?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In Europe, monitoring of breeding stock for Salmonella Pullorum (SP) or Salmonella Gallinarum (SG) infections is compulsory at the point of lay. Vaccinations against Salmonella Enteritidis (SE) and Salmonella Typhimurium (ST) are increasingly administered in Europe. These vaccines might induce cross-reactions in the rapid plate agglutination (RPA) SP/SG test due to shared O-antigens, possibly resulting in a lower test specificity. The extent to which the specificity of SP/SG serological tests is influenced by SE and/or ST vaccinations in the field has not been reported. In this paper, we report the diagnostic and flock specificity of the commercially available RPA SP/SG test using 1:2–1:16 serum dilutions on four panels of sera: SPF sera, field sera from flocks of varying age and SE/ST vaccination status, and reference sera from an international proficiency testing scheme. The results showed that the use of live SE/ST vaccines did not influence the specificity of the RPA SP/SG test. Inactivated vaccines showed a drop of the diagnostic specificity to 96.54% and a flock specificity of 34.1% when the 1:2 serum dilution was used. The 1:8 serum dilution showed a diagnostic specificity of 99.41% and a flock specificity of 86.4%. In conclusion, the use of SE/ST vaccines has either no effect or a modest effect on the specificity of the RPA SP/SG test used to monitor flocks. The main factors are the type of vaccine, and the serum dilution used for testing and a cut-off.
Introduction
Fowl typhoid and pullorum disease are OIE-listed poultry diseases caused by Salmonella Gallinarum (SG) and Salmonella Pullorum (SP). SG and SP are indistinguishable by normal serotyping and are generally regarded as biotypes of Salmonella enterica subsp. enterica serovar Gallinarum (Christensen et al., Citation1993). The SG serovar is non-motile in contrast to most zoonotic Salmonella serovars and can be horizontally and vertically transmitted and cause systemic disease in chickens and other avian species (OIE, Citation2018a). Since the first description more than 100 years ago, SG and SP have had a significant economic impact on the sustainability of commercial poultry production. For this reason, control and eradication programmes for both biotypes have already been running for many decades (Schat et al., Citation2021). In some parts of the world, including Europe, these programmes have shown a substantial decrease in the prevalence of both biotypes (Shivaprasad, Citation2000; Barrow & Freitas Neto, Citation2011; Wigley, Citation2017). However, both biotypes are still endemic in other parts of the world (Barrow & Freitas Neto, Citation2011; Wigley, Citation2017; European Commission, Citation2019).
In Europe, monitoring breeding stock at the point of lay is compulsory, and trade of hatching eggs or day-old chicks of SP- or SG-positive breeding flocks is prohibited within the EU (European Commission, Citation2019). The compulsory monitoring programme aims to prevent the spread of SP or SG from clinically healthy parent birds to their offspring (Shivaprasad, Citation2000; Wigley et al., Citation2001; Proux et al., Citation2002). Serological monitoring of healthy flocks for subclinical, chronic SP and SG infections is more sensitive than bacteriological culturing of caecal content (Wigley et al., Citation2001) or environmental samples and faeces (Gast, Citation1997; Shivaprasad, Citation2000; Proux et al., Citation2002; Wigley, Citation2017). The OIE recommends serological monitoring by a Rapid Plate Agglutination (RPA) test based on polyvalent SP antigens (OIE, Citation2018a). Developing a serovar-specific serological test for SP/SG is a challenge due to cross-reactions with other Salmonella serovars (Williams & Whittemore, Citation1979; Gast & Beard, Citation1990; Waltman & Horne, Citation1993). Cross-reactions with salmonellas that share O-antigens with SP and SG, such as other serogroup D Salmonella serotypes (O-antigens 1, 9, 12) like Salmonella Enteritidis (SE) or serogroup B Salmonella serotypes (O-antigens: 1, 4, [5] and 12) like Salmonella Typhimurium (ST), are a particular concern.
