ABSTRACT
Chickens represent a globally ubiquitous food animal underpinning many aspects of human nutrition and health. Consumption of chicken meat continues to surge, representing a cheaper, healthier, low-carbon alternative to other livestock meats. Despite this importance, we are still unable to define what lives within the chicken gut microbiome. This complex community bridges poultry diet, health and productivity as well as providing a reservoir for zoonotic pathogens. Even with decades of intensive study, we are still discovering novel microbial species within this environment, each of which has the potential to provide an avenue for commercial microbiome modulation. The chicken gut truly represents an exhilarating challenge in turning new-found knowledge into new-won power to improve the health and wealth of poultry and people.
The challenge: understanding and exploiting the chicken gut microbiome
Sustainable chicken production underpins global food security, providing a low-carbon alternative to protein from other livestock. Increasing demand has led to improved feed conversion and growth rate in poultry production, maximizing meat yield and quality while minimizing costs.
The chicken gut acts as the interface between dietary inputs and the outputs of production, mediating links between genetic potential and crucial environmental variables (Borda-Molina et al., Citation2018; Shang et al., Citation2018; Du et al., Citation2020; Glendinning et al., Citation2020). The chicken gut is home to a densely populated and taxonomically rich microbial community (Zhu et al., Citation2002; Wei et al., Citation2013) - the chicken gut microbiome - that includes bacteria, archaea, viruses and microbial eukaryotes. This microbiome acts as a biological powerhouse in digestion (Pan & Yu, Citation2014) and absorption of food (Mahmood & Guo, Citation2020), pathogen exclusion and immune development (Clavijo & Flórez, Citation2018). The community is predominantly bacterial and experiences successional (Jurburg et al., Citation2019) and temporal (Sekelja et al., Citation2012) variation. The chicken gut microbiome also represents a reservoir for important foodborne zoonotic pathogens such as Campylobacter, Salmonella and E. coli (Thames & Theradiyil Sukumaran, Citation2020).
Prophylactic supplementation of bird feed with sub-therapeutic concentrations of antibiotics has seen extensive use in poultry production as a way of improving the health and growth of livestock (Poole & Sheffield, Citation2013). However, the use of antibiotics as growth promoters in livestock has been associated with the emergence of antimicrobial resistance genes and their spread into the human population, leading to widespread bans on their use (Levy et al., Citation1976; Ma et al., Citation2020). Global efforts now focus on the development of safe and sustainable alternatives to antibiotics in chickens and other livestock. As the beneficial effects of antibiotics are presumed to be mediated through the gut microbiome (Kumar et al., Citation2018), research into this microbial community presents a promising route to the development of alternative biological therapeutics.
Fresh opportunities in discovery research
The last five decades have seen steady improvements in our understanding of the chicken gut microbiome, building on early use of conventional culture-dependent methods (Barnes et al., Citation1972; Salanitro et al., Citation1974) (). However, despite advances using molecular barcodes to track microbes, until recently, a significant portion of the chicken gut microbiome remained taxonomically undefined, collectively described as “microbial dark matter”.
Figure 1. Progression of poultry microbiome research and its benefit to commercial industry. Commonly utilised laboratory techniques for studying the chicken gut microbiome and in the development of modulatory interventions.
