ABSTRACT
Avian pathogenic Escherichia coli (APEC) cause extra-intestinal infections called colibacillosis, which is the dominant bacterial disease in broilers. To date, given the diversity of APEC strains and the need for an acceptable level of protection in day-old chicks, no satisfactory commercial vaccine is available. As part of a French nationwide project, we selected three representative strains among several hundred APEC that cause colibacillosis disease. We first performed experiments to develop colibacillosis in vivo models, using an inoculum of 3 × 107 CFU of each E. coli strain per chick. Two APEC strains (19-381 and 19-383-M1) were found to be highly virulent for day-old chicks, whereas the third strain (19-385-M1) induced no mortality nor morbidity.
We then produced an autogenous vaccine using the (Llyod, 1982; MaCQueen, 1967) 19–381 and 19-383-M1 APEC strains and a passive immunization trial was undertaken. Specific-pathogen-free Leghorn hens were vaccinated twice 2 weeks apart, the control group receiving a saline solution. The vaccinated and control hens exhibited no clinical signs, and egg production and fertility of both groups were similar. Fertile eggs were collected for 2 weeks after the second vaccination and chicks were obtained. After challenge with each APEC (19-381 and 19-383-M1), chicks appeared to be partially protected from infection with the 19-383-M1 strain, with 40% mortality compared with 80% for the non-vaccinated chicks. No protection was found when the chicks were challenged with the 19–381 strain. Now, further work is needed to consider some aspects: severity of the pathogen challenge model, persistence of the protection, number of APEC strains in the autogenous vaccine, choice of adjuvants, and heterologous protection by the vaccine made from strain 19-383-M1.
RESEARCH HIGHLIGHTS
Three APEC strains were characterized and selected to develop in vivo models of colibacillosis.
A bivalent autogenous vaccine was produced and a passive immunization trial was carried out.
Protection of chicks was demonstrated when challenged with the 19-383-M1 APEC strain (homologous challenge).
Further work is needed in particular to evaluate the protection against heterologous challenge.
Introduction
Avian pathogenic Escherichia coli (APEC) cause extra-intestinal infections called colibacillosis, which is the dominant bacterial disease on Gallus gallus farms (broilers and laying hens, in particular). Colibacillosis causes high mortality, high morbidity and high rates of carcass condemnations at the slaughterhouse; the resulting decrease in production leads to significant economic losses for the farmer. It also causes salpingitis and septicaemia in breeders. Moreover, APEC may be a potential foodborne zoonotic pathogen and a source or reservoir of extra-intestinal infections in humans (Moulin-Schouleur et al., Citation2007; Mellata, Citation2013; Liu et al., Citation2018). To control the disease, antibiotics such as beta-lactams, colistin and fluoroquinolones are used, most often by the oral route and administered in the drinking water. These treatments constitute a proven risk of selection and dissemination of antimicrobial resistance genes and resistant bacterial strains, also leading to public health concerns.
APEC harbour numerous virulence factors that cause colibacillosis. Two recent publications (Christensen et al., Citation2021; Kathayat et al., Citation2021) inventoried these virulence factors, which include adhesins, invasins, protectins, iron acquisition systems, toxins, a quorum-sensing system, transcriptional regulators, and genes associated with metabolism; this list is not exhaustive. Moreover, APEC produce extracellular polymeric substances with more than 500 different proteins that may interact with the host, combined with other bacteria, E. coli or others (Eboigbodin & Biggs, Citation2008). Another more recent paper (Delannoy et al., Citation2021) evaluated the genetic diversity of E. coli strains isolated from 80 broiler flocks, monitored from before chick placement to colibacillosis outbreaks. The E. coli isolates were characterized using high-throughput qPCR to screen genetic markers related to 23 serogroups, five phylogroups and 66 virulence factors and to determine genetic profiles. In addition to other findings, the study highlighted the huge diversity among avian E. coli with, for example, some flocks for which day-old chicks harboured the genetic profile of colibacillosis cases identified in other flocks, but nevertheless remained healthy.
