVs found in other countries, was not readily accepted, because the clinical diseases were radically different. Birds were depressed, had a hunched stance, stopped eating and suffered a notable loss of weight, and were dehydrated, and the litter was wet with urate deposits, but respiratory signs such as coughing and gasping were not seen consistently (Cumming, Citation1963; Condron et al., Citation1985). Subsequent studies have described in detail the distribution of virus in the tissues of chickens infected with nephropathogenic IBV strains (Doherty, Citation1967; Cumming, Citation1969a), as well as the pathogenic changes and factors that influence the pathogenesis and mortalities (Cumming, Citation1969a; Silller et al., Citation1974; Purcell et al., Citation1976; Chong et al., Citation1982; Ignjatovic et al., Citation2003).
Inoculation of 2-week-old specific-pathogen-free chicks with 15 classical IBV strains isolated from cases of uraemia between 1962 and 1976 confirmed field observations that the majority of IBV strains predominating at that time were nephropathogenic. All strains grew efficiently in both the kidney and the trachea, the majority induced histological lesions in both tissues (14/15 strains) and signs of clinical nephritis (10/15 strains). Experimental infection with these strains resulted in mortality rates of 30% to 94% (Ignjatovic et al., Citation1991; Ignjatovic et al., Citation2002), with four strains (N1/62, Q1/65, N8/74, and N9/74) highly lethal, causing death in 80% to 94% of chicks. The only exception was the N3/62 strain, which did not grow in the kidneys, or cause mortalities in inoculated chicks (Sapats et al., Citation1996b).
In contrast, only 3/13 (23%) classical strains isolated between 1981 and 1994 caused histological lesions in the kidneys and the mortality rates in experimentally infected chicks ranged from 5% to 37%. The majority (10/13 strains) caused histological lesions only in the trachea, and did not replicate in the kidneys, or cause death in experimentally infected chicks (Ignjatovic et al., Citation2002). Classical subgroup 1 respiratory strains continued to be isolated from commercial poultry until at least 2013 (V5/90, Q1/99, V1/02 and V2/02) with some also able to grow in the kidney (Hewson et al., Citation2010)
Subgroup 2 and 3 strains
All novel, subgroup 2 strains (N1/88, Q3/88, V18/91, V2/96, V2/93 and V3/93) are respiratory strains that replicate only in the trachea, causing pathological changes similar to those induced by classical strains (Mardani et al., Citation2008). The origin of the strains belonging to this subgroup remains unknown, but it is believed that they could have emerged as a result of recombination between IBV strains already circulating in Australia and an avian CoV of unknown origin (Quinteros et al., Citation2016). A similar recombination event between IBV strains and another unknown CoV appears to have resulted in the emergence of turkey-CoV (Lin et al., Citation2004; Jackwood et al., Citation2010).
Subgroup 2 strains reach a significantly lower titre in the trachea of infected chicks; at 5 days post-infection, the tracheas of chicks infected with classical strains contained 104 (Q1/76) or 105 (N1/62) median egg infective doses (EID50,) but those of chicks infected with the subgroup 2 strains contained only 102 (Q3/88, V18/91, and V2/96) or 103 (N1/88) EID50. The novel strain N1/08 is an exception; it replicates in the trachea and is also detected in the kidneys, the proventriculus and the caecal tonsils of some chickens (Quinteros, Citation2019). However, N1/08 is a recombinant derived from subgroup 2 (S1 from N1/88) and subgroup 3 strains (Hewson et al., Citation2014; Quinteros et al., Citation2016), which may be the reason for its ability to grow in kidney tissue (Quinteros, Citation2019). The novel subgroup 3 strains, such as N4/02, are also respiratory strains, are detected only in the trachea and cause no mortalities in specific-pathogen-free chicks (Sapats, Citation2009).
Reproductive tract disease and Australian IBV strains
Although it has been reported that IBV infection at an early age can cause permanent damage to the oviduct (Jones et al., Citation1972; de Wit, Nieuwenhuisen-van Wilgen et al., Citation2011), the mechanism underlying the effect of IBV on levels of egg production is yet to be fully understood. Strains such as N1/62 and N1/88 can cause cellular changes in the upper parts of the oviduct and affect the internal quality of eggs (Chousalkar & Roberts, Citation2007b). Australian strains of IBV can cause a loss of shell colour (Samiullah et al., Citation2016) or reduce albumen quality (Hewson et al., Citation2014). Although there are low levels of replication of these strains in the regions responsible for egg shell formation (Chousalkar & Roberts, Citation2007a), there is little evidence to suggest that infection with Australian IBV strains results in rough or corrugated egg shells. Clinical observations suggest that infection of adult laying hens with Australian strains does not result in “false layers” (Tom Grimes, personal communication), unlike infection with strains from the European Union (QX and QX-like strains), the United States (Massachusetts strains), China and other parts of the world (de Wit, Nieuwenhuisen-van Wilgen et al., Citation2011b; Amin et al., Citation2012; Chen et al., Citation2017). Vaccination with the VicS strain can also result in the loss of egg shell colour (Chousalkar et al., Citation2009). A recent study has found that infection with IBV affects the expression of genes involved in calcium transportation across the cell membrane and protoporphyrin synthesis (Khan et al., Citation2017).
Changes over time
Overall, all the major biological properties of Australian IBVs, their pathogenicity, tissue tropism and virulence, have changed over time, with highly nephropathogenic strains replaced by less pathogenic, respiratory strains (Ignjatovic et al., Citation1991; Ignjatovic et al., Citation2002; Mardani et al., Citation2008).
There may be several factors that have led to this change in the pathogenicity of prevailing IBV strains. Nutrition has been implicated in the aetiology of uraemia in chickens (Jackwood et al., Citation2020). One study concluded that diet affected the incidence of uraemia and suggested that the sodium-potassium balance in diets was associated with the aetiology of the disease (Beilharz et al., Citation1960), but others were unable to substantiate this finding (Gartner et al., Citation1966; Cumming, Citation1967). Pryor and Woo (Citation1964) concluded that a high meat meal content and high-energy diets probably contributed to mortality due to uraemia. In addition to changes in commercial poultry diets over time, the other profound change has been the genetic constitution of broiler breeds used for commercial meat production. It has been shown that mortality rates after infection with the most lethal IBV strains, such as N1/62, are dependent on the breed of chicken (Ignjatovic et al., Citation2003; Asif et al., Citation2007). The third factor is genetic changes in IBV strains circulating in the chicken population. The vaccine strain VicS and the respiratory strainV5/90 are almost identical genetically, with 99.9% nucleotide identity across their complete genomes (Quinteros, Citation2019). However, V5/90 does not replicate in the kidneys, unlike VicS, which is a nephropathogenic strain that causes histological lesions in kidneys (Sapats et al., Citation1996b). Changes in tissue tropism have been reported to result from random mutations in the coding sequences of several CoV, including IBV during propagation in chicken embryos (Ammayappan et al., Citation2009). The S1gp is considered the major determinant of cell tropism of IBVs (Casais et al., Citation2003). However, evidence is accumulating that other genes also contribute to the pathotype of IBVs (Weiss et al., Citation2005). Deletion of the individual accessory genes – 3a, 3b, 5a and 5b – in IBV strain H52 was shown to attenuate the phenotype of the virus in vitro, in ovo and in vivo, (Laconi et al., Citation2018). The genotypes of these attenuated H52 mutants are similar to the genotype of the novel subgroup 2 viruses, which are naturally occurring strains that lack the 3a, 4c, and 5a accessory genes (see below).
