ABSTRACT
Chicken astrovirus (CAstV) has for over a decade been associated with runting stunting syndrome, severe kidney disease and visceral gout, and white chick syndrome. However, knowledge of the molecular characteristics and pathogenicity of the virus in day-old specific pathogen-free (SPF) chicks is scarce. This study focused on the characterization of near-complete genome of three Malaysian CAstV isolates following virus propagation in SPF embryonated chicken eggs and pathogenicity in day-old SPF chicks. The three isolates demonstrated unique features including a point mutation in their intergenic regions and an additional stem-loop II-like motif (s2m) in ORF-2. Pairwise sequence comparison and phylogenetic analysis of the ORF-2 amino acid sequence of the three isolates revealed an identity share of 86–91% with group B CAstVs while forming a new subgroup in addition to the known four subgroups (Bi, Bii, Biii and Biv) that exhibit high identity of between 95% and 100% within the subgroups. In the pathogenicity study, birds in the infected and exposed sentinel groups exhibited lethargy and diarrhoea 3 days post-inoculation (dpi) that declined by 6 dpi, and 20% growth retardation by 9 dpi. Mild lymphocytic aggregates in the duodenum, tubular degeneration and interstitial nephritis were observed in the intestines and kidneys, respectively, in both groups. In addition, the mean virus copy numbers of the cloacal swabs were log10 13.23 at 3 dpi and log10 9.04 at 6 dpi for the infected and exposed sentinels, respectively. The study suggests that the Malaysian isolates should be assigned to a new subgroup, Bv within group B CAstV.
A single run of NGS protocol is capable of generating a near-complete genome sequence of CAstV.
The Malaysian CAstV isolates cluster together and exhibit 86–91% identity with published group B CAstVs.
The Malaysian CAstVs encode an additional stem-loop II-like motif (s2m) in ORF-2.
The isolates are pathogenic to day-old SPF chicks with lesions mainly in the intestine and kidneys.
RESEARCH HIGHLIGHTS
Introduction
Chicken astrovirus (CAstV) is an emerging poultry virus affecting chickens, especially the broiler breeds, across the globe (Baxendale & Mebatsion, Citation2004; Smyth, Citation2017). The virus belongs to the avastrovirus genus of the Astroviridae family and has a genomic structure and other characteristics similar to all known astroviruses (Baxendale & Mebatsion, Citation2004; Kang et al., Citation2012b). Structurally, the virus has a linear, short, positive-sense, single-stranded RNA genome of approximately 7.5 kb. The viral genome codes three major serially arranged open-reading frames (ORFs); two non-structural proteins including a trypsin-like serine protease and a viral protein-linked genome (VPg) in ORF-1a, and an RNA-dependent RNA polymerase (RdRp) in ORF-1b. The third ORF (ORF-2) codes for the capsid protein, a structural protein that exhibits a high variability in its C-terminal region, and equally regulates interactions between the virus, host cells, and antibodies (Arias & Dubois, Citation2017). In addition, the capsid protein demarcates the virus, based on antigenicity and amino acid genetic distances, into two major groups (A and B) with further subgrouping into three subgroups for Group A (Ai, Aii and Aiii), and four subgroups for Group B (Bi, Bii, Biii and Biv) (Smyth et al., Citation2012; Smyth, Citation2017).
Over the years, reports have shown a strong correlation between CAstV and disease conditions or syndromes in young broiler chickens. Presently, the three significant syndromes of CAstV include runting stunting syndrome (RSS), kidney disease and visceral gout, and white chick syndrome (WCS) or “white chicks” hatchery disease (Bulbule et al., Citation2013; Kang et al., Citation2018; Palomino-Tapia et al., Citation2020). In RSS, young broiler chickens exhibit growth-related problems and uneven flock performance as a result of maldigestion, malabsorption and enteritis (Baxendale & Mebatsion, Citation2004; de Wit et al., Citation2011; Kang et al., Citation2018), leading to a high culling rate and reduction in turnover. The condition is mostly characterized by intestinal cysts and villus atrophy, microscopically (Kang et al., Citation2012a; Devaney et al., Citation2016; Smyth, Citation2017). Depending on the CAstV strain, the pathogenicity of the virus in SPF chickens can be mild as in the case of 612 strain of Group A, or severe as in the case of FP3 strain of Group B (Smyth et al., Citation2007). On the other hand, kidney disease and visceral gout is typified by a mortality rate of up to 40% in young broiler chicks (Bulbule et al., Citation2013). The strains responsible for this condition are mostly of Indian origin and belong to the subgroup Biii CAstV (Bulbule et al., Citation2013). In addition, broiler and SPF chicks inoculated with the Indian CAstV kidney homogenates had 67.5% and 100% mortality, respectively. Lesions were extensive urate deposits in the kidneys and ureters, and severe visceral gout (Bulbule et al., Citation2013).
WCS, caused by CAstV subgroup Biv, has been reported to cause a temporal but significant decline in egg production, hatchability drops in susceptible breeders, and a mid to late death-in-shell embryo. In young hatched broiler chicks, the condition is characterized by small-size, weakness, short shanks, white plumage and distended abdomen; these chicks often do not survive the first few days of life (Sajewicz-Krukowska et al., Citation2016; Smyth, Citation2017; Long et al., Citation2017; David Citation2020). In addition to lesions of RSS and kidney disease, broilers with WCS present mottled to bright green livers and subcutaneous oedema at post-mortem examination (Smyth, Citation2017; Palomino-Tapia et al., Citation2020). Other notable lesions are hepatocellular vacuolar degeneration and accumulation of glycogen with interstitial nephritis (David Citation2020). The strains responsible for this condition are of Group B origin, subgroup Biv to be specific, and have been reported in Canada, Brazil and most of the Scandinavian countries (Smyth et al., Citation2013; Nuñez et al., Citation2020; Palomino-Tapia et al., Citation2020).
Recently, Raji et al. (Citation2019), reported the detection of CAstV in broiler chickens in Malaysia based on a reverse transcriptase polymerase chain reaction (RT–PCR) protocol. Additionally, a serological screening of broiler breeder sera using CAstV Group B ELISA confirmed widespread infection amongst broiler breeder flocks in Peninsular Malaysia (Raji et al., unpublished). However, little is known about the molecular characteristics and pathogenicity of the Malaysian CAstV isolates. Here we report the sequence characteristics of three Malaysian CAstVs (IBS503/2017, UPM1019/2018 and IBS543/2017) and the pathogenicity of one of the isolates (UPM1019/2018) in 1-day-old SPF chickens.
Materials and methods
Ethics statement
The chicken trial procedure was approved by the Institutional Animal Care and Use Committee (IACUC) Faculty of Veterinary Medicine, Universiti Putra Malaysia under reference number UPM/IACUC/AUP-R021/2019. The trial was carried out strictly in accordance with the approved recommendations.
Viruses used in the study
Three Malaysia CAstV isolates, IBS503/2017, IBS543/2017 and UPM1019/2018, that were successfully isolated and propagated after four passages in specific-pathogen-free (SPF) embryonated chicken eggs (ECE), were used in the study. These three viruses were isolated from tissue samples of broiler chicken flocks from three different states in Peninsular Malaysia. The flocks exhibited varying clinical signs including uneven growth and post-mortem lesions such as swollen kidneys with urate deposits, and visceral gout. An RT-PCR test, as described by Smyth et al. (Citation2009), was used to confirm the presence of CAstV in the tissue samples. Similarly, based on PCR and RT–PCR, the samples were tested against avian rotavirus, chicken parvovirus, avian reovirus, avian nephritis virus, infectious bronchitis virus, Newcastle disease virus and fowl adenovirus as described by Nunez et al. (Citation2015).
