ABSTRACT
Infectious bursal disease virus (IBDV) induces one of the most important immunosuppressive diseases in chickens leading to high economic losses due to increased mortality and condemnation rates, secondary infections and the need for antibiotic treatment. Over 400 publications have been listed on PubMed.gov in the last 5 years pointing out the research interest in this disease and the development of improved preventive measures. While B cells are the main target cells of the virus, other immune and non-immune cell populations are also affected, leading to a multifaceted impact on the normally well-orchestrated immune system in IBDV-infected birds. Recent studies clearly revealed the contribution of innate immune cells as well as T cells to a cytokine storm and subsequent death of affected birds in the acute phase of the disease. Transcriptomics identified differential regulation of immune-related genes between different chicken genotypes as well as virus strains, which may be associated with a variable disease outcome. The recent availability of primary B cell culture systems allowed a closer look into virus-host interactions during IBDV infection. The new emerging field of research with transgenic chickens will also open up new opportunities to understand the impact of IBDV on the host under in vivo conditions, which will help to understand the complex virus-host interactions further.
Introduction
The infectious bursal disease virus was first described in 1962 in Gumboro, Delaware, USA (Cosgrove, Citation1962; Eterradossi & Saif, Citation2020) and belongs to the family of Birnaviridae (Dobos et al., Citation1979). Today, different IBDV strains, antigenic variants and reassortants as well as recombinant viruses can be detected in chicken flocks almost all over the world (reviewed in Brown Jordan et al. (Citation2018); Islam et al. (Citation2021); Deorao et al., Citation2021; Feng et al., Citation2021). Recently, a new unified nomenclature was proposed allowing the classification of isolates based on both genome segments, specifically the regions encoding the viral proteins (VP) 2 and VP1 (Michel & Jackwood, Citation2017; Jackwood et al., Citation2018; Islam et al., Citation2021). While the sequence of VP1 is encoded in one open reading frame on segment B of the bisegmented double-stranded RNA (Azad et al., Citation1985; Morgan et al., Citation1988), segment A consists of two partially overlapping open reading frames (Müller, Scholtissek, et al., Citation1979). The smaller ORF encodes VP5 (Mundt et al., Citation1995), and the larger ORF encodes the polyprotein precursor (p) VP2-VP4-VP3 (Azad et al., Citation1985; Hudson et al., Citation1986). Therefore, eight genogroups based on segment A within serotype 1, and one genogroup of serotype 2 have now been confirmed. Based on segment B, five genogroups were identified. Due to currently known possible combinations of the two segments, a total of 15 genogroups with different lineages were determined (Islam et al., Citation2021). Recently, additional new genogroups were suggested based on novel VP2 sequences identified in Europe and Asia (Wang et al., Citation2021c; Legnardi et al., Citation2022). Ongoing monitoring is required to identify newly emerging IBDV strains and variants in the field as continuous selection pressure will lead to immune escape strains and viruses with functional advantages for segment B (Pikula et al., Citation2021).
Virulence is not always clearly associated with a genogroup (Lu et al., Citation2015; Jackwood et al., Citation2018; Islam et al., Citation2021). Live vaccine virus strains show different degrees of attenuation from mild to intermediate plus. In many countries, very virulent strains circulate (Tammiranta et al., Citation2018; Ekiri et al., Citation2021; Jiang et al., Citation2021), which may lead to high morbidity and mortality rates. Antigenic variants can also be detected and lead to subclinical infections associated with strong immunosuppression (Myint et al., Citation2021; Thai et al., Citation2021).
The overall goal in the field is to protect chickens against IBDV through prophylactic strategies, including biosecurity as well as vaccination of parent flocks and progeny (Müller et al., Citation2012). Current efforts are directed towards the development of more efficient vaccines, which match the circulating field viruses as the emergence of new variants may limit the efficacy of current vaccination approaches (Yang & Ye, Citation2020; Qiao et al., Citation2021; Yang et al., Citation2021). In addition, live attenuated vaccines may induce transient immunosuppression making the birds vulnerable to other invading pathogens (Prandini et al., Citation2016; Thomrongsuwannakij et al., Citation2021), and therefore new generation vaccines may provide suitable alternatives (Hein et al., Citation2021; Qiao et al., Citation2021). To improve prophylactic measures, the understanding of the immunosuppressive mechanisms of the virus and possible host-associated factors influencing the outcome of infectious bursal disease (IBD) is an important requirement. In this article, we will review the current knowledge of the interaction of IBDV with the immune system of the host leading to different degrees of immunosuppression in affected birds.
Virus proteins and their interaction with the host
IBDV is a non-enveloped virus. The icosahedral capsid is single-layered and its diameter is from 50 to 70 nm, differing by virus strain. The single layer of the capsid consists of the two main structural proteins VP2 and VP3 (Dobos et al., Citation1979; Jackwood et al., Citation1984). They are processed by VP4, the viral serine-lysine protease (Birghan et al., Citation2000; Lejal et al., Citation2000). VP4 cleaves the polyprotein pVP2-VP4-VP3 into single proteins (reviewed in Qin & Zheng, Citation2017). Additionally, VP4 may suppress the host’s antiviral immune response by interacting with glucocorticoid-induced leucine zipper in a strain-dependent manner (reviewed in Dulwich et al., Citation2020; ).
Table 1. Overview of the functions of IBDV viral proteins (VP) and their interaction with host factors.
pVP2 is further divided into VP2 and four small peptides (Da Costa et al., Citation2002; Irigoyen et al., Citation2009). These small peptides play different or unknown roles in virus replication and capsid assembly (Chevalier et al., Citation2005). The outer layer of the viral capsid is composed of VP2 trimers (Böttcher et al., Citation1997) and, therefore, VP2 is the main target for neutralizing antibodies (reviewed by Fahey et al., Citation1989). Different amino acid compositions at the variable region of VP2 lead to different antigenic characteristics and epitopes. These can be used to differentiate strains and genogroups (reviewed in Jackwood et al., Citation2018), and therefore need to be considered for the generation of vaccines (Huo et al., Citation2019; Li et al., Citation2020; Wang et al., Citation2020; Guo et al., Citation2021). Lazarus et al. (Citation2008) speculated that the variable region for binding neutralizing antibodies and the regions associated with virulence are encoded at different locations within the VP2 sequences.
In in vitro studies, it was shown that VP2 induces apoptosis by the reduction of oral cancer overexpressed 1 (Qin et al., Citation2017; Qin & Zheng, Citation2017). VP2 may also be detected by host intracellular sentinels, which leads to apoptosis and virus release (reviewed by Busnadiego et al., Citation2012; Li et al., Citation2012). It is only detectable by intracellular host sentinels if it is not connected to VP3 (Busnadiego et al., Citation2012).
VP3 forms the inner surface of the viral capsid (Böttcher et al., Citation1997). Additionally, VP3 interacts with VP1 for morphogenesis and viral replication (reviewed in Maraver et al., Citation2003, ). VP3 binds not only to VP2 but also to single-stranded and double-stranded (ds) RNA as well as single-stranded DNA (Kochan et al., Citation2003). Therefore, it may not only interfere with VP2- but also double-stranded RNA-dependent protein kinase (PKR)-induced apoptosis, the latter being activated through dsRNA (reviewed in Cubas-Gaona et al. (Citation2018)). In cell culture, VP3 also inhibits autophagy (Zhang et al., Citation2020) and protects viral replication by inhibition of melanoma differentiation-associated gene 5-dependent interferon (IFN)-ß production through binding to the deSUMOlyated apoptosis inhibitor 5 (Deng et al., Citation2021).
VP1 represents the RNA-dependent RNA polymerase of IBDV (Müller, Scholtissek, et al., Citation1979; Morgan et al., Citation1988). It is not only important for mRNA synthesis and viral replication (Felice et al., Citation2017) but also for virus encapsidation (reviewed in van den Berg, Citation2000). Recent studies demonstrated that VP1 also contributes to the virulence of an IBDV strain and, therefore, may contribute to the genetic shift of the virus and the evolution of new variants with variable virulence (Deorao et al., Citation2021).
VP5 is a non-structural protein of IBDV with structural similarities to host Toll-like receptor 3 (Mundt et al., Citation1995; Ganguly & Rastogi, Citation2018; ). It accumulates at the plasma membrane of IBDV-infected cells (Mendez et al., Citation2015). By binding to the p85α subunit of phosphatidylinositol 3-kinase, followed by activation of NF-κB and subsequent inhibition of the caspase cascade, VP5 mediates the inhibition of early apoptosis (Wei et al., Citation2011). In the early phase of virus infection, this step is important to ensure replication (Liu & Vakharia, Citation2006). On the other hand, VP5 can also induce apoptosis at later stages of replication to allow virus release. It is suggested that VP5 binds to the voltage-dependent anion channel 2 and receptor of activated protein C kinase 1 in the mitochondria in host cells, which leads to the activation of the caspase-activated apoptosis (Liu & Vakharia, Citation2006; Li et al., Citation2012; Lin et al., Citation2015). Comparing recombinant viruses expressing or not expressing the VP5 polypeptide revealed that this virus protein is required for non-lytic release of the virus from infected cells, which additionally enhances the dissemination speed between cells (Mendez et al., Citation2017). Therefore, VP5 can be considered a virulence factor, supported by the fact, that VP5-knockouts show strong attenuation of the virus (Yao et al., Citation1998).