Beyond the concern related to colibacillosis, the strong regulatory, scientific and societal pressure to reduce the use of antibiotics in poultry farming has led to search for other strategies to control this disease, including the development of an efficient vaccine. Despite plenty of vaccine candidates demonstrating efficacy in chickens in experimental studies, only one vaccine is currently commercially available in most regions worldwide (Galal et al., Citation2018; Chrétien et al., Citation2021). It is an attenuated O78 E. coli strain and provides effective protection against a challenge with the O78 wild strain (Koutsianos et al., Citation2020). However, according to the summary of the characteristics of this vaccine, the onset of immunity in chickens is established 2 weeks after vaccination with a reduction in colibacillosis lesions. Except for a few publications and under specific conditions (Mombarg et al., Citation2014, where the overall mortality was very high), the efficacy of the vaccine was not established to reduce mortality (as shown in the summary of the product characteristics at http://ircp.anmv.anses.fr/results.aspx), although mortality appears to be one of the most common clinical manifestations of colibacillosis (Kemmett et al., Citation2014). In addition, according to some studies (Ghunaim et al., Citation2014; Guabiraba & Schouler, Citation2015), this type of vaccine is less efficient against E. coli heterologous strains (e.g. those belonging to other serogroups or phylogroups). Therefore, this vaccine sometimes suffers from limited efficacy, given the diversity of APEC strains and the difficulty of obtaining a satisfactory level of protection as early as hatch time.
Autogenous vaccines may be one way to address and respond to this diversity of strains. They are produced from APEC strains isolated from the affected flock in which the autogenous vaccine is to be administered. This type of vaccine can be used either directly on target animals (Landman & van Eck, Citation2017; Kromann et al., Citation2021) or via a passive immunization strategy (i.e. administration of the vaccine to the broiler breeders to protect the day-old chicks by means of antibodies transmitted by their mother). Although it has yet to be demonstrated that autogenous vaccines can protect against heterologous APEC strains, it is easier to adapt their composition compared to that of a commercial vaccine, due to the heaviness of the drug marketing authorization process. Because of this, autogenous vaccines can more easily solve the diversity of APEC strains. To our knowledge, no satisfactory vaccine is available against colibacillosis in broilers and, to date, only two publications have reported possible protection of day-old chicks using passive immunization (Rosenberger et al., Citation1985; Heller et al., Citation1990).
Here, we describe two experiments. In the first, three APEC strains that have been characterized (Delannoy et al., Citation2021) in terms of virulence factor content, serogroup and phylogroup were used to develop in vivo models of colibacillosis for day-old chicks in two chicken breeds: the ANSES’ own Leghorn breed, and the Ross 308 breed (Aviagen®), and is the most common broiler breed in Europe. The use of two chicken breeds made it possible to check the sensitivity of our specific-pathogen-free (SPF) Leghorn breed to the E. coli challenge compared with that of Ross 308. This comparison allowed us to validate the use of our Leghorn SPF hens for the second experiment rather than the Ross 308 SPF hens, which were not available. In the second experiment, a passive immunization challenge study was then performed in broilers using a bivalent autogenous vaccine administered to breeder hens.
Materials and methods
Selection and characterization of APEC strains and preparation of mutants and inocula
Selection of three representative strains
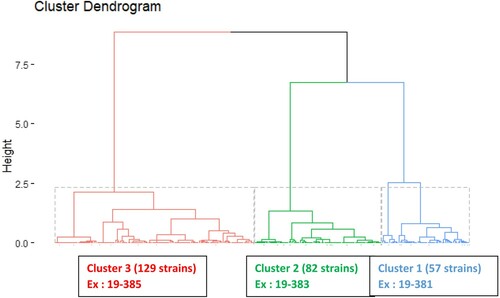
The experiments described in this manuscript are part of a French nationwide project that has been partly published recently (Delannoy et al., Citation2021) and for which the original data involved 1050 environmental or clinical E. coli isolates on which 68 variables were measured (i.e. phylogroup markers, serogroups and 66 virulence markers). Our first aim was to select three representative strains that cause colibacillosis disease for the experimental trials; therefore, statistical analyses were applied to a set of 269 E. coli strains from colibacillosis-diseased chickens only. First, non-informative variables (i.e. same value for all the strains) were discarded. Second, categorical variables were quantified and summarized using a multiple correspondence analysis (MCA) (Greenacre, Citation1984). The most informative MCA components were used as variables instead of the original ones. Third, a hierarchical clustering (Sokal & Sneath, Citation1963) approach was applied to the selected MCA components to choose the optimal number of clusters. Fourth, clustering was optimized using the k-means method (MacQueen, Citation1967; Lloyd, Citation1982) applied to the optimal number of clusters. Finally, the most representative strains (i.e. closest to the mode of the cluster) of each k-means cluster were identified. The R functions “MCA” and “hclust” of the “FactoMiner” package (Lê et al., Citation2008), and the “kmeans” function were used. At the end of the process, three clusters were obtained and three APEC strains representative of each cluster were selected: E. coli 19–381 (cluster 1), 19–383 (cluster 2) and 19–385 (cluster 3) as shown in .
