 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Salmonella Heidelberg (SH) has been reported in broiler flocks of many countries. The ability of some SH strains of poultry origin to cause foodborne infections in humans is a concern. Usually, infection of broiler flocks by SH occurs in the first days of life. Therefore, control measures should start early post-hatch. One of the strategies is to generate high titres of anti-Salmonella IgY in breeders by using bacterins to provide passive immunity to their progeny. In this study, three broiler breeder flocks were submitted to three Salmonella vaccination regimes (two doses of vaccine 1, two doses of vaccine 2, and non-vaccinated). When breeders were 30 or 55 weeks old, some of their offspring were separated and challenged with an SH strain at 3 days of age. Dissemination to organs, caecal colonization, and faecal excretion of SH were evaluated over a period of 20 days. Chicks from vaccinated 30-week-old breeders presented lower amounts of SH in caecal contents at 1, 3, and 6 days post-infection, correlating with high titres of maternal anti-Salmonella IgY in their yolk. In contrast, there were no differences in counts of SH in caecal contents of chicks when their parents were 55 weeks old and titres of IgY were reduced. Amounts of SH in liver and spleen were low and there were no differences among birds throughout the experiment. Progeny from a 30-week-old flock vaccinated with vaccine 1 also showed lower SH faecal shedding than the remaining birds. Apparently, the maternal IgY was associated with reduction in intestinal infection by SH.
Progeny from vaccinated 30-week-old breeders presented less SH in caecal content compared to the control.
High titres of maternal anti-Salmonella IgY could be associated with lower SH faecal shedding.
RESEARCH HIGHLIGHTS
Introduction
Salmonella enterica subspecies enterica serovar Heidelberg (SH) remains a serious burden to the broiler industry and public health worldwide (Gieraltowski et al., Citation2016; Rodrigues et al., Citation2020). SH can be vertically or horizontally transmitted to the birds (Collineau et al., Citation2020), persisting in the environment of the poultry house for several months. When SH-contaminated litter is recycled without an effective treatment, day-old broiler chicks can be infected early after arriving on the farm (Voss-Rech et al., Citation2019).
The absence of a complex gut microbiota, and an immature immune system, make the chicks much more susceptible to colonization by paratyphoid Salmonella serovars during the first 2 weeks of life (Crhanova et al., Citation2011). The earlier the Salmonella colonization of the intestinal tract of the chick, the longer the duration of infection and shedding of this pathogen (Inoue et al., Citation2008). For this reason, it is crucial to any control programme to start preventing Salmonella infection early post-hatch.
Attempts to control this pathogen by administering antibiotic therapy to the poultry flocks have led to the selection of multi-resistant strains of SH (Souza et al., Citation2020) which can reach broiler meat and other related products (Carson et al., Citation2019). In addition to antimicrobial multi-resistance, SH strains have genetic traits that confer the ability to provoke severe foodborne infection in humans (Monte et al., Citation2019), representing a significant threat to public health. Consequently, alternative measures are necessary to control and reduce the presence of SH at the farm level.
High-titre specific maternal antibodies can be successfully induced in chicks throughout breeder vaccination, especially when intramuscular administration of inactivated vaccines is included in the programme (Young et al., Citation2007). Circulating antibodies are transferred to the egg yolk as immunoglobulin Y (IgY) and are mobilizable by the progeny after hatching (Murai, Citation2013). Although passive immunity is thought to provide some protection against the early infection by Salmonella in chicks, few studies have indeed evaluated this effect (Armwood et al., Citation2019; Inoue et al., Citation2008; Young et al., Citation2007).
In order to investigate the protective properties of the passive immunity against Salmonella, the present study was carried out. Herein, broiler chicks originated from three breeder flocks at the start and at the end of the production cycle previously submitted to different Salmonella vaccination regimes using commercial vaccines containing killed cells of Salmonella serovars Enteritidis and Typhimurium were experimentally infected with SH. Enumeration of SH in caecal contents, liver, and spleen was performed over a period of 20 days. SH faecal shedding was also monitored by cloacal swabs. Moreover, levels of Salmonella-specific IgY were measured in the serum and yolk sac of the day-old chicks.
Materials and methods
The experiment was carried out at the facilities of the Avian Diseases Laboratory of the Department of Preventive Veterinary Medicine of Federal University of Minas Gerais (UFMG).