IBDV replication
Various virus receptors and receptor complexes were suggested to be involved in the recognition and attachment of IBDV (). Most of these tentative receptors were identified in vitro with only a few in in vivo studies (Nieper & Muller, Citation1996; Ogawa et al., Citation1998; Lin et al., Citation2007; Delgui et al., Citation2009; Luo et al., Citation2010; Liu et al., Citation2020, Citation2022). It was speculated that different virus strains might use different receptor complex compositions for attachment. How IBDV enters host cells is still not fully clear; while in vitro investigations suggested endocytosis (Yip et al., Citation2012), more recent studies provided evidence for macropinocytosis (Gimenez et al., Citation2015; Ye et al., Citation2017).
Table 2. Host receptors for binding IBDV.
IBDV needs to enter endosomes to release ribonucleoprotein (RNP) complexes (reviewed in Gimenez et al., Citation2021) consisting of the IBDV dsRNA, VP1 and VP3. In cell culture, the RNP is stabilized by voltage-dependent anion channel 1 (Han et al., Citation2017), protecting the complex from host sentinel proteins (Valli et al., Citation2012; Gimenez et al., Citation2021). But RNPs can also be recognized by tripartite motif 25, which is involved in a host antiviral feedback mechanism. Tripartite motif 25 targets Lys854 of VP3 and leads to ubiquitination and degradation (Wang, Yu, et al., Citation2021).
VP3 can bind to endosomal phosphatidylinositol-3-phophate on early endosomal membranes (Gimenez et al., Citation2015, Citation2021). This step is probably needed for the RNPs to travel and bind to the Golgi complex allowing RNPs to enter (Gimenez et al., Citation2022). In addition, VP3 induces a conformational change in VP1 to take off the steric blockade of the active part. Subsequently, VP1, the viral RNA polymerase, starts the replication (Garriga et al., Citation2007). Chicken eukaryotic translation elongation factor 1α may support VP1 polymerase activity of very virulent IBDV strains (Yang et al., Citation2020). Virus replication is promoted through the function of the Rab1b – Golgi-specific BFA resistance factor 1 – ADP ribosylation factor 1 axis. While the Golgi complex is rearranged, its secretory functionality is not affected (Gimenez et al., Citation2022). During replication and transcription, chicken heat shock protein 70 may interact with the dsRNA of IBDV (Meng et al., Citation2021). The viral assembly also takes place in the Golgi complex (Delgui et al., Citation2013; Gimenez et al., Citation2021). IBDV is subsequently released by the induction of apoptosis as described above (reviewed in Qin & Zheng, Citation2017; Li & Zheng, Citation2020).
Pathogenesis and impact of the host genotype
After oral infection, IBDV can be detected in the gut, especially in the caecum, and at 4 hours post-infection (hpi), the virus appears in the caecal tonsils. IBDV may infect macrophages and lymphocytes of the gut-associated lymphoid tissue (GALT) along the whole intestine without damaging the enterocytes. IBDV-positive macrophages and lymphocytes enter the bloodstream and reach the liver via the portal vein by 5 hpi (reviewed in Eterradossi & Saif, Citation2020). Kupffer cells may contribute to the control of the virus in the liver (Müller, Käufer, et al., Citation1979). If virus-positive cells enter the bloodstream, they are spread to other lymphoid tissues including the main target organ, the bursa of Fabricius (BF) by 11 hpi (reviewed in Eterradossi & Saif, Citation2020). The pathogenesis might slightly vary if the route of infection is changed, as after intranasal infection virus-positive cells may initially also be found in the follicle-associated epithelium and nasal-associated lymphoid tissue of the nasal cavity before virus spread to lymphoid tissues through the blood or intestinal route (Shah et al., Citation2021).
IgM-bearing B lymphocytes are the main target cells of IBDV, and massive virus replication leads to the destruction of these cells. A second viraemia may occur (Müller, Käufer, et al., Citation1979), and further lymphoid organs are affected, including the thymus and the spleen (Müller, Käufer, et al., Citation1979; Tanimura et al., Citation1995; Vervelde & Davison, Citation1997; Rautenschlein et al., Citation2007). Viral particles are also found in other cell types, such as in epithelial reticular cells in the thymus and in monocytes in bone marrow, but there is probably no replication in these cells (Tanimura et al., Citation1995). Recent studies demonstrated changes in the expression pattern of various antiviral factors, and cytokines associated with macrophage and T-cell activity early after IBDV-infection (Chen et al., Citation2022). Also, intestinal villi structure and mast cell activity, as well as mucous production, may be modified (Wang, Liu, et al., Citation2009; Li et al., Citation2018). Subsequently, IBDV-infection associates with changes in the gut microbiota composition (Li et al., Citation2018). Depending on the virulence of the virus, recovery will start at 7–10 days post-infection (dpi).
There are differences in the severity of IBD between different chicken breeds and lines (Dobner et al., Citation2020), which may be associated with variable immune reactions. IBDV-infected layer-type chickens often show more severe clinical signs than meat-type chickens (Nielsen et al., Citation1998; Aricibasi et al., Citation2010; Silva et al., Citation2016), possibly due to a stronger systemic innate immune response leading to a “cytokine storm” and death. Meat-type birds showed a higher magnitude of T cell reactions in the BF, which may correlate with better control of viral replication and faster recovery (Aricibasi et al., Citation2010). The association of a fast and vigorous innate immune response in some genotypes with clinical disease and bursa lesions was also confirmed by transcriptome analysis (Farhanah et al., Citation2018; Mohd Isa et al., Citation2020; Asfor et al., Citation2021). In the BF of more susceptible chicken lines, Asfor et al. (Citation2021) demonstrated an up-regulation of pathways involved in inflammation, cytoskeletal regulation by Rho GTPases, nicotinic acetylcholine receptor signalling, and Wnt signalling compared to other genotypes less susceptible to clinical disease.
The data on the impact of the MHC haplotype on IBDV pathogenesis are controversial (Hudson et al., Citation2002). There seems to be an association between the MHC haplotype and the quality of the anti-IBDV immune response (Hudson et al., Citation2002), which was mainly shown in vaccination experiments (Juul-Madsen et al., Citation2006; Butter et al., Citation2013).
B cells in IBDV immunopathogenesis and immunosuppression
The most susceptible age for IBD is between 3 and 6 weeks of age, when the main target organ, the BF, reaches its peak of development, and B cell differentiation has advanced (Zhao et al., Citation2016, ). The non-specific clinical signs during the acute phase of IBD include distress, ruffled feathers, anorexia and depression, possibly associated with diarrhoea and dehydration (reviewed in Ingrao et al., Citation2013). The extent of clinical signs may vary between infecting strains; the more virulent the strains, the higher the risk for the development of clinical disease and death (reviewed in Eterradossi & Saif, Citation2020; Lupini et al., Citation2020; Wang, Huang, et al., Citation2021). The acute phase of the clinical disease is accompanied by a macroscopically visible bursal oedema followed by bursa atrophy due to the destruction of the bursal follicles (reviewed in Eterradossi & Saif, Citation2020). Haemorrhages may occur and are speculated to be due to the cytokine storm (reviewed in Ingrao et al., Citation2013). Changes in the B cell genomic methylation and loss of genome integrity during the infection process may contribute to B cell death (Ciccone et al., Citation2017). Further lesions may occur, such as mottling of the spleen, and are described elsewhere in more detail (Mahgoub et al., Citation2012; Eterradossi & Saif, Citation2020). Older birds, or birds infected with less virulent strains, may show a fast, microscopically but not macroscopically full recovery of the bursa within only a few weeks (Kim et al., Citation1999; Rautenschlein et al., Citation2003; Felföldi et al., Citation2021).
Figure 1. Schematic overview of key immunological events in the bursa of Fabricius within the context of IBDV infection up to the time of beginning recovery. Created with BioRender.com
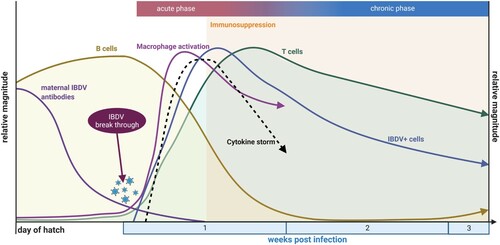
The loss of developing B lymphocytes associates with immunosuppression, which leads to an enhanced susceptibility for secondary infections and a poor vaccine response (reviewed in Naqi et al., Citation2001; Hashemzade et al., Citation2019; Eterradossi & Saif, Citation2020). If surviving birds are infected very early in life, they may suffer lifelong immunosuppression due to incomplete recovery of the B cell compartment (reviewed in Kim et al., Citation1999; Ingrao et al., Citation2013). Interestingly, the humoral immune response against IBDV is not affected, and birds develop high titres of circulating anti-IBDV antibodies. They may already be detected between 5 and 7 dpi and may contribute to viral clearance (Aricibasi et al., Citation2010) via the various antibody-mediated mechanisms, including opsonization, antibody-dependent cell-mediated or complement-dependent cytotoxicity.