Preparation of mutants and inocula
To facilitate the recovery of the strains from internal organs during in vivo assays, spontaneous rifampicin-resistant mutants of E. coli 19-381, 19–383 and 19–385 were obtained by culturing the strains on Mueller-Hinton (MH) medium containing rifampicin (250 mg/l). No mutant was obtained for strain 19-381. The mutants were compared with parental strains, in terms of their phylogenetic group (Clermont et al., Citation2000) and their antimicrobial susceptibility determined by a broth micro-dilution method on EUVSEC plates (Sensititre, ThermoFisher Scientific, Dardilly, France). Then the E. coli strain (19-381) and the obtained mutants (19-383-M1 and 19-385-M1) were cultured overnight in MH broth at 37°C under agitation. The cultures were centrifuged and re-suspended in peptone buffer to obtain a titre of approximately 3 × 108 colony forming units (CFU)/ml for both in vivo trials. The objective was to be in conformity with Schouler et al. (Citation2012) where the dose inoculated per chick was about 5 × 107 CFU. The titres were determined by plating decimal dilutions on MH agar plates.
Whole genome sequencing and characterization of the three strains
Whole genome sequencing (WGS) was performed on a Novaseq 6000 system with the Nextera kit. The raw reads were processed using the shovill method (https://github.com/tseemann/shovill, not published yet) with the “–trim” option. This method cleaned raw reads using trimmomatic (Bolger et al., Citation2014) and assembled the reads using Spades to generate contigs (Prjibelski et al., Citation2020). The de novo contigs were then screened against Megablast (Chen et al., Citation2015) on a local nucleotide database. All contigs belonged to E. coli strains. The contigs shorter than 200 nucleotides or with a k-mer coverage lower than 2 were filtered out. The sequences were analysed using the tools from the Center for Genomic Epidemiology (CGE, http://genomicepidemiology.org/) to determine the main characteristics of the strains (serotype, sequence type (ST), resistance genes and E. coli virulence genes) and the ClermonTyper (http://clermontyping.iame-research.center/) to determine phylogroups. The susceptibility of the three strains was studied by disc diffusion assay according to the AFNOR NF U47-107 (Citation2012).
