ABSTRACT
Avian chlamydiosis is an acute or chronic disease of birds after infection by Chlamydia. Although Chlamydia psittaci is the primary agent of the disease, two additional species, Chlamydia avium and Chlamydia gallinacea, have also been recognized as potential disease agents. Therefore, the diagnosis of avian chlamydiosis requires differential identification of these avian Chlamydia species. The objective of the present study was to develop a multiplex real-time polymerase chain reaction (PCR) assay to rapidly differentiate between these three species of avian Chlamydia (C. psittaci, C. avium, and C. gallinacea) as well as to detect the genus Chlamydia. Specific genetic regions of the three species were identified by comparative analysis of their genome sequences. Also, the genus-specific region was selected based on 23S rRNA sequences. PCR primers and probes specific to the genus and each species were designed and integrated in the multiplex real-time PCR assay. The assay was highly efficient (94.8–100.7%). It could detect fewer than 10 copies of each target sequence of the genus and each species. Twenty-five Chlamydia control and field DNA samples were differentially identified while 20 other bacterial strains comprising 10 bacterial genera were negative in the assay. This assay allows rapid, sensitive, and specific detection of the genus and the three species of avian Chlamydia in a single protocol that is suitable for routine diagnostic purposes in avian diagnostic laboratories.
Introduction
Avian chlamydiosis is an acute or chronic bacterial infectious disease that occurs in various pet birds, wild birds, and poultry after infection by Chlamydia (OIE, Citation2018; Vanrompay, Citation2020). Currently, 11 species in genus Chlamydia have been recognized. Chlamydia psittaci, Chlamydia avium, and Chlamydia gallinacea are commonly isolated from birds (Borel et al., Citation2018; Vanrompay, Citation2020). C. psittaci infection was originally reported in psittacine birds and humans who had contact with these birds, and was originally called psittacosis or parrot fever (OIE, Citation2018; Vanrompay, Citation2020). It is also found in a variety of mammals and many other bird species (Vanrompay, Citation2020). This systemic disease can cause significant economic damage to psittacine birds and domestic poultry farms, although its clinical signs can vary in severity depending on the species and age of birds and the virulence of the agent (van Buuren et al.; Citation1994; Vanrompay, Citation2020).
Two other Chlamydia species, C. avium and C. gallinacea, can also be found in birds (Sachse et al., Citation2014). Although their clinical and epidemiological importance remains unclear, C. avium has been isolated from pigeons and psittacines and C. gallinacea has been found in poultry and occasionally psittacines (Borel et al., Citation2018; Guo et al., Citation2016; Sachse et al., Citation2014; Stokes et al., Citation2019). C. gallinacea is associated with chlamydiosis in humans as well, although this needs to be further confirmed (Guo et al., Citation2016; Hulin et al., Citation2015). It is likely that C. avium can cause respiratory disease in psittacine birds and pigeons, and C. gallinacea is implicated in reduced body weight gains of chickens and is pathogenic in embryonated chicken eggs (Borel et al., Citation2018; Guo et al., Citation2016; Heijne et al., Citation2021). These two species are hypothesized agents of avian chlamydiosis and the diagnostic approach to the disease includes species-specific assays to identify the agent unambiguously (OIE, Citation2018; Sachse & Laroucau, Citation2015; Vanrompay, Citation2020).
C. psittaci can be isolated from cell culture or embryonated eggs. However, organism isolation is not the preferred method for the identification of avian chlamydiosis because isolation of the organism is time-consuming and risky to laboratory personnel (OIE, Citation2018; Vanrompay, Citation2020). In addition, it requires high-quality samples (Andersen & Vanrompay, Citation2008; OIE, Citation2018; Vanrompay, Citation2020). Moreover, some strains cannot grow in vitro (OIE, Citation2018; Vanrompay, Citation2020). Therefore, molecular methods such as conventional polymerase chain reaction (PCR), real-time PCR, and DNA microarray are recommended for the identification of avian Chlamydia to confirm infection. Currently, real-time PCR assays are commonly used to detect three avian Chlamydia species, C. psittaci, C. avium, and C. gallinacea, in diagnostic laboratories because it is a highly rapid and accurate method with potential for quantification (OIE, Citation2018; Vanrompay, Citation2020). The recommended procedure is to conduct a Chlamydiaceae-specific PCR, followed by PCR assays for specific detection of C. psittaci, C. avium, and C. gallinacea (OIE, Citation2018).