Primer design, viral RNA extraction and amplification
For the identification of the most similar genome sequences of CAstV, the Basic Local Alignment Search Tool (BLAST) was employed. Three complete genome sequences of CAstV (GA2011, CkP5 and CC_CkAstV) showing the highest similarities were downloaded from the NCBI database. Using a multiple sequence alignment tool on the three downloaded CAstV genomes, three sets of primers spanning the entire genome of the virus were designed and validated using Primer-BLAST software tools. The first two fragments (frgmnts-I and II) cover 3.4 and 1.6 kb spanning from ORF-1a to ORF-1b and to the first few nucleotide bases in ORF-2 (). The third primer set, situated at the 3’-end of ORF-1b to the near end of ORF-2, is a published primer set designed by Smyth et al. (Citation2009).
Table 1. Primer sets used in the amplification of the whole genome.
After propagation of the three isolates in ECE, total RNA was extracted from enteric and liver homogenate from the SPF embryos using TRIzol™ Reagent (Invitrogen, California, USA) according to the manufacturer’s instructions after three cycles of freeze-thawing at −80°C and room temperature.
The three designed primer pairs were used to amplify viral RNA using SuperScript IV One-Step RT–PCR System with the 2X Platinum™ SuperFi™ DNA Polymerase kit (Invitrogen). Briefly, 2.5 µl of the extracted viral RNA was used as a template for a one-step RT–PCR. Each reaction mix contained 1 µM of each primer, 1× reaction buffer, enzyme mix 1 µl, RNA 2.5 µl and autoclaved double-distilled H2O to 50 µl. Bio-Rad C1000 Touch Thermal Cycler (Bio-Rad, California, USA) was used for the amplification, commencing with an RT step at 50°C for 10 min, then an initial denaturation of 98°C for 2 min. This was followed by 40 cycles of denaturation at 98°C for 10 s, using an annealing temperature for 10 s at 66.5°C for frgmnt_I, 67.9°C for frgmnt_II and at 67.7°C for frgmnt_III, and an extension of 72°C for 2, 1 and 1.5 min, respectively. The final extension was set at 72°C for 7 min. The PCR products were electrophoresed using 1% agarose gel and the gel was then viewed using a Bio-Rad Gel Doc XR (Bio-Rad). The PCR products were gel-purified using ReliaPrep™ DNA clean-up and concentration system (Promega, Madison, WI, USA) and stored at −20°C until the downstream application.
Library preparation and sequencing
The purified PCR fragments originating from the isolates were quantified using Qubit™ dsDNA HS Assay Kit (Invitrogen, Waltham, MA, USA) and normalized with nuclease-free water to 30 µl. A total of 350 ng of DNA was subjected to library preparation using Nextera DNA Flex Library Prep Kit (Illumina Inc., San Diego, CA, USA) based on the manufacturer’s protocol. Briefly, the genomic (g)DNA was tagmented using bead-linked transposomes in a single reaction step. The tagmented DNA was then cleaned up and used as a template in a 50 μl PCR with five cycles and processed as detailed in the Illumina DNA Prep. Furthermore, a double-sided bead was used to purify the amplified library for use on the Illumina MiSeq platform. A post library quality check was then carried out to quantify the DNA fragment size and library concentration using 2100 Bioanalyzer (Agilent Technologies, CA, USA). Subsequently, DNA libraries were normalized to 4 nM and were pooled in equal volumes based on the libraries unique indexes. Pooled libraries were then denatured and diluted with 0.2N NaOH, and pre-chilled hybridization buffer (HT1) to produce a denatured 12 pM library in 1 mM NaOH solution. Finally, the library was sequenced using MiSeq (Illumina Inc.) with a read length of 2 × 151 bp.
Data analyses
Generated raw reads from the sequencing were automatically separated into individual NGS libraries originating from each isolate of the CAstV based on the unique combination of indexes tagged using the MiSeq reporter software (Illumina Inc.). Overlapping paired-end reads of each library within a FastQ file were filtered on a Phred quality (Q) score (Q33) and imported into BBDuk (BBTools version 36) http://jgi.doe.gov/data-and-tools/bbtols/ for the trimming of adapters and de novo assembly of the paired-end reads to contigs with a targeted depth of 150x. Using the NCBI database, the contigs were subjected to BLAST analysis (BLASTN), and the reference genomes were detected based on the sequence highest and lowest, identity and E-value, respectively. Contigs with low coverage were excluded, and partial but overlapping contigs were combined where necessary. The genome sequences were further analysed for the prediction of putative open reading frames (ORF) by the ORF finder tool of the NCBI (https://www.ncbi.nlm.nih.gov/orffinder/).
Sequence and phylogenetic analyses
Genome sequences of the three Malaysian isolates and 24 other CAstV genomes available in GenBank were aligned using Clustal W, and phylogenetic trees were built using MEGA-X. A suitable model for phylogenetic analysis was selected based on a model with the lowest Bayesian Information Criterion value (Kumar et al., Citation2018). Rapid bootstrapping and searching for the best-scoring maximum likelihood (ML) tree with 1000 bootstrap replicates was applied. A similar phylogenetic analysis was carried out on ORF-1a and ORF-1b. The phylogenetic analysis of the capsid gene (ORF-2) of the three isolates, together with those of 42 published CAstV isolates (Supplementary Table 1) from different geographic locations, was conducted based on amino acid sequences. Identity analysis was determined using the BioEdit sequence identity matrix. The P-distances (P-dist) amongst and within CAstV lineages were calculated using MEGA-X PASC software. The classification of the CAstV isolates was based on a phylogenetic tree generated by MEGA-X ML on a 1000 bootstrap replicate of the genome sequences, complete and C-terminal end amino acid sequences of the capsid gene.
Pathogenicity evaluation
Determination of embryo infective dose (EID50)
Virus titre in SPF-ECEs for the homogenates of liver and intestines of the fourth passaged UPM1019/2019 was determined by chicken embryo infectivity assay. A serial 10-fold dilution of the suspension of isolate UPM1019/2019 (0.2 ml/embryo) was inoculated into the yolk sac of 5-day-old SPF-ECEs. The eggs were then incubated at 37°C for 10 days under 50% humidity. Embryo mortality within 24 h was considered non-specific. Embryos that died within 2–10 days post-inoculation (dpi) and those that survived up to 10 dpi were subjected to necropsy examination for specific lesions. The infectivity titre was calculated as EID50 based on the Reed-Muench method and was expressed as the median embryo infective dose (EID50)/ml.
Pathogenicity of isolate UPM1019/2018
A chicken pathogenicity trial was carried out in an attempt to reproduce the clinical manifestations observed in the field, virus re-isolation and possible spread of the virus to exposed sentinel chickens. Briefly, 45 1-day-old SPF chickens of both sexes were randomly allotted to three groups: infected (group 1) (n=15), exposed sentinels (group 2) (n=15) and control (group 3) (n=15). The birds were fed and provided drinking water ad libitum. The infected group were transferred to an experimental chicken infectious unit and each chicken was orally administered with 0.2 ml of UPM1019/2018 virus 105 EID50. Exposed sentinel chickens were introduced 24 h after the inoculation of group 1, while group 3 were administered 0.2 ml of PBS orally. The birds were monitored every day for clinical manifestations throughout the study which lasted for 15 days from the day of inoculation. On 3, 6, 9, 12 and 15 dpi, three chickens each from groups 1, 2 and 3 were weighed individually, cloacal swabs and blood samples were taken, and the chickens were euthanized for post-mortem examination. The weight of each individual chicken was used to calculate the percentage growth retardation as described by McNeilly et al. (Citation1994). Cloacal swabs were placed in sterile PBS for RNA extraction for viral load quantification. Sera were harvested from the blood samples for antibody detection at different time-points. Tissue samples from the duodenum and kidney were examined at necropsy and sections were taken for histopathologic investigations and virus detection by RT–PCR. The tissues were divided into two; a segment placed in a tube containing 10% neutral buffered formalin (Sigma-Aldrich, Missouri, US, Singapore) for fixation before further tissue processing and evaluation under the microscope using haematoxylin and eosin (H & E staining); the second segment of the tissue was placed in sterile PBS, homogenized, and stored in −80°C ultra-low freezer for RT–PCR.