Felföldi et al. (Citation2021) suggested that the secretory glycoproteins released by the secretory dendritic cells (BSDC), which are located in the medulla of the bursa follicles, may allow first IBDV binding to the BSDCs, and later also to B cells. The infected BSDCs may transform into macrophage-like cells, which migrate into the cortex, where inflammation is induced. Therefore, IBDV infection inhibits the glycoprotein release, which leads to B cell apoptosis (Oláh et al., Citation2022). Bursal epithelial cells, especially basal cells, accumulate large numbers of IBDV particles (Shah et al., Citation2021). Depending on the virulence of the IBDV strain, cystic cavities develop in the follicular medulla, and fibroplasia is observed microscopically. In the bursal recovery phase, B cells repopulate the bursal follicles (Vervelde & Davison, Citation1997; Kim et al., Citation1999; Withers et al., Citation2005, ). This recovery process may depend on the number of remaining BSDC, as IBDV-mediated exhaustion of its precursors prevents the establishment of the necessary microenviroment for B cell maturation and/or survival (Oláh et al., Citation2022). Recent studies suggested the involvement of C-X-C motif chemokine (CXC) ligand 12 and CXC ligand 13, which are highly expressed in the bursa of IBDV-infected chickens, in the recruitment of immature B cells into the bursa mesenchyme. CXC receptor 5 /CXC receptor 13 interaction may subsequently induce the B cell migration into the follicles (Shah et al., Citation2021). Withers et al. (Citation2005) described two types of recovering bursal follicles: large fully rebuilt follicles and small underdeveloped follicles, which lack the normal anatomic architecture. Chickens with mostly underdeveloped follicles mounted a lower antibody response to secondary pathogens than chickens with mostly recovered follicles. Nevertheless, both types produced lower antibody levels than IBDV uninfected birds (Vervelde & Davison, Citation1997; Withers et al., Citation2005). In contrast, Kim et al. (Citation1999) showed that chickens infected at 1-week post-hatch, returned to an almost normal humoral immune response between 7 and 12 weeks post-infection, depending on the virulence of the infecting strain. Inflammatory foci were observed in the recovering bursa with high numbers of T cells, a few B lymphocytes and γδT cells, and a low number of diffusely distributed macrophages as well as aggregates of CD40+ cells in the foci centre (Withers et al., Citation2005). The recovery process was shortened if fibrotic lesions were less pronounced and the expression of transforming growth factor β and the matrix metalloproteinase 9 was reduced (Yu, Xu, et al., Citation2021).
IgA+ B cells may increase while IgM+ B cell populations decrease in the bursa follicles (Shah et al., Citation2021), suggesting an acceleration of IgA secretion in the bursa of IBDV-infected birds. A recently established primary B-cell model allowed elucidation of the gene expression profiles in IBDV-infected B cells (Dulwich et al., Citation2017). It was demonstrated that both an attenuated strain and a very virulent strain down-regulated the expression of key genes in B-cell activation and signalling, including tumour necrosis factor superfamily member 13B, CD72 and Grb2-related adaptor protein. On the other hand, the extent of upregulation of antiviral type I IFNs and IFN-stimulated genes in these IBDV-infected B cell cultures was associated with viral virulence (Dulwich et al., Citation2017). Pretreatment of cell cultures with type I IFN induced downregulation of the dsRNA-dependent protein kinase, which may suppress subsequent IBDV infection. The addition of IFN to infected cultures exacerbated apoptosis, suggesting a significant role of the IBDV genomic dsRNA in the apoptotic response and pathogenesis (Cubas-Gaona et al., Citation2018). Other studies confirmed that the type I IFN pathway is not a sufficient antiviral defence mechanism in IBDV-infected chickens to control virus infection (Shah et al., Citation2021).
Depending on the virulence of the infecting strain, B cells in other lymphoid tissues may also be affected, contributing to the suppression of the humoral branch of the chicken immune system. IBDV infection may also account for a transient suppression of B lymphocytes in the spleen, blood (Shah et al., Citation2021) and the GALT within the caecal tonsils. Interestingly, the B cells at these locations may recover faster than in the BF (Li et al., Citation2018).
T cells in IBDV-immunopathogenesis and immunosuppression
T lymphocytes are commonly considered not to be susceptible to virus infection but play a significant role in the immunopathogenesis. Mahgoub et al. (Citation2012) also demonstrated viral antigen closely associated with CD8αα+ TCR2+ T cells. Chemokine (C–C motif) ligand 19, which is involved in immunoregulatory and inflammatory processes, is upregulated in the BF of IBDV-infected birds and attracts T cells from blood and lymph vessels to infiltrate into the BF (Tanimura & Sharma, Citation1997; Wang et al., Citation2019, ) and caecal tonsils (Mahgoub et al., Citation2012). The majority of infiltrating T cells are TCR2+ (Tanimura & Sharma, Citation1997). CD4+ as well as CD8+ T cells may infiltrate the BF starting from 1-4 dpi, depending on the replication pattern of the virus (Kim et al., Citation2000; Withers et al., Citation2005; Aricibasi et al., Citation2010), while reports vary about the quantity. Some studies observed more CD8+ (Tanimura & Sharma, Citation1997; Dobner et al., Citation2020) than CD4+ T cells and others more CD4+ T cells in the BF (Ruan et al., Citation2020). This is influenced by the bird’s genotype as well as the IBDV strain (Tanimura & Sharma, Citation1997; Aricibasi et al., Citation2010; Tippenhauer et al., Citation2013). T cells express IFN-γ (Tanimura & Sharma, Citation1997; Rauw et al., Citation2007) and activate macrophages. Macrophages are also stimulated by the p38 mitogen-activated protein kinases or NF-κB pathway, which subsequently leads to a cytokine storm (Khatri & Sharma, Citation2006).
In the acute phase of IBD, T cells control viral replication. This is possibly mediated through the release of proinflammatory cytokines, e.g. interleukin (IL)-2 and IFN-γ, which induce apoptosis and other cell-death pathways. In addition, cytotoxic T cells induce lysis of infected cells and uninfected bystander cells (Kim et al., Citation2000; Rautenschlein et al., Citation2002). The CD8+ cells putatively use the Fas-FasLigand and/ or perforin-granzyme A cytolytic pathway to destroy cells (Rauf et al., Citation2011, Citation2012).
It was speculated that some resident bursal CD4+ T cells might stimulate B cells to produce antibodies against IBDV before the infection of the B lymphocyte occurs, but the main antibody production was suggested to take place beyond the BF (Tanimura & Sharma, Citation1997). After this rapid infiltration of T cells, numbers decline again. T cells may interfere with tissue recovery in the chronic phase (Kim et al., Citation2000; Rautenschlein et al., Citation2002). Dobner et al. (Citation2020) demonstrated that meat-type birds showed a faster bursal recovery with an earlier decrease in IL-10 mRNA expression and reduction in the number of T cells in the BF than layer-type birds (Dobner et al., Citation2020). Layer-type chickens had overall higher levels of IL-10 and CD4+ T cells, which could be regulatory T cells, than the broilers. On the other hand, the meat-type birds had higher numbers of CD8+ T cells, which may be involved in an earlier viral clearance and, thereafter, earlier recovery (Dobner et al., Citation2020). Genotype influences were also confirmed by Mohd Isa et al. (Citation2020), who demonstrated variable regulation of T-cell immunity associated genes between birds developing more severe compared to birds with less bursa lesions. The expression levels of, for example, CD86 and CTLA4 as well as Th1-associated cytokines varied, and, most strikingly, the expression of IL12B was observed only in the more susceptible line, compared to the expression of IL15R, which was only detected in the more resistant birds (Mohd Isa et al., Citation2020). More resistant birds also showed lower levels of B cell genomic modification after IBDV infection. This reduced the numbers of infiltrating T cells and reduced expression levels of CD40L and FasL. Therefore, less immunopathology is observed in more resistant birds compared to more susceptible birds (Ciccone et al., Citation2017). Intrabursal T cells of IBDV-infected birds were demonstrated to inhibit the mitogenic response of splenocytes, also indicating immunomodulatory activities of this cell population (Kim & Sharma, Citation2000).
Also, the GALT undergoes changes in the T cell compartment after IBDV infection. Recent in vivo studies demonstrated a decrease of CD4+ cells in the caecal tonsils of infected birds, while CD8+ T cells were not affected. Interestingly, the T cell subsets were differentially modulated in different lymphoid tissues. While in the caecal tonsils IFN-β and CTLA-4 mRNA expression was upregulated, in the BF the changes in the IFN-γ, IL-10 and T cell checkpoint receptor LAG-3 mRNA expression were more prominent (Ruan et al., Citation2020).
Innate immune reactions contributing to IBDV immunopathogenesis and immunosuppression
The interactions between the host’s innate immune system and invading IBDV are complex and not fully elucidated yet. The differential activation of pattern recognition receptors was suggested to initiate the expression of antiviral and inflammatory cytokines in various cells of the innate immune systems but also other host cells (Yu et al., Citation2019). Heterophils are the first line of defence, and they are detected already within hours after virus inoculation but wane beyond the acute phase of the disease. In vitro studies demonstrated alteration of their functions after IBDV infection, which contributes to the overall immunosuppression due to the loss of phagocytic activity (Lam, Citation1998).
Macrophages were identified as possible target cells for IBDV (Khatri & Sharma, Citation2007; Lee et al., Citation2015). Their numbers change, therefore, not only in the bursa of Fabricius but also in the spleen during the infection (Palmquist et al Citation2006; Aricibasi et al., Citation2010). Some authors described a transient increase in the relative proportion of these cells in the BF, which may later on also be due to the loss of B cells (Khatri et al., Citation2005; Aricibasi et al. Citation2010). Virus and virus particles are detectable in the cytoplasm of macrophages (reviewed in Khatri & Sharma, Citation2007). Activated macrophages release proinflammatory cytokines, e.g. IL-6, inducible nitric oxide synthase, IL-1ß and tumour necrosis factor α (Khatri et al., Citation2005; Eldaghayes et al., Citation2006; Rautenschlein et al., Citation2007, ) to destroy virus-infected cells by apoptosis, cytotoxicity and phagocytosis. This strong inflammatory response may contribute to the cytokine storm.