Nevertheless, the majority of currently available real-time PCR assays target a species of avian Chlamydia individually. There are no reports of multiplex real-time PCR assays being used to differentiate between all three avian Chlamydia species, although a duplex PCR was used to detect C. avium and C. gallinacea (Heijne et al., Citation2018). The diagnosis of avian chlamydiosis can be facilitated greatly with reduced sample handling time if relevant Chlamydia species can be simultaneously examined in samples, particularly psittacines, pigeons, and poultry birds. Thus the objective of this study was to identify genetic regions specific to the genus Chlamydia and three avian Chlamydia species (C. psittaci, C. avium and C. gallinacea) using comparative analysis of their genome sequences. Subsequently, a multiplex real-time PCR assay was developed to allow rapid differential diagnosis of avian chlamydiosis.
Materials and methods
Bacterial strains and DNA samples
Twenty-five DNA samples of Chlamydia and 20 DNA samples of other bacteria were used for sensitivity and specificity analyses in this study (). Control DNA samples of C. psittaci 6BC, C. pneumoniae CM-1 and C. trachomatis ATCC VR-902B were purchased from Vircell Microbiologists (Granada, Spain). Genomic DNAs of C. avium 10DC88 and C. gallinacea 08DC63 were kindly provided by Dr. Christiane Schnee (Friedrich-Loeffler-Institut, Jena, Germany). Twenty DNA samples of Chlamydia field samples were confirmed as C. psittaci (n = 14), C. avium (n = 3), or C. gallinacea (n = 3) using individual real-time PCR assays for Chlamydia spp., C. psittaci, C. avium, and C. gallinacea reported by Ehricht et al. (Citation2006), Menard et al. (Citation2006), Zocevic et al. (Citation2013) and Laroucau et al. (Citation2015), respectively, and ompA or 16S rRNA sequencing (Kaltenboeck et al., Citation1993; Pudjiatmoko et al. Citation1997). Twenty other DNA samples from non-chlamydial reference strains comprised 10 bacterial genera commonly found in poultry. These DNA samples were prepared using a DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. DNA preparations were stored at −20°C until use. All DNA samples of Chlamydia from field samples () were part of a DNA collection obtained by a project approved by the Animal Ethics Committee in the Animal and Plant Quarantine Agency (permission number 2021-577), Korea.
Design of primers and probes
Complete genome sequences of C. psittaci 6/BC (GenBank accession no. CP002586), C. avium 10DC88 (GenBank accession no. CP006571) and C. gallincea 08-1274/3 (GenBank accession no. CP015840) were downloaded from NCBI genome database (NCBI Resource Coordinators, Citation2015). Repeat sequences in the genomes were masked using Tandem Repeats Finder (Benson, Citation1999) and RepeatMasker (Jurka, Citation2000). Genome sequences were aligned using the Mauve program (Darling et al., Citation2010). Strain-specific sequences were extracted using BBmap (Bushnell, Citation2014) and EMBOSS package (Rice et al., Citation2000). Candidate regions specific to each of the three species of Chlamydia were selected using BLAST search (Altschul et al., Citation1997) and retrieved with 500 bp flanking length (). These retrieved sequences were used for designing primers and probes specific to each species. Also, 23S rRNA gene sequences of the genus Chlamydia were collected from the NCBI database. These sequences were aligned and analyzed to identify regions specific to the genus using CLC Main Workbench 6.9.1 software (Qiagen, Aarhus, Denmark). Specific regions were used for designing genus-specific primers and probes. All primers and probes were designed using Primer3 (Untergasser et al., Citation2012). Specificities of primer and probe sequences were tested by searching for homologous sequences in the NCBI database using BLAST.