Genetic characteristics
Genetic analysis has become the method of choice for the characterization of IBV as it unambiguously establishes the strain identity, the degree of change in the functionally important domains of the genome, and the likely origin (lineage) of the virus. The S1gp sequence is used as the principal criterion for differentiation, as it induces protective immunity (Ignjatovic & Galli, Citation1994b; Sapats et al., Citation1996b; Mardani et al., Citation2008; Valastro et al., Citation2016). The sequencing of genes for the other structural (the S2, N and M) and accessory (3a, 3b, 4b, 4c, 5a and 5b) proteins, which also have a role in immune responses and virus replication (Sapats et al., Citation1996a; Mardani et al., Citation2008; Mardani et al., Citation2010; Hewson et al., Citation2011), as well as the sequencing of two polymerase genes (1a and 1b) have also been useful for characterization of IBVs (Quinteros et al., Citation2015; Quinteros et al., Citation2016; Quinteros, Citation2019).
The combined sequence analyses of Australian IBVs have confirmed the antigenic classification of strains into three separate subgroups of classical subgroup 1, novel subgroup 2 and novel subgroup 3 strains (Ignjatovic et al., Citation2006). Most Australian strains belong to classical subgroup 1 (Sapats et al., Citation1996b; Mardani et al., Citation2008; Hewson et al., Citation2011), represented by the N1/62 and VicS strains (). The S1gp amino acid sequences of strains within this subgroup differ by 3% to 18%, while the S2, N and M proteins are more conserved (1% to 9%, 3% to 7%, and 1% to 12% difference, respectively), as are the accessory proteins 3a, 3b, 4a (E), 4c, 4d, 5a and 5b (Mardani et al., Citation2008). Strains isolated between 1962 and 1974, before widespread vaccination (N9/74, N3/62, N2/75, V1/71, V2/72, Q1/73), have greater sequence variability in the S1gp (12% to 19%), while strains isolated in more recent years, since 1990 (V5/90, Q1/99, Q4/99, V2/03) are usually variants of serotype B and C vaccine viruses, from which they differ by 2% to 5%. Full genome sequencing supports the grouping of classical subgroup 1 strains in a single cluster, distinct from the subgroup 2 and 3 strains (Quinteros et al., Citation2015; Quinteros et al., Citation2016). It also shows that the genomic organization of classical subgroup 1 strains is similar to that of other gamma-CoVs, (5′ Pol 1ab, S1, S2, 3a, 3b, E, M, 4b, 4c, 5a, 5b, N, 6b, 3′), with serotype B vaccine strains missing the 6b coding region (Mardani et al., Citation2006; Mardani et al., Citation2008; Hewson et al., Citation2011; Quinteros et al., Citation2016).
The S1gp amino acid sequences of the subgroup 2 strains (N1/88, Q3/88, V18/91, V6/92) () differ markedly (33% to 39%) from those of the classical strains, as do the sequences of the other structural proteins, S2, M and N (by 17% to 37%). Full genome sequencing has confirmed the distinct nature of these strains; the regions coding for accessory proteins are drastically altered, with four genes deleted (3a, 4c, 5a and 6b), the 4b gene translocated to another position and its sequence altered, and a new coding region of 159 base pairs inserted upstream of the 3b gene. The genomic organization of subgroup 2 strains is therefore unique – 5′ Pol 1ab, S1, S2, 4b, 2X, 3b, E, M 5b, N 3′ (Mardani et al., Citation2008; Hewson et al., Citation2011). In addition, the sequence of 5b accessory gene is altered in the V18/91 and V6/92 strains, with these strains having a smaller genome (26, 938 nucleotides) than the other strains (27, 669 nucleotides for the N1/62 strain) (Quinteros, Citation2019). Strain N1/08 is an exception. The S1gp sequence places this strain with the novel subgroup 2 strains, as its S1gp nucleotide sequence has 98% identity with that of N1/88. However, the remainder of the genome of N1/08 has only 69% identity with those the other novel subgroup 2 strains, with the sequences of the part of the S2 gene and the remainder of the 3′ end of the genome, which encodes the N, M and accessory proteins genes, similar to those of strains in subgroup 3 (Hewson et al., Citation2014). As a result, the genomic organization of N1/08 differs from that of the other subgroup 2 strains, and instead is similar to that of the classical strains (5′ Pol 1ab, S1, S2, 3a, 3b, E, M, 4b, 4c, 5a, 5b, N, 6b, 3′).
The novel subgroup 3, represented by N1/03, comprises 12 strains isolated between 2002 and 2009 in NSW, V1/07, which was isolated in Victoria in 2007, and Q1/13, which was isolated in Queensland in 2013. Their S1gp sequence is also unique and distinct from those of both the subgroup 1 and 2 strains (38% to 46%). However, their S2, N and M protein sequences are more similar to those of the classical strains (90% to 97%) (Hewson et al., Citation2010, Citation2011; Quinteros et al., Citation2016). Phylogenetic analysis using the N gene sequences reveals a high level of similarity between N1/03 and the VicS strain, while the 3′ end of the genome is very similar to the A3 vaccine. Recombination analysis of the entire genome of N1/03 reveals multiple recombination points along the genome, with the parental strains that included an unknown strain, which donated the S1 gene, and the A3 and VicS vaccine viruses (GI-5 and GI-6 genotypes), which jointly donated the majority of the N1/03 genome (Quinteros et al., Citation2016).
Phylogenetic relationships between IBVs from Australia and those from other parts of the world
In phylogenetic analyses, the ancestral relationships and evolutionary history of IBV strains are inferred using sequences from the conserved polymerase, the S1gp or the N gene regions (Vijaykrishna et al., Citation2007; Valastro et al., Citation2016). Phylogenetic analysis of Australian IBVs based on their S1gp gene sequences separated the strains into three separate phylogenetic subgroups (1, 2 and 3) (Sapats et al., Citation1996b; Ignjatovic et al., Citation2006; Mardani et al., Citation2010; Quinteros et al., Citation2016) suggesting distinct and separate evolution of each subgroup.