Bacteria
Spontaneous nalidixic acid-resistant strain of Salmonella enterica subsp. enterica serovar Heidelberg (SH Nalr) was used to challenge the birds. SH Nalr was provided by Professor Angelo Berchieri Junior from the State University of São Paulo, Jaboticabal campus. It had been previously isolated from a broiler flock from Brazilian South region (SISGEN accession: A968A81).
Broiler chicks
Day-old broiler chicks originated from three distinct Cobb500™ (Cobb-Vantress) breeder flocks were obtained from a Brazilian poultry company at two time- points, when breeders were about 30 and 55 weeks old, respectively. In brief, the three breeder flocks had the same age and were reared in the same property but in distinct barns located at the same nucleus of production and receiving the same feeding programme. Except for Salmonella vaccination, the three breeder flocks were submitted to the same vaccination scheme which includes vaccines against Marek's disease, fowl pox, infectious bursal disease, infectious bronchitis, Newcastle disease, chicken anaemia, encephalomyelitis, orthoreovirus and coccidiosis. Breeder flocks were also submitted to an official programme of periodic monitoring for Mycoplasma spp. and Salmonella spp. (Brazil, Citation2009) and they remained free from these pathogens. Flocks were sampled at 1, 12, 24, 36, 40, and 52 weeks of age for the Salmonella monitoring scheme. Samples of faeces, cloacal swabs, drag swabs, organs and offspring were submitted to bacteriological procedures for Salmonella spp. isolation and serovar identification. Blood serum samples of the unvaccinated breeders were also collected at 12, 24, 36, 40, and 52 weeks of age and tested by rapid slide agglutination test with Salmonella Pullorum-stained antigen. In case of positive reaction, serum samples were also evaluated by a microagglutination test. Additionally, they were monitored for other viruses, including aviadenovirus, avian metapneumovirus, avian leukosis, EDS-76 virus, and avian reticuloendotheliosis virus, and none of them were diagnosed.
Upon arrival at the laboratory, samples of meconium were collected from the cardboard transport boxes and processed to assure that the birds were free of Salmonella spp. In addition, in both the experiments, 10 birds of each breeder flock were maintained as negative controls in a separate room and were monitored for Salmonella spp. twice each week. Samples of meconium and pools of caecal faeces were examined according to Zancan et al. (Citation2000).
Experimental design
Two experiments were carried out. In the first, chicks from 30-week-old broiler breeders were divided into three groups of 40 birds each (A, B, and C). Meanwhile, in the second experiment, chicks from the same breeders at 55 weeks of age were divided into three groups of 40 birds each (D, E, and F). All groups of birds were housed in three distinct battery cages placed in isolating acclimatized rooms. Feed and water were provided ad libitum. Birds of groups A and D originated from broiler breeders vaccinated with two doses (at 12 and 16 weeks) of an inactivated Salmonella vaccine 1 (Gallimune® SE + ST, Boehringer Ingelheim Animal Health, containing killed cells of Salmonella serovars Enteritidis and Typhimurium plus oil adjuvant) and birds of groups B and E originated from breeders previously vaccinated with two doses of an inactivated Salmonella vaccine 2 (Nobilis® Salenvac T, MSD Animal Health, formulated with killed cells of Salmonella serovars Enteritidis and Typhimurium plus aluminium hydroxide gel adjuvant). Birds of groups C and F originated from breeders that had not been vaccinated against Salmonella spp.
At 3 days of age, birds of all infected groups received 0.2 ml of an inoculum containing 5 × 103 colony forming units (CFU) of SH Nalr by oral gavage. The experiments were carried out in accordance with the recommendations in the Ethical Principles on Animal Experimentation (CEUA) of the National Council for the Control of Animal Experimentation (CONCEA). The protocol was approved by the Ethical Committee on Animal Experimentation from the Veterinary School of Federal University of Minas Gerais on 16 April 2020 (Permit Number: 68/2020).
Bacteriology
At 1, 3, 6, 12, and 20 days post-infection (dpi), five birds from each infected group (25 birds in total) were humanely euthanized. Samples of spleens, livers, and caecal contents were collected for bacterial enumeration. Bacterial shedding in faeces was also monitored by cloacal swabs twice each week. All bacteriological procedures followed the methodology described by Berchieri et al. (Citation2001). Briefly, enumeration of Salmonella strains in the samples was estimated by plating aliquots of decimal dilutions onto brilliant green agar (BGA) (Oxoid, London, UK) plates, containing 100 μg/ml of nalidixic acid (Sigma-Aldrich, Saint Louis, MO, USA). The first dilution of each sample was added to an equal volume of double-strength selenite broth (Oxoid, London, UK) and incubated. Plates and selenite enrichment cultures were also incubated for 24 h at 37°C.