Table 1. Genetic regions specific to three avian Chlamydia species based on comparative genomic analysis.
These probes were labelled with reporter dyes HEX (genus Chlamydia), FAM (C. psittaci), Texas Red (C. avium) and Cy5 (C. gallinacea) (Bioneer, Daejeon, Korea). Primer and probe sequences are shown in .
Table 2. Primers and probes for the multiplex real-time PCR assay for simultaneous detection of the genus of avian Chlamydia and its three species.
Real-time PCR assay
Multiplex real-time PCR assays were performed using CFX96 real-time PCR Detection Systems (Bio-Rad, Hercules, CA, USA) in a total volume of 20 μl in duplicate or triplicate. The reaction mixture contained 10 μl iQ Multiplex Powermix (Bio-Rad), 0.5 μg/μl of bovine serum albumin, 0.3 µM of each primer, 0.2 µM of each probe, and 1–2 µl of template DNA.
Amplification conditions consisted of an initial denaturation at 95°C for 3 min followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. Fluorescence signals were detected using HEX, FAM, Texas Red, and Cy5 channels. Individual real-time PCR reactions specific to the genus and each of three species were the same as those for Chlamydia spp., C. psittaci, C. avium, and C. gallinacea reported by Ehricht et al. (Citation2006), Menard et al. (Citation2006), Zocevic et al. (Citation2013), and Laroucau et al. (Citation2015), respectively. They were used for comparison with the multiplex real-time PCR assay described above.
Limit of detection and amplification efficiency
The detection limit and amplification efficiency of the multiplex real-time PCR assay were determined using plasmids containing the target sequences specific to the genus and three species of Chlamydia. Each target sequence was amplified by PCR using Platinum Taq DNA polymerase high fidelity (Invitrogen, Carlsbad, CA, USA) with specific primers () and DNA samples of C. psittaci 6/BC, C. avium 10DC88, and C. gallincea 08-1274/3. Amplicons were cloned into pCR2.1 (Invitrogen) according to the manufacturer’s instructions. Ten-fold serial dilutions (100–106copies/μl) of purified plasmids were prepared and used to determine the lowest number of copies that could be detected with the assay. Standard curves were also constructed to calculate the amplification efficiency of the assay.
Results
Genetic regions specific to the genus Chlamydia and its three species
Comparative analysis of complete genome sequences of C. psittaci 6/BC, C. avium 10DC88, and C. gallincea 08-1274/3 revealed four sequence regions specific to C. psittaci, six regions specific to C. avium, and 10 regions specific to C. gallinacea. GenBank accession numbers, sequence regions, and coding proteins of these sequence regions are presented in . Also, 23S rRNA gene sequence regions specific to the genus Chlamydia were identified by multiple sequence alignment (data not shown).
Establishment of the multiplex real-time PCR assay
Primers and probes specific to the genus Chlamydia and each of its three species (C. psittaci, C. avium, and C. gallinacea) were designed based on their specific regions found in this study (). The selected target regions were highly conserved within genus or species and had lowest similarity to other bacterial species based on multiple sequence alignments and BLAST searches. The multiplex real-time PCR using the combination of all primer sets and probes with HEX, FAM, Texas Red, and Cy5 channels was capable of detecting all target genes of the genus Chlamydia and its three species (C. psittaci, C. avium, and C. gallinacea) cloned into plasmids.
The efficiency and detection limit of the assay
Ten-fold serial dilutions (100–106 copies/μl) of plasmids harboring each target sequence were used to determine the efficiency and the detection limit of this multiplex real-time PCR assay. Experiments were carried out in triplicate to determine the reproducibility of the multiplex real-time PCR. The assay efficiently detected all target genes of the three species as well as the genus Chlamydia. Its amplification efficiency was 94.8% for genus Chlamydia, 99.8% for C. psittaci, 100.7% for C. avium, and 99.7% for C. gallinacea with high linear correlations (R2 > 0.99) (). This multiplex real-time PCR had a detection limit of less than 10 copies of each target sequence of the genus and each species ().
