ABSTRACT
Avian pathogenic Escherichia coli (APEC) is the bacterial pathogen of poultry colibacillosis, which causes significant economic losses to the poultry industry. The lack of an effective vaccine against multiple serotypes and the emergence of multi-resistant isolates have made the control of avian colibacillosis troublesome. To identify conserved potential vaccine candidates, 58 genomes of APEC were obtained (54 sequenced by our laboratory and four downloaded from NCBI). A reverse vaccinology (RV) method based on the pangenome – called Pan-RV analysis – was performed in APEC-protective protein mining for the first time. Finally, four proteins were selected, and their immunoreactivity with anti-O1, O2, and O78 serum was verified by western blotting. Our in silico method of analysis will pave the way for rapid screening of vaccine candidates and will lay the foundation for the development of a highly effective subunit vaccine controlling APEC infection.
Pan-RV analysis was used for the first time in the discovery of APEC-protective proteins.
A total of 53 potential protective proteins were screened out.
Four proteins were verified as potential vaccine candidates using western blotting.
RESEARCH HIGHLIGHTS
Introduction
Escherichia coli (E. coli) is a normal bacterium in the gastrointestinal tract of humans and animals. Avian pathogenic Escherichia coli (APEC) is a type of extraintestinal E. coli (ExPEC), that can cause disease in avian species (Moulin & Fairbrother, Citation1999). APEC is the pathogen of colibacillosis, causing air sacculitis, pericarditis, perihepatitis, peritonitis and septicaemia (Matter et al., Citation2011). O1, O2, and O78 are recognized as the most prevalent serotypes (Ewers et al., Citation2003). China is the largest commercial duck-breeding country (Dziva & Stevens, Citation2008). Intensive breeding has been widely applied, and outbreaks of poultry colibacillosis have been reported frequently and have caused significant economic losses (Vandekerchove et al., Citation2004).
The control of bacterial disease has relied heavily on medical treatment. However, the abuse of antibiotics has led to an increasing level of drug resistance in APEC (Szmolka & Nagy, Citation2013). On the other hand, drug residue on poultry products is a serious problem that needs to be given sufficient attention (Rabie & Amin Girh, Citation2020). Moreover, some organisms are naturally resistant to most antibiotics without inducing resistance (Nakae et al., Citation1997), and all these reasons lead to the urgent need for an immunological method to control this bacterial disease. Vaccination may be a good choice. Traditional whole-cell inactivated bacterial vaccines can provide satisfactory protection against homologous serotypes (Melamed et al., Citation1991; Yaguchi et al., Citation2009) but poorly activate cross-protection against heterogeneous isolates (Kwaga et al., Citation1994; Kariyawasam et al., Citation2004). Subunit vaccines prepared from one or a few antigens are an alternative method for addressing the problem of cross-protection. A multivalent pilus vaccine that was developed by Gyimah et al. (Citation1986) addressed the problem of cross-protection. A bivalent vaccine derived from attenuated Salmonella expressing O-antigen polysaccharide protected against avian pathogenic E. coli O1 and E. coli O2 infection (Han et al., Citation2018). Bao et al., (Citation2013) identified chaperonin GroEL as a novel phylogenetically conserved protein that was observed to provide effective protection against heterologous isolates using immunoproteomics. Therefore, the selection of subunit vaccine components is extremely important for the effectiveness of vaccination. Antigen selection based on empirical screening methods is laborious and expensive (Yasmin & Yaqoub, Citation2018), and a more rational and comprehensive approach is needed to discover potential antigen candidates.
In contrast with conventional methods, the reverse vaccinology (RV) approach is an in silico method that does not require any culture or empirical screening, and uses only bacterial genomic data to identify protective antigens. This method was first used successfully by Pizza et al. (Citation2000) in the development of the serogroup B meningococcal vaccine. Subsequently, the RV approach was applied successfully for many pathogens, such as Streptococcus pneumoniae (Wizemann et al., Citation2001), Helicobacter pylori (Chakravarti et al., Citation2000), Chlamydia pneumoniae (Montigiani et al., Citation2002) and Bacillus anthracis (Ariel et al., Citation2002). However, a major limitation of these studies is that they only used one strain or plasmid sequence data to perform RV analysis. Due to the diversity of the strain genome, it has become essential to identify the core genome of a given bacterial species or genus through pangenome analysis and find the desired universal protective antigens (Donati & Rappuoli, Citation2013).
In this study, we first performed a pangenome analysis of 58 APEC genomes (four were downloaded from NCBI and 54 were sequenced by our laboratory), and then performed RV analysis based on the core genome. It is an improved RV version, so-called Pan-RV analysis. We aimed to find the desired conserved protective antigen candidates and assess their potential ability to protect against the O1, O2, and O78 isolates (the three major serotypes of APEC).