CAstV-specific antibody determination
A Chicken Astrovirus Group B Antibody test kit-based enzyme-linked immunosorbent assay (ELISA) (BioChek, Reeuwijk, Netherlands) was employed to determine antibody titre from the serum of the SPF birds based on the manufacturer's protocol. The serum samples were collected in line with the FAO protocol and stored at 4°C until analysis. Briefly, the blood was left in the syringe for 2 h at room temperature. The serum was then centrifuged for 30 s in a microcentrifuge at 3000×g to remove red blood cells by pelleting. The serum was then transferred into a second tube, labelled, and stored at 4°C until analysis.
Viral load determination using RT-qPCR
The real-time RT–PCR assay was performed as described by Smyth et al. (Citation2010) with modification. Briefly, the reaction was performed using a TaqMan based quantitative RT–PCR (EXPRESS One-Step SuperScript® qRT-PCR kit) (Invitrogen), forward primer, 5’-GCYGCTGCTGAAGAWATACAG-3’, reverse primer 5’-CATCCCTCTACCAGATTTTCTGAAA-3’ and a probe 5’-(6-FAM)-CAGAAGTCGGGCCC-(MGB)-3’ with the cycling programme set at 50°C for 15 min hold (cDNA synthesis) 95°C for 2 min hold, and 40 cycles of 95°C for 15 s, 60°C for 1 min. The reaction run was conducted on a CFX96 Real-Time System (Bio-Rad).
Statistical analysis
The data obtained for the viral shedding from the real-time PCR were analysed using Minitab 16.2.4 software (Minitab, Coventry, UK). Differences between means of the virus load at different time-points were analysed using ANOVA with Tukey post-hoc test (P < 0.05). All quantitative data were expressed as mean ± standard deviation.
Results
Amplification with the three designed CAstV primers
The RNA of the three isolates extracted from a pool of intestines and liver homogenates of SPF-ECE was amplified, and displayed three DNA fragments on agarose gel electrophoresis of approximately 3.4 kb (frgmnt_I), 1.6 kb (frgmnt_II) and 2.2 kb (frgmnt_III) which were visible in the three CAstV (Supplementary Figure 1).
Genome sequence analyses of the three isolates
In this study, we used a protocol that generated a consensus level of 98% coverage of the CAstV genome. The generated output by the Illumina MiSeq NGS platform was a total read of 89,749, 90,753 and 93,423 for IBS543/2017, UPM1019/2018 and IBS503/2017, respectively, at an average length of 151 bp paired ends. After trimming and removing host-specific reads, low quality and adapter reads, a total of 80,817 reads were used for the assembly (). The generated and edited consensus sequence lengths of the isolates were 7424 nucleotide (nt) for IBS503/2017, 7397 nt for UPM1019/2018, and 7379 nt for IBS543/2017. The three Malaysian CAstV isolate sequences are accessible under the following accession number in GenBank: MT491730 for IBS503/2017, MT491731 for UPM1019/2018, and MT49132 for IBS543/2017.
Figure 1. Genomic organization of the Malaysian CAstV isolates, IBS503/2017, UPM1019/2018 and IBS543/2017. TM helix, transmembrane; NLS, nuclear localization signal; RFS, ribosomal frameshift; s2m, stem-loop II-like motif; VPg, viral protein genome linked; RdRp, RNA-dependent RNA polymerase and ORF, open reading frame.
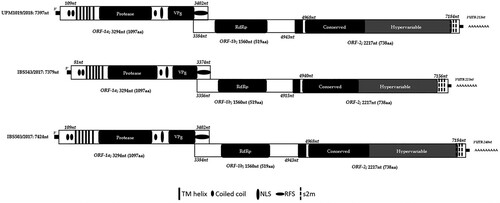
Genome sequence analysis of the three isolates exhibited a genomic organization similar to other astroviruses where each of the genomes consisted of three open reading frames (ORF-1a, ORF-1b and ORF-2) (). The genomes were observed to have both ORF-1b and ORF-2 in the +1-reading frame typical of CAstVs. Due to the deficiency of the sequencing reaction, analysis of the three isolates revealed a missing 21 nt of the untranslated regions (UTR) and first few nt at the 5’-end when compared to other CAstV complete genomes. Meanwhile, the 3’-end UTRs were 239 (7185-7424), 212 (7185-7397) and 223 (7156-7379) nt long for IBS503/2017, UPM1019/2018 and IBS543/2017, respectively (). shows features of the three isolates and a comparison between them and other published CAstV genomes. A protein family (Pfam) (http://pfam.xfam.org) analysis revealed that ORF-1a of the three Malaysian isolates encodes a trypsin-like serine protease, and with a region that has homology to a peptidase domain (). In addition, the amino acid motifs contained a bipartite nuclear localization signal (NLS) and six possible transmembrane domains (). Also encoded in the ORF-1a, is a viral protein genome-linked (VPg) at the 5’-end possessing a TEEEY-like residue (SEEEY) that is associated with viral infection in human astroviruses (Supplementary Figure 2). An overlap of 18 nt was observed between ORF-1a and ORF-1b with a conserved heptamer (AAAAAAC) positioned within the ribosomal frameshift signal (RFS) at positions 3393 to 3399 nt for IBS503/2017 and UPM1019/2018, and 3365 to 3371 nt for IBS543/2017 (Supplementary Figure 2). Equally, RNA-fold analysis (http://rfam.xfam.org) predicted the presence of a stem-loop downstream of the heptameric sequence.
Table 2. Genome structure of the three Malaysian isolates obtained following next-generation sequencing using Illumina platform.
As with most astroviruses, ORF-1b of the three isolates was observed to start with a start codon (ATG) and was positioned upstream of the heptamer and ORF-1a stop codon (TAG) (Supplementary Figure 2). Analysis of the ORF-1b revealed an RNA-dependent RNA polymerase (RdRp) domain based on Pfam analysis ().
The comparison of the aligned amino acid sequences of the three Malaysian isolates revealed an aa change in ORF-1a (P706L) and two aa change in ORF-2 (Y23H and V493I). These changes were also detected in the reference GA2011 aa sequences (Supplementary Figure 3). Thus, these variations lead to an amino acid identity of 99.0–99.1% and 99.6–100% between the ORF-1a and ORF-1b of the three isolates, respectively.
A 24 nt “spacer sequence”, also referred to as intergenic region, which is typical of some astroviruses and present in CAstV, was noticed between the end of ORF-1b and the start of ORF-2. The region also harbours a highly conserved pentamer (CCGAA) as observed in other strains of the avian astroviruses (Supplementary Figure 2). Downstream of the pentamer with four nucleotides was a point mutation from G to T in the three isolates relative to all the CAstV strains presently characterized.