Vaccination of layers and parent flocks against SE and/or ST has become popular in Europe as an additional tool to biosecurity to improve the control of these zoonotic salmonellas in commercial poultry. SE and ST vaccines might induce cross-reactions in the RPA SP/SG test due to the shared O-antigens, showing a potential lower specificity of the RPA SP/SG test. However, quantification of this potential effect on the specificity of the RPA SP/SG test under field conditions has not been reported.
This study reports the diagnostic specificity and the flock specificity of a commercially available RPA SP/SG test on four panels of sera: 579 SPF sera, 62,419 sera from 1641 layer, parent and grandparent flocks of varying age, and 11,504 field sera from young parent flocks with a known vaccination programme for SE and ST obtained from March 2020 to January 2021. Additionally, the results of the RPA SP/SG test of sera tested in an international proficiency testing scheme (PTS) for detecting antibodies against Salmonella in serum are reported.
Materials and methods
Rapid plate agglutination test
All sera were tested against two experimentally raised SP-antibody-positive sera and a negative control serum, according to ISO 17025 requirements. For screening, sera without previous heat treatment were diluted 1:2 in 0.5 M phosphate-buffered saline (PBS), pH 7.2. Subsequently, 25 µl volumes of 1:2 diluted serum were mixed with the same volume of antigen (Biovac, Beaucouzé, France) on a white Perspex plate gently swirling the mixture for 2 minutes. Sera with agglutination reactions within 2 minutes were considered suspect. A maximum of 10 suspect sera was retested per flock, using the sera with the strongest reactions. These sera were subsequently inactivated by heating for 30 min at 56°C, centrifuged and serially diluted in twofold steps from 1:2 to 1:32 in PBS and retested in the RPA SP/SG test, as described. If agglutination was shown in the serum in the 1:2 and 1:4 dilution after inactivation it was considered negative. Similar to the Mg and Ms RPA test used for the Dutch monitoring programmes of Mycoplasma gallisepticum and Mycoplasma synoviae, the RPA SP/SG test was considered specific positive when agglutination occurred at serum dilution 1:8 or higher.
Panels of sera
Sera obtained from specified pathogen-free (SPF) birds, from field flocks and a PTS were analysed in the RPA SP/SG test. The diagnostic specificity of the RPA SP/SG test was assessed based on all sera, and the flock specificity was only assessed on field sera. The field and SPF sera were obtained from March 2020 to January 2021. The field sera also included sera that were collected within the frame of the compulsory Dutch SP/SG monitoring programme. From each parent or grandparent flock of 19-24 weeks of age, 1% of the birds were sampled with a minimum of 30 and a maximum of 60 samples (Kamp, Citation2014), aiming at detecting seroconversion in 10–5% of the birds, respectively, with 95% confidence (Thrusfield, Citation2001). All field sera from flocks were regarded as SP/SG, SE and ST negative as no infections were detected in these flocks or their progeny during the whole production period. Moreover, no SP or SG vaccines were used in these flocks.
Panel of SPF sera
A total of 579 sera from SPF layers and broiler breeders (animal facility of Royal GD) were included in the study ().
Table 1. Results of RPA test for antibodies to Salmonella Pullorum and Salmonella Gallinarum on 579 SPF sera and 62,419 field sera from 1641 Dutch layer, breeder and grandparent flocks of varying age and unknown vaccination programmes against Salmonella Enteritidis and Salmonella Typhimurium.
Panel of field sera of 1641 Dutch chicken flocks of varying age with unknown SE/ST vaccination status
From March 2020 to January 2021, 62,419 sera from 1641 submissions of blood samples from Dutch layer, breeder and grandparent flocks varying from 18 to 98 weeks of age were tested in the RPA SP/SG test (). Most of the layer and parent flocks had been vaccinated against SE and ST during rearing; however, this information on individual flock level was not available.
Sera of 206 young parent flocks with a known SE/ST vaccination status
A separate analysis was made for the results of 11,504 sera from 206 young parent flocks with known vaccination status for Salmonella, sampled according to the Dutch national SP/SG monitoring programme at 19–24 weeks of age as mentioned above. Four vaccines had been used: live vaccine AviPro® Salmonella DUO (Elanco GmbH, Cuxhaven, Germany), two inactivated vaccines with SE and ST antigens: Nobilis Salenvac T (Intervet Nederland B.V., Boxmeer, the Netherlands), and Gallimune SE + ST (Boehringer Ingelheim Animal Health Netherlands B.V., Alkmaar, the Netherlands), and an inactivated vaccine with SE, ST and S. Infantis antigens: Nobilis Salenvac ETC (Intervet Nederland B.V.).