There is limited and controversial information on the impact of natural killer (NK) cells on IBDV infection (Sharma & Lee, Citation1983). Infection with an IBDV vaccine or a very virulent strain of in vitro cultures of intraepithelial lymphocyte NK cells led to either up- or down-regulation of the expression of various surface antigens as well as NK-lysin, respectively (Jahromi et al., Citation2018). A transcriptome analysis indicated that as early as 3 dpi, genes were differentially regulated in the intraepithelial lymphocyte NK cells of the duodenum of IBDV-inoculated compared to virus-free birds. Some genes were associated with the recruitment of NK cells to the infected area and some with the downregulation of the antiviral activity of NK cells, suggesting a contribution to the compromised local immunity in the gut (Boo et al., Citation2020).
Anti-inflammatory compounds such as dexamethasone may protect IBDV-infected birds from severe clinical disease development emphasizing the role of inflammatory immune reaction in IBDV-immunosuppression (Shin et al., Citation2021).
Besides immune cell populations, other cell types may contribute to the innate immune response and/or immunopathogenesis of IBDV. Recently, melanocytes were identified to be susceptible to IBDV infection, and upregulated Toll-like receptor signalling pathways as well as antiviral factors were observed after in vitro infection (Han et al., Citation2021).
Interestingly, new studies also indicate that IBDV may interact with factors of the innate immune system to evade host immunity. The PTEN, a dual-specificity phosphatase, is involved in various survival strategies of the host (Worby & Dixon, Citation2014). During the early phase after IBDV infection, PTEN is downregulated, allowing the virus to replicate (Yu, Li, et al., Citation2021). Another innate host factor supporting virus replication is optineurin. IBDV infection induces endogenous optineurin expression, which subsequently suppresses the MDA5-mediated IFNβ production, allowing more efficient virus replication (Li et al., Citation2021). On the other hand, the pryin domain-containing 3 inflammasome activation was shown to interfere with IBDV replication in cell culture through the induction of pro-inflammatory cytokines and can therefore be suggested as an antiviral mechanism of the innate immune system (He et al., Citation2021).
Consequences of immunosuppression on intervention strategies
Not only IBDV may circulate in the field. Other pathogens, including Marek’s disease virus, chicken infectious anaemia virus and non-infectious factors including mycotoxins, may additionally challenge the immune system and exacerbate the IBDV-induced immunosuppression (reviewed by Hoerr, Citation2010; Gimeno & Schat, Citation2018). Depending on the combination of immunosuppressive factors, different branches of the immune system are compromised (Gimeno & Schat, Citation2018).
Just recently, it was shown that co-infection of variant IBDV with fowl adenoviruses promoted the pathogenicity of fowl adenoviruses and enhanced virus shedding (Xu et al., Citation2021). Similar observations were made with IBDV in combination with other pathogens infecting different host target tissues within the host (for example, Gallardo et al., Citation2012; Kulappu Arachchige et al., Citation2021). But the destruction of target cells and/or induction of the antiviral state may also affect co-infecting pathogens; these mechanisms are non-specific, and culminate in antigenic effects (Jackwood, Citation2011). IBDV-induced immunosuppression is therefore based on compromised systemic innate and acquired immune mechanisms as well as reduced local, specifically mucosal-associated immunity demonstrating the complexity of IBD (Wang, Zhou, et al., Citation2009; Gallardo et al., Citation2012; Li et al., Citation2018; Xu et al., Citation2021).
Therefore, vaccination programmes have to be carefully adjusted to the field situation. If IBDV-infected birds are immunocompromised, protection will be difficult to achieve.
Conclusions and outlook
The recent availability of transgenic chicken models will help to investigate research questions in the in vivo context, which will help to also advance our knowledge on IBD (Sid & Schusser, Citation2018). Further understanding of the immunopathogenic mechanisms of IBDV will help to improve current vaccination strategies in the field. Multivalent recombinant vaccines are increasing, conquering the market with different types of recombinant viruses (Qiao et al., Citation2021), of which some are suitable for the in ovo application route (Hein et al., Citation2021). Also, other alternative approaches to attenuated vaccine strains or inactivated full-antigen vaccines are under investigation, such as plant-based subunit vaccines (Marusic et al., Citation2021; Yang et al., Citation2021). This vaccine research has to be supported by the identification of new adjuvants to further direct and enhance the specific immunity (Ebrahimi et al., Citation2019; Sachan et al., Citation2019; Lu et al., Citation2020).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aricibasi, M., Jung, A., Heller, E.D. & Rautenschlein, S. (2010). Differences in genetic background influence the induction of innate and acquired immune responses in chickens depending on the virulence of the infecting infectious bursal disease virus (IBDV) strain. Veterinary Immunology and Immunopathology, 135, 79–92.
- Asfor, A.S., Nazki, S., Reddy, V., Campbell, E., Dulwich, K.L., Giotis, E.S., Skinner, M.A. & Broadbent, A.J. (2021). Transcriptomic analysis of inbred chicken lines reveals infectious bursal disease severity is associated with greater bursal inflammation in vivo and more rapid induction of pro-inflammatory responses in primary bursal cells stimulated ex vivo. Viruses, 13, 933.
- Azad, A.A., Barrett, S.A. & Fahey, K.J. (1985). The characterization and molecular cloning of the double-stranded RNA genome of an Australian strain of infectious bursal disease virus. Virology, 143, 35–44.
- Birghan, C., Mundt, E. & Gorbalenya, A.E. (2000). A non-canonical lon proteinase lacking the ATPase domain employs the ser-Lys catalytic dyad to exercise broad control over the life cycle of a double-stranded RNA virus. The EMBO Journal, 19, 114–123.
- Boo, S.Y., Tan, S.W., Alitheen, N.B., Ho, C.L., Omar, A.R. & Yeap, S.K. (2020). Transcriptome analysis of chicken intraepithelial lymphocyte natural killer cells infected with very virulent infectious bursal disease virus. Scientific Reports, 10, 18348.
- Böttcher, B., Kiselev, N.A., Stel'Mashchuk, V.Y., Perevozchikova, N.A., Borisov, A.V. & Crowther, R.A. (1997). Three-dimensional structure of infectious bursal disease virus determined by electron cryomicroscopy. Journal of Virology, 71, 325–330.
- Brown Jordan, A., Gongora, V., Hartley, D. & Oura, C. (2018). A review of eight high-priority, economically important viral pathogens of poultry within the Caribbean region. Veterinary Sciences, 5, 14.
- Busnadiego, I., Maestre, A.M., Rodríguez, D. & Rodríguez, J.F. (2012). The infectious bursal disease virus RNA-binding VP3 polypeptide inhibits PKR-mediated apoptosis. PLoS One, 7, e46768.
- Butter, C., Staines, K., van Hateren, A., Davison, T.F. & Kaufman, J. (2013). The peptide motif of the single dominantly expressed class I molecule of the chicken MHC can explain the response to a molecular defined vaccine of infectious bursal disease virus (IBDV). Immunogenetics, 65, 609–618.
- Chen, R., Chen, J., Xiang, Y., Chen, Y., Shen, W., Wang, W., Li, Y., Wei, P. & He, X. (2022). Differential modulation of innate antiviral profiles in the intestinal lamina propria cells of chickens infected with infectious bursal disease viruses of different virulence. Viruses, 14, 393.
- Chevalier, C., Galloux, M., Pous, J., Henry, C., Denis, J., Da Costa, B., Navaza, J., Lepault, J. & Delmas, B. (2005). Structural peptides of a nonenveloped virus are involved in assembly and membrane translocation. Journal of Virology, 79, 12253–12263.
- Ciccone, N.A., Smith, L.P., Mwangi, W., Boyd, A., Broadbent, A.J., Smith, A.L. & Nair, V. (2017). Early pathogenesis during infectious bursal disease in susceptible chickens is associated with changes in B cell genomic methylation and loss of genome integrity. Developmental & Comparative Immunology, 73, 169–174.
- Cosgrove, A.S. (1962). An apparently new disease of chickens: avian nephrosis. Avian Diseases, 6, 385–389.
- Cubas-Gaona, L.L., Diaz-Beneitez, E., Ciscar, M., Rodriguez, J.F. & Rodriguez, D. (2018). Exacerbated apoptosis of cells infected with infectious bursal disease virus upon exposure to interferon alpha. Journal of Virology, 92, e00364-18.
- Da Costa, B., Chevalier, C., Henry, C., Huet, J.C., Petit, S., Lepault, J., Boot, H. & Delmas, B. (2002). The capsid of infectious bursal disease virus contains several small peptides arising from the maturation process of pVP2. Journal of Virology, 76, 2393–2402.
- Delgui, L., Oña, A., Gutiérrez, S., Luque, D., Navarro, A., Castón, J.R. & Rodriguez, J.F. (2009). The capsid protein of infectious bursal disease virus contains a functional α4β1 integrin ligand motif. Virology, 386, 360–372.
- Delgui, L.R., Rodríguez, J.F. & Colombo, M.I. (2013). The endosomal pathway and the Golgi complex are involved in the infectious bursal disease virus life cycle. Journal of Virology, 87, 8993–9007.
- Deng, T., Hu, B., Wang, X., Yan, Y., Zhou, J., Lin, L., Xu, Y., Zheng, X. & Zhou, J. (2021). DeSUMOylation of apoptosis inhibitor 5 by Avibirnavirus VP3 supports virus replication. mBio, 12, e0198521.
- Deorao, C.V., Rajasekhar, R., Ravishankar, C., Nandhakumar, D., Sumod, K., Palekkodan, H., John, K. & Chaithra, G. (2021). Genetic variability in VP1 gene of infectious bursal disease virus from the field outbreaks of Kerala, India. Tropical Animal Health and Production, 53, 407.
- Dobner, M., Auerbach, M., Mundt, E., Icken, W. & Rautenschlein, S. (2020). Genotype-associated differences in bursal recovery after infectious bursal disease virus (IBDV) inoculation. Veterinary Immunology and Immunopathology, 220, 109993.