Pathogenicity of three E. coli strains (19-381, 19-383-M1 and 19-385-M1) on day-old chicks
The virulence of the selected APEC strains was evaluated in a lethality assay by subcutaneous inoculation into day-old chicks and for two chicken breeds (Leghorn and Ross 308). For this experiment, we used chicks hatched from the eggs of 45-week-old and 40-week-old hens for SPF Leghorn and Ross 308 hens, respectively. The experimental design is shown in . Four groups were defined for each chicken breed. Leghorn groups were: L-NI (non-infected), L-381 (infected with 19-381), L-383M (infected with 19-383-M1), and L-385M (infected with 19-385-M1). Ross 308 groups were: R-NI (non-infected), R-381 (infected with 19-381), R-383M (infected with 19-383-M1), and R-385M (infected with 19-385-M1). The experiment was performed in accordance with French animal welfare regulations and the protocol was approved by the ANSES/ENVA/UPEC Ethics Committee and the French Ministry for Higher Education, Research and Innovation (APAFIS #21978-2019091215094222V1). The experiment was conducted at the ANSES Ploufragan animal facilities. Four rooms and two pens per room were used. The chicks were tagged with unique numbers and housed in negative pressure, air-filtered, level-2 containment rooms in floor pens with wood shavings as bedding material. The day-old chicks were distributed in the rooms in such a way to obtain similar average weights across the different experimental groups. They were then inoculated subcutaneously (0.1 ml per chick, at the neck level between the two wings) according to the experimental design outlined in . Daily mortality and clinical status (normal, slightly depressed, prostrate) were monitored until the end of the experiment, 8 days after inoculation. Body weight was assessed before inoculation and at the end of the experiment. In case of mortality, or for 10 live birds at the end of the experiment, colibacillosis lesions were determined and liver and spleen samples were collected. All liver and spleen samples were grown on MacConkey (MC) media. The samples from birds inoculated with E. coli 19-383-M1 or E. coli 19-385-M1 were grown on MC media supplemented with rifampicin (250 mg/l), and those from birds inoculated with E. coli 19–381 or E. coli 19-383-M1 were grown on MC supplemented with ciprofloxacin (0.25 mg/l). Hence, the choice of the antibiotic used for selection was based on the resistance of the different strains. Samples from the non-inoculated birds were grown on all three media. For each positive sample, one randomly chosen colony was identified with an E. coli-specific PCR (Furet et al., Citation2009), and its phylogenetic group was determined (Clermont et al., Citation2000; Peebles et al., Citation2005).
Table 1. Experimental design for the pathogenicity experiment
APEC autogenous vaccine
The autogenous vaccine used was an inactivated and adjuvanted vaccine produced by an authorized laboratory (Labocea, Ploufragan, France). The vaccine included the APEC 19–381 and 19-383-M1 strains, because the 19-385-M1 strain induced no mortality nor clinical signs (see Results). The antigenic fraction was composed of the corresponding whole bacterial cells, whose culture was carried out in broth then in agar medium (PPLO agar base, Difco, Le Pont De Claix, France). The bacterial cells were harvested by adding 0.9% NaCl physiological serum for injection (Fresenius Kabi, Sevres, France), supplemented with 0.5% of a 37% formaldehyde solution (Sigma Aldrich, Darmstadt, Germany) to inactivate the bacteria. The concentration of bacterial cells in the aqueous phase collected was between 108 and 109 CFU/ml. This aqueous phase was then emulsified with an oily adjuvant ISA25 (SEPPIC, Castres, France) to produce an oil/water vaccine, the adjuvant representing 25% (vol/vol) of the final mixture. The inactivation of the bacteria and the sterility of the autovaccine were checked using tests on broths and subcultures on agar medium with a final reading at 14 days, in accordance with the guidelines of the European Pharmacopoeia. Each breeder hen was inoculated in the pectoral muscle with 0.3 ml of the vaccine, as described below.
Passive immunization experiment
This second experiment was also performed in accordance with the same welfare and ethics regulations (authorization number APAFIS #24443-2020030217596117V3). The experiment was conducted at the ANSES Ploufragan animal facilities. Forty SPF Leghorn layers from ANSES Ploufragan, housed in furnished cages, were randomly distributed into two groups and two separate rooms: 20 layer hens were vaccinated twice at 20 and 22 weeks of age, and the other 20 layer hens received a saline solution at the same ages (control group). Each room also housed four non-vaccinated SPF Leghorn roosters, which were previously distributed at random. Fertile eggs were collected for 2 weeks after the second vaccination: these eggs were thus collected from 22- to 24-week-old hens. The hens were observed for clinical evaluation from the first injection until the end of egg collection. Fertile eggs from both hen groups were incubated in the same incubator, but care was taken to avoid mixing them. Day-old chicks were then obtained from vaccinated and non-vaccinated hens. Six groups of 20 chicks were then randomly formed so as to obtain similar average weights (and standard deviation) in the different groups (). All chicks were tagged with unique numbers. The chicks of a given group were housed in negative-pressure level-2 isolators with a volume of 1.36 m3 each (these isolators are made to order for our institute). The same parameters as for the pathogenicity experiment were recorded: daily mortality and clinical status until the end of the experiment, body weight before inoculation and at the end of the experiment. In case of mortality or for all live birds at the end of the experiment, colibacillosis lesions were determined, and liver and spleen samples were collected.