Chicks were also examined by cloacal swabs at 2, 7, 10, 14, 17, and 20 dpi. In total, 115 swabs were taken from birds of each group. Swabs were plated on BGA and further incubated in selenite broth. Those samples for which no bacteria grew on BGA were re-streaked onto new BGA plates from the enriched cultures.
Anti-Salmonella IgY detection in vitelline sac and serum
At 1 day of age, five birds of each group were euthanized. Blood serum and yolk content of the vitelline sac were collected for anti-Salmonella antibody detection using specific indirect ELISA for antibodies against serogroup B Salmonella serovars (BioChek, Gouda, The Netherlands). Yolk contents were previously subjected to a purification step described by Murai et al. (Citation2016). In summary, 1 g of the yolk was collected and transferred into a 50-ml polypropylene tube. The yolk sample was diluted with five volumes of PBS and stored at 4°C overnight, then centrifuged at 10,000 × g for 25 min at 4°C. The supernatant was collected and filtered through Whatman number 2 filter paper. This final solution was used for the determination of the IgY concentrations. Sample dilutions and all procedures for antibody detection were performed according to the manufacturer’s instructions (BioChek, Gouda, The Netherlands). After a final incubation step, ELISA microplates were immediately analysed in a Bio-Rad absorbance reader, model 680 (Bio-Rad, Hercules, CA, USA), set at a wavelength of 405 nm and the readings analysed using the BioChek II Diagnostic Software version 2018 (BioChek).
The relative amounts of antibodies in the samples (serum or egg yolk) were calculated with reference to the positive control sample (provided in the kit). This relationship was expressed as S/P ratio (tested sample to positive control) which was calculated using the following equation:
The antibody titres were calculated using a subsequent equation which relates the S/P ratio of the tested samples at a 1:100 dilution to end-point titres.
Log10 titre = 1.13 * Log10 (S/P) + 3.156. The titre value is the antilog of the obtained number. Geometric mean titres (GMT) were calculated using the following equation:
Statistical analysis
Mean counts data of Salmonella strains recovered from caecal contents, livers and spleens were first submitted to normality test. Bacterial counts data that showed a normal distribution (experiment 1, mean counts of SH in caecal contents at 12 dpi) were analysed by means of two-way ANOVA and compared by Tukey’s test. On the contrary, non-Gaussian distribution (the remaining bacterial count data) was evaluated by Mann–Whitney U test. Data on faecal shedding obtained by cloacal swabs (primary or delayed secondary enrichment) were converted to binary numbers (1, 0) and were compared by Chi-Square test. Geometric mean titres of IgY from progeny yolk and serum were compiled and compared using Tukey’s test. P < 0.05 was considered significant. Statistical analyses were performed using GraphPad Prism version 8.0.1 (GraphPad Software, US).
Results
Experiment 1: progeny from 30-week-old broiler breeders
Bacteriology
Bacteriological examination of the cardboard transport boxes and of the uninfected control chicks indicated that experimental birds were free of Salmonella spp.
The results of SH enumeration in livers, spleens, and caecal contents of birds belonging to groups A (originated from breeders immunized with vaccine 1 and challenged with SH), B (originated from breeders immunized with vaccine 2 and challenged with SH), and C (originated from unvaccinated breeders and challenged with SH) are shown in . There was no statistically significant difference among the bacterial numbers in livers and spleens over the experiment (P > 0.05).
Table 1. Mean counts of Salmonella Heidelberg Nalr (SH) of five birds in spleen, liver, and caecal contents at 1, 3, 6, 12, and 20 days post-infection (dpi).
At 1, 3, and 6 dpi, SH counts in caecal contents were lower in birds of group A, when compared with birds of group C (P < 0.05). On the contrary, at 12 and 20 dpi, counts in caecal contents were similar in birds of all groups (P < 0.05). SH shedding was monitored by cloacal swabs of birds of groups A, B, and C and the results are displayed in . The number of positive cloacal swabs in birds of group A was lower than that in birds of groups B and C throughout the experiment (P < 0.01). The differences in SH faecal shedding were seen at 2, 7, and 10 dpi.