- Dobos, P., Hill, B.J., Hallett, R., Kells, D.T., Becht, H. & Teninges, D. (1979). Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. Journal of Virology, 32, 593–605.
- Dulwich, K.L., Giotis, E.S., Gray, A., Nair, V., Skinner, M.A. & Broadbent, A.J. (2017). Differential gene expression in chicken primary B cells infected ex vivo with attenuated and very virulent strains of infectious bursal disease virus (IBDV). Journal of General Virology, 98, 2918–2930.
- Dulwich, K.L., Asfor, A., Gray, A., Giotis, E.S., Skinner, M.A. & Broadbent, A.J. (2020). The stronger downregulation of in vitro and in vivo innate antiviral responses by a very virulent strain of infectious bursal disease virus (IBDV), compared to a classical strain, is mediated, in part, by the VP4 protein. Frontiers in Cellular and Infection Microbiology, 10, 315.
- Ebrahimi, M.M., Shahsavandi, S. & Shayan, P. (2019). TIR-TLR7 as a molecular adjuvant: simultaneous enhancing humoral and cell-mediated immune responses against inactivated infectious bursal disease virus. Viral Immunology, 32, 252–257.
- Ekiri, A.B., Armson, B., Adebowale, K., Endacott, I., Galipo, E., Alafiatayo, R., Horton, D.L., Ogwuche, A., Bankole, O.N., Galal, H.M., Maikai, B.V., Dineva, M., Wakawa, A., Mijten, E., Varga, G. & Cook, A.J.C. (2021). Evaluating disease threats to sustainable poultry production in Africa: Newcastle disease, infectious bursal disease, and avian infectious bronchitis in commercial poultry flocks in Kano and Oyo states, Nigeria. Frontiers in Veterinary Science, 8, 730159.
- Eldaghayes, I., Rothwell, L., Williams, A., Withers, D., Balu, S., Davison, F. & Kaiser, P. (2006). Infectious bursal disease virus: strains that differ in virulence differentially modulate the innate immune response to infection in the chicken bursa. Viral Immunology, 19, 83–91.
- Eterradossi, N. & Saif, Y.M. (2020). Infectious bursal disease. In D.E. Swayne, M. Boulianne, C.M. Logue, L.R. McDougald, V. Nair & D.L. Suarez (Eds.), Diseases of poultry (14th ed., pp. 257–283). Wiley-Blackwell.
- Fahey, K.J., Erny, K. & Crooks, J. (1989). A conformational immunogen on VP-2 of infectious bursal disease virus that induces virus-neutralizing antibodies that passively protect chickens. Journal of General Virology, 70, 1473–1481.
- Farhanah, M.I., Yasmin, A.R., Mat Isa, N., Hair-Bejo, M., Ideris, A., Powers, C., Oladapo, O., Nair, V., Khoo, J.S., Ghazali, A.K., Yee, W.Y. & Omar, A.R. (2018). Bursal transcriptome profiling of different inbred chicken lines reveals key differentially expressed genes at 3 days post-infection with very virulent infectious bursal disease virus. Journal of General Virology, 99, 21–35.
- Felföldi, B., Bódi, I., Minko, K., Benyeda, Z., Nagy, N., Magyar, A. & Oláh, I. (2021). Infection of bursal disease virus abrogates the extracellular glycoprotein in the follicular medulla. Poultry Science, 100, 101000.
- Felice, V., Franzo, G., Catelli, E., Di Francesco, A., Bonci, M., Cecchinato, M., Mescolini, G., Giovanardi, D., Pesente, P. & Lupini, C. (2017). Genome sequence analysis of a distinctive Italian infectious bursal disease virus. Poultry Science, 96, 4370–4377.
- Feng, X., Zhu, N., Cui, Y., Hou, L., Zhou, J., Qiu, Y., Yang, X., Liu, C., Wang, D., Guo, J., Sun, T., Shi, Y., Han, N., Mo, M. & Liu, J. (2021). Characterization and pathogenicity of a naturally reassortant and recombinant infectious bursal disease virus in China. Transboundary and Emerging Diseases, 1–13.
- Gallardo, R.A., van Santen, V.L. & Toro, H. (2012). Effects of chicken anaemia virus and infectious bursal disease virus-induced immunodeficiency on infectious bronchitis virus replication and genotypic drift. Avian Pathology, 41, 451–458.
- Ganguly, B. & Rastogi, S.K. (2018). Structural and functional modeling of viral protein 5 of infectious bursal disease virus. Virus Research, 247, 55–60.
- Garriga, D., Navarro, A., Querol-Audí, J., Abaitua, F., Rodríguez, J.F. & Verdaguer, N. (2007). Activation mechanism of a noncanonical RNA-dependent RNA polymerase. Proceedings of the National Academy of Sciences, 104, 20540–20545.
- Gimenez, M.C., Aguirre, J.F.R., Colombo, M.I. & Delgui, L.R. (2015). Infectious bursal disease virus uptake involves macropinocytosis and trafficking to early endosomes in a Rab5-dependent manner. Cellular Microbiology, 17, 988–1007.
- Gimenez, M.C., Issa, M., Sheth, J., Colombo, M.I., Terebiznik, M.R. & Delgui, L.R. (2021). Phosphatidylinositol 3-phosphate mediates the establishment of infectious bursal disease virus replication complexes in association with early endosomes. Journal of Virology, 95, e02313-20.
- Gimenez, M.C., Frontini-Lopez, Y.R., Pocognoni, C.A., Roldán, J.S., García Samartino, C., Uhart, M., Colombo, M.I., Terebiznik, M.R., Delgui, L.R. & López, S. (2022). Rab1b-GBF1-ARF1 secretory pathway axis is required for birnavirus replication. Journal of Virology, 96, e0200521.
- Gimeno, I.M. & Schat, K.A. (2018). Virus-induced immunosuppression in chickens. Avian Diseases, 62, 272–285.
- Guo, X., Sun, W., Wei, L., Wang, X., Zou, Y., Zhang, Y., Li, S., Wang, N., Jiang, M., Zhao, H., Qu, E., Pang, Y., Yin, J. & Ren, G. (2021). Development and evaluation of a recombinant VP2 neutralizing epitope antigen vaccine candidate for infectious bursal disease virus. Transboundary and Emerging Diseases, 68, 3658–3675.
- Han, C., Zeng, X., Yao, S., Gao, L., Zhang, L., Qi, X., Duan, Y., Yang, B., Gao, Y., Liu, C., Zhang, Y., Wang, Y. & Wang, X. (2017). Voltage-dependent anion channel 1 interacts with ribonucleoprotein complexes to enhance infectious bursal disease virus polymerase activity. Journal of Virology, 91, e00584-17.
- Han, D., Tai, Y., Hua, G., Yang, X., Chen, J., Li, J. & Deng, X. (2021). Melanocytes in black-boned chicken have immune contribution under infectious bursal disease virus infection. Poultry Science, 100, 101498.
- Hashemzade, F., Mayahi, M., Shoshtary, A., Shapouri, M.R.S.A & Ghorbanpoor, M. (2019). Effect of experimental infectious bursal disease virus on clinical signs and pathogenesis of avian influenza virus H9N2 in turkey by real time PCR. Veterinary Research Forum, 10, 293–297.
- He, Z., Chen, X., Fu, M., Tang, J., Li, X., Cao, H., Wang, Y. & Zheng, S.J. (2018). Infectious bursal disease virus protein VP4 suppresses type I interferon expression via inhibiting K48-linked ubiquitylation of glucocorticoid-induced leucine zipper (GILZ). Immunobiology, 223, 374–382.
- He, Z.Y., Ma, Y.L., Wu, D.X., Feng, W.H. & Xiao, J. (2021). Protective effects of the NLRP3 inflammasome against infectious bursal disease virus replication in DF-1 cells. Archives of Virology, 166, 1943–1950.
- Hein, R., Koopman, R., Garcia, M., Armour, N., Dunn, J.R., Barbosa, T. & Martinez, A. (2021). Review of poultry recombinant vector vaccines. Avian Diseases, 65, 438–452.
- Hoerr, F.J. (2010). Clinical aspects of immunosuppression in poultry. Avian Diseases, 54, 2–15.
- Hudson, J.C., Hoerr, E.J., Parker, S.H. & Ewald, S.J. (2002). Quantitative measures of disease in broiler breeder chicks of different major histocompatibility complex genotypes after challenge with infectious bursal disease virus. Avian Diseases, 46, 581–592.
- Hudson, P.J., Mckern, N.M., Power, B.E. & Azad, A.A. (1986). Genomic structure of tbe large RNA segment of infectious bursal disease virus. Nucleic Acids Research, 14, 5001–5012.
- Huo, S., Zhang, J., Fan, J., Wang, X., Wu, F., Zuo, Y. & Zhong, F. (2019). Co-expression of chicken IL-2 and IL-7 enhances the immunogenicity and protective efficacy of a VP2-expressing DNA vaccine against IBDV in chickens. Viruses, 11, 476.
- Ingrao, F., Rauw, F., Lambrecht, B. & van den Berg, T. (2013). Infectious bursal disease: a complex host-pathogen interaction. Developmental & Comparative Immunology, 41, 429–438.
- Irigoyen, N., Garriga, D., Navarro, A., Verdaguer, N., Rodríguez, J.F. & Castón, J.R. (2009). Autoproteolytic activity derived from the infectious bursal disease virus capsid protein. Journal of Biological Chemistry, 284, 8064–8072.