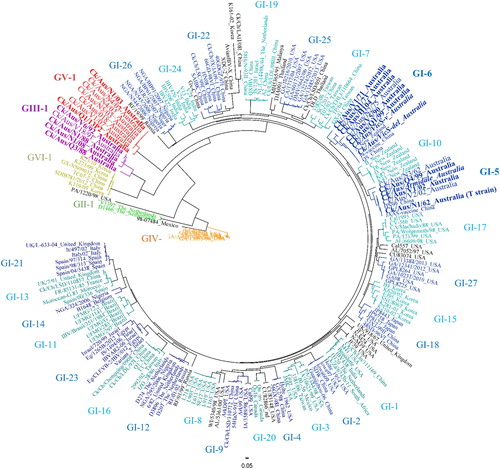
Recent phylogenetic analysis of the S1gp gene sequences of 1518 IBV strains isolated throughout the world has allowed development of the first comprehensive genetic classification of these viruses and a comparison of all known strains isolated from poultry (Valastro et al., Citation2016). All IBVs are grouped into six genotypes (GI, GII, GIII, GIV, GV and GVI), and the majority of IBV strains belong to one genotype, GI (). In 2016, when this lineage classification was proposed, the lineages belonging to this genotype GI were 27 (Valastro et al., Citation2016). These lineages can be found in . However, new lineages have been identified over the years and have been classified as GI-28, GI-29, and GI-30 (Chen et al., Citation2017; Xu et al., Citation2018; Brown Jordan et al., Citation2020). Some of these GI lineages are commonly found throughout the world, while others are locally circulating. The other five genotypes are all unique and were previously designated Dutch D1466 (GII), Australian novel subgroup 2 strains (GIII), USA Delaware variants (GIV), Australian novel subgroup 3 strains (GV), and strains from Korea and China isolated between 2007 and 2012 (GVI). Genotypes III and V are exclusively composed of Australian strains (). Phylogenetic analysis has demonstrated how different these Australian strains are from those found throughout the rest of the world, as it can be seen in (Quinteros et al., Citation2015; Quinteros et al., Citation2016). It has been speculated that this differentiation could have been a result of the geographic separation of Australia, allowing local strains of IBV to evolve independently. Other factors, such as import barriers, could have also contributed to this biological isolation (Quinteros et al., Citation2015; Quinteros et al., Citation2016). This provides an extraordinary opportunity for studies of the evolution of IBV. This understanding could be extrapolated to understanding the potential past and future evolution of other coronaviruses, including SARS-CoV-2, the causative agent of the current global pandemic referred to as coronavirus disease 2019 (COVID-19).
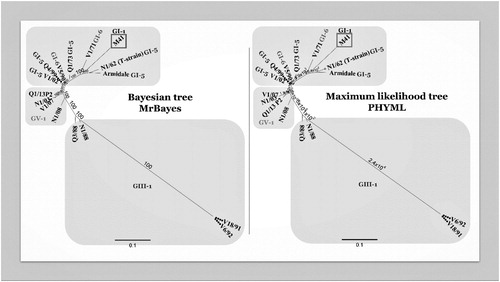
Figure 2. Comparison of two phylogenetic trees constructed with the same multiple alignment of the complete genome sequences of Australian strains of IBV. The multiple alignment was performed using MAFFT, and the phylogenetic trees using PHYML (maximum likelihood) and MrBayes (Bayesian) in Geneious v10.2.6. Two phylogenetic trees were included using two different algorithms to increase reliability of the results. Originally, the complete genome nucleotide sequences of 19 Australian strains were included in this tree. However, some strains were removed to increase clarity, as they were highly similar or identical to other strains. The strains not included were H104 (100% similarity to Q1/73), Q1/99, VicS-del and VicS-v (99.9%, 99.7% and 99.9% of similarity with V5/90). The grey squares depict the different genotypes GI, GIII and GV.
Australian classical strains are separated into two lineages within the GI genotype, GI-5, and GI-6, represented by the strain N1/62 and VicS vaccine virus, respectively (). The same grouping of the Australian classical strains into two lineages, GI-5 and GI-6, was also obtained using their complete genome sequences and different phylogenetic models, increasing confidence in the proposed separation of Australian classical strains into two separate lineages, as can be seen in (Quinteros, Citation2019). The N1/62 and VicS strains were isolated at about the same time, in 1962, but at distant locations, in NSW and Victoria, respectively. Their S1 gene sequences are similar (83%), but the separation into two lineages indicates a separate ancestry, or that, by the time of isolation, N1/62 and VicS had diverged sufficiently from a parental strain to form genetically separate lineages. Interestingly, the GI-5 and GI-6 lineages also contain strains from China and New Zealand (Quinteros, Citation2019). The GI-5 lineage includes the strains JAAS, a vaccine strain, and HN99, both isolated in China, while the GI-6 lineage includes the strains QS and J-9 (a vaccine strain), both from China, and strain A from New Zealand. These four Chinese strains are probably re-isolates of the two Australian IBV vaccines, Armidale and VicS, which have been used in parts of China to control nephropathogenic strains of IBV (Wu et al., Citation1998; Liu et al., Citation2006)
Figure 3. Radial phylogenetic tree based on the complete nucleotide sequence of the IBV surface protein S1. Due to its complexity, GI genotype was subdivided in 27 lineages. Different colours represent different genotypes (light and dark blue are considered the same colour, both belonging to GI genotype). The names of unclassified strains are in black. The names of the Australian strains are magnified. Only Australian strains with a complete nucleotide sequence of the S1 gene were included. The vaccine strains A3 (Armidale or N1/62) and VicS (represented by the two subpopulations VicS-v and VicS-del) are rendered in italics.
In the GIII genotype, the Australian subgroup 2 strains N1/88, V18/91, V6/92 and N1/08 comprise one lineage, GIII-1. The Q3/88 strain, which is also a subgroup 2 strain, lies outside the GIII-1 lineage, but still clusters with the other subgroup 2 strains on the GIII phylogenetic branch, suggesting that it has an origin distinct from that of the N1/88-like strains (Valastro et al., Citation2016). Phylogenetic analysis using the entire genome sequences of all the subgroup 2 strains has confirmed that the Q3/88 strain is distant from N1/88 and belongs to a separate lineage, and also showed that V18/91 and V6/92 are similarly distant from N1/88, indicating that they have a separate ancestral relationship with N1/88 (Quinteros, Citation2019). Therefore, the phylogenetic analysis based on the full genome sequence suggests that there are three separate lineages within the GIII genotype, GIII-1 (represented by N1/88), GIII-2 (represented by Q3/88), and GIII-3 (represented by V18/91 and V6/92), and that these three groups of strains have different origins (). Phylogenetic analyses of the N1/08 strain differ, depending on whether the S1gp gene alone or the entire genome sequence is used. In the S1gp analysis it lies within GIII (), while analysis using the complete genome places it in genotype GV () (Hewson et al., Citation2014; Quinteros et al., Citation2016). The different outcomes of these analyses reflect the recombinant origins of N1/08 and demonstrate that the results of phylogenetic analyses of IBVs can be affected by the gene targeted for analysis.
Genotype GV includes only the Australian subgroup 3 strains, represented by the N1/03 strain. The unique nature of this group of viruses is indicated by further phylogenetic analyses. Analysis based on the N gene sequences places the N1/03-like strains in the GI -5 genotype (Ignjatovic et al., Citation2006), while analyses based on the full genome or the S1gp sequences place these strains in the genotype GV (Quinteros et al., Citation2016), suggesting that they also have a separate origin from those in genotypes GI and GIII.