Of the 11,504 sera, 7168 sera were obtained from 128 flocks vaccinated with live SE/ST vaccine three times (126 flocks) or four times (two flocks). Another 1151 samples were obtained from 20 flocks vaccinated three times with a live SE/ST vaccine and boosted once (six flocks) or twice (14 flocks) with an inactivated SE/ST vaccine. A further 1216 samples were obtained from 24 flocks vaccinated twice with an inactivated SE/ST vaccine (21 flocks) or an inactivated SE/ST/Group C vaccine (three flocks). Finally, 1969 samples were obtained from 34 flocks that had not been vaccinated for Salmonella ( and ).
Table 2. Results of RPA test for antibodies to Salmonella Pullorum and Salmonella Gallinarum on 11,504 sera from 206 young parent flocks with a known vaccination programme against Salmonella Enteritidis and Salmonella Typhimurium that all had been sampled according to the Dutch monitoring programme at 19–24 weeks of age.
Table 3. Results of RPA test for antibodies to Salmonella Pullorum and Salmonella Gallinarum at flock level on 11,504 sera from 206 young parent flocks with a known vaccination programme against Salmonella Enteritidis and Salmonella Typhimurium that all had been sampled according to the Dutch monitoring programme at 19–24 weeks of age.
Sera proficiency testing schemes
As part of the quality control system of the ISO 17025-accredited RPA SG/SP test, Royal GD has participated annually since 2010 in an ISO 17043-accredited International PTS for the detection of antibodies against Salmonella in chicken serum. In 2010–2021, 24 different sera had been included in this PTS. The 24 sera included sera obtained from SPF birds, which were challenged with S. Virchow, S. Infantis, SE field strain or live vaccine, ST field strain or live vaccine, SP, SG field strain or live SG vaccine, or were mock challenged. For the PTS, all samples had to be tested twice on separate days. An overview of the sera and the results are shown in .
Table 4. Results of RPA SP/SG test international PTS Royal GD from 2010 to 2020.
Statistics
The diagnostic specificity, flock specificity and their 95% confidence intervals were calculated. The diagnostic specificity of the test is the proportion of true negatives that are detected. If the diagnostic specificity is given for a specific serum dilution, samples that showed agglutination at that serum dilution were considered positive.
The flock specificity was the proportion of flocks from which all sera were negative in the RPA SP/SG test for the given cut-off.
The diagnostic specificity and the flock specificity were calculated according to the following formula:
The 95% confidence intervals were determined using the following method for confidence intervals of proportions that approach 100% (Thrusfield, Citation2007):
Results
SPF sera
All 579 SPF sera were negative in the RPA SP/SG test showing a diagnostic specificity of 100% at serum dilution 1:2 ().
Field sera of 1641 Dutch chicken flocks of varying age with unknown SE/ST vaccination status
In total, 99.65% of the 62,419 1:2 diluted sera of 1641 Dutch layer, breeder and grandparent flocks of varying age and unknown Salmonella vaccination status tested negative in the RPA SG/SP-test (). The remaining 219 sera showed a RPA SP/SG titre of 1:2 (145×), 1:4 (58×), 1:8 (15×) or 1:16 (1×). The diagnostic specificity of the RPA SP/SG test for serum dilutions higher than 1:2 was 99.88%, 99.97% and ≅100.00% for the 1:4, 1:8 and 1:16 dilutions, respectively. Sera of 1565 flocks tested negative in the RPA (1:2 diluted sera). The number of positive sera of the remaining 76 flocks varied from 1 to 10 per flock. The flock specificities for the different dilutions, as cut-off varied from 95.37% to 99.94% (). The 95% confidence intervals of the specificities are listed in .