- Islam, M.R., Nooruzzaman, M., Rahman, T., Mumu, T.T., Rahman, M.M., Chowdhury, E.H., Eterradossi, N. & Müller, H. (2021). A unified genotypic classification of infectious bursal disease virus based on both genome segments. Avian Pathology, 50, 190–206.
- Jackwood, D.J., Saif, Y.M. & Hughes, J.H. (1984). Nucleic acid and structural proteins of infectious bursal disease virus isolates belonging to serotypes I and II. Avian Diseases, 28, 990–1006.
- Jackwood, D.J. (2011). Viral competition and maternal immunity influence the clinical disease caused by very virulent infectious bursal disease virus. Avian Diseases, 55, 398–406.
- Jackwood, D.J., Schat, K.A., Michel, L.O. & de Wit, S. (2018). A proposed nomenclature for infectious bursal disease virus isolates. Avian Pathology, 47, 576–584.
- Jahromi, M.Z., Bello, M.B., Abdolmaleki, M., Yeap, S.K., Hair-Bejo, M. & Omar, A.R. (2018). Differential activation of intraepithelial lymphocyte-natural killer cells in chickens infected with very virulent and vaccine strains of infectious bursal disease virus. Developmental & Comparative Immunology, 87, 116–123.
- Jiang, N., Wang, Y., Zhang, W., Niu, X., Huang, M., Gao, Y., Liu, A., Gao, L., Li, K., Pan, Q., Liu, C., Zhang, Y., Cui, H., Wang, X. & Qi, X. (2021). Genotyping and molecular characterization of infectious bursal disease virus identified in important poultry-raising areas of China during 2019 and 2020. Frontiers in Veterinary Science, 8, 759861.
- Juul-Madsen, H.R., Dalgaard, T.S., Røntved, C.M., Jensen, K.H. & Bumstead, N. (2006). Immune response to a killed infectious bursal disease virus vaccine in inbred chicken lines with different major histocompatibility complex haplotypes. Poultry Science, 85, 986–998.
- Khatri, M., Palmquist, J.M., Cha, R.M. & Sharma, J.M. (2005). Infection and activation of bursal macrophages by virulent infectious bursal disease virus. Virus Research, 113, 44–50.
- Khatri, M. & Sharma, J.M. (2006). Infectious bursal disease virus infection induces macrophage activation via p38 MAPK and NF-kappa B pathways. Virus Research, 118, 70–77.
- Khatri, M. & Sharma, J.M. (2007). Modulation of macrophages by infectious bursal disease virus. Cytogenetic and Genome Research, 117, 388–393.
- Kim, I.J., Gagic, M. & Sharma, J.M. (1999). Recovery of antibody-producing ability and lymphocyte repopulation of bursal follicles in chickens exposed to infectious bursal disease virus. Avian Diseases, 43, 401–413.
- Kim, I.J., You, S.K., Kim, H., Yeh, H.Y. & Sharma, J.M. (2000). Characteristics of bursal T lymphocytes induced by infectious bursal disease virus. Journal of Virology, 74, 8884–8892.
- Kim, I.J. & Sharma, J.M. (2000). IBDV-induced bursal T lymphocytes inhibit mitogenic response of normal splenocytes. Veterinary Immunology and Immunopathology, 74, 47–57.
- Kochan, G., Gonzalez, D. & Rodriguez, J.F. (2003). Characterization of the RNA-binding activity of VP3, a major structural protein of infectious bursal disease virus. Archives of Virology, 148, 723–744.
- Kulappu Arachchige, S.N., Kanci Condello, A., Zhu, L., Shil, P.K., Tivendale, K.A., Underwood, G.J., Noormohammadi, A.H., Browning, G.F. & Wawegama, N.K. (2021). Effects of immunosuppression on the efficacy of vaccination against Mycoplasma gallisepticum infection in chickens. Veterinary Microbiology, 260, 109–182.
- Lam, K.M. (1998). Alteration of chicken heterophil and macrophage functions by the infectious bursal disease virus. Microbial Pathogenesis, 25, 147–155.
- Lazarus, D., Pasmanik-Chor, M., Gutter, B., Gallili, G., Barbakov, M., Krispel, S. & Pitcovski, J. (2008). Attenuation of very virulent infectious bursal disease virus and comparison of full sequences of virulent and attenuated strains. Avian Pathology, 37, 151–159.
- Lee, C.C., Wu, C.C. & Lin, T.L. (2015). Role of chicken melanoma differentiation-associated gene 5 in induction and activation of innate and adaptive immune responses to infectious bursal disease virus in cultured macrophages. Archives of Virology, 160, 3021–3035.
- Legnardi, M., Franzo, G., Tucciarone, C.M., Koutoulis, K., Duarte, I., Silva, M., Le Tallec, B. & Cecchinato, M. (2022). Detection and molecular characterization of a new genotype of infectious bursal disease virus in Portugal. Avian Pathology, 51, 97–105.
- Lejal, N., Da Costa, B., Huet, J.C. & Delmas, B. (2000). Role of Ser-652 and Lys-692 in the protease activity of infectious bursal disease virus VP4 and identification of its substrate cleavage sites. Journal of General Virology, 81, 983–992.
- Li, G., Kuang, H., Guo, H., Cai, L., Chu, D., Wang, X., Hu, J. & Rong, J. (2020). Development of a recombinant VP2 vaccine for the prevention of novel variant strains of infectious bursal disease virus. Avian Pathology, 49, 557–571.
- Li, J. & Zheng, S.J. (2020). Role of microRNAs in host defense against infectious bursal disease virus (IBDV) infection: a hidden front line. Viruses, 12, 543.
- Li, L., Kubasova, T., Rychlik, I., Hoerr, F.J. & Rautenschlein, S. (2018). Infectious bursal disease virus infection leads to changes in the gut associated-lymphoid tissue and the microbiota composition. PLoS One, 13, e0192066.
- Li, Y., Jiang, N., Mao, Y., Zhang, W., Xiao, J., Wu, X. & Wu, H. (2021). Chicken optineurin suppresses MDA5-mediated interferon β production. Poultry Science, 100, 9–18.
- Li, Z.H., Wang, Y.Q., Xue, Y.F., Li, X.Q., Cao, H. & Zheng, S.J.J. (2012). Critical role for voltage-dependent anion channel 2 in infectious bursal disease virus-induced apoptosis in host cells via interaction with VP5. Journal of Virology, 86, 1328–1338.
- Lin, T.-W., Lo, C.-W., Lai, S.-Y., Fan, R.-J., Lo, C.-J., Chou, Y.-M., Thiruvengadam, R. & Wang, A.H.-J. (2007). Chicken heat shock protein 90 is a component of the putative cellular receptor complex of infectious bursal disease virus. Journal of Virology, 81, 8730–8741.
- Lin, W., Zhang, Z., Xu, Z., Wang, B., Li, X., Cao, H., Wang, Y. & Zheng, S.J. (2015). The association of receptor of activated protein kinase C 1(RACK1) with infectious bursal disease virus viral protein VP5 and voltage-dependent anion channel 2 (VDAC2) inhibits apoptosis and enhances viral replication. Journal of Biological Chemistry, 290, 8500–8510.
- Liu, A., Pan, Q., Li, Y., Yan, N., Wang, J., Yang, B., Chen, Z., Qi, X., Gao, Y., Gao, L., Liu, C., Zhang, Y., Cui, H., Li, K., Wang, Y. & Wang, X. (2020). Identification of chicken CD74 as a novel cellular attachment receptor for infectious bursal disease virus in bursa B lymphocytes. Journal of Virology, 94, e01712-19.
- Liu, A., Pan, Q., Wang, S., Zhang, Y., Li, Y., Wang, Y., Qi, X., Gao, L., Liu, C., Zhang, Y., Cui, H., Li, K., Wang, X. & Gao, Y. (2022). Identification of chicken CD44 as a novel B lymphocyte receptor for infectious bursal disease virus. Journal of Virology, 96, e00113-22.
- Liu, M.H. & Vakharia, V.N. (2006). Nonstructural protein of infectious bursal disease virus inhibits apoptosis at the early stage of virus infection. Journal of Virology, 80, 3369–3377.
- Lu, H., Zhang, X., Wang, Y., Zong, Y., Wang, Y., Zhang, X., Xia, X. & Sun, H. (2020). Superior adjuvanticity of the genetically fused D1 domain of Neisseria meningitides Ag473 lipoprotein among three toll-like receptor ligands. Bioscience Reports, 40, BSR20193675.
- Lu, Z., Zhang, L., Wang, N., Chen, Y., Gao, L., Wang, Y., Gao, H., Gao, Y., Li, K., Qi, X. & Wang, X. (2015). Naturally occurring reassortant infectious bursal disease virus in northern China. Virus Research, 203, 92–95.
- Luo, J., Zhang, H., Teng, M., Fan, J.-M., You, L.-M., Xiao, Z.-J., Yi, M.-L., Zhi, Y.-B., Li, X.-W., & Zhang, G.-P. (2010). Surface IgM on DT40 cells may be a component of the putative receptor complex responsible for the binding of infectious bursal disease virus. Avian Pathology, 39, 359–365.
- Lupini, C., Felice, V., Silveira, F., Mescolini, G., Berto, G., Listorti, V., Cecchinato, M. & Catelli, E. (2020). Comparative in vivo pathogenicity study of an ITA genotype isolate (G6) of infectious bursal disease virus. Transboundary and Emerging Diseases, 67, 1025–1031.
- Luque, D., Saugar, I., Rejas, M.T., Carrascosa, J.L., Rodríguez, J.F. & Castón, J.R. (2009). Infectious bursal disease virus: ribonucleoprotein complexes of a double-stranded RNA virus. Journal of Molecular Biology, 386, 891–901.