Evolution and origin of Australian IBV
The origin of IBV remains speculative. It is only since the emergence of severe acute respiratory syndrome-related virus strains (SARS)-1 and -2 that there has been an interest in determining the origin of coronaviruses, and this interest has primarily focussed on the human CoVs, although a few studies have also included some IBV strains (M41, Baudette, Holte and Con) (Woo et al., Citation2012; Wertheim et al., Citation2013). From these studies it was concluded that CoVs are an ancient viral lineage, with bats as the oldest natural host and the source of all CoVs found in other animal species, humans, and birds (Vijaykrishna et al., Citation2007). Divergence from the most recent common ancestor of all CoVs was estimated, initially, to have been around 10,000 years ago, that of avian CoVs to have been around 5,000 years ago (Woo et al., Citation2012), and that of IBV strain M41 to have been between 1887 and 1941 (Vijaykrishna et al., Citation2007). However, more recent molecular clock analysis suggests that CoVs are much older, and have possibly existed since the time that these animal species diverged from each other, about 300 million years ago (Wertheim et al., Citation2013). CoVs of bats and birds show the greatest variation in their sequences compared to CoVs found in other animals and humans and, for that reason, it has been speculated that avian CoVs originated from a single introduction of an ancestral bat CoV, possibly via raptorial species of birds (Vijaykrishna et al., Citation2007; Mikula et al., Citation2016; Chamings et al., Citation2018). Since that time, avian CoVs have co-existed and co-evolved with their hosts, with birds becoming the natural hosts and likely reservoirs of all avian CoVs. Bats are, on the other hand, the reservoirs of human CoVs such as SARS-1 and SARS-2 and other mammalian CoVs (Vijaykrishna et al., Citation2007; Woo et al., Citation2012; Wertheim et al., Citation2013). This long association of CoVs with their hosts was shown, in bats, to lead to the establishment of a unique host–pathogen interaction that ensures co-existence and survival of both (Wertheim et al., Citation2013). At present, from our understanding of avian CoV ecology, this long-time relationship appears to have been established in wild birds, but not in Galliformes, and, in particular, not in commercial poultry.
The introduction of CoVs into birds has resulted in diversification into a range of species and two distantly related phylogenetic lineages, the gamma-CoVs, which infect many wild species including pigeons, peafowl, parrots, quail, penguins and waterfowl, but also Galliformes (chickens, turkeys, partridges, pheasants, guinea fowl), and the delta-CoVs, which are found mainly in wild birds, but also in pigs (Woo et al., Citation2012; Wille et al., Citation2020). Comparisons of IBVs with other gamma-CoVs have suggested that IBV-like viruses found in other birds do not contribute significantly to the diversification of IBVs in commercial poultry. Although an IBV similar to the vaccine strain VicS has been isolated from pigeons in Australia (Barr et al., Citation1988), and there has been an unconfirmed report of infection of magpies with IBV (Cumming, Citation1969b), these appear most likely to have resulted from accidental acquisition through co-habitation of wild birds with vaccinated poultry, rather than a result of sustained transmission in wild bird populations. Delta-CoVs have been detected in many species of wild birds, including in Australia (Woo et al., Citation2009; Woo et al., Citation2012; Chamings et al., Citation2018). They are genetically distinct from gamma-CoVs, including IBV, with a distinct genome organization, and could be a source of new CoVs that could infect domestic poultry (Woo et al., Citation2012). However, sequence and phylogenetic analyses of IBVs and delta-CoVs have not yet detected any cross-species transmission of delta-CoVs from wild birds into domestic chickens (Woo et al., Citation2012).
The majority of IBV strains isolated worldwide are genetically and phylogenetically similar and belong to one genotype, GI. This genotype contains 30 different lineages, in different geographic regions of the world, many of which are country-specific (Valastro et al., Citation2016; Chen et al., Citation2017; Xu et al., Citation2018; Brown Jordan et al., Citation2020). Despite this diversity, there appears to be limited divergence from the ancestral GI IBV, as all 30 lineages cluster closely together. Australian classical strains isolated in the early 1960s, in the GI-5 and GI-6 lineages, which have presumably diverged since their introduction into Australia, also cluster closely with the other lineages. A large number of IBVs isolated in Australia over the last 60 years also belong to the GI-5 and GI-6 lineages, and these isolates differ, almost exclusively, as a result of point mutations, with only occasional changes resulting from deletions in the accessory protein gene regions (Sapats et al., Citation1996b; Mardani et al., Citation2008; Hewson et al., Citation2012).
The origin of the IBV strains in the GIII genotype (N1/88, Q3/88 and V18/91), on the other hand, is less clear. There are several features that suggest that all three of these strains are of exogenous origin. That is, that they were introduced into commercial poultry from a different species of bird. All three viruses emerged suddenly at the location from which they were isolated, causing disease (Ignjatovic et al., Citation1997). The growth properties in vitro and in vivo, and the antigenic properties, of all three strains differ profoundly from those of strains in the GI-5 and GI-6 genotypes (Ignjatovic et al., Citation1997; Mardani et al., Citation2008). The sequence of the genome of these viruses, including the S1 gene sequence, is distinctly different from those of other gamma-CoVs, or of the delta-CoVs (Woo et al., Citation2012; Quinteros, Citation2019). In addition, the arrangement of the structural genes in the GIII virus genomes differs from the characteristic arrangements seen in other gamma-CoVs, or the delta-CoVs (Mardani et al., Citation2008; Hewson et al., Citation2011), with a number of accessory genes missing, resulting in a smaller genome. These changes in N1/88, Q3/88 and V18/91 are so extensive that the origins of these IBVs could not have been a result of direct divergence from the strains in GI-5 and GI-6 genotypes that were previously circulating in Australian poultry. Although N1/88, Q3/88 and V18/91 emerged at distinct and distant locations, and N1/88 and Q3/88 emerged in the same year, their phylogenetic relationships indicate that they have diverged from a common ancestor into three separate genetic lineages. The conserved, non-structural protein 12 of strains V18/92 and V6/92, is phylogenetically equally distant from both gamma- and delta-CoV from wild birds, indicating that they may have originated from, yet unknown, wild birds (Quinteros, Citation2019). However, the origin of strain N1/08 (which is also in the GIII-1 lineage, but was isolated 10 years after the isolation of N1/88) can be attributed to recombination between two parental viruses, an N1/88-like virus from genotype GIII-1, which donated the S1gp gene (94% amino acid sequence identity), and N1/03 from genotype GV, which donated the other structural genes (with 96% amino acid sequence identity) (Hewson et al., Citation2014; Quinteros et al., Citation2016).
The origin of the GV genotype strains is also ambiguous. The N1/03 strain has characteristics of a recombinant derived from an unknown parental strain, which donated the S1gp gene, and at least another two parental strains, from genotypes GI-5 and GI-6, which donated the remainder of the genome. However, a large number of recombination points in the genome of this virus, and the contribution of at least three distinct parental strains, suggests that it is unlikely that it resulted from a single recombinational event. The donor strain for the S1gp gene has not been detected in commercial poultry before, or since, the emergence of the genotype GV strains (Quinteros et al., Citation2016). It was initially hypothesized that this group of strains could have originated from a recombinational event between subgroup 1 and 2 strains (Mardani et al., Citation2010). However, whole genome similarity plot analysis has demonstrated that this is not the case, so the donor of the S1gp gene remains unknown, and seems likely to have been introduced into commercial poultry from another host, possibly wild birds (Quinteros et al., Citation2016). Alternatively, it is possible that the recombination events that resulted in this virus occurred in a wild bird that was infected with GI-5 and GI-6 viruses acquired from commercial chickens. The polymerase genes of N1/03 contain segments that have a high sequence identity with those of the VicS vaccine virus, while other sections have a high sequence identity with the A3, or Armidale, vaccine virus. As these segments are scattered throughout the polymerase genes (seven sections), it is possible that multiple recombination events between these two vaccines have contributed to the emergence of this virus (Quinteros et al., Citation2016). The likelihood of this is supported by the simultaneous administration of both these vaccines as standard practice in some poultry flocks in Australia.