Materials and methods
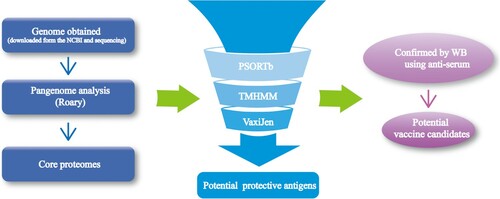
The workflow of our Pan-RV analysis is illustrated in . In the first stage of analysis, we obtained genomes from the NCBI database and sequenced them. Then, we extracted the core proteome after pangenome analysis. In the second stage, RV analysis was performed to predict subcellular localization, transmembrane helices, and antigenicity. Potential vaccine candidates were obtained in the last stage, in which predicted conserved protective antigens were expressed by E. coli BL21 and confirmed by western blot test using O1, O2, and O78 antiserum.
Figure 1. The workflow of Pan-RV analysis in this study to select conserved potential vaccine candidates for APEC.
All the scripts used in our Pan-RV pipeline have uploaded to the GitHub (https://github.com/WZH999999999/Scripts-in-PanRV-pipeline.git).
Isolates
Fifty-four APEC isolates were isolated from Jiangsu and Guangxi provinces in China, and the information on these isolates is listed in Table S1. All were isolated from diseased and dead ducks, which had the typical clinical signs of avian colibacillosis before death, and characteristic pathological changes upon post-mortem examination. Only the internal tissues of the brain and liver were separated and used as samples for bacterial isolation, and only the isolates exhibiting the growth characteristics of E. coli (convex, circular, smooth, grey colonies on blood agar, and round red colonies on MacConkey agar medium) and profuse pure growth were selected for 16S rRNA sequencing.
Data retrieval
The 54 isolates in this study were recently sequenced and uploaded to NCBI, and the remaining four genomes were downloaded from NCBI (Table S1).
Pangenome analysis
The CDSs of 58 genome sequences were annotated using Prokka 1.14 (Seemann, Citation2014), and GFF3-format files of all genome sequences were generated for pangenome analysis. Fifty-eight proteomes were analyzed using Roary version 3.13.0 (Page et al., Citation2015) to identify the core proteomes with default settings (genes that are found in 99–100% of bacteria are considered to be core genes).
Subcellular localization
In this step, the core proteomes outputted from the pangenome analysis were uploaded to PSORTb (3.0.2; http://www.psort.org/psortb/) (Yu et al., Citation2010) to predict subcellular localization; this method based on machine learning algorithm generates prediction results for five major localizations for Gram-negative bacteria (cytoplasm, cytoplasmic membrane, periplasm, outer membrane and extracellular membrane), and all settings were default except for selecting “Negative” in the “Choose Gram stain” option. Only the outer membrane and extracellular proteins could be screened out to enter the next step of analysis.
Transmembrane topology
The TMHMM Server (2.0; http://www.cbs.dtu.dk/services/TMHMM/) (Krogh et al., Citation2001) was used to predict transmembrane helices in the outer membrane proteins and the extracellular proteins screened out above. This method for predicting membrane protein topology based on a hidden Markov model could correctly predict 97–98% of the transmembrane helices. Proteins with multiple transmembrane regions were excluded, and only proteins with transmembrane helices ≤2 were selected. Meanwhile, a molecular weight filter was set to “<110KD” in this step.
Prediction of antigenicity
The proteins filtered in previous steps were then uploaded to VaxiJen v2.0 (http://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html) (Doytchinova & Flower, Citation2007) to make antigenicity predictions. This antigenicity prediction method was solely based on the physicochemical properties of proteins without recourse to sequence alignment, the precision rate of which ranged from 70% to 89%. The default threshold was 0.4, and proteins with a prediction score >0.4 were regarded as probable antigens.
Cloning, expression and purification of recombinant potential antigens
To express and purify the recombinant potential vaccine candidates, APEC strain EC164 (O78) genomic DNA was used as the template for PCR with PrimeSTAR HS DNA Polymerase (Takara, Dalian, China). The designed primers and related information are listed in Table S2. The PCR products of all proteins selected in the previous Pan-RV analysis were connected to the pET28a vectors transformed into E. coli BL21 (DE3). The cloned PCR products were confirmed by DNA sequencing. The proteins were not induced at 37°C for 6 h with 1 mM IPTG until the OD600 of the cells reached 0.6. Cell cultures were harvested by centrifugation at 12,000 × g at 4°C for 10 min. Proteins were purified using Ni-TrapTM columns (GE Health care, Uppsala, Sweden).