- Mahgoub, H.A., Bailey, M. & Kaiser, P. (2012). An overview of infectious bursal disease. Archives of Virology, 157, 2047–2057.
- Maraver, A., Oña, A., Abaitua, F., González, D., Clemente, R., Ruiz-Díaz, J.A., Castón, J.R., Pazos, F. & Rodriguez, J.F. (2003). The oligomerization domain of VP3, the scaffolding protein of infectious bursal disease virus, plays a critical role in capsid assembly. Journal of Virology, 77, 6438–6449.
- Marusic, C., Drissi Touzani, C., Bortolami, A., Donini, M., Zanardello, C., Lico, C., Rage, E., Fellahi, S., El Houadfi, M., Terregino, C. & Baschieri, S. (2021). The expression in plants of an engineered VP2 protein of infectious bursal disease virus induces formation of structurally heterogeneous particles that protect from a very virulent viral strain. PLoS One, 16, e0247134.
- Mendez, F., de Garay, T., Rodriguez, D. & Rodriguez, J.F. (2015). Infectious bursal disease virus VP5 polypeptide: a phosphoinositide-binding protein required for efficient cell-to-cell virus dissemination. PLoS One, 10, e0123470.
- Mendez, F., Romero, N., Cubas, L.L., Delgui, L.R., Rodriguez, D. & Rodriguez, J.F. (2017). Non-lytic egression of infectious bursal disease virus (IBDV) particles from infected cells. PLoS One, 12, e0170080.
- Meng, Y.F., Yu, X.X., You, C.X., Zhang, W.J., Sun, Y.F., Li, L.A., Jin, T.M., Pan, P.Y. & Xie, A.L. (2021). Chicken heat shock protein 70 Is an essential host protein for infectious bursal disease virus infection in vitro. Pathogens, 10, 664.
- Michel, L.O. & Jackwood, D.J. (2017). Classification of infectious bursal disease virus into genogroups. Archives of Virology, 162, 3661–3670.
- Mohd Isa, F., Ahmed Al-Haj, N., Mat Isa, N., Ideris, A., Powers, C., Oladapo, O., Nair, V. & Omar, A.R. (2020). Differential expression of immune-related genes in the bursa of Fabricius of two inbred chicken lines following infection with very virulent infectious bursal disease virus. Comparative Immunology, Microbiology and Infectious Diseases, 68, 101399.
- Morgan, M.M., Macreadie, I.G., Harley, V.R., Hudson, P.J. & Azad, A.A. (1988). Sequence of the small double-stranded RNA genomic segment of infectious bursal disease virus and its deduced 90-kDa product. Virology, 163, 240–242.
- Müller, H., Scholtissek, C. & Becht, H. (1979). The genome of infectious bursal disease virus consists of two segments of double-stranded RNA. Journal of Virology, 31, 584–589.
- Müller, H., Mundt, E., Eterradossi, N. & Islam, M.R. (2012). Current status of vaccines against infectious bursal disease. Avian Pathology, 41, 133–139.
- Müller, R., Käufer, I., Reinacher, M. & Weiss, E. (1979). Immunofluorescent studies of early virus propagation after oral infection with infectious bursal disease virus (IBDV). Zentralblatt Fur Veterinarmedizin Reihe B-Journal of Veterinary Medicine Series B-Infectious Diseases Immunology Food Hygiene Veterinary Public Health, 26, 345–352.
- Mundt, E., Beyer, J. & Muller, H. (1995). Identification of a novel viral protein in infectious bursal disease virus-infected cells. Journal of General Virology, 76, 437–443.
- Myint, O., Suwanruengsri, M., Araki, K., Izzati, U.Z., Pornthummawat, A., Nueangphuet, P., Fuke, N., Hirai, T., Jackwood, D.J. & Yamaguchi, R. (2021). Bursa atrophy at 28 days old caused by variant infectious bursal disease virus has a negative economic impact on broiler farms in Japan. Avian Pathology, 50, 6–17.
- Naqi, S., Thompson, G., Bauman, B. & Mohammed, H. (2001). The exacerbating effect of infectious bronchitis virus infection on the infectious bursal disease virus-induced suppression of opsonization by Escherichia coil antibody in chickens. Avian Diseases, 45, 52–60.
- Nielsen, O.L., Sørensen, P., Hedemand, J.E., Laursen, S.B. & Jørgensen, P.H. (1998). Inflammatory response of different chicken lines and B haplotypes to infection with infectious bursal disease virus. Avian Pathology, 27, 181–189.
- Nieper, H. & Muller, H. (1996). Susceptibility of chicken lymphoid cells to infectious bursal disease virus does not correlate with the presence of specific binding sites. Journal of General Virology, 77, 1229–1237.
- Ogawa, H., Yamaguchi, T., Setiyono, A., Ho, T., Matsuda, H., Furusawa, S., Fukushi, H. & Hirai, K. (1998). Some characteristics of a cellular receptor for virulent infectious bursal disease virus by using flow cytometry. Archives of Virology, 143, 2327–2341.
- Oláh, I., Felföldi, B., Benyeda, Z., Kovács, T., Nagy, N. & Magyar, A. (2022). The bursal secretory dendritic cell (BSDC) and the enigmatic chB6(+) macrophage-like cell (Mal). Poultry Science, 101, 101727.
- Palmquist, J.M., Khatri, M., Cha, R.M., Goddeeris, B.M., Walcheck, B. & Sharma, J.M. (2006). In vivo activation of chicken macrophages by infectious bursal disease virus. Viral Immunology, 19, 305–315.
- Pikula, A., Lisowska, A., Jasik, A. & Perez, L.J. (2021). The novel genetic background of infectious bursal disease virus strains emerging from the action of positive selection. Viruses, 13, 396.
- Prandini, F., Simon, B., Jung, A., Pöppel, M., Lemiere, S. & Rautenschlein, S. (2016). Comparison of infectious bursal disease live vaccines and a HVT-IBD vector vaccine and their effects on the immune system of commercial layer pullets. Avian Pathology, 45, 114–125.
- Qiao, Q., Song, M., Song, C., Zhang, Y., Wang, X., Huang, Q., Wang, B., Yang, P., Zhao, S., Li, Y., Wang, Z. & Zhao, J. (2021). Single-dose vaccination of recombinant chimeric Newcastle disease virus (NDV) LaSota vaccine strain expressing infectious bursal disease virus (IBDV) VP2 gene provides full protection against genotype VII NDV and IBDV challenge. Vaccines, 9, 1483.
- Qin, Y., Xu, Z., Wang, Y., Li, X., Cao, H. & Zheng, S.J. (2017). VP2 of infectious bursal disease virus induces apoptosis via triggering oral cancer overexpressed 1 (ORAOV1) protein degradation. Frontiers in Microbiology, 8, 1351.
- Qin, Y. & Zheng, S.J. (2017). Infectious bursal disease virus-host interactions: multifunctional viral proteins that perform multiple and differing jobs. International Journal of Molecular Sciences, 18, 161.
- Rauf, A., Khatri, M., Murgia, M.V. & Saif, Y.M. (2011). Expression of perforin-granzyme pathway genes in the bursa of infectious bursal disease virus-infected chickens. Developmental & Comparative Immunology, 35, 620–627.
- Rauf, A., Khatri, M., Murgia, M.V. & Saif, Y.M. (2012). Fas/FasL and perforin-granzyme pathways mediated T cell cytotoxic responses in infectious bursal disease virus infected chickens. Results in Immunology, 2, 112–119.
- Rautenschlein, S., Yeh, H.-Y., Njenga, M.K. & Sharma, J.M. (2002). Role of intrabursal T cells in infectious bursal disease virus (IBDV) infection: T cells promote viral clearance but delay follicular recovery. Archives of Virology, 147, 285–304.
- Rautenschlein, S., Yeh, H.Y. & Sharma, J.M. (2003). Comparative immunopathogenesis of mild, intermediate, and virulent strains of classic infectious bursal disease virus. Avian Diseases, 47, 66–78.
- Rautenschlein, S., von Samson-Himmelstjerna, G. & Haase, C. (2007). A comparison of immune responses to infection with virulent infectious bursal disease virus (IBDV) between specific-pathogen-free chickens infected at 12 and 28 days of age. Veterinary Immunology and Immunopathology, 115, 251–260.
- Rauw, F., Lambrecht, B. & van den Berg, T. (2007). Pivotal role of ChIFNgamma in the pathogenesis and immunosuppression of infectious bursal disease. Avian Pathology, 36, 367–374.
- Ruan, Y.N., Wang, Y., Guo, Y.P., Xiong, Y.W., Chen, M.M., Zhao, A.Y. & Liu, H.B. (2020). T cell subset profile and inflammatory cytokine properties in the gut-associated lymphoid tissues of chickens during infectious bursal disease virus (IBDV) infection. Archives of Virology, 165, 2249–2258.
- Sachan, S., Dhama, K., Latheef, S.K., Samad, H.A., Mariappan, A.K., Munuswamy, P., Singh, R., Singh, K.P., Malik, Y.S. & Singh, R.K. (2019). Immunomodulatory potential of Tinospora cordifolia and CpG ODN (TLR21 agonist) against the very virulent, infectious bursal disease virus in SPF chicks. Vaccines, 7, 106.
- Shah, A.U., Li, Y., Ouyang, W., Wang, Z., Zuo, J., Shi, S., Yu, Q., Lin, J. & Yang, Q. (2021). From nasal to basal: single-cell sequencing of the bursa of Fabricius highlights the IBDV infection mechanism in chickens. Cell & Bioscience, 11, 212.