Sera of 206 Dutch young parent flocks with a known SE/ST vaccination status
The results and the diagnostic specificity of the RPA SP/SG test for the sera of the young parent flocks with a known vaccination programme against SE and ST are summarized in . shows the results of the flock specificity of the RPA SP/SG test at the flock level.
All 1151 sera obtained from 34 unvaccinated flocks were negative at the 1:2 serum dilution. This was comparable to the RPA SP/SG test results on the 7168 sera obtained from 128 flocks that had been vaccinated with live SE/ST vaccine. Only one serum sample showed agglutination in the RPA SP/SG test at the 1:2 serum dilution, but not at higher dilutions. The diagnostic specificity of the RPA SP/SG test using the 1:2 serum dilution as a cut-off was 99.99% for sera of flocks that had been vaccinated with live SE/ST vaccines or were unvaccinated. The corresponding flock specificity was 99.4% and based on a single positive sample in a single flock ().
Most of the sera (96.54%) from the flocks vaccinated with inactivated SE/ST vaccines, with or without live vaccines, tested negative in the RPA SP/SG test at the 1:2 serum dilution. Of the untreated 1:2 diluted sera, a maximum of 10 sera per submission reacted in the test. After inactivation, 1.48% of the sera were positive up to serum dilution 1:2, 1.39% up to serum dilution 1:4, 0.59% up to serum dilution 1:8 and 0.0% at serum dilution 1:16 or higher. shows the corresponding diagnostic specificities, ranging from 96.54% (1:2 dilution as cut-off) to 100% (1:16 as cut-off). shows the corresponding flock specificities, ranging from 34.1% (1:2 dilution as cut-off) to 100% (1:16 as cut-off).
Sera proficiency testing schemes
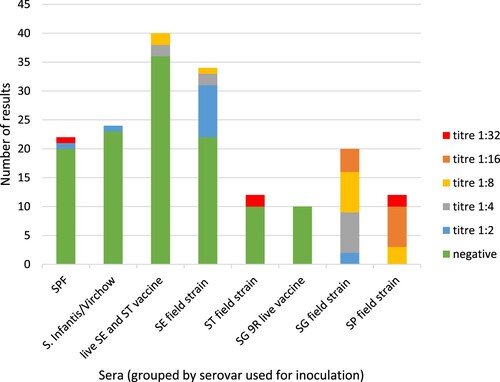
The results of the international PTS showed that 159 out of 174 tests (91.4%) were correct using serum dilution 1:8 as a cut-off (). Six results were false-positive and included the test performed on one SPF serum, one SE vaccine serum, one SE field strain serum and two ST field strain sera. The mean RPA SP/SG titres were up to 0.3 in the mock sera, the S. Infantis and S. Virchow sera, the SE and/or ST vaccine sera and the SG vaccine sera (). For the SE and ST field strain sera, the mean titres were 0.8 and 1.0, respectively. In the SP or SG field strain sera, the mean titres were 3.9 and 2.7, respectively. shows the division of the individual titres found within the samples included in the international PTS. The results show that only in the SP and SG sera most of the samples were positive at the 1:8 and 1:16 dilutions for SG and the 1:8, 1:16 and 1:32 dilution for SP, respectively. For SE and/or ST vaccine and field strain sera, most of the samples already tested negative in the 1:2 dilution.
Discussion
Serological monitoring of young parent flocks using the RPA SP/SG test for antibodies against SP/SG is recommended to prevent the spread of SP or SG from clinically healthy SP/SG carrier parent birds to their offspring (Shivaprasad, Citation2000; Wigley et al., Citation2001; Proux et al., Citation2002; Wigley, Citation2017). The OIE recommends serological monitoring by an RPA test based on polyvalent SP antigens (OIE, Citation2018a). The increased use of vaccines against SE and/or ST might hamper the serological monitoring due to cross-reactions in the RPA SP/SG test caused by the shared O-antigens between SE and ST with SG and SP, showing a lower specificity of the RPA SP/SG test. Here, we present the quantification of the influence of SE and ST vaccinations on the diagnostic and flock specificities of the RPA SP/SG test for the detection of antibodies to SG and SP under field conditions.