Western blotting of selected proteins and validation of immunoreactivity
The immunoreactivity of potential protective antigens in silico was confirmed by western blotting with anti-EC320 (O1), anti-EC165 (O2) and anti-EC164 (O78) hyperimmune serum (Zoonbio Biotechnology, Nanjing, China). Hyperimmune serum was prepared with formaldehyde-killed whole cells by immunizing New Zealand rabbits.
Recombinant proteins were separated using SDS-PAGE and then were electroblotted onto PVDF membranes. The PVDF membrane was blocked with 5% nonfat-dried milk (diluted with PBS) for 1 h at 37°C. After three washes with PBST, the membrane was incubated with hyperimmune serum (1:200 dilution) for 1 h at 37°C. And after three rinses with PBST, it was incubated with goat anti-rabbit IgG antibody (Biosharp, Hefei, China; 1:10,000 dilution). 3,3ʹ-Diaminobenzidine (DAB; Tiangen, Beijing, China) was used to develop the specific bands.
Accession numbers
The accession numbers are listed in Table S1.
Results
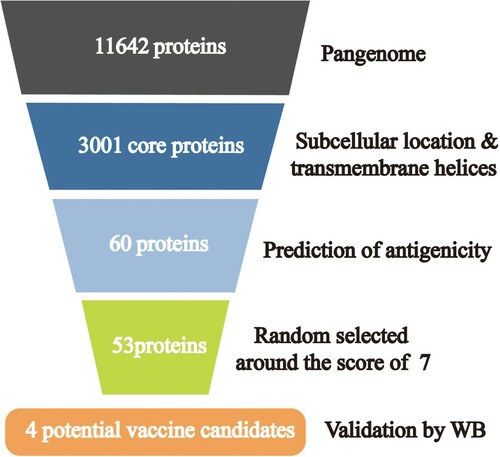
The results of the Pan-RV analysis performed to identify potential vaccine candidates against APEC isolates are summarized in . In total, 11,642 genes were identified after pangenome analysis. Among them, 3001 genes were identified as core genes. After the prediction of subcellular localization and transmembrane helices, 60 proteins were screened for prediction of antigenicity. Ultimately, it was predicted that 53 proteins were potential protective antigens, using the VaxiJen server, and four of them were validated using western blotting.
Figure 2. Summary of protein selection results in each step of the Pan-RV analysis. A total of 11,642 genes were identified after pangenome analysis in the first step; 3,001 genes were identified as core genes in the second step; after prediction of subcellular localization and transmembrane helices, 60 proteins were screened out in the third step; 53 proteins were predicted as potential protective antigens using VaxiJen server in the fourth step; four proteins were validated as the potential candidates using western blot in the last step.
Pangenome analysis
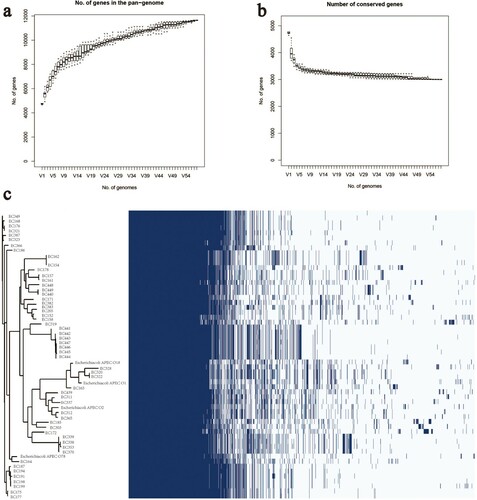
In total, of 58 genomes were annotated using Prokka, and all genome FASTA formats were transformed to GFF3 format and used to perform pangenome analysis using Roary. The results showed that a total of 11,642 genes were identified in the genome data, and among them, 3001 genes were identified as core genes. We found that the pangenome was open, based on the shape of the curve, which is consistent with the results of Rasko et al. (Citation2008). Due to this feature, the size of the pangenome reservoir in our study showed varying degrees of similarity to previous studies (Rasko et al., Citation2008; McNally et al., Citation2016). The number of conserved genes decreased and tended to be stable as the number of isolates increased ().
Reverse vaccinology (RV) analysis
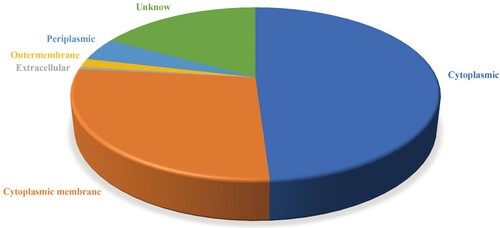
The core proteomes generated from the pangenome analysis were submitted to RV analysis. Subcellular localization screening using PSORTb v3.0 showed that 1470 proteins out of the core proteomes were cytoplasmic, 829 proteins were cytoplasmic membrane, 130 were periplasmic, 15 were extracellular, 48 were outer membrane and 509 were unknown (). The extracellular and outer membrane proteins were selected to enter the next filtration of the pipeline.