- Sharma, J.M. & Lee, L.F. (1983). Effect of infectious bursal disease on natural killer cell activity and mitogenic response of chicken lymphoid cells: role of adherent cells in cellular immune suppression. Infection and Immunity, 42, 747–754.
- Shin, S.Y., Han, T.H., Kwon, H.J., Kim, S.J. & Ryu, P.D. (2021). Dexamethasone reduces infectious bursal disease mortality in chickens. Journal of Veterinary Science, 22, e33.
- Sid, H. & Schusser, B. (2018). Applications of gene editing in chickens: a new era is on the horizon. Frontiers in Genetics, 9, 456.
- Silva, M.S.E., Rissi, D.R. & Swayne, D.E. (2016). Very virulent infectious bursal disease virus produces more-severe disease and lesions in specific-pathogen-free (SPF) Leghorns than in SPF broiler chickens. Avian Diseases, 60, 63–66.
- Tammiranta, N., Ek-Kommonen, C., Rossow, L. & Huovilainen, A. (2018). Circulation of very virulent avian infectious bursal disease virus in Finland. Avian Pathology, 47, 520–525.
- Tanimura, N., Tsukamoto, K., Nakamura, K., Narita, M. & Maeda, M. (1995). Association between pathogenicity of infectious bursal disease virus and viral antigen distribution detected by immunohistochemistry. Avian Diseases, 39, 9–20.
- Tanimura, N. & Sharma, J.M. (1997). Appearance of T cells in the bursa of Fabricius and cecal tonsils during the acute phase of infectious bursal disease virus infection in chickens. Avian Diseases, 41, 638–645.
- Thai, T.N., Jang, I., Kim, H.A., Kim, H.S., Kwon, Y.K. & Kim, H.R. (2021). Characterization of antigenic variant infectious bursal disease virus strains identified in South Korea. Avian Pathology, 50, 174–181.
- Thomrongsuwannakij, T., Charoenvisal, N. & Chansiripornchai, N. (2021). Comparison of two attenuated infectious bursal disease vaccine strains focused on safety and antibody response in commercial broilers. Veterinary World, 14, 70–77.
- Tippenhauer, M., Heller, D.E., Weigend, S. & Rautenschlein, S. (2013). The host genotype influences infectious bursal disease virus pathogenesis in chickens by modulation of T cells responses and cytokine gene expression. Developmental & Comparative Immunology, 40, 1–10.
- Valli, A., Busnadiego, I., Maliogka, V., Ferrero, D., Castón, J.R., Rodríguez, J.F. & García, J.A. (2012). The VP3 factor from viruses of Birnaviridae family suppresses RNA silencing by binding both long and small RNA duplexes. PLoS One, 7, e45957.
- van den Berg, T.P. (2000). Acute infectious bursal disease in poultry: a review. Avian Pathology, 29, 175–194.
- Vervelde, L. & Davison, T.F. (1997). Comparison of the in situ changes in lymphoid cells during infection with infectious bursal disease virus in chickens of different ages. Avian Pathology, 26, 803–821.
- Wang, D., Zhou, X., She, R., Xiong, J., Sun, Q., Peng, K., Liu, L. & Liu, Y. (2009). Impaired intestinal mucosal immunity in specific-pathogen-free chickens after infection with very virulent infectious bursal disease virus. Poultry Science, 88, 1623–1628.
- Wang, D., Liu, Y., She, R., Xu, J., Liu, L., Xiong, J., Yang, Y., Sun, Q. & Peng, K. (2009). Reduced mucosal injury of SPF chickens by mast cell stabilization after infection with very virulent infectious bursal disease virus. Veterinary Immunology and Immunopathology, 131, 229–237.
- Wang, Q., Ou, C., Wei, X., Yu, Y., Jiang, J., Zhang, Y., Ma, J., Liu, X. & Zhang, G. (2019). CC chemokine ligand 19 might act as the main bursal T cell chemoattractant factor during IBDV infection. Poultry Science, 98, 688–694.
- Wang, S., Yu, M., Liu, A., Bao, Y., Qi, X., Gao, L., Chen, Y., Liu, P., Wang, Y., Xing, L., Meng, L., Zhang, Y., Fan, L., Li, X., Pan, Q., Zhang, Y., Cui, H., Li, K., Liu, C., He, X., Gao, Y. & Wang, X. (2021). TRIM25 inhibits infectious bursal disease virus replication by targeting VP3 for ubiquitination and degradation. PLoS Pathogens, 17, e1009900.
- Wang, W., Huang, Y., Zhang, Y., Qiao, Y., Deng, Q., Chen, R., Chen, J., Huang, T., Wei, T., Mo, M., He, X. & Wei, P. (2021). The emerging naturally reassortant strain of IBDV (genotype A2dB3) having segment A from Chinese novel variant strain and segment B from HLJ 0504-like very virulent strain showed enhanced pathogenicity to three-yellow chickens. Transboundary and Emerging Diseases, 1–1.
- Wang, Y., Jiang, N., Fan, L., Niu, X., Zhang, W., Huang, M., Gao, L., Li, K., Gao, Y., Liu, C., Cui, H., Liu, A., Pan, Q., Zhang, Y., Wang, X. & Qi, X. (2021c). Identification and pathogenicity evaluation of a novel reassortant infectious bursal disease virus (genotype A2dB3). Viruses, 13, 1682.
- Wang, Z., Mi, J., Wang, Y., Wang, T., Qi, X., Li, K., Pan, Q., Gao, Y., Gao, L., Liu, C., Zhang, Y., Wang, X. & Cui, H. (2020). Recombinant Lactococcus expressing a novel variant of infectious bursal disease virus VP2 protein can induce unique specific neutralizing antibodies in chickens and provide complete protection. Viruses, 12, 1350.
- Wei, L., Hou, L., Zhu, S., Wang, J., Zhou, J. & Liu, J. (2011). Infectious bursal disease virus activates the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway by interaction of VP5 protein with the p85alpha subunit of PI3K. Virology, 417, 211–220.
- Withers, D.R., Young, J.R. & Davison, T.F. (2005). Infectious bursal disease virus-induced immunosuppression in the chick is associated with the presence of undifferentiated follicles in the recovering bursa. Viral Immunology, 18, 127–137.
- Worby, C.A. & Dixon, J.E. (2014). PTEN. Annual Review of Biochemistry, 83, 641–669.
- Xu, A.H., Sun, L., Tu, K.H., Teng, Q.Y., Xue, J. & Zhang, G.Z. (2021). Experimental co-infection of variant infectious bursal disease virus and fowl adenovirus serotype 4 increases mortality and reduces immune response in chickens. Veterinary Research, 52, 61.
- Yang, B., Yan, N., Liu, A., Li, Y., Chen, Z., Gao, L., Qi, X., Gao, Y., Liu, C., Zhang, Y., Cui, H., Li, K., Pan, Q., Wang, Y. & Wang, X. (2020). Chicken eEF1alpha is a critical factor for the polymerase complex activity of very virulent infectious bursal disease virus. Viruses, 12, 249.
- Yang, D., Zhang, L., Duan, J., Huang, Q., Yu, Y., Zhou, J. & Lu, H. (2021). A single vaccination of IBDV subviral particles generated by Kluyveromyces marxianus efficiently protects chickens against novel variant and classical IBDV strains. Vaccines, 9, 1443.
- Yang, H. & Ye, C. (2020). Reverse genetics approaches for live-attenuated vaccine development of infectious bursal disease virus. Current Opinion in Virology, 44, 139–144.
- Yao, K., Goodwin, M.A. & Vakharia, V.N. (1998). Generation of a mutant infectious bursal disease virus that does not cause bursal lesions. Journal of Virology, 72, 2647–2654.
- Ye, C.J., Han, X.P., Yu, Z.L., Zhang, E.L., Wang, L.J. & Liu, H.B. (2017). Infectious bursal disease virus activates c-Src to promote alpha 4 beta 1 integrin-dependent viral entry by modulating the downstream Akt-RhoA GTPase-actin rearrangement cascade. Journal of Virology, 91, e01891-16.
- Yip, C.W., Hon, C.C., Zeng, F. & Leung, F.C. (2012). Cell culture-adapted IBDV uses endocytosis for entry in DF-1 chicken embryonic fibroblasts. Virus Research, 165, 9–16.
- Yu, Y., Cheng, L., Li, L., Zhang, Y., Wang, Q., Ou, C., Xu, Z., Wang, Y. & Ma, J. (2019). Effects of IBDV infection on expression of chTLRs in chicken bursa. Microbial Pathogenesis, 135, 103632.
- Yu, Y., Li, L., Sun, R., Xu, Z., Wang, Q., Ou, C., Zhang, Y., Gao, P. & Ma, J. (2021). Tissue distribution and developmental changes of PTEN in the immune organs of chicken and effect of IBDV infection on it. Poultry Science, 100, 101356.
- Yu, Y., Xu, Z., Ou, C., Wang, Q., Zhang, Y., Guo, F., Gao, P. & Ma, J. (2021). The effect of ghrelin on the fibrosis of chicken bursa of Fabricius infected with infectious bursal disease virus. General and Comparative Endocrinology, 303, 113705.
- Zhang, Y., Hu, B., Li, Y., Deng, T., Xu, Y., Lei, J. & Zhou, J. (2020). Binding of Avibirnavirus VP3 to the PIK3C3-PDPK1 complex inhibits autophagy by activating the AKT-MTOR pathway. Autophagy, 16, 1697–1710.
- Zhao, S., Jia, Y., Han, D., Ma, H., Shah, S.Z., Ma, Y. & Teng, K. (2016). Influence of the structural development of bursa on the susceptibility of chickens to infectious bursal disease virus. Poultry Science, 95, 2786–2794.