Table 2. Recovery of Salmonella Heidelberg Nalr (SH) from birds by cloacal swabs taken at 2, 7, 10, 14, 17, and 20 days post-infection (dpi).
Anti-Salmonella IgY detection – progeny from 30-week-old broiler breeders
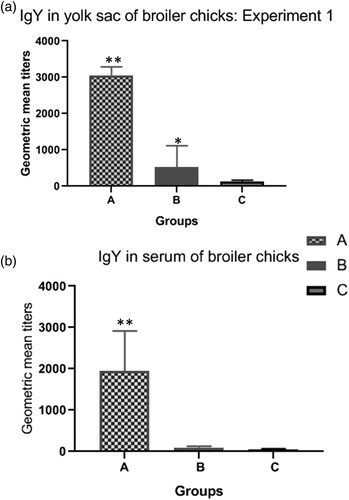
Geometric mean titres (GMT) of IgY anti-Salmonella serogroup B in vitelline content and blood serum of 1-day-old broiler chicks are shown in . Birds of groups A and B produced higher levels of IgY than birds originated from unvaccinated breeders. However, titres were much higher in birds of group A compared to group B (P < 0.01).
Figure 1. Antibody titres in progeny of broiler breeders submitted to different regimes of vaccination against Salmonella. Group A: birds originated from breeders vaccinated with vaccine 1. Group B: birds originated from breeders vaccinated with vaccine 2. Group C: birds originated from breeders unvaccinated against Salmonella. The antibody titres (GMT) were measured in the yolk contents (a) and blood serum (b) of day-old broiler chicks. Significant differences are indicated by asterisks (*P < 0.05; **P < 0.01).
Experiment 2: progeny from 55-week-old broiler breeders
Bacteriology
The results of SH enumeration in livers, spleens, and caecal contents of birds belonging to groups D (originated from breeders vaccinated with vaccine 1 and challenged with SH), E (originated from breeders immunized with vaccine 2 and challenged with SH), and F (originated from unvaccinated breeders and challenged with SH) are shown in . There was no statistically significant difference among the bacterial numbers in livers, spleens, and caecal contents throughout the experiment (P > 0.05). SH shedding was monitored by cloacal swabs of birds of groups D, E, and F, and results are displayed in . The number of positive cloacal swabs in birds of group D was lower than that in birds of groups E and F throughout the experiment (P < 0.01). However, these differences were concentrated at 14 and 17 dpi.
Table 3. Mean counts of Salmonella Heidelberg Nalr (SH) of five birds in spleen, liver, and caecal contents at 1, 3, 6, 12, and 20 days post-infection (dpi).
Table 4. Recovery of Salmonella Heidelberg Nalr (SH) from birds by cloacal swabs taken at 2, 7, 10, 14, 17, and 20 days post-infection (dpi).
Anti-Salmonella IgY detection – progeny from 55-week-old broiler breeders
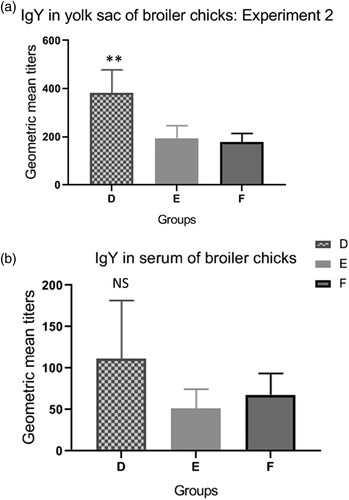
GMT of IgY in yolk sac and blood serum of 1-day-old broiler chicks are shown in . Although the overall titres of IgY were low in birds of all groups, birds of group D produced higher levels of IgY (P < 0.01) in yolk content than birds of groups E and F. There were no significant differences among levels of IgY in blood serum of birds of the three groups.
Figure 2. Antibody titres in progeny of broiler breeders submitted to different regimes of vaccination against Salmonella. Group D: birds originated from breeders vaccinated with vaccine 1. Group E: birds originated from breeders vaccinated with vaccine 2. Group F: birds originated from breeders unvaccinated against Salmonella. The antibody titres (GMT) were measured in the yolk contents (a) and blood serum (b) of day-old broiler chicks. Significant differences are indicated by asterisks (P < 0.01).