The transmembrane helix topology of the selected proteins was analyzed using TMHMM Server v2.0. Only transmembrane helices between 0 and 1 with a molecular weight <110 kDa were screened out.
In total, of 60 proteins were filtered in the last filtration, and their antigenicity was predicted using VaxiJen v2.0. Fifty-three potentially protective antigens were selected using the default threshold (0.4) (). The total number of proteins was too great to work with, and we found that higher scores are associated with poor prediction in most cases due to the lower molecular weight. Therefore, from the predicted pool, we randomly selected four proteins, which had a score close to seven. Information on these four proteins was found at Uniprot (https://www.uniprot.org/) and is listed in .
Table 1. Potential protective antigens predicted by Pan-RV analysis.
Table 2. Description of four potential vaccine candidates.
Recombinant protein expression and purification
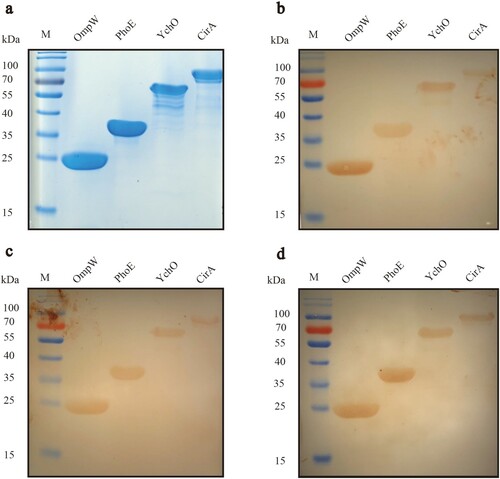
Probable antigens identified from the Pan-RV analysis were expressed in vitro using the pET-28a expression vector. All four proteins were expressed successfully. The expression products of the four genes were run on the four pieces of SDS-PAGE gels; one was used to check the bands of interest and the effect of purification ((a)), and the other three gels were used to confirm immunoreactivity.
Immunoreactivity of potential protective antigens
To confirm the immunoreactivity of predicted antigens in silico, another three pieces of the SDS-PAGE gels mentioned above were used for western blotting. The four purified expression products were electroblotted onto three PVDF membranes, and the photographs showed immunoreactivity with the following primary antibodies: anti-O1 ((b)), anti-O2 ((c)), and anti-O78 serum ((d)).
Based on the results, all four proteins were found to be immunoreactive with the antiserum of the major epidemic APEC isolates (O1, O2, and O78). OmpW had a relatively stronger reaction with anti-O1, O2, and O78 serum than the other three proteins. Meanwhile, PhoE had a relatively stronger reaction with anti-O78 serum. Due to their immunoreactivity with all three antisera, these four proteins were regarded as potential vaccine candidates.
Discussion
Avian pathogenic Escherichia coli (APEC) is one of the most harmful pathogens causing serious economic losses to the poultry industry (Ghunaim et al., Citation2014). As a member of the ExPEC family, the APEC typing system is large and complex. Based on a multiplex PCR assay, the pathogen can be classified into eight phylogenetic groups (A, B1, B2, C, D, E, F and cryptic clade I) (Clermont et al., Citation2000; Clermont et al., Citation2013). In addition, APEC has numerous ST types and serotypes. Among them, some specific phylogroups/types are closely related to pathogenicity and prevalence. For example, the poultry-source E. coli isolates in phylogroup B2 were recognized as the major source of avian colibacillosis (Johnson et al., Citation2012; Zhu Ge et al., Citation2014). ST117 was one of the STs identified in the present study as highly virulent avian E. coli (Dissanayake et al., Citation2014), and O1, O2, and O78 were considered the predominant APEC serotypes responsible for outbreaks in many countries (Cloud et al., Citation1985; Ewers et al., Citation2004).
Control of the APEC epidemic relied heavily on antibiotics. However, drug resistance among APEC isolates has become increasingly severe (Szmolka & Nagy, Citation2013). In addition, the drug residue problem is an important factor to consider for our modern healthy lives. In such a context, vaccines are a better choice than drug therapy.