Similar to all known astroviruses, ORF-2 of the three isolates encode a structural polyprotein referred to as the capsid protein precursor and share a 100% identity. With a length of 2217 nt and coding 738 aa, Rfam (http://rfam.xfam.org) evaluation of the ORF-2 showed the existence of three coronavirus like s2m comparable to the s2m motifs belonging to some members of the family s2m (Accession number: RF00164) at 3’-end of the three isolates. The s2m nt sequences were 43 nt in length with a 56 nt space between them similar to all known CAstV. Unlike previously characterized CAstV which have two s2m motif, the three Malaysian isolates were predicted to have a third s2m motif at the 3’-end of their genomes at the following positions, 7161 to 7203 nt, 7258–7299 nt and 7354–7395 nt for IBS503/2017 and UPM1019/2018 while positions 7133–7175 nt, 7230 to 7271 nt and 7326 to 7367 nt were observed in IBS543/2017 ().
Based on the complete nucleotide sequence of the genomes, pairwise comparison of the three isolates revealed 73–87% identity with 24 published CAstV genomes (). The highest genome sequence identity of 87% was observed between the three Malaysian isolates and two US strains (CC_CkAstV and CkP5) and 11 Canadian strains (CA-AB/14-1235a, CA-AB/14-1235b, CA-AB/14-1235c, CA-AB/14-1235d, CA-AB/15-1262a, CA-AB/15-1262b, CA-AB/15-1262c, CA-AB/15-1262d, CA-AB/17-0773a, CA-AB/17-0823, CA-SK/18-0942). There was 73% identity between the three Malaysian isolates and the only characterized CAstV group A genome (PL/G059/2014) of Poland. Analysis of ORF-1a and ORF-1b nt and aa sequences revealed an identity share of 74–89% nt and 84–94%, and 77–95% and 86–98%, respectively ().
Table 3. Comparisons of the genome, ORF-1a and ORF-1b nucleotide and amino acid sequences of the three Malaysian isolates with published CAstV isolates.
However, based on capsid protein sequences encoded in ORF-2, IBS503/2017, UPM1019/2018, and IBS543/2018 expressed a homology with group B at 86–91% sequence identity (). Accordingly, the three isolates shared the following identities with members of subgroup B; 86% with GA2011 and VF08-3 both of Bii; 87% with 05V150/152/154 of Bii and PDRC/526/North_Zone of Biii; 88% with HBLP717-1/2018 of Bi; 89% with CHN/2017/NJ1701, GDYHTJ718-6/2018, CHN/2018/CZ1801, FP3 of Bi, and ANAND/2016 and PDRC/573/West_Zone of Biii; 90% with 11522 and 1010 of Bi, and CC_CkAstV, USP748-12, USP748-16, USP748-18, USP748-20 and all the 14 Canadian strains of Biv; and a 91% identity share with CkP5 of Biv (). A low homology (37–38% sequence identity) was equally observed between the three isolates and group A CAstVs (). Supplementary Table 2 describes the intra-subgroup percentage identity share of 97–100% within Bi, 95–96% within Bii, 97–98% within Biii, and 97–100% within Biv. The capsid gene amino acid sequences mean genetic distances (P-dist) with other CAstV strains belonging to group B are as follows: 0.09 with the CkP5; 0.10 with 11522, 1010, CC_CkAstV, all the 14 Canadian strains and four of the Brazilian strains; 0.11 with CHN/2017/NJ1701, GDYHTJ718-6/2018, CHN/2018/CZ1801, FP3, PDRC/573/West_Zone, PDRC/526/North Zone, ANAND/2016; 0.12 with HBLP717-1/2018; 0.13 with VF08-3 and 05V150/152/154; 0.14 with GA2011 ().
Table 4. Comparisons of the capsid protein (ORF-2) nucleotide and amino acid sequences of the three Malaysian isolates with published CAstV isolates.
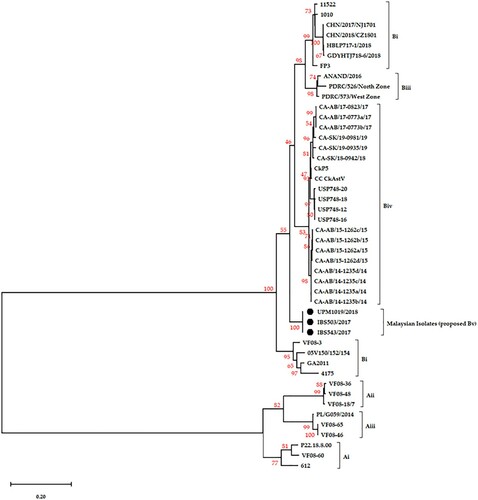
Phylogenetic analysis of the three isolates
Phylogenetic analysis of the complete nucleotide sequences of the three isolates, nucleotide and amino acid sequences of the ORFs was carried out to investigate the relationship of the isolates with other CAstVs. Based on the complete nucleotide sequences, the three Malaysian isolates formed a new subgroup on the constructed phylogenetic tree (). A similar topology was observed when ORF-1a and ORF-1b phylogenetic trees were constructed (not shown). On the other hand, the capsid protein sequences presented a topology of two distinct groups (Group A and Group B) in line with the earlier described identity shares (). The three isolates clustered together within Group B CAstV and appear to have diverged from a common ancestor of the Bi, Biii and Biv strains as compared to Bii (). Their complete capsid protein sequences placed them independently sharing a common ancestral root with members of subgroup Bi of UK (FP3, 1010 and 11522) and Chinese (CHN/2017/NJ1701, HBLP717-1/2018, GDYHTJ718-6/2018 and CHN/2018/C1801) origins; Biii comprising of all Indian strains (ANAND/2016, PDRC/526/North_Zone and PDR/C573/West_Zone); and Biv which is made-up of two US strains (CC_CkAstV and CkP5) and 14 Canadian strains (). In addition, when the analysis was performed using the C-terminal end of the capsid protein sequence (416–738 aa residues), a similar topology tree was obtained (not shown).
Figure 2. The phylogenetic relationship, based on the genome of the three Malaysian isolates (dark circles) and other published CAstV genomes. The analysis is based on a complete nucleotide sequence, and the tree was built using the ML method with bootstrap replicates of 1000 in MEGA-X. The bootstrap values are indicated on the tree branches.
Pathogenicity of the Malaysian CAstV in SPF chickens
CAstV used in the study
The isolate UPM1019/2018 used in the pathogenicity study was confirmed positive for CAstV only and negative for avian rotavirus, chicken parvovirus, avian reovirus, avian nephritis virus, infectious bronchitis virus, Newcastle disease virus and fowl adenovirus by PCR or RT–PCR as described earlier.
Clinical signs
Chickens in both infected (group 1) and exposed sentinel (group 2) presented clinical signs that included somnolence, lethargy, depression, ruffled feathers, and diarrhoea at 3 dpi. Although a decline in the clinical manifestations was noticed on 6 dpi, one chicken in each of the infected and exposed sentinel groups was observed to be depressed on 9 dpi. Retardation in growth compared to the control group (group 3) was seen in groups 1 and 2. At 3, 6, 9, 12 and 15 dpi, group 1 exhibited 2.71%, 15%, 20%, 12.94%, and 17.58% growth retardation, respectively, while group 2 showed 0.65%, 15%, 19.9%, 13.%3 and 18.63% retardation, respectively (Supplementary Table 3).