Discussion
Salmonella Heidelberg (SH) is a concern to the broiler industry of many countries (Antunes et al., Citation2016; Armwood et al., Citation2019). The continued isolation of strains of SH which are resistant to multiple antimicrobial agents (Souza et al., Citation2020) adds to the importance of studying ways to mitigate the risk of this organism in broiler flocks. Consequently, control measures that could help to reduce this pathogen are welcome.
Similarly to other paratyphoid Salmonella serovars, the introduction of SH into the broiler chain involves several players including contaminated litter, vertical transmission from parent flocks to their progeny, rodents and insect infestations, contaminated feed, inefficient cleaning and disinfection procedures, circulation of personnel, and contaminated fomites among others (Collineau et al., Citation2020; Gast & Porter, Citation2020). In most cases, infections of chicks by SH occur very early, in the first days of life (Sivaramalingam et al., Citation2013). Therefore, control measures should start as early as possible.
One of the strategies explored by broiler companies to provide early protection against Salmonella is the transmission of passive immunity to the progeny (Dórea et al., Citation2010; Inoue et al., Citation2008). There are different commercial inactivated Salmonella vaccines available to be used in breeders with the purpose to illicit high titres of specific circulating antibodies to be transferred to the progeny. The majority is formulated using inactivated cells of Salmonella serovar Enteritidis (SE) and/or Typhimurium (STM) plus adjuvant (El-Enbaawy et al., Citation2013; Crouch et al., Citation2020). SH has somatic (O) and flagellar (H) antigenic composition which is very similar to that of STM, differing only in the flagellar phase 1 antigen (Grimont & Weill, Citation2007). Therefore, cross-protection against SH is expected using vaccines formulated with STM.
In the present study, passive immunity in progeny of broiler breeders receiving three Salmonella vaccination regimes was evaluated. Broiler chicks were challenged with an SH field strain at 3 days old and invasion of organs, caecal colonization and faecal excretion of the strain were evaluated at 20 days. It was observed that chicks from vaccinated 30-week-old breeders (groups A and B) presented lower amounts of SH in caecal contents at 1, 3, and 6 dpi (), indicating correlation between the reductions in caecal colonization and high titres of maternal anti-Salmonella IgY present in the yolk contents of these chicks (), although the mechanisms behind maternal IgY protection against gut colonization of pathogenic bacteria are not fully understood (Härtle et al., Citation2014). By contrast, when progeny from 55-week-old breeders were challenged (experiment 2), titres of maternal antibodies were low () and no differences were found in SH enumeration in caecal contents of chicks from all groups (). Similar results have been described by Armwood et al. (Citation2019), which evaluated the progeny of breeder flocks with high and low levels of IgY and found a higher percentage of SH in caeca and liver/spleen of chicks from breeders with reduced levels of IgY. Inoue et al. (Citation2008) also demonstrated early protective effects of maternal antibodies in broiler chicks challenged with SE.
Although it was not the aim of this study to investigate differences between vaccines, it was possible to see variations between antibody titres, duration, and protection conferred by the two vaccines used in the present study. Possibly, differences in formulations including adjuvant inactivation method, among other manufacturing details, could be related to these differences (Rabie & Girh, Citation2020).
The SH counts in liver and spleen were low in both the experiments and there were no statistical differences between birds of all groups. Differences in the ability of paratyphoid Salmonella strains to cause systemic infection in chicks have been described for other serovars (Hinton et al., Citation1990; Martelli et al., Citation2014) and this also seems to be the case with SH. The low SH counts in liver and spleen indicates that the strain used in this study showed limited ability to establish systemic infection. Consequently, herein, it was not possible to properly assess the effects of circulating maternal antibodies on systemic infection by SH.
In this study, birds of groups A (progeny from 30-week-old breeders vaccinated with vaccine 1) also presented reductions in SH faecal shedding throughout the experiment (). It was observed that reductions in faecal shedding were concentrated mainly in the first week of testing, correlating with the data of caecal counts and the levels of IgY in these birds. Considering that reductions of Salmonella faecal shedding are important as part of any control programme (FAO, Citation2002; Van Immerseel et al., Citation2009), the data of the present study indicate that maternal-derived antibodies could contribute to the control of this pathogen.