In general, the specificity of the RPA SP/SG test was 99.65% on the 62,419 1:2 diluted sera of 1641 Dutch layer, breeder and grandparent flocks. Specificity was practically 100% when the 1:8 dilution was used as a cut-off. Most of these sera were from SE- and/or ST-vaccinated flocks;, however, the vaccination status of the individual flocks was unknown. Therefore, more detailed information was obtained from the results of the subset of sera from 206 young breeder flocks with known SE/ST vaccination programmes and a fixed sampling age of 19–24 weeks of age. In this period, breeder flocks are monitored for antibodies against SP/SG and, in the same period, the peak of the antibody level against SE/ST is to be expected shortly after the last vaccination, applied at the end of the rearing period. The diagnostic specificity of the RPA SP/SG test was not influenced by the use of live SE and ST vaccines as it was similar in unvaccinated flocks (average specificity of 99.99%).
The inactivated vaccines, with or without priming with live vaccines, developed to induce high levels of antibodies, resulted in a drop of the diagnostic specificity by 3.45 per cent points (pp) from 99.99% to 96.54% when serum dilution 1:2 was used as a cut-off. The corresponding flock specificity dropped from 99.4% to 34.1%. At a serum dilution of 1:8, the diagnostic and flock specificty were 100% for the sera of non-vaccinated flocks and flocks vaccinated with SE live vaccines. For flocks vaccinated with SE inactivated vaccine at a serum dilution of 1:8, the diagnostic specificity was 99.41% while flock specificity was 86.4%. When the 1:16 dilution was used as a cut-off, the specificity increased to 100%. A higher serum dilution as a cut-off for the RPA test is also recommended and used for the Mycoplasma gallisepticum (MG) and Mycoplasma synoviae (MS) RPA test. The studies showed that agglutination at a dilution of 1:8 can be regarded as specific positive as agglutination at a dilution lower than 1:8 occurred due to cross-reactions with mycoplasma species other than MG and MS or other nonspecific factors (Feberwee et al., Citation2005; OIE, Citation2018b; Feberwee et al., Citation2020).
As for MG and MS (OIE, Citation2018b), besides the dilution of choice as a cut-off of the test, criteria for the percentage of suspect and positive samples might help with the interpretation of the RPA SP/SG test results. This could also be included for interpretation of SP/SG RPA resulst as in none of the SE/ST vaccinated flocks as in none of the SE/ST-vaccinated flocks were more than 12.5% of the sera positive at the 1:2 dilution. However, experimentally infected birds at 2 and 6 weeks after infection with SP showed a high percentage (50–80%) of reactors in the RPA SP/SG test (Williams & Whittemore, Citation1979; Gast, Citation1997). In our study, 1:2 diluted positive sera were detected in 29 of 44 flocks vaccinated with inactivated SE/ST vaccines. In these 29 flocks, the average number of positive sera was 2.8. Resampling and retesting after 4 weeks can be used to confirm the absence of antibodies against SP/SG, as, in those cases, the number of positive samples decreases (personal communication, data not shown).
In conclusion, the diagnostic specificity and the flock specificity of the RPA SP/SG test were high and were not noticeably influenced by using live SE/ST vaccines. The use of inactivated SE/ST vaccine resulted in a drop of 3.45 pp in diagnostic specificity when the 1:2 serum dilution was used as a cut-off. This drop of 3.45 pp resulted in a significant drop in flock specificity from 96.54% to 34.1%. Using the 1:8 serum dilution for these sera as a cut-off, the diagnostic specificity was 99.41% showing a flock specificity of 86.4%. The 1:16 serum dilution increased the specificity to 100%. These results imply that using the RPA SP/SG test as a monitoring tool in chickens vaccinated with live SE/ST vaccine is not hampered by the loss of specificity. When using the RPA SG/SP test in hens vaccinated with inactivated SE/ST vaccine, a low percentage of reactions can be expected in serum dilutions 1:8 or higher. A positive result should be interpreted keeping the lower flock specificity in mind and might require retesting of the flock.