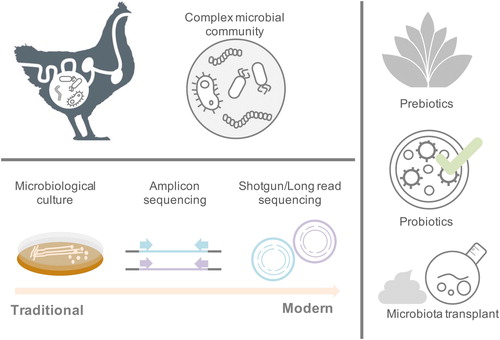
In recent years, rapid advances in “omics” approaches have taken our understanding of the gut microbiota far beyond that revealed by culture approaches alone (Shaufi et al., Citation2015; Lagier et al., Citation2016; Almeida et al., Citation2019; Zhang et al., Citation2019). In particular, analysis of DNA extracted from faecal or caecal samples en masse (“shotgun metagenomics”) (Sergeant et al., Citation2014; Glendinning et al., Citation2020) has allowed us to overcome the biases and bottlenecks associated with culture. Through the use of both culture-based and sequence-based strategies, we have recently uncovered vast genetic, functional and taxonomic diversity in this setting (Glendinning et al., Citation2020; Gilroy et al., Citation2021), documenting millions of genes, thousands of bacterial genomes and hundreds of new species.
Avenues for translational research
Prebiotics (hard-to-digest carbohydrates) and probiotics (mono- or mixed microbial cultures) provide natural alternatives to antibiotics as growth promoters and have seen remarkable commercial uptake over recent years (Al-Khalaifa et al., Citation2019; Krysiak et al., Citation2021). When delivered to the host at key developmental stages, these products indirectly or directly modulate the gut microbiota and significantly influence the trajectory of successional development (Kabir, Citation2009; Ramírez et al., Citation2020). While no standardized commercial probiotic formulation exists, lactic acid bacteria (e.g. Lactobacillus, Enterococcus) and spore-formers (e. g. Bacillus) are typical components of polymicrobial mixtures associated with resistance to salmonellosis in poultry (Tellez et al., Citation2012; Khan & Chousalkar, Citation2020; Hai et al., Citation2021). Probiotics have been linked to a reduction in adhesion and proliferation of opportunistic enteric pathogens alongside increases in host nutrient acquisition and, as a result, growth and performance (Hernandez-Patlan et al., Citation2020).
Poor reproducibility of effects remains a source of doubt in the technical viability of pre- and probiotics (Deng et al., Citation2020). This probably stems from considerable bird-to-bird variation in the composition of the gut microbiome. An improved understanding of the complex microbe-microbe interactions within this environment is likely to generate a refined list of bacterial candidates for inclusion in probiotics.
Drawing on longstanding use of microbiota transplantation in chickens and other livestock, together with its recent success in human medicine for C. difficile infection (Bakken et al., Citation2011), development of approaches to storing and delivering a standardized gut microbiota promises to be a hot topic in poultry microbiome research (Ramírez et al., Citation2020).
Enzymes that degrade hard-to-digest components of the chicken diet (e.g. xylans, phytate) are widely used as feed additives (Kiarie et al., Citation2013). This opens up another avenue for exploitation of the gut microbiome: bioprospecting for enzymes and other bio-molecules with useful properties that can be identified through bioinformatics searches of metagenomic sequences and then cloned and expressed in the lab, before commercialization.
Linking discovery to translation
Our own microbiome research has opened up new horizons in discovering, documenting and characterizing a microbial parts list for the chicken gut (Gilroy et al., Citation2021). The resulting datasets greatly expand the known diversity of the chicken gut microbiome and provide a key resource for future high-resolution taxonomic and functional studies on the chicken gut microbiome. However, given that, even after decades of intensive study, new species are still being discovered in the human gut, we can be confident that our work presents far from the last word on this topic.
Much remains to be discovered, while we also face the exhilarating challenge of turning new-found knowledge into new-won power to improve the health and wealth of poultry and people.
Ethical statement
This research did not involve human beings and/or animals.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Al-Khalaifa, H., Al-Nasser, A., Al-Surayee, T., Al-Kandari, S., Al-Enzi, N., Al-Sharrah, T., Ragheb, G., Al-Qalaf, S. & Mohammed, A. (2019). Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poultry Science, 98, 4465–4479.
- Almeida, A., Mitchell, A.L., Boland, M., Forster, S.C., Gloor, G.B., Tarkowska, A., Lawley, T.D. & Fin, R.D. (2019). A new genomic blueprint of the human gut microbiota. Nature, 568, 499–504.