By contrast, in experiment 2, although an overall reduction in SH faecal shedding was observed in birds of group D compared to others, these differences indeed happened at 14 and 17 dpi (). Taking into account the low levels of maternal IgY in these birds and the differences in SH shedding were observed in late stages of the trial, they are likely related to other unmeasured factors rather than maternal antibodies. Altogether, these results suggest that passive immunity elicited by the inactivated Salmonella vaccines used in this study was not maintained for the whole production life of the breeder flocks. By contrast, Crouch et al. (Citation2020) reported high titres of specific IgY in 56-week-old broiler breeders vaccinated at 10 and 17 weeks of age with a Salmonella-inactivated vaccine, but passive immunity and protection against Salmonella were not assessed in the offspring of these breeders.
According to Kowalczyk et al. (Citation1985), the total amount of IgY absorbed by the chick represents about 10% of that deposited into the egg yolk, and the fate of the remaining 90% is not known. The IgY delivered to the intestine when the remaining contents of the yolk sac are transferred through the yolk stalk is probably much more important for the early protection against gut infections than the circulating IgY (Härtle et al., Citation2014; Tan et al., Citation2019; Xu et al., Citation2011). Indeed, in the present study, even with low titres of circulating IgY, reductions in caecal colonization were observed in birds of group B (), suggesting little or no effect of circulating IgY in this process.
It has been hypothesized that IgY enables agglutination of pathogens (viral, bacterial, and fungal), leading to their immobilization, which facilitates their removal from the gut (Tan et al., Citation2019). In vitro studies proved that inhibition of adhesion is a key mechanism for IgY action against Salmonella spp. It was demonstrated that IgY is able to bind to exposed factors on the surface of Gram-negative bacteria, such as fimbriae (or pili), flagella, lipopolysaccharides, and outer membrane proteins (Xu et al., Citation2011), impairing the function of these growth-related components of the bacteria (Lee et al., Citation2002). Possibly, this mechanism also takes place in vivo, although additional studies are necessary to confirm.
Our results suggest some level of protection conferred by maternal-derived antibodies against intestinal infection by SH in broiler chicks under experimental conditions. We believe these results could be extrapolated to the field conditions and this tool (passive immunity) could indeed contribute to the control of SH by reducing its prevalence in the broiler farms. The positive effects of this strategy were already demonstrated in two field studies that evaluated the impact of Salmonella vaccination of breeders on the prevalence of this pathogen in their broiler progeny (Berghaus et al., Citation2011; Dórea et al., Citation2010). However, in order to yield satisfactory results, maternal immunity should be adopted as part of a rigorous biosecurity programme, including suitable cleaning and disinfection procedures in the hatchery and barns, rodent control, and additional tools devoted to reduce the contamination by Salmonella on broiler farms as much as possible (Gast & Porter, Citation2020; Van Immerseel et al., Citation2009).
In summary, high titres of maternal antibodies transferred by the 30-week-old breeders immunized with inactivated vaccines containing STM to their progeny could be associated with reductions in caecal colonization by SH. Day-old chicks with the highest levels of IgY in yolk content also presented the lowest SH faecal shedding over 20 days of the experiment. Levels of IgY transferred by the vaccinated breeders to their progeny were low when they reached 55 weeks of age, increasing the susceptibility of their offspring to SH infections. Additional studies that include evaluation of a more complete vaccination programme, and also assessing progeny of breeders of various ages, are needed to determine the ideal Salmonella-specific IgY titres in the chicken to maintain reasonable passive protection for a longer period.
Acknowledgements
National Council of Technological and Scientific Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES) research grants are gratefully acknowledged for the scholarships.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Antunes, P., Mourao, J., Campos, J. & Peixe, L. (2016). Salmonellosis: the role of poultry meat. Clinical Microbiology and Infection, 22, 110–121.
- Armwood, B.T., Rieth, A., Baldwin, L., Roney, C.S., Barbieri, N.L. & Logue, C.M. (2019). Assessing the ability of maternal antibodies to protect broiler chicks against colonization by Salmonella Heidelberg. Avian Diseases, 63, 289–293.
- Berchieri, A., Murphy, C.K., Marston, K. & Barrow, P.A. (2001). Observations on the persistence and vertical transmission of Salmonella enterica serovars Pullorum and Gallinarum in chickens: effect of bacterial and host genetic background. Avian Pathology, 30, 221–231.
- Berghaus, R.D., Thayer, S.G., Maurer, J.J. & Hofacre, C.L. (2011). Effect of vaccinating breeder chickens with a killed Salmonella vaccine on Salmonella prevalences and loads in breeder and broiler chicken flocks. Journal of Food Protection, 74, 727–734.