Figure 1. Efficiency of the multiplex real-time PCR assay. Amplification curves (A) and standard curves (B) of the assay for detecting Chlamydia positive control DNA were generated using the optimal amplification conditions in triplicate. Amplification efficiencies of the assay based on the standard curves ranged from 94.8% to 100.7% with high linear correlations (R2 > 0.99).
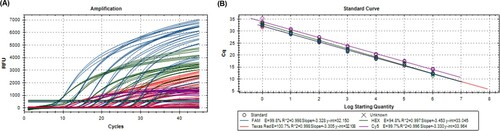
Table 3. Limits of detection of the multiplex real-time PCR assay.
The sensitivity and specificity of the assay
Using DNAs of five species of Chlamydia as references, 20 field DNA samples validated by individual real-time PCR and ompA or 16S rRNA sequencing for the genus of Chlamydia and its three species reported previously (Ehricht et al., Citation2006; Menard et al., Citation2006; Zocevic et al., Citation2013; Laroucau et al., Citation2015) were used to test the sensitivity of the assay. Also, 20 genomic DNA extracts of non-chlamydial bacteria representing 10 genera commonly found in poultry were used to test the specificity of the assay. Reference DNAs of C. psittaci 6BC, C. avium 10DC88, C. gallinacea 08DC63, C. pneumoniae CM-1, and C. trachomatis ATCC VR-902B, 13 C. psittaci, three C. avium, and three C. gallinacea samples were all successfully detected by the assay with Ct values comparable to those of known individual assays used for validation of samples (). The results also showed that 20 non-chlamydial bacterial DNAs were all negative with the assay ().
Table 4. Results of multiplex real-time assays for Chlamydia and non-Chlamydia strains and DNA samples.
Discussion
C. psittaci is the primary agent of avian chlamydiosis. It causes respiratory disease in psittacines, pigeons, and poultry among domesticated birds as well as many other species of birds (van Buuren et al.; Citation1994; Vanrompay, Citation2020; Vorimore et al., Citation2015). However, its clinical signs are usually nonspecific with various severity levels (van Buuren et al.; Citation1994; Vanrompay, Citation2020). Many birds including older psittacine birds and poultry might be asymptomatic, often shedding the organism in faeces and nasal discharges (Donati et al., Citation2018; Piasecki et al., Citation2012; Popelin-Wedlarski et al., Citation2020). C. avium has been mainly found in pigeons, which are asymptomatic, and has also been found in clinical cases of pigeons and psittacines (Borel et al., Citation2018; Pisanu et al., Citation2018; Popelin-Wedlarski et al., Citation2020). C. gallinacea is commonly identified in poultry flocks and occasionally psittacines, although its virulence in birds remains unclear (Frutos et al., Citation2015; Guo et al., Citation2016; Hulin et al., Citation2015; Stokes et al., Citation2019). Therefore, it is necessary to diagnose avian chlamydiosis using appropriate methods capable of specifically identifying avian Chlamydia species.
Species-specific detection of avian Chlamydia can be executed using molecular methods. PCR is the most commonly used method to directly detect Chlamydia species in tissue samples from sick birds and in cloacal samples or faeces from asymptomatic birds (OIE, Citation2018; Vanrompay, Citation2020). Real-time PCR is the preferred method validated for diagnostic purposes (OIE, Citation2018). Individual real-time PCR assays specific for Chlamydiaceae and avian Chlamydia species, C. psittaci, C. avium, and C. gallinacea, are available as described previously (Ehricht et al., Citation2006; Laroucau et al., Citation2015; Menard et al., Citation2006; Zocevic et al., Citation2013). The Chlamydiaceae-specific PCR assay is based on sequences of 23S rRNA of the family that includes only one genus, Chlamydia (Ehricht et al., Citation2006). Species-specific assays are based on sequences of the outer membrane protein A (OmpA) or the incA gene of C. psittaci (Menard et al., Citation2006; Pantchev et al., Citation2009), the enoA gene of C. avium (Zocevic et al., Citation2013), and the enoA gene and 16S rRNA of C. gallinacea (Laroucau et al., Citation2015; Zocevic et al., Citation2012). The currently recommended approach includes a Chlamydiaceae-specific PCR, followed by PCR assays for specific detection of C. psittaci, C. avium, and C. gallinacea (OIE, Citation2018).