In summary, the majority of Australian IBV strains, those in genotypes GI-5 and GI-6, originated from a single common gamma-CoV ancestor, from which the majority of IBVs in other countries have also evolved. However, over the last 30 years, there have been four separate introductions of divergent lineages, which belong to two new genotypes of IBV from as yet unidentified host(s). These new IBV genotypes (GIII and GV) caused disease and had a significant economic impact in commercial poultry flocks and required development of new vaccines for their control. The incursion of these novel recombinants, the ongoing uncertainty about their origins, and the differences in their tropism and pathogenicity illustrate the complexities of CoV ecology and our lack of understanding of factors that facilitate the emergence of new strains, as has been the case with recently emerged SARS-CoV-1 and CoV-2 viruses.
Vaccines developed and used in Australia
A timeline with the date of introduction of each IBV vaccine developed and used in Australia is shown in .
The efficacy of IB vaccination and disease control has been one of the perpetual concerns of the Australian poultry industry. From the early 1960s, the concern was to control mortalities caused by nephropathogenic strains, and from the early 1980s to control losses due to respiratory disease. The IBV strains circulating between the 1960s and late 1980s varied in pathogenicity, serotype and genotype, but the choice of strains for vaccine development was, at the time, random. Initially, several experimental live-attenuated vaccines were produced and used on a limited number of sites (Wadey & Faragher, Citation1981b). Two vaccines developed from these early IB strains (the VicS and N2/62 strains) were registered for use in 1965 and 1978, respectively, and are still in use today. The VicS virus, isolated from a case of nephritis in broilers in the early 1960s (Wadey & Faragher, Citation1981b; Klieve & Cumming, Citation1988b) is antigenically similar to the other serotype B nephropathogenic strains (Ignjatovic et al., Citation2006) and belongs to genotype GI-6. It was later found to contain a mixture of two viral populations that differ in pathogenicity (Hewson et al., Citation2012). Vaccine A3, derived from the nephropathogenic strain N2/62 by cloning in chicken embryo kidney cells, is serotype C, has an S1gp amino acid sequence that has only 81% identity with that of VicS (Ignjatovic et al., Citation2006) and belongs to genotype GI-5. Two other serotype B vaccines, Steggles (with the synonyms S, Vac5 and VacB3) and Inghams (with the synonyms I, Vac4 and VacB2), were developed in the mid-1980s and 1990s, respectively, initially for exclusive use by two different commercial poultry producers. Both strains are antigenically and genetically almost identical to VicS (Ignjatovic et al., Citation2006). An additional three IB vaccines have since been developed, Q and N in 1992 for control of genotype GIII viruses, and N5 in 2006 for control of genotype GV viruses. Use of these three vaccines was limited to specific sites, in combination with serotype B and C vaccines (T. Grimes, personal communication).
The protective capacity of IB vaccines available in Australia has been assessed in several studies (Klieve & Cumming, Citation1988a; Endo-Munoz & Faragher, Citation1989a; Arvidson et al., Citation1990; Klieve et al., Citation1990; Arvidson et al., Citation1991; Sapats, Citation2009). The results are not always comparable, largely because the methods of assessment and the criteria used to evaluate the level of protection differ between studies. For example, vaccines VicS, I, S, and A3 (from genotypes GI-5 and GI-6) all protected against challenge with the highly pathogenic N1/62 strain, but not against N9/74, although both challenge strains are genotype GI-5. Vaccine I provided better protection against heterologous challenge with IBVs of five different serotypes (from genotypes GI-5 and GI-6) than the genetically identical VicS and S vaccines. IB vaccines from genotypes GI-5 and GI-6 did not protect against challenge with GIII and GV genotype strains and, conversely, GIII and GV vaccines did not protect against challenge with GI-5 and GI-6 viruses.
Similarly, although the A3 and VicS vaccines were developed from nephropathogenic GI-5 and GI-6 strains, respectively, these vaccines are not effective against nephropathogenic strains from China, which belong to different genetic lineages (Wu et al., Citation1998). Conversely, unconfirmed reports suggest that inactivated M41-type genotype GI-1 vaccines are not effective against challenge with GI-5 Australian strains (N1/62).
Overall, notwithstanding the need for use of variant vaccines on some sites, and continuing concerns over the efficacy of existing IB vaccines, the serotype B and C (genotypes GI-6 and GI-5) IB vaccines, which were developed over 40 years ago, have been continually in use and have remained sufficiently effective for control of the pathogenic and antigenic variants of GI-5 and GI-6 viruses that have emerged in Australia since the mid-1960s. This extended efficacy of these attenuated vaccines against viruses that have evolved in the same genetic lineages offers some assurance that vaccines developed to control other coronaviruses, including the human CoVs, may provide lasting protection for many years, in the absence of incursion of novel variants from distinct genetic lineages, either by introduction from a novel host or by recombination with a distantly-related virus.
Acknowledgements
The authors wish to thank Dr Tom Grimes and Dr Clive Jackson for information about the Australian IBV vaccines and their comments on this manuscript. We also thank Dr Trevor Faragher and Dr Mike McDermott for information on historical IBV strains and vaccines.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Amin, O., Valastro, V., Salviato, A., Drago, A., Cattoli, G. & Monne, I. (2012). Circulation of QX-like infectious bronchitis virus in the Middle East. Veterinary Record, 171, 530–530.
- Ammayappan, A., Upadhyay, C., Gelb, J.J. & Vakharia, V.N. (2009). Identification of sequence changes responsible for the attenuation of avian infectious bronchitis virus strain Arkansas DPI. Archives of Virology, 154, 495–499.
- Anonymous. (1958). Infectious bronchitis of chickens. Australian Veterinary Journal, 34, 28–29.
- Arvidson, Y., Tannock, G.A., Senthilselvan, A. & Zerbes, M. (1990). A model for determining immunogenic relationships between avian infectious bronchitis viruses. Archives of Virology, 111, 227–238.
- Arvidson, Y., Tannock, G.A., Zerbes, M. & Ignjatovic, J. (1991). Efficacy of Australian vaccines against recent isolates of avian infectious bronchitis viruses. Australian Veterinary Journal, 68, 211–212.
- Asif, M., Lowenthal, J.W., Ford, M.E., Schat, K.A., Kimpton, W.G. & Bean, A.G. (2007). Interleukin-6 expression after infectious bronchitis virus infection in chickens. Viral Immunology, 20, 479–486.
- Avellaneda, G.E., Villegas, P., Jackwood, M.W. & King, D.J. (1994). In vivo evaluation of the pathogenicity of field isolates of infectious bronchitis virus. Avian Diseases, 38, 589–597.
- Awad, F., Chhabra, R., Baylis, M. & Ganapathy, K. (2014). An overview of infectious bronchitis virus in chickens. World’s Poultry Science Journal, 70, 375–384.
- Bagust, T. (1989). An overview of Australia’s poultry industry in 1989. Australian Veterinary Journal, 66, 416.
- Bande, F., Arshad, S.S., Omar, A.R., Hair-Bejo, M., Mahmuda, A. & Nair, V. (2017). Global distributions and strain diversity of avian infectious bronchitis virus: a review. Animal Health Research Reviews, 18, 70–83.