- Brazil – Ministry of Agriculture, Livestock and Food Supply. (2009). Normative Instruction n.78 of November 3, 2003. In Legislation Manual (pp. 200–207). National Animal Health Programs in Brazil. http://ww3.panaftosa.org.br/Comp/MAPA/ManuaisTecnicos/ManualLegislacao/LegislationManualOK.pdf.
- Carson, C., Li, X.Z., Agunos, A., Loest, D., Chapman, B., Finley, R., Mehrotra, M., Sherk, L.M., Gaumond, R. & Irwin, R. (2019). Ceftiofur-resistant Salmonella enterica serovar Heidelberg of poultry origin – a risk profile using the Codex framework. Epidemiology and Infection, 4, 1–20.
- Collineau, L., Phillips, C., Agunos, A., Carson, C., Chapman, B., Fazil, A., Reid-Smith, R. & Smith, B.A. (2020). A within-flock model of Salmonella Heidelberg transmission in broiler chickens. Preventive Veterinary Medicine, 174, 1–13.
- Crhanova, M., Hradecka, H., Faldynova, M., Matulova, M., Havlickova, H., Sisak, F. & Rychlik, I. (2011). Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar Enteritidis infection. Infection and Immunity, 79, 2755–2763.
- Crouch, C.F., Nell, T., Reijnders, M., Donkers, T., Pugh, C., Patel, A., Davis, P., van Hulten, M.C.W. & Vries, S.P.W. (2020). Safety and efficacy of a novel inactivated trivalent Salmonella enterica vaccine in chickens. Vaccine, 38, 6741–6750.
- Dórea, F.C., Cole, D.J., Hofacre, C., Zamperini, K., Mathis, D., Doyle, M.P., Lee, M.D. & Maurer, J.J. (2010). Effect of Salmonella vaccination of breeder chickens on contamination of broiler chicken carcasses in integrated poultry operations. Applied and Environmental Microbiology, 76, 7820–7825.
- El-Enbaawy, M.I., Ahmed, Z.A.M., Sadek, M.A. & Ibrahim, H.M. (2013). Protective efficacy of Salmonella local strains representing groups B, C, D and E in a prepared polyvalent formalin inactivated oil adjuvant vaccine in layers. International Journal of Microbiological Research, 4, 288–295.
- FAO (Food and Agriculture Organization of the United Nations). (2002). Risk characterization of Salmonella in broilers. In: Risk assessments of Salmonella in eggs and broiler chickens, Microbiological Risk Assessment Series 2 (pp. 277–296).
- Gast, R.K. & Porter Jr, R.E. (2020). Salmonella infections. In M. Bouliane, C.M. Logue, L.R. McDougald, V. Nair & D.L. Suarez (Eds.), Diseases of poultry (pp. 719–753). Iowa: Wiley-Blackwell.
- Gieraltowski, L., Higa, J., Peralta, V., Green, A., Schwensohn, C., Rosen, H., Libby, T., Kissler, B., Marsden-Haug, N., Booth, H., Kimura, A., Grass, J., Bicknese, A., Tolar, B., Defibaugh-Chávez, S., Williams, I. & Wise, M. (2016). National outbreak of multidrug resistant Salmonella Heidelberg infections linked to a single poultry company. PLoS One, 11, 1–13.
- Grimont, P.A. & Weill, F.X. (2007). Antigenic formulae of the Salmonella serovars (p. 168). Paris: WHO Collaborating Centre Reference Research of Salmonella..
- Härtle, S., Magor, K.E., Göbel, T.W., Davison, F. & Kaspers, B. (2014). Structure and evolution of avian immunoglobulins. In K.A. Schat, B. Kaspers & P. Kaiser (Eds.), Avian immunology (pp. 103–120). Waltham: Academic Press (Elsevier).
- Hinton, M., Threlfall, E.J. & Rowe, B. (1990). The invasive potential of Salmonella Enteritidis phage types for young chickens. Letters in Applied Microbiology, 10, 237–239.
- Inoue, A.Y., Berchieri, A., Bernardino, A., Paiva, J.B. & Sterzo, E.V. (2008). Passive immunity of progeny from broiler breeders vaccinated with oil-emulsion bacterin against Salmonella enteritidis. Avian Diseases, 52, 567–571.
- Kowalczyk, K., Daiss, J., Halpern, J. & Roth, T.F. (1985). Quantitation of maternal-fetal IgG transport in the chicken. Immunology, 54, 755–762.