- Bakken, J.S., Borody, T., Brandt, L.J., Brill, J.V., Demarco, D.C., Franzos, M.A., Kelly, C., Khoruts, A., Louie, T., Martinelli, L.P. & Moore, T.A. (2011). Treating Clostridium difficile infection with fecal microbiota transplantation. Clinical Gastroenterology and Hepatology, 9, 1044–1049.
- Barnes, E.M., Mead, G.C., Barnuml, D.A. & Harry, E.G. (1972). The intestinal flora of the chicken in the period 2 to 6 weeks of age, with particular reference to the anaerobic bacteria. British Poultry Science, 13, 311–326.
- Borda-Molina, D., Seifert, J. & Camarinha-Silva, A. (2018). Current perspectives of the chicken gastrointestinal tract and its microbiome. Computational and Structural Biotechnology Journal, 16, 131–139.
- Clavijo, V. & Flórez, M.J.V. (2018). The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poultry Science, 97, 1006–1021.
- Deng, W., Dittoe, D.K., Pavlidis, H.O., Chaney, W.E., Yang, Y. & Ricke, S.C. (2020). Current perspectives and potential of probiotics to limit foodborne campylobacter in poultry. Frontiers in Microbiology, 11, 583429.
- Du, W., Deng, J., Yang, Z., Zeng, L. & Yang, X. (2020). Metagenomic analysis reveals linkages between cecal microbiota and feed efficiency in Xiayan chickens. Poultry Science, 99, 7066–7075.
- Gilroy, R., Ravi, A., Getino, M., Pursley, I., Horton, D.L., Alikhan, N.F., Baker, D., Gharbi, K., Hall, N., Watson, M., Adriaenssens, E.M., Foster-Nyarko, E., Jarju, S., Secka, A., Antonio, M., Oren, A., Chaudhuri, R.R., La Ragione, R., Hildebrand, F. & Pallen, M.J. (2021). Extensive microbial diversity within the chicken gut microbiome revealed by metagenomics and culture. PeerJ, 9, e10941.
- Glendinning, L., Stewart, R.D., Pallen, M.J., Watson, K.A. & Watson, M. (2020). Assembly of hundreds of novel bacterial genomes from the chicken caecum. Genome Biology, 21, 1–16.
- Hai, D., Lu, Z., Huang, X., Lv, F. & Bie, X. (2021). In vitro screening of chicken-derived lactobacillus strains that effectively inhibit salmonella colonization and adhesion. Foods, 10, 569.
- Hernandez-Patlan, D., Solis-Cruz, D., Hargis, B.M. & Tellez, G. (2020). The use of probiotics in poultry production for the control of bacterial infections and aflatoxins. In E. Franco-Robles & J. Ramirez-Emiliano (Eds.), Prebiotics and Probiotics-Potential Benefits in Nutrition and Health (pp. 217–238). London: IntechOpen.
- Jurburg, S.D., Brouwer, M.S.M., Ceccarelli, D., van der Goot, J., Jansman, A.J.M. & Bossers, A. (2019). Patterns of community assembly in the developing chicken microbiome reveal rapid primary succession. MicrobiologyOpen, 8, e00821.
- Kabir, S.M. (2009). The role of probiotics in the poultry industry. International Journal of Molecular Sciences, 10, 3531–3546.
- Khan, S. & Chousalkar, K.K. (2020). Salmonella Typhimurium infection disrupts but continuous feeding of bacillus based probiotic restores gut microbiota in infected hens. Journal of Animal Science and Biotechnology, 11, 1–16.
- Kiarie, E., Romero, L.F. & Nyachoti, C.M. (2013). The role of added feed enzymes in promoting gut health in swine and poultry. Nutrition Research Reviews, 26, 71–88.