Among the traditional vaccines, the inactivated vaccine was the earliest attempt to control APEC through vaccination in 1957, and the formalin-inactivated vaccine successfully provided protection against challenge (Gross, Citation1957). After this attempt, many studies focused on the killed vaccine (Deb & Harry, Citation1978; Panigraphy et al., Citation1984; Gyimah et al., Citation1985) but, generally, inactivated vaccines provided protection against homologous challenge only. Live attenuated vaccines may have the potential to protect against heterologous challenges (Frommer et al., Citation1994), but may also cause many side effects, such as disease signs (Arp, Citation1980; Frommer et al., Citation1994) or reduced weight gain (Fernandes Filho et al., Citation2013). Compared with traditional vaccines, subunit vaccines are an ideal choice due to their increased heterologous protection and safety (Gyimah et al., Citation1986; Kariyawasam et al., Citation2002; Lynne et al., Citation2012). However, conventional methods for subunit selection are mostly based on experience and immunoproteomics (Bao et al., Citation2013), which is laborious and expensive.
In this study, an improved RV version, which combines pangenome analysis on the basis of rational filtration steps built by reverse vaccinology, was used for the first time to identify the potential protective and universal antigens of APEC. A total of 58 APEC genomes were used for prediction. Finally, four proteins were selected through Pan-RV analysis and confirmed by western blot. To our surprise, all four proteins had reactivity with all three antisera of major serotypes (O1, O2, and O78), although the degree of reaction varied. These results, in turn, proved the feasibility of our method.
Among these four proteins, OmpW is an outer membrane protein, which has been reported as a vaccine candidate in many species, such as Aeromonas hydrophila (Maiti et al., Citation2012), Burkholderia pseudomallei (Casey et al., Citation2016), and Klebsiella pneumoniae (Kurupati et al., Citation2006). In this article, western blot analysis showed that OmpW has stronger reaction than other proteins with all three anti-sera, and outer-membrane PhoE protein has been used as a carrier for foreign antigenic determinants in many studies (Agterberg, Adriaanse, Barteling, et al., Citation1990; Agterberg, Adriaanse, Lankhof, et al., Citation1990; Tommassen et al., Citation1993; Janssen et al., Citation1994). This protein has a relatively strong reaction with anti-O78 serum. CirA is considered to be a member of the regulatory network in response to glucose concentration changes in E. coli (Yang et al., Citation2011), and the YchO gene has been identified through in silico research as an important virulence factor mediating the adhesion, invasion, biofilm formation and motility of APEC (Pilatti et al., Citation2016). Both CirA and YchO reacted with antiserum in this research.
Overall, these four proteins selected through Pan-RV analysis in this study are novel potential vaccine candidates for controlling three major serotypes of APEC. Because the analyzed genomes included other serotypes, they may have the ability to further protect against other serotypes. Unfortunately, for various reasons, it was not possible to perform an in vivo protection study, though it is worthy of further verification by peers.
Our in silico method of analysis will pave the way for the rapid screening of vaccine candidates, with sequencing becoming low-cost and common. This study will lay the foundation for the development of an effective subunit vaccine controlling APEC infection.
Supplemental Material
Download MS Excel (12 KB)Supplemental Material
Download MS Excel (9.7 KB)Supplemental Material
Download JPEG Image (1.7 MB)Supplemental Material
Download JPEG Image (73.4 KB)Supplemental Material
Download JPEG Image (2.2 MB)Supplemental Material
Download JPEG Image (1,004.3 KB)Supplemental Material
Download JPEG Image (90.5 KB)Acknowledgements
This work was supported by the Key Laboratory of Biological Products and Chemical Drugs for Animals, Ministry of Agriculture, China Animal Husbandry Industry Co., Ltd, the Jiangsu Modern Agriculture (waterfowl) Industrial Technology System Disease Prevention and Control Innovation Team (JATS[2018]222) and the Key Research and Development Plan of Jiangsu Province (BE2019304).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Agterberg, M., Adriaanse, H., Barteling, S., van Maanen, K. & Tommassen, J. (1990). Protection of guinea-pigs against foot-and-mouth disease virus by immunization with a PhoE-FMDV hybrid protein. Vaccine, 8, 438–440.
- Agterberg, M., Adriaanse, H., Lankhof, H., Meloen, R. & Tommassen, J. (1990). Outer membrane PhoE protein of Escherichia coli as a carrier for foreign antigenic determinants: immunogenicity of epitopes of foot-and-mouth disease virus. Vaccine, 8, 85–91.
- Ariel, N., Zvi, A., Grosfeld, H., Gat, O., Inbar, Y., Velan, B., Cohen, S. & Shafferman, A. (2002). Search for potential vaccine candidate open reading frames in the Bacillus anthracis virulence plasmid pXO1: in silico and in vitro screening. Infection and Immunity, 70, 6817–6827.