Ethical statement
The study was performed on data acquired from samples sent to Royal GD for the Dutch National monitoring programme. No permission from an Animal Welfare Body was needed. The data are presented anonymously to ensure the privacy of the farmers.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Barrow, P.A. & Freitas Neto, O.C. (2011). Pullorum disease and fowl typhoid–new thoughts on old diseases: a review. Avian Pathology, 40, 1–13.
- Christensen, J.P., Olsen, J.E. & Bisgaard, M. (1993). Ribotypes of Salmonella enterica serovar Gallinarum biovars gallinarum and pullorum. Avian Pathology, 22, 725–738.
- Commission, E. (2019). Commission delegated regulation (EU) 2019/2035 of 28 June 2019 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as regards rules for establishments keeping terrestrial animals and hatcheries, and the traceability of certain kept terrestrial animals and hatching eggs.
- Feberwee, A., Dijkman, R., Wiegel, J., Ter Veen, C., Bataille, H., Bouwstra, R. & de Wit, S. (2020). Rate of false positive reactions in 11 M. gallisepticum and M. synoviae serological tests in samples obtained from SPF birds inoculated with heterologous mycoplasma species. Avian Pathology, 1–6.
- Feberwee, A., Mekkes, D.R., de Wit, J.J., Hartman, E.G. & Pijpers, A. (2005). Comparison of culture, PCR, and different serologic tests for detection of Mycoplasma gallisepticum and Mycoplasma synoviae infections. Avian Diseases, 49, 260–268.
- Gast, R.K. & Beard, C.W. (1990). Serological detection of experimental Salmonella enteritidis infections in laying hens. Avian Diseases, 34, 721–728.
- Gast, R.K. (1997). Detecting infections of chickens with recent Salmonella Pullorum isolates using standard serological methods. Poultry Science, 76, 17–23.
- Kamp, H.J.G. (2014). Regeling van de Minister van Economische Zaken van 10 december 2014, nr WJZ/14139630, houdende wijziging van diverse regelingen in verband met de opheffing van bedrijfslichamen en de overname van taken. [Regulation of the Ministery of Economical Affairs of December 10th 2014, no WHZ/14139630, concerning the changes of various regulations in regards to the abolishment of the product boards and taking over of tasks.] ‘s Gravenhage: Staatscourant.
- OIE. (2018a). Chapter 2.3.11 Fowl Typoid and Pullorum Disease.
- OIE. (2018b). Chapter 3.3.5. Avian Mycoplasmosis (Mycoplasma gallisepticum and Mycoplasma synoviae).
- Proux, K., Humbert, F., Jouy, E., Houdayer, C., Lalande, F., Oger, A. & Salvat, G. (2002). Improvements required for the detection of Salmonella Pullorum and Gallinarum. Canadian Journal of Veterinary Research, 66, 151–157.
- Schat, K.A., Nagaraja, K.V. & Saif, Y.M. (2021). Pullorum disease: evolution of the eradication strategy. Avian Diseases, 65, 227–236.
- Shivaprasad, H.L. (2000). Fowl typhoid and pullorum disease. Revue Scientifique et Technique, 19, 405–424.
- Thrusfield, M. (2007). Diagnostic testing veterinary epidemiology. 3rd ed. (pp. 317–330). Oxford: Blackwell.
- Waltman, W.D. & Horne, A.M. (1993). Isolation of Salmonella from chickens reacting in the pullorum-typhoid agglutination test. Avian Diseases, 37, 805–810.
- Wigley, P. (2017). Salmonella enterica serovar Gallinarum: addressing fundamental questions in bacteriology sixty years on from the 9R vaccine. Avian Pathology, 46, 119–124.
- Wigley, P., Berchieri, A., Jr., Page, K.L., Smith, A.L. & Barrow, P.A. (2001). Salmonella enterica serovar Pullorum persists in splenic macrophages and in the reproductive tract during persistent, disease-free carriage in chickens. Infection and Immunology, 69, 7873–7879.
- Williams, J.E. & Whittemore, A.D. (1979). Serological response of chickens to Salmonella thompson and Salmonella Pullorum infections. Journal of Clinical Microbiology, 9, 108–114.