Additionally, a one-way analysis of variance (ANOVA) of the bodyweight gains of the two challenged groups (1 and 2) showed statistically significant differences beginning from 3 dpi, (F (4, 10) = 47.43, P = 0.000) for the infected group and (F (4, 10) = 31.49, P = 0.000) for the exposed sentinel group. A Tukey post-hoc test showed a statistically significant difference between the groups, especially when infected and exposed sentinel groups were compared with the control group. Similarly, the growth retardation rate was very noticeable in both the infected and exposed sentinel (20%) groups at 9 dpi when compared to the control group (Supplementary Table 3).
Gross pathology and histopathology
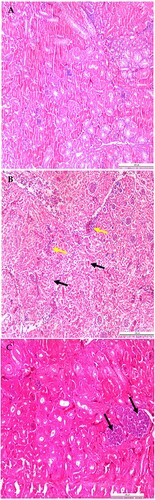
At necropsy, chickens in both the infected and exposed sentinel groups were cachexic with a visible, prominent keel bone, air-filled empty crop with distended intestines at 3 dpi. Swollen kidneys, enlarged ureters with extensive urate deposits, visceral gout, heart covered with white chalky urate deposits, and thin white membranous urate layer covering the liver and coelomic cavity were observed in one out of three chickens euthanized in group 1 on 6 dpi, and in one out of three chickens euthanized in groups 1 and 2 on 9 dpi (Supplementary Figure 4A) (Supplementary Table 4). Histopathologically, cross-sectional evaluation of the duodenum on 6 dpi revealed a cyst-like formation in the crypts of Lieberkühn (cystic crypt), and mild cellular debris was seen in the crypt lumen ((B,C)). Similarly, histopathology of the kidney samples from two out of three infected chickens on 6 and 9 dpi, and one kidney sample out of three kidney samples from group 2 showed urate deposits, lymphocytic infiltrations, and interstitial nephritis (). Generally, microscopic lesions observed in the intestine were mild. Tissue samples from the control group were grossly and histologically normal.
Figure 4. Gross and histologic lesions observed in chickens infected with CAstV isolate UPM1019/2018 at 6 and 9 dpi. (A) Chalky white urate deposit on the heart (black arrow) with visceral gout thin membranous covering (red arrow). (B) Cyst-like formation (black arrow) in the crypt of Lieberkühn. (C) Cellular like debris in the crypt with adjacent degenerate cells (circle). Stained with H & E; Scale bar C = 100 µm; D = 50 µm.
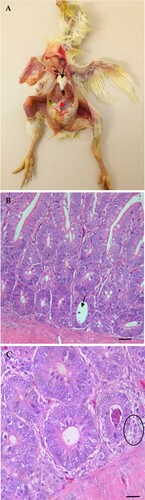
Figure 5. Microscopic lesions in kidneys of CAstV- infected and exposed sentinel chickens at 6 and 9 dpi. (A) Normal kidney control. (B) Urate deposits (black arrows) and interstitial inflammatory infiltrates (yellow arrows) with compressed glomeruli. (C) Tubular necrosis with the accumulation of cellular debris evolving into a tophus (black arrows), urate deposits, degeneration and necrosis of tubules. Stained with H & E; scale bar A, B and C = 200 μm.
Antibody detection
Both CAstV-infected and exposed sentinel groups elicited specific antibody titres against CAstV. On the other hand, birds in the control group administered PBS orally were negative throughout the study. The peak positive (1570.33 ± 807.57) estimated mean time in the infected group was observed on 12 dpi with a subsequent decline (1110.33 ± 819.96) on 15 dpi, while the exposed sentinel group was positive on 15 dpi at an estimated mean time of 1387.67 ± 141.72 (Supplementary Figure 5). Consequently, results from the two-way ANOVA indicated that the means of the antibodies between the CAstV infected and exposed sentinel groups did not significantly differ (P ≥ 0.05).
Viral load quantitation
Swab samples collected from the cloaca of the CAstV-infected and exposed sentinel SPF chickens on 3, 6, 9, 12 and 15 dpi were processed and analysed for virus shedding. Based on the standard curve generated, virus copy numbers were deduced by utilizing the formula (x = y − c/m); where x is the virus copy number, c is the intercept on Y-axis, m is the slope of the standard curve and y is the cq value. At each time-point, the generated viral copy number was calculated as log10, and one-way ANOVA and Tukey post-hoc tests were used for the analyses of the mean significant difference between the groups. Chicken astrovirus was detected at all the time-points at varying quantities in the two groups. However, in the CAstV infected group, the mean viral load (13.23 ± 0.05) was significantly high on 3 dpi compared to other days and the viral load of exposed sentinels (). The quantity of shedding continued to decline in both groups with a statistically significant (P < 0.05) difference in mean viral shedding between the groups, except for 12 and 15 dpi that were not comparable.
Table 5. Mean virus copy numbers of CAstV-infected and exposed sentinel SPF chickens at different time-points.
Discussion
Undoubtedly, the poultry industry contributes significantly to the provision of animal protein globally. Driven by the growing demand for poultry meat and eggs worldwide, it is projected that the industry will continue to witness a modest rate of increase for the next 10 years (IndexBox, Citation2020). However, emerging and re-emerging poultry viral pathogens continue to be of great concern to the industry (David Citation2020). Amongst these viral pathogens is an array of enteric viruses including CAstV which has, over about two decades, been incriminated in cases of enteritis, varying illnesses and syndromes in chickens especially the commercial broiler chickens (Kang & Gray, Citation2013; Smyth, Citation2017).
In an earlier study, we described the detection and propagation of the Malaysian CAstV isolates, and the initial characterization of the virus isolates (based on partial polymerase gene amino acid sequences), and CAstV seroprevalence in broiler breeder sera (Raji et al., unpublished). In this present paper, we describe a sequencing protocol that is capable of generating a near-complete genome of three Malaysian CAstVs followed by molecular analyses and characterization. In addition, the pathogenicity of one isolate (UPM1019/2018) out of three characterized isolates was determined in day-old SPF chickens.
The sequencing approach described in this study utilizes only three primer sets that cover over 98% of CAstV genome length. With a quick turnaround period, the procedure could allow multiplexing of 96 samples by tagging unique dual barcodes (index adapters) on both ends of a sample over a single sequencing run, which significantly reduces the cost and arduous work required for genome sequencing. Because the adopted technique employed a unique combination of indexes for each CAstV sample in the course of multiplexing, and the raw sequenced data was filtered to conform to the barcoded indexes, produced sequences originated purely from the CAstV isolates obtained from positive tissue samples. Of interest, different sequencing platforms such as Sanger (Kang et al., Citation2012b, Citation2018), Illumina (Sajewicz-Krukowska & Domanska-Blicharz, Citation2016) and Ion Torrent (Patel et al., Citation2017) have been utilized in the sequencing of CAstV genomes available in NCBI GenBank.
The lengths of the assembled genomes, IBS503/2017 (7424 nt), UPM1019/2018 (7397 nt) and IBS543/2017 (7379 nt) excluding the 5’-end UTR, the first few nt of ORF-1a, and the poly-A tail were comparable to those of published astroviruses (Finkbeiner et al., Citation2008; Kang et al., Citation2012b; Kang et al., Citation2018; Sajewicz-Krukowska & Domanska-Blicharz, Citation2016; Patel et al., Citation2017; Palomino-Tapia et al., Citation2020) ().
As described in other astroviruses, the three Malaysian CAstV genomes exhibited the presence of three ORFs encoding three different proteins: trypsin-like serine protease in ORF-1a, RdRp in ORF-1b, and capsid protein precursor in ORF-2. Similarly, ORFs-1b and -2 of the three isolates were found to be in +1 reading frame () as reported in turkey astroviruses (TAstV-1 and 2), other CAstVs and most mammalian astrovirus genomes. Although the sizes of the ORFs are similar in all the characterized CAstVs, the absence of 21 nt UTR at the 5’-end; shortness of 40 nt in IBS503/2017 and UPM1019/2018, and 80 nt in IBS543/2017 was due to the inability of the NGS sequencing protocols to capture the 5’-end UTR flanking region and the first few nucleotides of the genomes of the three isolates ().