- Arp, L.H. (1980). Consequences of active or passive immunization of turkeys against Escherichia coli O78. Avian Diseases, 24, 808–815.
- Bao, Y., Zhai, Z., Wang, S., Ma, J., Zhang, W. & Lu, C. (2013). Chaperonin GroEL: a novel phylogenetically conserved protein with strong immunoreactivity of avian pathogenic Escherichia coli isolates from duck identified by immunoproteomics. Vaccine, 31, 2947–2953.
- Casey, W.T., Spink, N., Cia, F., Collins, C., Romano, M., Berisio, R., Bancroft, G.J. & McClean, S. (2016). Identification of an OmpW homologue in Burkholderia pseudomallei, a protective vaccine antigen against melioidosis. Vaccine, 34, 2616–2621.
- Chakravarti, D.N., Fiske, M.J., Fletcher, L.D. & Zagursky, R.J. (2000). Application of genomics and proteomics for identification of bacterial gene products as potential vaccine candidates. Vaccine, 19, 601–612.
- Clermont, O., Bonacorsi, S. & Bingen, E. (2000). Rapid and simple determination of the Escherichia coli phylogenetic group. Applied and Environmental Microbiology, 66, 4555–4558.
- Clermont, O., Christenson, J.K., Denamur, E. & Gordon, D.M. (2013). The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environmental Microbiology Reports, 5, 58–65.
- Cloud, S.S., Rosenberger, J.K., Fries, P.A., Wilson, R.A. & Odor, E.M. (1985). In vitro and in vivo characterization of avian Escherichia coli. I. Serotypes, metabolic activity, and antibiotic sensitivity. Avian Diseases, 29, 1084–1093.
- Deb, J.R. & Harry, E.G. (1978). Laboratory trials with inactivated vaccines against Escherichia coli (O2:K1) infection in fowls. Research in Veterinary Science, 24, 308–313.
- Dissanayake, D.R., Octavia, S. & Lan, R. (2014). Population structure and virulence content of avian pathogenic Escherichia coli isolated from outbreaks in Sri Lanka. Veterinary Microbiology, 168, 403–412.
- Donati, C. & Rappuoli, R. (2013). Reverse vaccinology in the 21st century: improvements over the original design. Annals of the New York Academy of Sciences, 1285, 115–132.
- Doytchinova, I.A. & Flower, D.R. (2007). Vaxijen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics, 8, 4.
- Dziva, F. & Stevens, M.P. (2008). Colibacillosis in poultry: unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathology, 37, 355–366.
- Ewers, C., Janssen, T., Kiessling, S., Philipp, H.C. & Wieler, L.H. (2004). Molecular epidemiology of avian pathogenic Escherichia coli (APEC) isolated from colisepticemia in poultry. Veterinary Microbiology, 104, 91–101.
- Ewers, C., Janssen, T. & Wieler, L.H. (2003). Avian pathogenic Escherichia coli (APEC). Berliner und Münchener Tierärztliche Wochenschrift, 116, 381–395.
- Fernandes Filho, T., Favaro, C., Jr., Ingberman, M., Beirao, B.C., Inoue, A., Gomes, L. & Caron, L.F. (2013). Effect of spray Escherichia coli vaccine on the immunity of poultry. Avian Diseases, 57, 671–676.
- Frommer, A., Freidlin, P.J., Bock, R.R., Leitner, G., Chaffer, M. & Heller, E.D. (1994). Experimental vaccination of young chickens with a live, non-pathogenic strain of Escherichia coli. Avian Pathology, 23, 425–433.
- Ghunaim, H., Abu-Madi, M.A. & Kariyawasam, S. (2014). Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: potentials and limitations. Veterinary Microbiology, 172, 13–22.
- Gross, W.B. (1957). Pathological changes of an Escherichia coli infection in chickens and turkeys. American Journal of Veterinary Research, 18, 724–730.
- Gyimah, J.E., Panigrahy, B. & Williams, J.D. (1986). Immunogenicity of an Escherichia coli multivalent pilus vaccine in chickens. Avian Diseases, 30, 687–689.
- Gyimah, J.E., Panigrapy, B., Hall, C.F. & Williams, J.D. (1985). Immunogenicity of an oil-emulsified Escherichia coli bacterin against heterologous challenge. Avian Diseases, 29, 540–545.
- Han, Y., Liu, Q., Willias, S., Liang, K., Li, P., Cheng, A. & Kong, Q. (2018). A bivalent vaccine derived from attenuated Salmonella expressing O-antigen polysaccharide provides protection against avian pathogenic Escherichia coli O1 and O2 infection. Vaccine, 36, 1038–1046.