- Krysiak, K., Konkol, D. & Korczyński, M. (2021). Overview of the use of probiotics in poultry production. Animals, 11, 1620.
- Kumar, S., Chen, C., Indugu, N., Werlang, G.O., Singh, M., Kim, W.K. & Thippareddi, H. (2018). Effect of antibiotic withdrawal in feed on chicken gut microbial dynamics, immunity, growth performance and prevalence of foodborne pathogens. PLoS One, 13, e0192450.
- Lagier, J.-C., Khelaifia, S., Alou, M.T., Ndongo, S., Dione, N., Hugon, P., Caputo, A., Cadoret, F., Traore, S.I. & Dubourg, G. (2016). Culture of previously uncultured members of the human gut microbiota by culturomics. Nature Microbiology, 1, 1–8.
- Levy, S.B., Fitzgerald, G.B. & Macone, A.B. (1976). Spread of antibiotic-resistant plasmids from chicken to chicken and from chicken to man. Nature, 260, 40–42.
- Ma, F., Xu, S., Tang, Z., Li, Z. & Zhang, L. (2020). Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosafety and Health, 3, 32–38.
- Mahmood, T. & Guo, Y. (2020). Dietary fiber and chicken microbiome interaction: where will it lead to? Animal Nutrition, 6, 1–8.
- Pan, D. & Yu, Z. (2014). Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes, 5, 108–119.
- Poole, T. & Sheffield, C. (2013). Use and misuse of antimicrobial drugs in poultry and livestock: mechanisms of antimicrobial resistance. Pakistan Veterinary Journal, 33, 266-271.
- Ramírez, G.A., Richardson, E., Clark, J., Keshri, J., Drechsler, Y., Berrang, M.E., Meinersmann, R.J., Cox, N.A. & Oakley, B.B. (2020). Broiler chickens and early life programming: microbiome transplant-induced cecal community dynamics and phenotypic effects. PLoS One, 15, e0242108.
- Salanitro, J.P., Fairchilds, I.G. & Zgornicki, Y.D. (1974). Isolation, culture characteristics, and identification of anaerobic bacteria from the chicken cecum. Applied Microbiology, 27, 678–687.
- Sekelja, M., Rud, I., Knutsen, S.H., Denstadli, V., Westereng, B., Naes, T. & Rudi, K. (2012). Abrupt temporal fluctuations in the chicken fecal microbiota are explained by its gastrointestinal origin. Applied and Environmental Microbiology, 78, 2941–2948.
- Sergeant, M.J., Constantinidou, C., Cogan, T.A., Bedford, M.R., Penn, C.W. & Pallen, M.J. (2014). Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS One, 9, e91941.
- Shang, Y., Kumar, S., Oakley, B. & Kim, W.K. (2018). Chicken gut microbiota: importance and detection technology. Frontiers in Veterinary Science, 5, 254.
- Shaufi, M.A.M., Sieo, C.C., Chong, C.W., Gan, H.M. & Ho, Y.W. (2015). Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathogens, 7, 1–12.
- Tellez, G., Pixley, C., Wolfenden, R.E., Layton, S.L. & Hargis, B.M. (2012). Probiotics/direct fed microbials for Salmonella control in poultry. Food Research International, 45, 628–633.
- Thames, H.T. & Theradiyil Sukumaran, A. (2020). A review of Salmonella and Campylobacter in broiler meat: emerging challenges and food safety measures. Foods, 9, 776.
- Wei, S., Morrison, M. & Yu, Z. (2013). Bacterial census of poultry intestinal microbiome. Poultry Science, 92, 671–683.
- Zhang, X., Li, L., Butcher, J., Stintzi, A. & Figeys, D. (2019). Advancing functional and translational microbiome research using meta-omics approaches. Microbiome, 7, 1–12.
- Zhu, X.Y., Zhong, T., Pandya, Y. & Joerger, R.D. (2002). 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Applied and Environmental Microbiology, 68, 124–137.