- Barr, D.A., Reece, R.L., O’Rourke, D., Button, C. & Faragher, J.T. (1988). Isolation of infectious bronchitis virus from a flock of racing pigeons. Australian Veterinary Journal, 65, 228–228.
- Beilharz, R.G. & McDonald, M.W. (1960). Possible effect of sodium potassium balance on development of uremia. Australian Veterinary Journal, 36, 89–90.
- Benyeda, Z., Szeredi, L., Mató, T., Süveges, T., Balka, G., Abonyi-Tóth, Z., Rusvai, M. & Palya, V. (2010). Comparative histopathology and immunohistochemistry of QX-like, Massachusetts and 793/B serotypes of infectious bronchitis virus infection in chickens. Journal of Comparative Pathology, 143, 276–283.
- Brown Jordan, A., Fusaro, A., Blake, L., Milani, A., Zamperin, G., Brown, G., Carrington, C.V.F., Monne, I. & Oura, C.A.L. (2020). Characterization of novel, pathogenic field strains of infectious bronchitis virus (IBV) in poultry in Trinidad and Tobago. Transboundary and Emerging Diseases, 67, 2775–2788.
- Casais, R., Dove, B., Cavanagh, D. & Britton, P. (2003). Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. Journal of Virology, 77, 9084–9089.
- Cavanagh, D. (2001). A nomenclature for avian coronavirus isolates and the question of species status. Avian Pathology, 30, 109–115.
- Chamings, A., Nelson, T.M., Vibin, J., Wille, M., Klaassen, M. & Alexandersen, S. (2018). Detection and characterisation of coronaviruses in migratory and non-migratory Australian wild birds. Scientific Reports, 8, 5980.
- Chen, Y., Jiang, L., Zhao, W., Liu, L., Zhao, Y., Shao, Y., Li, H., Han, Z. & Liu, S. (2017). Identification and molecular characterization of a novel serotype infectious bronchitis virus (GI-28) in China. Veterinary Microbiology, 198, 108–115.
- Chong, K.T. & Apostolov, K. (1982). The pathogenesis of nephritis in chickens induced by infectious bronchitis virus. Journal of Comparative Pathology, 92, 199–211.
- Chousalkar, K.K., Cheetham, B.F. & Roberts, J.R. (2009). Effects of infectious bronchitis virus vaccine on the oviduct of hens. Vaccine, 27, 1485–1489.
- Chousalkar, K.K. & Roberts, J.R. (2007a). Ultrastructural observations on effects of infectious bronchitis virus in eggshell-forming regions of the oviduct of the commercial laying hen. Poultry Science, 86, 1915–1919.
- Chousalkar, K.K. & Roberts, J.R. (2007b). Ultrastructural study of infectious bronchitis virus infection in infundibulum and magnum of commercial laying hens. Veterinary Microbiology, 122, 223–236.
- Chubb, R. C., & Vanessa, M. (1974). Some observations on the propagation of avian infectious bronchitis virus in tissue culture. Australian Veterinary Journal, 50, 63-68.
- Chubb, R.C., Wells, B.A. & Cumming, R.B. (1976). Some immunological aspects of a recent Australian isolate of infectious bronchitis virus. Australian Veterinary Journal, 52, 378–381.
- Condron, R.J. & Marshall, A.T. (1985). Pathogenesis of infectious bronchitis nephritis. 2. Studies of water and electrolyte balance in colostomised chickens. Avian Pathology, 14, 509–520.
- Cumming, R.B. (1962). The aetiology of “uraemia” of chickens. Australian Veterinary Journal, 38, 554–554.
- Cumming, R.B. (1963). Infectious avian nephrosis (uraemia) in Australia. Australian Veterinary Journal, 39, 145–147.
- Cumming, R.B. (1967). Dietary sodium and potassium in infectious bronchitis of chickens. Australian Veterinary Journal, 43, 156–156.
- Cumming, R.B. (1969a). Studies on avian infectious bronchitis virus 1. Distribution and survival of the virus in tissues of affected chickens and studies on the carrier status. Australian Veterinary Journal, 45, 305–308.
- Cumming, R.B. (1969b). Studies on avian infectious bronchitis virus 3. Attempt to infect other species. Australian Veterinary Journal, 45, 312–314.
- Cumming, R.B. (1969c). Studies on avian infectious bronchitis virus. 2. Incidence of the virus in broiler and layer flocks, by isolation and serological methods. Australian Veterinary Journal, 45, 309–311.
- Cumming, R.B. (1970). Studies on Australian infectious bronchitis virus IV. Appparent farm-to-farm airborne transmission of infectious bronchitis virus. Avian Pathology, 14, 191–195.
- Dawson, P. & Gough, R. (1971). Antigenic variation in strains of avian infectious bronchitis virus. Archiv fur die Gesamte Virusforschung, 34, 32.
- de Wit, J.J., Cazaban, C., Dijkman, R., Ramon, G. & Gardin, Y. (2018). Detection of different genotypes of infectious bronchitis virus and of infectious bursal disease virus in European broilers during an epidemiological study in 2013 and the consequences for the diagnostic approach. Avian Pathology, 47, 140–151.
- de Wit, J.J., Cook, J.K.A. & van der Heijden, H.M.J.F. (2011). Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathology, 40, 223–235.
- de Wit, J.J., Nieuwenhuisen-van Wilgen, J., Hoogkamer, A., van de Sande, H., Zuidam, G.J. & Fabri, T.H. (2011). Induction of cystic oviducts and protection against early challenge with infectious bronchitis virus serotype D388 (genotype QX) by maternally derived antibodies and by early vaccination. Avian Pathology, 40, 463–471.
- Doherty, P.C. (1967). Titration of avian infectious bronchitis virus in the tissues of experimentally infected chickens. Australian Veterinary Journal, 43, 575–578.
- Endo-Munoz, L.B. & Faragher, J.T. (1989a). Avian infectious bronchitis: cross-protection studies using different Australian subtypes. Australian Veterinary Journal, 66, 345–348.
- Endo-Munoz, L.B. & Faragher, J.T. (1989b). A fluorescence test in allantoic cells for the detection of infectious bronchitis virus. Australian Veterinary Journal, 66, 338–340.
- Fabricant, J. (1998). The early history of infectious bronchitis. Avian Diseases, 42, 648–650.
- Faragher, J.T. (1987). A haemagglutination inhibition test for avian infectious bronchitis virus antibody. Australian Veterinary Journal, 64, 250–252.
- Gartner, R.J.W., Newton, L.G. & Burton, H.W. (1966). Relationships between dietary sodium and potassium, level of meat and bone meal and infectious bronchitis in chickens. Australian Veterinary Journal, 42, 357–363.
- Geering, W.A. & Bruce, A.I. (1970). Reference collection of strains of avian infectious bronchitis virus. Australian Veterinary Journal, 46, 76–76.
- Gilchrist, P.T. (1962). Avian respiratory diseases. Australian Veterinary Journal, 38, 495–499.
- Gilchrist, P.T. (1963). A survey of avian respiratory diseases. Australian Veterinary Journal, 39, 140–144.
- Gilchrist, P.T. (1968). The organisation of the Australian poultry industry and its influence on disease. Australian Veterinary Journal, 44, 420.
- Gough, R., Randall, C., Dagless, M., Alexander, D., Cox, W. & Pearson, D. (1992). A ‘new’ strain of infectious bronchitis virus infecting domestic fowl in Great Britain. The Veterinary Record, 130, 493–494.