- Janssen, R., Wauben, M., van der Zee, R. & Tommassen, J. (1994). Immunogenicity of a mycobacterial T-cell epitope expressed in outer membrane protein PhoE of Escherichia coli. Vaccine, 12, 406–409.
- Johnson, J.R., Porter, S.B., Zhanel, G., Kuskowski, M.A. & Denamur, E. (2012). Virulence of Escherichia coli clinical isolates in a murine sepsis model in relation to sequence type ST131 status, fluoroquinolone resistance, and virulence genotype. Infection and Immunity, 80, 1554–1562.
- Kariyawasam, S., Wilkie, B.N. & Gyles, C.L. (2004). Construction, characterization, and evaluation of the vaccine potential of three genetically defined mutants of avian pathogenic Escherichia coli. Avian Diseases, 48, 287–299.
- Kariyawasam, S., Wilkie, B.N., Hunter, D.B. & Gyles, C.L. (2002). Systemic and mucosal antibody responses to selected cell surface antigens of avian pathogenic Escherichia coli in experimentally infected chickens. Avian Diseases, 46, 668–678.
- Krogh, A., Larsson, B., von Heijne, G. & Sonnhammer, E.L. (2001). Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. Journal of Molecular Biology, 305, 567–580.
- Kurupati, P., Teh, B.K., Kumarasinghe, G. & Poh, C.L. (2006). Identification of vaccine candidate antigens of an ESBL producing Klebsiella pneumoniae clinical strain by immunoproteome analysis. Proteomics, 6, 836–844.
- Kwaga, J.K., Allan, B.J., Hurk, J., Seida, H. & Potter, A.A. (1994). A carAB mutant of avian pathogenic Escherichia coli serogroup O2 is attenuated and effective as a live oral vaccine against colibacillosis in turkeys. Infection and Immunity, 62, 3766.
- Lynne, A.M., Kariyawasam, S., Wannemuehler, Y., Johnson, T.J., Johnson, S.J., Sinha, A.S., Lynne, D.K., Moon, H.W., Jordan, D.M., Logue, C.M., Foley, S.L. & Nolan, L.K. (2012). Recombinant Iss as a potential vaccine for avian colibacillosis. Avian Diseases, 56, 192–199.
- Maiti, B., Shetty, M., Shekar, M., Karunasagar, I. & Karunasagar, I. (2012). Evaluation of two outer membrane proteins, Aha1 and OmpW of Aeromonas hydrophila as vaccine candidate for common carp. Veterinary Immunology and Immunopathology, 149, 298–301.
- Matter, L.B., Barbieri, N.L., Nordhoff, M., Ewers, C. & Horn, F. (2011). Avian pathogenic Escherichia coli MT78 invades chicken fibroblasts. Veterinary Microbiology, 148, 51–59.
- McNally, A., Oren, Y., Kelly, D., Pascoe, B., Dunn, S., Sreecharan, T., Vehkala, M., Välimäki, N., Prentice, M.B., Ashour, A., Avram, O., Pupko, T., Dobrindt, U., Literak, I., Guenther, S., Schaufler, K., Wieler, L.H., Zhiyong, Z., Sheppard, S.K., McInerney, J.O. & Corander, J. (2016). Combined analysis of variation in core, accessory and regulatory genome regions provides a super-resolution view into the evolution of bacterial populations. PLoS Genetics, 12, e1006280.
- Melamed, D., Leitner, G. & Heller, E.D. (1991). A vaccine against avian colibacillosis based on ultrasonic inactivation of Escherichia coli. Avian Diseases, 35, 17–22.
- Montigiani, S., Falugi, F., Scarselli, M., Finco, O., Petracca, R., Galli, G., Mariani, M., Manetti, R., Agnusdei, M., Cevenini, R., Donati, M., Nogarotto, R., Norais, N., Garaguso, I., Nuti, S., Saletti, G., Rosa, D., Ratti, G. & Grandi, G. (2002). Genomic approach for analysis of surface proteins in Chlamydia pneumoniae. Infection and Immunity, 70, 368–379.
- Moulin, M.D. & Fairbrother, J.M. (1999). Avian pathogenic Escherichia coli (APEC). Veterinary Research, 30, 299.
- Nakae, M., Sugahara, Y., Sasaki, H., Yasui, H. & Shibasaki, K. (1997). Serotypes and drug susceptibility of Pseudomonas aeruginosa isolated from clinical specimens. The Japanese Journal of Antibiotics, 50, 187–194.