In the present study, comparative analysis of genome sequences of C. psittaci, C. avium, and C. gallinacea available in the NCBI database revealed genetic regions specific to each species and useful for species differentiation. Selected sequence regions were highly conserved within species although they encode hypothetical proteins. Also, the analysis of 23S rRNA gene sequences available in the database showed specific sequence regions that could be used for identifying the genus Chlamydia. The development of the multiplex real-time PCR assay based on these specific sequence regions in the present study was intended to perform all gene-specific and species-specific detections in one reaction. The assay was highly efficient. It could be used to detect less than 10 copies of each target DNA sequence of the genus and each species. This assay could also specifically identify avian Chlamydia without any amplification of non-chlamydial species. Although numbers of positive and negative samples tested in this study were limited due to the difficulty in obtaining them, the sensitivity and specificity of this assay were at least comparable to those of individual assays reported previously (Ehricht et al., Citation2006; Laroucau et al., Citation2015; Menard et al., Citation2006; Zocevic et al., Citation2013).
Specific identification of avian Chlamydia species including C. psittaci, C. avium, and C. gallinacea has become necessary for the diagnosis of avian chlamydiosis in birds, such as domesticated birds. The multiplex real-time PCR assay developed in this study can be used to detect and differentiate avian Chlamydia species in bird samples more efficiently than the current sequential approach using individual assays. Therefore, this new rapid and accurate assay will be very useful for routine diagnosis of avian chlamydiosis in avian diagnostic laboratories, facilitating quicker identification of C. psittaci, a zoonotic agent capable of causing severe disease, with reduced sample handling time. Nevertheless, there was a limitation in that this assay was validated with limited numbers of Chlamydia control samples and geographically restricted field samples. Testing of the field samples from only one host species for each Chlamydia species could be another limitation of the assay, given the high levels of genetic diversity in the avian Chlamydia species, particularly C. psittaci and C. gallinacea. Further validation using local samples might be necessary to adopt this assay in diagnostic laboratories in other countries.
Acknowledgements
The authors would like to thank Dr. Christiane Schnee (Friedrich-Loeffler-Institut, Jena, Germany) for providing genomic DNAs of C. avium 10DC88 and C. gallinacea 08DC63. The authors also thank Kyung-Yun Kim (Insilicogen, Yongin, Korea) for help with comparative genome sequence analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W. & Lipman, D.J. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research, 25, 3389–3402.
- Andersen, A.A. & Vanrompay, D. (2008). Chlamydiosis. In L. Dufour-Zavala, D.E. Swayne, J.R. Glisson, J.E. Pearson, W.M. Reed, M.W. Jackwood & P.R. Woolcock (Eds.), A laboratory manual for the isolation, identification and characterization of avian pathogens, 5th edn (pp. 65–74). Jacksonville, FL: American Association of Avian Pathologists.
- Benson, G. (1999). Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Research, 27, 573–580.
- Borel, N., Polkinghorne, A. & Pospischil, A. (2018). A review on chlamydial diseases in animals: still a challenge for pathologists? Veterinary Pathology, 55, 374–390.
- Bushnell, B. (2014). BBMap: short read aligner, and other bioinformatic tools. https://sourceforge.net/projects//bbmap/.
- Darling, A.E., Mau, B. & Perna, N.T. (2010). Progressive mauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One, 5, e11147.