- Hart, L. (1946). Diseases of the respiratory tract in fowls. Australian Veterinary Journal, 22, 125–131.
- Hewson, K.A., Browning, G.F., Devlin, J.M., Ignjatovic, J. & Noormohammadi, A.H. (2010). Application of high-resolution melt curve analysis for classification of infectious bronchitis viruses in field specimens. Australian Veterinary Journal, 88, 408–413.
- Hewson, K.A., Ignjatovic, J., Browning, G.F., Devlin, J.M. & Noormohammadi, A.H. (2011). Infectious bronchitis viruses with naturally occurring genomic rearrangement and gene deletion. Archives of Virology, 156, 245–252.
- Hewson, K.A., Noormohammadi, A.H., Devlin, J.M., Browning, G.F., Schultz, B.K. & Ignjatovic, J. (2014). Evaluation of a novel strain of infectious bronchitis virus emerged as a result of spike gene recombination between two highly diverged parent strains. Avian Pathology, 43, 249–257.
- Hewson, K.A., Noormohammadi, A.H., Devlin, J.M., Mardani, K. & Ignjatovic, J. (2009). Rapid detection and non-subjective characterisation of infectious bronchitis virus isolates using high-resolution melt curve analysis and a mathematical model. Archives of Virology, 154, 649–660.
- Hewson, K.A., Scott, P.C., Devlin, J.M., Ignjatovic, J. & Noormohammadi, A.H. (2012). The presence of viral subpopulations in an infectious bronchitis virus vaccine with differing pathogenicity – A preliminary study. Vaccine, 30, 4190–4199.
- Hunt, S. (1965). Programmes of disease control on commercial poultry farms. Australian Veterinary Journal, 41, 101–103.
- Ignjatovic, J., Ashton, D.F., Reece, R., Scott, P. & Hooper, P. (2002). Pathogenicity of Australian strains of avian infectious bronchitis virus. Journal of Comparative Pathology, 126, 115–123.
- Ignjatovic, J. & Ashton, F. (1996). Detection and differentiation of avian infectious bronchitis viruses using a monoclonal antibody-based ELISA. Avian Pathology, 25, 721–736.
- Ignjatovic, J. & Galli, L. (1994a). The S1 glycoprotein but not the N or M proteins of avian infectious bronchitis virus induces protection in vaccinated chickens. Archives of Virology, 138, 117–134.
- Ignjatovic, J. & Galli, L. (1994b). Structural proteins of avian infectious bronchitis virus: Role in immunity and protection. In H. Laude & J.F. Vautherot (Eds.), Coronaviruses. Advances in Experimental Medicine and Biology, vol 342. Boston, MA: Springer. doi:https://doi.org/10.1007/978-1-4615-2996-5_71.
- Ignjatovic, J. & Galli, L. (1995). Immune responses to structural proteins of avian infectious bronchitis virus. Avian Pathology, 24, 313–332.
- Ignjatovic, J., Gould, G. & Sapats, S. (2006). Isolation of a variant infectious bronchitis virus in Australia that further illustrates diversity among emerging strains. Archives of Virology, 151, 1567–1585.
- Ignjatovic, J. & McWaters, P.G. (1991). Monoclonal antibodies to three structural proteins of avian infectious bronchitis virus: characterization of epitopes and antigenic differentiation of Australian strains. Journal of General Virology (United Kingdom), 72, 2915–2922.
- Ignjatovic, J., Reece, R. & Ashton, F. (2003). Susceptibility of three genetic lines of chicks to infection with a nephropathogenic T strain of avian infectious bronchitis virus. Journal of Comparative Pathology, 128, 92–98.
- Ignjatovic, J., Sapats, S.I. & Ashton, F. (1997). A long-term study of Australian infectious bronchitis viruses indicates a major antigenic change in recently isolated strains. Avian Pathology, 26, 535–552.
- Jackson, C.A.W. (2001). Fifty years of poultry disease research and control in the Australian poultry industry. Paper presented at the Western Poultry Disease Conference.
- Jackson, C.A.W., Kingston, D.J. & Hemsley, L.A. (1972). A total mortality survey of nine batches of broiler chickens. Australian Veterinary Journal, 48, 481–487.
- Jackwood, M.W. (2012). Review of infectious bronchitis virus around the world. Avian Diseases, 56, 634–641.
- Jackwood, M.W., Boynton, T.O., Hilt, D.A., McKinley, E.T., Kissinger, J.C., Paterson, A.H., Robertson, J., Lemke, C., McCall, A.W., Williams, S.M., Jackwood, J.W. & Byrd, L.A. (2010). Emergence of a group 3 coronavirus through recombination. Virology, 398, 98–108.
- Jackwood, M.W. & de Wit, J.J. (2013). Infectious bronchitis. In D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez & V.L. Nair (Eds.), Diseases of Poultry 13th ed. (pp. 139–159). Aimes, IA: Wiley.
- Jackwood, M.W. & de Wit, S. (2020). Infectious bronchitis. In D. Swayne (Ed.), Diseases of Poultry 14th ed. (pp. 167–188). Hoboken, NJ: Wiley InterScience.
- Jones, R. & Jordan, F. (1972). Persistence of virus in the tissues and development of the oviduct in the fowl following infection at day old with infectious bronchitis virus. Research in Veterinary Science, 13, 52–60.
- Khan, S., Roberts, J. & Wu, S.B. (2017). Reference gene selection for gene expression study in shell gland and spleen of laying hens challenged with infectious bronchitis virus. Scientific Reports, 7, 14271.
- Klieve, A.V. & Cumming, R.B. (1988a). Immunity and cross-protection to nephritis produced by Australian infectious bronchitis viruses used as vaccines. Avian Pathology, 17, 829–839.
- Klieve, A.V. & Cumming, R.B. (1988b). Infectious bronchitis: safety and protection in chickens with maternal antibody. Australian Veterinary Journal, 65, 396–397.
- Klieve, A.V. & Cumming, R.B. (1990). Respiratory disease and immunity to challenge produced by Australian strains of infectious bronchitis virus. Avian Pathology, 19, 305–312.
- Laconi, A., van Beurden, S.J., Berends, A.J., Krämer-Kühl, A., Jansen, C.A., Spekreijse, D., Chénard, G., Philipp, H.-C., Mundt, E. & Rottier, P.J. (2018). Deletion of accessory genes 3a, 3b, 5a or 5b from avian coronavirus infectious bronchitis virus induces an attenuated phenotype both in vitro and in vivo. Journal of General Virology, 99, 1381.
- Lin, T.L., Loa, C.C. & Wu, C.C. (2004). Complete sequences of 3′ end coding region for structural protein genes of turkey coronavirus. Virus Research, 106, 61–70.
- Liu, S.W., Zhang, Q.X., Chen, J.D., Han, Z.X., Liu, X., Feng, L., Shao, Y.H., Rong, J.G., Kong, X.G. & Tong, G.Z. (2006). Genetic diversity of avian infectious bronchitis coronavirus strains isolated in China between 1995 and 2004. Archives of Virology, 151, 1133–1148.
- Lohr, J.E. (1976). Serologic differences between strains of infectious bronchitis virus from New Zealand, Australia, and the United States. Avian Diseases, 20, 478–482.