Alignment of ORF-1a of the three isolates with other CAstVs, revealed a group of conserved motifs, including non-structural polypeptides (nsps): trypsin-like serine proteases, transmembrane helices, putative bipartite NLS and coiled-coil domains. Essentially, the high sequence identity seen in the NLS motifs was identified as important for a putative VPg between the Malaysian CAstVs and other astroviruses indicating that the Malaysian CAstVs also encode a VPg as does other CAstV. The conserved VPg putative protein of IBS503/2017, UPM1019/2018 and IBS543/2017 contains positively charged residues and basic amino acids as described by Fuentes et al. (Citation2012) and harbours a conserved tyrosine residue in the modified SEEEY (TEEEY-like) domain (Supplementary Figure 2).
As in many other astroviruses (Sajewicz-Krukowska & Domanska-Blicharz, Citation2016; Patel et al., Citation2017; Kang et al., Citation2018), the three Malaysian CAstVs exhibit an overlap between ORF-1a and ORF-1b of 18 nt length, although, recently, a 33-nt long overlap was reported by Xue et al. (Citation2020), it still falls within the range of 12–45 overlap length reported for avastroviruses (Méndez et al., Citation2013). The overlapping region covers the heptanucleotide (AAAAAAC) sequence also referred to as the “slippery sequence” (Supplementary Figure 2), and known to be responsible for the translation of the ORF-1b encoded viral RNA polymerase via a frameshift mechanism (RFS) in astroviruses other than avastroviruses (Kang et al., Citation2012b). Immediately upstream of the heptanucleotide and downstream of ORF-1a stop codon, the ORF-1b of the three isolates as in other avastroviruses possessed a start codon (ATG). The frame (ORF-1b) has been reported to have a similar length in nucleotide (1560 nt) and amino acid (519 aa) sequences across astroviruses.
Also, there exists a conserved intergenic region of 24 nt between the stop of ORF-1b and the start of ORF-2 harbouring a conserved pentamer (CCGAA) at positions 4953–4957 nt sequences of IBS503/2017 and UPM1019/2018 and 4925–4929 nt sequence of IBS543/2017. This pentameric region is highly conserved amongst CAstVs with an identity share of 100%. Interestingly, 4 nt (position 4961 of IBS503/2017 and UPM1019/2018, and 4933 nt of IBS543/2017) downstream of the highly conserved pentamer, the three Malaysian isolates exhibited a point mutation from guanine (G) to thymine (T). A similar mutation immediately after the pentamer (5000 nt position) was observed in eight out of the 14 recently characterized Canadian CAstV strains that were documented to cause WCS in chickens (Palomino-Tapia et al., Citation2020).
Furthermore, IBS503/2017, UPM1019/2018 and IBS543/2017 ORF-2 codes a protein length of 738 aa which falls within the documented range of 670–744 aa in length across the various avastroviruses (Koci & Schultz-Cherry, Citation2002). As the ORF-2 capsid gene is studied extensively at the molecular level; much of what is known about the astroviruses and their relatedness comes from its analyses. Three significant domains exist within astroviruses based on a comparative analysis of the capsid protein gene: the RNA binding domain, the N-terminal and the C-terminal domains (Krishna, Citation2005). In line with previous studies, the RNA binding domain and the N-terminal are the most conserved of the three regions. The three Malaysian isolates shared the most similarities with available CAstV sequences in these two regions. The N-terminal of the studied isolates contained a preserved motif of repeats of SRSRSRSRSRSR with a high number of basic residues similar to those of CAstVs, DAstV and TAstv-2 (Koci & Schultz-Cherry, Citation2002) and exhibiting a much higher conserved sequence (not shown). The region is known to play a significant role in the encapsidation of astroviruses and virion formation (Krishna, Citation2005). Conversely, the C-terminal, which is made up of nearly 300 aa, is exposed to antibodies elicited by the host and potentially binds to new cells within the host. By contrast, the hypervariable domain exhibits lower identity shares. An identity share of 72–86% was observed at the C-terminal end of the Malaysian isolates and other strains of the CAstV, thus, indicating hypervariability and the clear divergence of the Malaysian CAstV isolates from subgroup B CAstVs.
The Rfam analysis of the three genomes predicted a third coronavirus s2m at the ORF-2. This is in addition to the two s2m that is present in the group B CAstV strains, including the three Malaysian isolates. The s2m is a highly conserved region in Astroviridae but reportedly missing in AstV-MLB1, rat, bat and turkey astroviruses (Pantin-Jackwood et al., Citation2006; Finkbeiner et al., Citation2008). While the absence of s2m suggests that it is a non-essential feature in the avastroviruses, it is proposed that the region evolved as a result of recombination with non-related viruses (Monceyron et al., Citation1997). Biologically, the significance of the absence or variations in s2m or ORF arrangement is uncertain but could indicate changes due to evolutionary pressure (Pantin-Jackwood et al., Citation2011). Two recently characterized Chinese strains, HBLP717-1/2018 and GDYHTJ718-6/2018 (Xue et al., Citation2020) were equally predicted using the Rfam analysis to have a third s2m as predicted for the Malaysian isolates in this study.
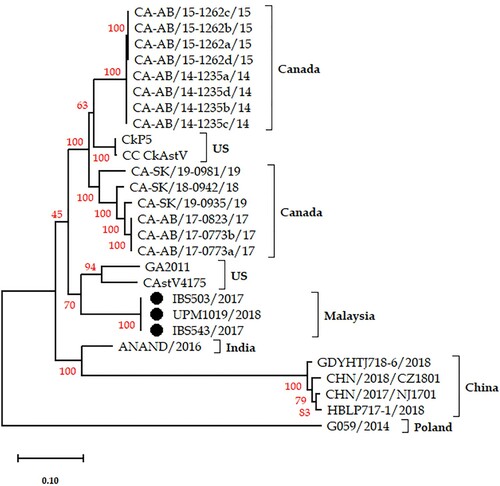
The assessment of the phylogenetic tree of IBS503/2017, UPM1019/2018 and IBS543/2017 based on the genome sequences confirmed that the isolates are novel and share a larger cluster with strains from the US (CkP5 and CC_CkAstV (Kang et al., Citation2018)), the 14 Canadian strains (Palomino-Tapia et al., Citation2020), India (ANAND/2016, Patel et al., Citation2017) and China (CHN/2017/NJ170 (Zhao et al., Citation2020), HBLP717-1/2018 and GDYHTJ718-6/2018 (Xue et al., Citation2020), and CHN/2018/CZ1801) (). The Poland strain, G059/2014 that belongs to group A (Sajewicz-Krukowska & Domanska-Blicharz, Citation2016) was placed farthest from the three Malaysian isolates signifying a distant relationship between the three isolates and the strain ( and ). The nucleotide sequences of the three near-complete genomes have the closest identity (86–87%) to subgroup Biv isolates originating from the US and Canada, followed by US strain GA2011 and Indian strain ANAND/2016 with an identity of 86%. However, a low identity of 75–76% was recorded between the three Malaysian isolates and the four Chinese strains and 4175 strain of the US (). In line with the International Committee on Taxonomy of Viruses (ICTV) criteria for avastroviruses demarcation, these P-dists are even lower than the proposed ICTV distance values of 0.576–0.742 between, and 0.204 to 0.284 within avastroviruses (). With this, the three Malaysian isolates (IBS503/2017, IBS543/2017 and UPM1019/2018) belong to the avastrovirus genus and within the B group.