- Donati, M., Laroucau, K., Guerrini, A., Balboni, A., Salvatore, D., Catelli, E., Lupini, C., Levi, A. & Di Francesco, A. (2018). Chlamydiosis in backyard chickens (Gallus gallus) in Italy. Vector Borne and Zoonotic Diseases, 18, 222–225.
- Ehricht, R., Slickers, P., Goellner, S., Hotzel, H. & Sachse, K. (2006). Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Molecular and Cellular Probes, 20, 60–63.
- Frutos, M.C., Monetti, M.S., Vaulet, L.G., Cadario, M.E., Fermepin, M.R., Ré, V.E. & Cuffini, C.G. (2015). Genetic diversity of Chlamydia among captive birds from central Argentina. Avian Pathology, 44, 50–56.
- Guo, W., Li, J., Kaltenboeck, B., Gong, J., Fan, W. & Wang, C. (2016). Chlamydia gallinacea, not C. psittaci, is the endemic chlamydial species in chicken (Gallus gallus). Scientific. Reports, 6, 19638.
- Heijne, M., van der Goot, J., Fijten, H., van der Giessen, J., Kuijt, E., Maassen, C., van Roon, A., Wit, B., Koets, A. & Roest, I. (2018). A cross sectional study on Dutch layer farms to investigate the prevalence and potential risk factors for different Chlamydia species. PLoS One, 13, e0190774.
- Heijne, M., Jelocnik, M., Umanets, A., Brouwer, M., Dinkla, A., Harders, F., Keuken, L., Roest, H., Schaafsma, F., Velkers, F., Van der Goot, J., Pannekoek, Y. & Koets, A. (2021). Genetic and phenotypic analysis of the pathogenic potential of two novel Chlamydia gallinacea strains compared to Chlamydia psittaci. Scientific Reports, 11, 16516.
- Hulin, V., Oger, S., Vorimore, F., Aaziz, R., de Barbeyrac, B., Berruchon, J., Sachse, K. & Laroucau, K. (2015). Host preference and zoonotic potential of Chlamydia psittaci and C. gallinacea in poultry. Pathogens and Disease, 73, 1–11.
- Jurka, J. (2000). Repbase update: a database and an electronic journal of repetitive elements. Trends in Genetics, 16, 418–420.
- Kaltenboeck, B., Kousoulas, K.G. & Storz, J. (1993). Structures of and allelic diversity and relationships among the major outer membrane protein (ompA) genes of the four chlamydial species. Journal of Bacteriology, 175, 487–502.
- Laroucau, K., Aaziz, R., Meurice, L., Servas, V., Chossat, I., Royer, H., de Barbeyrac, B., Vaillant, V., Moyen, J.L., Meziani, F., Sachse, K. & Rolland, P. (2015). Outbreak of psittacosis in a group of women exposed to Chlamydia psittaci-infected chickens. Euro Surveillance, 20, 21155.
- Ménard, A., Clerc, M., Subtil, A., Mégraud, F., Bébéar, C. & de Barbeyrac, B. (2006). Development of a real-time PCR for the detection of Chlamydia psittaci. Journal of Medical Microbiology, 55, 471–473.
- NCBI Resource Coordinators. (2015). Database resources of the National Center for biotechnology information. Nucleic Acids Research, 43, D6–D17.
- OIE (World Organization for Animal Health). (2018). Chapter 3.3.1. avian chlamydiosis. In OIE Biological Standards Commission, Manual of diagnostic tests and vaccines for terrestrial animals, 8th edn (pp. 783–795). Paris: OIE.
- Pantchev, A., Sting, R., Bauerfeind, R., Tyczka, J. & Sachse, K. (2009). New real-time PCR tests for species-specific detection of Chlamydophila psittaci and Chlamydophila abortus from tissue samples. The Veterinary Journal, 181, 145–150.
- Piasecki, T., Chrząstek, K. & Wieliczko, A. (2012). Detection and identification of Chlamydophila psittaci in asymptomatic parrots in Poland. BMC Veterinary Research, 8, 233.