- Lee, E.N., Sunwoo, H.H., Menninen, K. & Sim, J.S. (2002). In vitro studies of chicken egg yolk antibody (IgY) against Salmonella Enteritidis and Salmonella Typhimurium. Poultry Science, 81, 632–641.
- Martelli, F., Gosling, R., Kennedy, E., Rabie, A., Reeves, H., Clifton-Hadley, F., Davies, R. & La Ragione, R. (2014). Characterization of the invasiveness of monophasic and aphasic Salmonella Typhimurium strains in 1-day-old and point-of-lay chickens. Avian Pathology, 43, 269–275.
- Monte, D.F., Lincopan, N., Berman, H., Cerdeira, L., Keelara, S., Thakur, S., Fedorka-Cray, P.J. & Landgraf, M. (2019). Genomic features of high-priority Salmonella enterica serovars circulating in the food production chain, Brazil, 2000–2016. Scientific Reports, 9, 1–12.
- Murai, A. (2013). Maternal transfer of immunoglobulins into egg yolks of birds. Journal of Poultry Science, 50, 185–193.
- Murai, A., Kakiuchi, M., Hamano, T., Kobayashi, M., Tsudzuki, M., Nakano, M., Matsuda, Y. & Horio, F. (2016). An ELISA for quantifying quail IgY and characterizing maternal IgY transfer to egg yolk in several quail strains. Veterinary Immunology and Immunopathology, 175, 16–23.
- Rabie, N.S. & Girh, Z.M.S.A. (2020). Bacterial vaccines in poultry. Bulletin of the National Research Centre, 44, 1–7.
- Rodrigues, I.B.B.E., Silva, R.L., Menezes, J., Machado, S.C.A., Rodrigues, D.P., Pomba, C., Abreu, D.L.C., Nascimento, E.R., Aquino, M.H.C. & Pereira, V.L.A. (2020). High prevalence of multidrug-resistant nontyphoidal Salmonella recovered from broiler chickens and chicken carcasses in Brazil. Brazilian Journal of Poultry Science, 22, 1–6.
- Sivaramalingam, T., Pearl, D.L., McEwen, S.A., Ojkic, D. & Guerin, M.T. (2013). A temporal study of Salmonella serovars from fluff samples from poultry breeder hatcheries in Ontario between 1998 and 2008. Canadian Veterinary Journal Research, 77, 12–23.
- Souza, A.I.S., Saraiva, M.M.S., Casas, M.R.T., Oliveira, G.M., Cardozo, M.V., Benevides, V.P., Barbosa, F.O., Neto, O.C.F., Almeida, A.M. & Berchieri, A.J. (2020). High occurrence of beta-lactamase-producing Salmonella Heidelberg from poultry origin. PLoS One, 15, 1–11.
- Tan, X., Li, J., Li, Y., Li, J., Wang, Q., Fang, L., Ding, X., Huang, P., Yang, H. & Yin, Y. (2019). Effect of chicken egg yolk immunoglobulins on serum biochemical profiles and intestinal bacterial populations in early-weaned piglets. Journal of Animal Physiology and Animal Nutrition, 103, 1503–1511.
- Van Immerseel, F., De Zutter, L., Houf, K., Pasmans, F., Haesebrouck, F. & Ducatelle, R. (2009). Strategies to control Salmonella in the broiler production chain. World’s Poultry Science Journal, 65, 367–392.
- Voss-Rech, D., Kramer, B., Silva, V.S., Rebelatto, R., Abreu, P.G., Coldebella, A. & Vaz, C.S.L. (2019). Longitudinal study reveals persistent environmental Salmonella Heidelberg in Brazilian broiler farms. Veterinary Microbiology, 233, 118–123.
- Xu, Y., Li, X., Jin, L., Zhen, Y., Lu, Y., Li, S., You, J. & Wang, L. (2011). Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: a review. Biotechnology Advances, 29, 860–868.
- Young, S.C., Olusanya, O., Jones, K.H., Liu, T., Liljebjelke, K.A. & Hofacre, C.L. (2007). Salmonella incidence on broilers from breeders vaccinated with live and killed Salmonella. Journal of Applied Poultry Research, 16, 521–528.
- Zancan, F.B., Berchieri Junior, A., Fernandes, S.A. & Gama, N.M.S.Q. (2000). Salmonella spp. investigation in transport box of day old birds. Brazilian Journal of Microbiology, 31, 230–232.