With regards to the amino acid sequence of the capsid protein gene (ORF-2) of the three Malaysian isolates, the phylogenetic analysis depicted the existence of two distinct CAstV groups (A and B) as earlier described by Smyth et al. (Citation2012, Smyth, Citation2017) with the three Malaysian isolates forming a new subgroup within the group B CAstVs (). The clade of IBS503/2017, UPM1019/2018 and IBS543/2017 along with the capsid sequences of CAstV strains 4175, GA2011 (Kang et al., Citation2012b), CkP5, CC_CkAstV (Kang et al., Citation2018), ANAND/2016 (Patel et al., Citation2017), CHN/2017/NJ1701 (Zhao et al., Citation2020), GDYHTJ718-6/2018 and HBLP717-1/2018 (Xue et al., Citation2020), the 14 Canadian strains (Palomino-Tapia et al., Citation2020), and other capsid protein sequences obtained from GenBank were placed in group B (). Furthermore, phylogenetic analysis based on the C-terminal ends of the capsid proteins (416–738 aa residue) of the Malaysian isolates and those of subgroup B isolates presented a tree topology similar to the complete ORF-2 sequence tree (not shown). The three Malaysian isolates with an ORF-2 sequence identity of 100% clustered forming a new subgroup with an identity of 88–90% with Bi strains, 86–87% with Bii strains, 87–89% with Biii strains, and 90–91% with Biv strains (). However, amino acid identity shares within the subgroups were: Bi, 96–100%; Bii, 95–96%; Biii, 97–98%; and Biv, 97–100% (Supplementary Table 2). These high intragroup homologies exhibited by strains within the subgroups could suggest that the three Malaysian isolates are a new cluster considering the low identity (86–91%) between them and strains in the other subgroups (Supplementary Table 2). Thus, based on the phylogenetic tree, ORF-2 amino acid pairwise sequence comparison, and the predicted additional s2m of the Malaysian isolates suggest that, tentatively, the three isolates should be assigned into a new subgroup Bv within group B CAstV.
Given the high homology (100% sequence identity) between the three Malaysian isolates and their clustering within Group B CAstVs, one of the Malaysian isolates, UPM1019/2018, was used in the pathogenicity study in SPF chickens. The virus was mild to moderately pathogenic with the ability to cause gross and histologic lesions in the intestines and the kidneys of the infected and exposed sentinel SPF chickens. Although all the observable clinical manifestations including mild diarrhoea, listlessness, mild to moderate depression, anorexia and growth retardation are general clinical signs of most infections in chickens, they have been equally reported in CAstV infection as early as 3 dpi. A 20% retardation in growth was observed in the infected and exposed sentinel groups compared to the control group as early as 9 dpi. Of interest is the gouty lesion seen as an extensive white chalky covering on the heart, white thin membranous covering of the liver and the enlargement of the ureters and kidneys with both containing urates on 6 and 9 dpi. These lesions, although not severe, were as described by Bulbule et al. (Citation2013) in which they identified CAstV subgroup Biii as the cause of severe gout in broiler flocks in India.
Furthermore, the elicited antibody titre appears to have influenced the virus shedding; however, complete virus clearance was not observed due to the termination of the study by 15 dpi. A similar effect of antibody was previously reported by Sajewicz-Krukowska et al. (Citation2016), where they reported an extended period of virus clearance in infected and exposed birds. Notwithstanding, it is good to note that the onset, duration, and quantity of shedding can be influenced by strain, the quantity of virus, immune status, and type of bird at the time of infection (Smyth, Citation2017).
Comparably, FP3, the Indian strains, CkP5 and CC_CkAstV in subgroups Bi, Biii and Biv, respectively, have been documented to cause clinical manifestation and lesions similar to the Malaysian isolate (UPM1019/2018). The FP3 isolate was the first CAstV isolate documented to cause severe kidney disease, followed by the Indian isolates that were reported to cause visceral gout and severe kidney disease in SPF chickens (Smyth et al., Citation2007; Bulbule et al., Citation2013; Patel et al., Citation2017). Interestingly, these isolates (FP3 and the Indian isolates) were equally propagated in SPF-ECE, similar to the Malaysian isolate. On the other hand, CkP5 and CC_CkAstV are documented to be the aetiologic agent of runting and stunting in broiler chickens (Kang et al., Citation2018). In contrast, the Malaysian isolate did not cause mortality as reported in the Indian strain, but the severity and visceral gout were similar to both FP3 and the Indian isolates. Although experimental infection with CkP5 and CC_CkAstV was in broiler chickens (Kang et al., Citation2018), retardation in growth was similar to that in the Malaysian isolate. These findings provide direct evidence that the Malaysian isolate causes runting stunting syndrome, kidney disease and visceral gout phenotype as confirmed in the pathogenicity study. As a caution, lesions observed in pathogenicity or chicken trials cannot and do not suggest a possible CAstV isolate group or subgroup. For example, the recent grouping of 14 Canadian strains reported to cause WCS with two US strains CC-CkAstv and CkP5 that were responsible for RSS into Biv. Similarly, FP3 of Bi and the Indian strains of Biii are both reported to be responsible for kidney disease and visceral gout.
In conclusion, the present study describes the near-complete genomes of three CAstV propagated in SPF-ECE and responsible for runting stunting syndrome, kidney disease and visceral gout in commercial broiler chickens in Malaysia between 2017 to 2018. The molecular characterization, pairwise sequence comparison and phylogenetic study strongly suggest the tentative classification of the three Malaysian isolates into a new subgroup, Bv.
Supplemental Material
Download MS Excel (22.2 KB)Supplemental Material
Download MS Word (32.1 KB)Supplemental Material
Download TIFF Image (99.4 KB)Supplemental Material
Download TIFF Image (2.9 MB)Supplemental Material
Download TIFF Image (135.3 KB)Supplemental Material
Download TIFF Image (397.3 KB)Supplemental Material
Download TIFF Image (90.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arias, C.F. & Dubois, R.M. (2017). The astrovirus capsid: a review. Viruses, 9, 1–13.
- Baxendale, W. & Mebatsion, T. (2004). The isolation and characterisation of astroviruses from chickens. Avian Pathology, 33, 364–370.
- Bulbule, N.R., Mandakhalikar, K.D., Kapgate, S.S., Deshmukh, V.V., Schat, K.A. & Chawak, M.M. (2013). Role of chicken astrovirus as a causative agent of gout in commercial broilers in India. Avian Pathology, 42, 464–473.
- David, F. (2020). White chick syndrome. In Y.M. Saif, D.E. Swayne, M.J. Pantin-Jackwood, E. Spackman, T.J. Johnson, J.M. Day, D. French, E. Gingerich, S.F. Bilgili, K. Jones, G. Boggan & M. Markis (Eds.). Diseases of Poultry 14th edn (pp. 1393–1395). Hoboken, NJ: John Willey & Sons, Inc.
- de Wit, J.J., ten Dam, G.B., vande Laar, J.M.A.M., Biermann, Y., Verstegen, I., Edens, F. & Schrier, C.C. (2011). Detection and characterization of a new astrovirus in chicken and turkeys with enteric and locomotion disorders. Avian Pathology, 40, 453–461.
- Devaney, R., Trudgett, J., Trudgett, A., Meharg, C. & Smyth, V. (2016). A metagenomic comparison of endemic viruses from broiler chickens with runting-stunting syndrome and from normal birds. Avian Pathology, 45, 616–629.