- Mardani, K., Browning, G.F., Ignjatovic, J. & Noormohammadi, A.H. (2006). Rapid differentiation of current infectious bronchitis virus vaccine strains and field isolates in Australia. Australian Veterinary Journal, 84, 59–62.
- Mardani, K., Noormohammadi, A.H., Hooper, P., Ignjatovic, J. & Browning, G.F. (2008). Infectious bronchitis viruses with a novel genomic organization. Journal of Virology, 82, 2013.
- Mardani, K., Noormohammadi, A.H., Ignjatovic, J. & Browning, G.F. (2010). Naturally occurring recombination between distant strains of infectious bronchitis virus. Archives of Virology, 155, 1581–1586.
- Mikula, P., Morelli, F., Lučan, R.K., Jones, D.N. & Tryjanowski, P. (2016). Bats as prey of diurnal birds: a global perspective. Mammal Review, 46, 160–174.
- Newton, L.G. & Simmons, G.C. (1963). Avian nephritis and uraemia. Australian Veterinary Journal, 39, 135–139.
- Peisley, H.R. (1961). The development of the animal quarantine service of the commonwealth of Australia. Australian Veterinary Journal, 37, 243–252.
- Promkuntod, N. (2016). Dynamics of avian coronavirus circulation in commercial and non-commercial birds in Asia – a review. Veterinary Quarterly, 36, 30–44.
- Pryor, W. & Woo, K. (1964). Relationship between protein/energy levels and uraemia in chickens. Australian Veterinary Journal, 40, 236–237.
- Purcell, D.A., Tham, V.L. & Surman, P.G. (1976). The histopathology of infectious bronchitis in fowls infected with a nephrotropic “T” strain of virus. Australian Veterinary Journal, 52, 85–91.
- Quinteros, J.A. (2019). Genomics and Pathogenesis of Australian Avian Coronaviruses. (Doctor of Philosophy). Minerva: The University of Melbourne.
- Quinteros, J.A., Lee, S.W., Markham, P.F., Noormohammadi, A.H., Hartley, C.A., Legione, A.R., Coppo, M.J.C., Vaz, P.K. & Browning, G.F. (2016). Full genome analysis of Australian infectious bronchitis viruses suggests frequent recombination events between vaccine strains and multiple phylogenetically distant avian coronaviruses of unknown origin. Veterinary Microbiology, 197, 27–38.
- Quinteros, J.A., Markham, P.F., Lee, S.-W., Hewson, K.A., Hartley, C.A., Legione, A.R., Coppo, M.J.C., Vaz, P.K. & Browning, G.F. (2015). Analysis of the complete genomic sequences of two virus subpopulations of the Australian infectious bronchitis virus vaccine VicS. Avian Pathology, 44, 182–191.
- Ranby, P.D. (1965). Programmes of disease control on commercial poultry farms. Australian Veterinary Journal, 41, 104–106.
- Reid, G.G., Grimes, T.M., Eaves, F.W. & Blackall, P.J. (1984). A survey of disease in five commercial flocks of meat breeder chickens. Australian Veterinary Journal, 61, 13–16.
- Samiullah, S., Roberts, J. & Chousalkar, K. (2016). Infectious bronchitis virus and brown shell colour: Australian strains of infectious bronchitis virus affect brown eggshell colour in commercial laying hens differently. Avian Pathology, 45, 552–558.
- Sapats, S.I. (2009). Control of infectious bronchitis virus: improved diagnostics and disease surveillance through the application of genomic technology. Australian Poultry CRC, Poultry Hub.
- Sapats, S.I., Ashton, F., Wright, P.J. & Ignjatovic, J. (1996a). Novel variation in the N protein of avian infectious bronchitis virus. Virology, 226, 412–417.
- Sapats, S.I., Ashton, F., Wright, P.J. & Ignjatovic, J. (1996b). Sequence analysis of the S1 glycoprotein of infectious bronchitis viruses: identification of a novel genotypic group in Australia. Journal of General Virology, 77, 413–418.
- Schalk, A. (1931). An apparently new respiratory disease of baby chicks. Journal of the American Veterinary Medical Association, 78, 413–423.
- Silller, W.G. & Cumming, R.B. (1974). The histopathology of an interstitial nephritis in the fowl produced experimentally with infectious bronchitis virus. Journal of Pathology, 114, 163–173.
- Stephens, P. & Simmons, G.C. (1968). Neutralising antibodies for avian infectious bronchitis virus in Queensland poultry flocks. Australian Veterinary Journal, 44, 29–30.
- Valastro, V., Holmes, E.C., Britton, P., Fusaro, A., Jackwood, M.W., Cattoli, G. & Monne, I. (2016). S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification. Infection, Genetics and Evolution, 39, 349–364.
- Vijaykrishna, D., Smith, G.J.D., Zhang, J.X., Peiris, J.S.M., Chen, H. & Guan, Y. (2007). Evolutionary insights into the ecology of coronaviruses. Journal of Virology, 81, 4012–4020.
- Wadey, C.N. & Faragher, J.T. (1981a). Australian infectious bronchitis viruses: plaque formation and assay methods. Research in Veterinary Science, 30, 66–69.
- Wadey, C.N. & Faragher, J.T. (1981b). Avian infectious bronchitis viruses: identification of nine subtypes by a neutralisation test. Research in Veterinary Science, 30, 70–74.
- Watson, A.R.A. (1968). The economic significance of respiratory diseases in the Victorian chicken meat industry. Australian Veterinary Journal, 44, 251–253.
- Weiss, S.R. & Navas-Martin, S. (2005). Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiology and Molecular Biology Reviews, 69, 635–664.
- Wertheim, J.O., Chu, D.K.W., Peiris, J.S.M., Kosakovsky Pond, S.L. & Poon, L.L.M. (2013). A case for the ancient origin of coronaviruses. Journal of Virology, 87, 7039–7045.
- Wille, M. & Holmes, E.C. (2020). Wild birds as reservoirs for diverse and abundant gamma- and deltacoronaviruses. FEMS Microbiology Reviews, 44, 631–644.
- Woo, P.C.Y., Lau, S.K.P., Lam, C.S.F., Lai, K.K.Y., Huang, Y., Lee, P., Luk, G.S.M., Dyrting, K.C., Chan, K.H. & Yuen, K.Y. (2009). Comparative analysis of complete genome sequences of three avian coronaviruses reveals a novel group 3c coronavirus. Journal of Virology, 83, 908–917.
- Woo, P.C.Y., Lau, S.K.P., Lam, C.S.F., Lau, C.C.Y., Tsang, A.K.L., Lau, J.H.N., Bai, R., Teng, J.L.L., Tsang, C.C.C., Wang, M., Zheng, B.-J., Chan, K.-H. & Yuen, K.-Y. (2012). Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and Deltacoronavirus. Journal of Virology, 86, 3995–4008.
- Wu, Z., Yang, Q., Fu, C., Zhao, X. & Ignjatovic, J. (1998). Antigenic and immunogenic characterization of infectious bronchitis virus strains isolated in China between 1986 and 1995. Avian Pathology, 27, 578–585.
- Xu, L., Han, Z., Jiang, L., Sun, J., Zhao, Y. & Liu, S. (2018). Genetic diversity of avian infectious bronchitis virus in China in recent years. Infection, Genetics and Evolution, 66, 82–94.