- Pisanu, B., Laroucau, K., Aaziz, R., Vorimore, F., Le Gros, A., Chapuis, J.L. & Clergeau, P. (2018). Chlamydia avium detection from a ring-necked parakeet (Psittacula krameri) in France. Journal of Exotic Pet Medicine, 2, 68–74.
- Popelin-Wedlarski, F., Roux, A., Aaziz, R., Vorimore, F., Lagourette, P., Crispo, M., Borel, N. & Laroucau, K. (2020). Captive psittacines with Chlamydia avium infection. Avian Diseases, 64, 542–546.
- Pudjiatmoko, F., Fukushi, H., Ochiai, Y., Yamaguchi, T. & Hirai, K. (1997). Phylogenetic analysis of the genus Chlamydia based on 16S rRNA gene sequences. International Journal of Systemic Bacteriology, 47, 425–431.
- Rice, P., Longden, I. & Bleasby, A. (2000). EMBOSS: the European Molecular Biology Open Software Suite. Trends in Genetics, 16, 276–277.
- Sachse, K. & Laroucau, K. (2015). Two more species of Chlamydia–does it make a difference? FEMS Pathogens and Disease, 73, 1–3.
- Sachse, K., Laroucau, K., Riege, K., Wehner, S., Dilcher, M., Creasy, H.H., Weidmann, M., Myers, G., Vorimore, F., Vicari, N., Magnino, S., Liebler-Tenorio, E., Ruettger, A., Bavoil, P.M., Hufert, F.T., Rosselló-Móra, R. & Marz, M. (2014). Evidence for the existence of two new members of the family Chlamydiaceae and proposal of Chlamydia avium sp. and Chlamydia gallinacea sp. nov. Systemic Applied Microbiology, 37, 79–88.
- Stokes, H.S., Martens, J.M., Chamings, A., Walder, K., Berg, M.L., Segal, Y. & Bennett, A. (2019). Identification of Chlamydia gallinacea in a parrot and in free-range chickens in Australia. Australian Veterinary Journal, 10, 20478.
- Untergasser, A., Cutcutache, I., Koressaar, T., Ye, J., Faircloth, B.C., Remm, M. & Rozen, S.G. (2012). Primer3—new capabilities interfaces. Nucleic Acids Research, 40, e115.
- van Buuren, C.E., Dorrestein, G.M. & van Dijk, J.E. (1994). Chlamydia psittaci infections in birds: a review on the pathogenesis and histopathological features. The Veterinary Quarterly, 16, 38–41.
- Vanrompay, D. (2020). Avian chlamydiosis. In D.E. Swayne, M. Boulianne, C.M. Logue, L.R. McDougald, V. Nair & D.L. Suarez (Eds.), Diseases of poultry, 14th edn (pp. 1086–1107). Hoboken, NJ: Wiley-Blackwell.
- Vorimore, F., Thebault, A., Poisson, S., Cleva, D., Robineau, J., de Barbeyrac, B., Durand, B. & Laroucau, K. (2015). Chlamydia psittaci in ducks: a hidden health risk for poultry workers. FEMS Pathogens and Disease, 73, 1–9.
- Zocevic, A., Vorimore, F., Marhold, C., Horvatek, D., Wang, D., Slavec, B., Prentza, Z., Stavianis, G., Prukner-Radovcic, E., Dovc, A., Siarkou, V.I. & Laroucau, K. (2012). Molecular characterization of atypical Chlamydia and evidence of their dissemination in different European and Asian chicken flocks by specific real-time PCR. Environmental Microbiology, 14, 2212–2222.
- Zocevic, A., Vorimore, F., Vicari, N., Gasparini, J., Jacquin, L., Sachse, K., Magnino, S. & Laroucau, K. (2013). A real-time PCR assay for the detection of atypical strains of Chlamydiaceae from pigeons. PLoS One, 8, e58741.