- Finkbeiner, S.R., Kirkwood, C.D. & Wang, D. (2008). Complete genome sequence of a highly divergent astrovirus isolated from a child with acute diarrhea. Virology Journal, 5, 117.
- Fuentes, C., Bosch, A., Pintó, R.M. & Guix, S. (2012). Identification of human astrovirus genome-linked protein (VPg) essential for virus infectivity. Journal of Virology, 86, 10070–10078.
- IndexBox. (2020). Global poultry production to reach 137M tonnes in 2020, mainly driven by growth in China, the EU, and the UK. Global Trade Magazine.
- Kang, G. & Gray, J.J. (2013). Astroviruses. In A. Magill, D. Hill, T. Solomon & T.B.T. Edward (Eds.). Hunter’s Tropical Medicine and Emerging Infectious Disease 9th edn (pp. 286–288). London: W.B. Saunders.
- Kang, K.I., El-gazzar, M., Sellers, H.S., Dorea, F., Williams, S.M., Kim, T., Collett, S. & Mundt, E. (2012a). Investigation into the aetiology of runting and stunting syndrome in chickens. Avian Pathology, 41, 41–50.
- Kang, K.I., Icard, A.H., Linnemann, E., Sellers, H.S. & Mundt, E. (2012b). Determination of the full length sequence of a chicken astrovirus suggests a different replication mechanism. Virus Genes, 44, 45–50.
- Kang, K.I., Linnemann, E., Icard, A.H., Durairaj, V., Mundt, E. & Sellers, H.S. (2018). Chicken astrovirus as an aetiological agent of runting-stunting syndrome in broiler chickens. Journal of General Virology, 99, 512–524.
- Koci, M.D. & Schultz-Cherry, S. (2002). Avian astroviruses. Avian Pathology, 31, 213–227.
- Krishna, N.K. (2005). Identification of structural domains involved in astrovirus capsid biology. Viral Immunology, 18, 17–26.
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018). MEGA x: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549.
- Long, K.E., Hastie, G.M., Ojkić, D. & Brash, M.L. (2017). Economic impacts of white chick syndrome in Ontario, Canada. Avian Diseases, 61, 402–408.
- Mcneilly, F., Connor, T.J., Calvert, V.M., Smyth, J.A., Curran, W.L., Morley, A.J., Thompson, D., Singh, S., Mcferran, J.B., Adair, B.M. & McNulty, M.S. (1994). Studies on a new enterovirus-like virus isolated from chickens. Avian Pathology, 23, 313–327.
- Méndez, E., Murillo, A., Velázquez, R., Burnham, A. & Arias, C.F. (2013). Replication cycle of astroviruses. In S. Schultz-Cherry (Ed.). Astrovirus Research Essential Ideas, Everyday Impacts, Future Directions 1st edn (pp. 19–46). New York: Springer.
- Monceyron, C., Grinde, B. & Jonassen, T. (1997). Molecular characterisation of the 3’-end of the astrovirus genome. Archives of Virology, 142, 699–706.
- Nuñez, L.F.N, Parra, S.H., Mettifogo, E., Catroxo, M.H., Astolfi-Ferreira, C.S. & Piantino Ferreira, A.J.P. (2015). Isolation of chicken astrovirus from specific pathogen-free chicken embryonated eggs. Poultry Science, 94, 947–954.
- Nuñez, L.F.N., Santander-Parra, S.H., Kyriakidis, N.C., Astolfi-Ferreira, C.S., Buim, M.R., De la Torre, D. & Ferreira, A.J.P. (2020). Molecular characterization and determination of relative cytokine expression in naturally infected day-old chicks with chicken astrovirus associated to white chick syndrome. Animals, 10, 1–18.
- Palomino-Tapia, V., Mitevski, D., Inglis, T., van der Meer, F., Martin, E., Brash, M., Provost, C., Gagnon, C.A. & Abdul-Careem, M.F. (2020). Chicken astrovirus (CAstV) molecular studies reveal evidence of multiple past recombination events in sequences originated from clinical samples of white chick syndrome (WCS) in western Canada. Viruses, 12, 1–20.
- Pantin-Jackwood, M.J., Spackman, E. & Woolcock, P.R. (2006). Molecular characterization and typing of chicken and Turkey astroviruses circulating in the United States: implications for diagnostics. Avian Diseases, 50, 397–404.
- Pantin-Jackwood, M.J., Strother, K.O., Mundt, E., Zsak, L., Day, J.M. & Spackman, E. (2011). Molecular characterization of avian astroviruses. Archives of Virology, 156, 235–244.
- Patel, A.K., Pandit, R.J., Thakkar, J.R., Hinsu, A.T., Pandey, V.C., Pal, J.K., Prajapati, K.S., Jakhesara, S.J. & Joshi, C.G. (2017). Complete genome sequence analysis of chicken astrovirus isolate from India. Veterinary Research Communications, 41, 67–75.
- Raji, A.A., Omar, A.R., Ideris, A. & Bejo, M.H. (2019). Proceedings of the 31st Veterinary Association Malaysia (VAM) Congress (p. 41), Bangi, Malaysia.
- Sajewicz-Krukowska, J. & Domanska-Blicharz, K. (2016). Nearly full-length genome sequence of a novel astrovirus isolated from chickens with ‘white chicks’ condition. Archives of Virology, 161, 2581–2587.
- Sajewicz-Krukowska, J., Pać, K., Lisowska, A., Pikuła, A., Minta, Z., Króliczewska, B. & Domańska-Blicharz, K. (2016). Astrovirus-induced “white chicks” condition – field observation, virus detection and preliminary characterization. Avian Pathology, 45, 2–12.
- Smyth, V.J. (2017). A review of the strain diversity and pathogenesis of chicken astrovirus. Viruses, 9, 1–10.
- Smyth, J.A., Connor, T.J., McNeilly, F., Moffet, D.A., Calvert, V.M. & McNulty, M.S. (2007). Studies on the pathogenicity of enterovirus-like viruses in chickens. Avian Pathology, 36, 119–126.
- Smyth, V.J., Jewhurst, H.L., Adair, B.M. & Todd, D. (2009). Detection of chicken astrovirus by reverse transcriptase-polymerase chain reaction. Avian Pathology, 38, 293–299.
- Smyth, V.J., Jewhurst, H.L., Wilkinson, D.S., Adair, B.M., Gordon, A.W. & Todd, D. (2010). Development and evaluation of real-time TaqMan® RT-PCR assays for the detection of avian nephritis virus and chicken astrovirus in chickens. Avian Pathology, 39, 467–474.
- Smyth, V.J., Todd, D., Trudgett, J., Lee, A. & Welsh, M.D. (2012). Capsid protein sequence diversity of chicken astrovirus. Avian Pathology, 41, 151–159.
- Smyth, V.J., Trudgett, J., Wylie, M., Jewhurst, H., Conway, B., Welsh, M., Kaukonen, E. & Perko-Mäkelä, P. (2013). Chicken astrovirus detected in hatchability problems associated with ‘white chicks’. Veterinary Record, 173, 403–404.
- Xue, J., Han, T., Zhao, Y., Yang, H. & Zhang, G. (2020). Complete genome sequence and phylogenetic analysis of novel avastroviruses circulating in China from 2016 to 2018. Virus Research, 278, 197858.
- Zhao, W., Wu, Z., Yao, Y., Qin, A. & Qian, K. (2020). The isolation and molecular characterization of an astrovirus from “Yellow” chickens, China. Frontiers in Veterinary Science, 7, 1–10.