- Page, A.J., Cummins, C.A., Hunt, M., Wong, V.K., Reuter, S., Holden, M.T., Fookes, M., Falush, D., Keane, J.A. & Parkhill, J. (2015). Roary: rapid large-scale prokaryote Pan genome analysis. Bioinformatics (Oxford, England), 31, 3691–3693.
- Panigraphy, B., Gyimah, J.E., Hall, C.F. & Williams, J.D. (1984). Immunogenic potency of an oil-emulsified Escherichia coli bacterin. Avian Diseases, 28, 475–481.
- Pilatti, L., Paiva, J.D., Rojas, T., Leite, J.L., Conceição, R.A., Nakazato, G. & Wanderley, D. (2016). The virulence factor ychO has a pleiotropic action in an avian pathogenic Escherichia coli (APEC) strain. BMC Microbiology, 16, 35.
- Pizza, M., Scarlato, V., Masignani, V., Giuliani, M.M., Arico, B., Comanducci, M., Jennings, G.T., Baldi, L., Bartolini, E., Capecchi, B., Galeotti, C.L., Luzzi, E., Manetti, R., Marchetti, E., Mora, M., Nuti, S., Ratti, G., Santini, L., Savino, S., Scarselli, M., Storni, E., Zuo, P., Broeker, M., Hundt, E., Knapp, B., Blair, E., Mason, T., Tettelin, H., Hood, D.W., Jeffries, A.C., Saunders, N.J., Granoff, D.M., Venter, J.C., Moxon, E.R., Grandi, G. & Rappuoli, R. (2000). Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science, 287, 1816–1820.
- Rabie, N.S. & Amin Girh, Z.M.S. (2020). Bacterial vaccines in poultry. Bulletin of the National Research Centre, 44, 15.
- Rasko, D.A., Rosovitz, M.J., Myers, G.S., Mongodin, E.F., Fricke, W.F., Gajer, P., Crabtree, J., Sebaihia, M., Thomson, N.R., Chaudhuri, R., Henderson, I.R., Sperandio, V. & Ravel, J. (2008). The pangenome structure of Escherichia coli: comparative genomic analysis of E. coli commensal and pathogenic isolates. Journal of Bacteriology, 190, 6881–6893.
- Seemann, T. (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics, 30, 2068–2069.
- Szmolka, A. & Nagy, B. (2013). Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Frontiers in Microbiology, 4, 258.
- Tommassen, J., Agterberg, M., Janssen, R. & Spierings, G. (1993). Use of the enterobacterial outer membrane protein PhoE in the development of new vaccines and DNA probes. Zentralblatt Für Bakteriologie International Journal of Medical Microbiology, 278, 396–406.
- Vandekerchove, D., De Herdt, P., Laevens, H. & Pasmans, F. (2004). Colibacillosis in caged layer hens: characteristics of the disease and the aetiological agent. Avian Pathology, 33, 117–125.
- Wizemann, T.M., Heinrichs, J.H., Adamou, J.E., Erwin, A.L., Kunsch, C., Choi, G.H., Barash, S.C., Rosen, C.A., Masure, H.R. & Tuomanen, E. (2001). Use of a whole genome approach to identify vaccine molecules affording protection against Streptococcus pneumoniae infection. Infection and Immunity, 69, 1593–1598.
- Yaguchi, K., Ohgitani, T., Noro, T., Kaneshige, T. & Shimizu, Y. (2009). Vaccination of chickens with liposomal inactivated avian pathogenic Escherichia coli (APEC) vaccine by eye drop or coarse spray administration. Avian Diseases, 53, 245–249.
- Yang, J.N., Wang, C., Guo, C., Peng, X.X. & Li, H. (2011). Outer membrane proteome and its regulation networks in response to glucose concentration changes in Escherichia coli. Molecular Biosystems, 7, 3087–3093.
- Yasmin, H. & Yaqoub, A. (2018). Identification of cross-protective potential antigens against pathogenic Brucella spp. through combining Pan-genome analysis with reverse vaccinology. Journal of Immunology Research, 2018, 1474517.
- Yu, N.Y., Wagner, J.R., Laird, M.R., Melli, G., Rey, S., Lo, R., Dao, P., Sahinalp, S.C., Ester, M., Foster, L.J. & Brinkman, F.S. (2010). PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics, 26, 1608–1615.
- Zhu Ge, X., Jiang, J., Pan, Z., Hu, L., Wang, S., Wang, H., Leung, F.C., Dai, J. & Fan, H. (2014). Comparative genomic analysis shows that avian pathogenic Escherichia coli isolate IMT5155 (O2:K1:H5; ST complex 95, ST140) shares close relationship with ST95 APEC O1:K1 and human ExPEC O18:K1 strains. PLoS ONE, 9, e112048.