Table 2. Experimental design for the passive immunization experiment.
The experimental design is presented in . Six groups of chicks were defined: non-vaccinated and non-challenged (NVNC), vaccinated and non-challenged (VNC), two groups of non-vaccinated and challenged (NVC 381 and NVC 383M, respectively challenged with 19–381 and 19-383-M1) and two groups of vaccinated and challenged (VC 381 and VC 383M, respectively challenged with 19–381 and 19-383-M1). On their first day of life, the chicks from the NVC 381, NVC 383M, VC 381, and VC 383M groups were challenged as described for the first experiment. Mortality, clinical status and lesions of dead birds or birds sacrificed on day 9 were recorded as described above. Organs were cultured as for the first trial, but only one isolate was characterized from each organ.
Statistics
For both experiments, the qualitative variables (i.e. mortality, clinical status) were analysed using a Chi-square test, or Fisher’s exact test for small samples (n ≤ 5). The quantitative data were analysed using either an analysis of variance or a Wilcoxon test, depending on the number of observations and the parametric hypothesis checking. The level of significance was set to P ≤ 0.05.
Sequences
The sequences were deposited in GenBank and are available from the NCBI, BioProject PRJNA795346 (Accessions SRR17934378, SRR17934377 and SRR17934376).
Results
Characterization of the three representative E. coli strains and mutants obtained
The genetic characteristics of the colibacillosis isolates obtained were used to select three strains representative of the three clusters on the basis of the characteristics (i.e. phylogroup, serogroup and 66 virulence markers) of the 269 colibacillosis strains. A hierarchical clustering procedure was performed using 45 variables (variables with no variability were removed) and 268 strains (one atypical strain was discarded). Then, a k-means method was applied with k = 3 classes. The three k-means clusters, containing respectively 57, 82 and 129 strains, were the same as with those identified in the hierarchical clustering ().
Cluster 1 contained isolates mostly belonging to the B2 phylogroup, and to the O2:K1 serogroup, and possessing several plasmid virulence associated genes (e.g. iut, ompT, tsh), and also the ibeA gene, absent in most isolates of Clusters 2 and 3. Cluster 2 contained isolates belonging mostly to phylogroup F, and possessing the pic, fimA1 and ireA genes, which are absent in most isolates of Clusters 1 and 3. Cluster 3 included mostly isolates belonging to phylogroup B1 and possessing, unlike most isolates of Clusters 1 and 2, the genes ETT2.2, fepC, hcp and hra. Finally, for each class, the most representative strains (i.e. E. coli 19-381, 19–383 and 19-385) were selected. E. coli 19–381 shared all the characteristics presented in for strains of Cluster 1, except the iha and csgA3 genes. E. coli 19–383 shared the characteristics of Cluster 2, except the iutA and cma genes. Based on its genomic sequence, E. coli 19–383 was shown to belong to phylogroup G, a recently described phylogroup intermediate between the F and B2 phylogroups. Thirty out of 39 of the characteristics of Cluster 3 were present in E. coli 19-385.
Table 3. Modes of the 39 significant variables for each cluster obtained using the k-means method performed on 268 E. coli strains and 45 descriptive variables.
The three selected strains had been obtained from cases of early colibacillosis (i.e. broiler flocks of up to 10 days of age with a daily mortality rate higher than 0.3% and suspect clinical signs or typical colibacillosis lesions). Susceptibility tests showed that E. coli 19–381 was resistant to sulfamethoxazole, trimethoprim, tetracycline, ciprofloxacin and ampicillin; E. coli 19–383 was resistant to sulfamethoxazole, trimethoprim, tetracycline, ciprofloxacin, ampicillin and chloramphenicol; and E. coli 19–385 was resistant to sulfamethoxazole and tetracycline.
We were able to obtain rifampicin-resistant mutants for E. coli 19–383 and E. coli 19-385, but not for E. coli 19-381. The two mutants belonged to the same phylogenetic group as their parental strains and susceptibility testing showed that, in addition to rifampicin resistance, the mutants E. coli 19-383-M1 and E. coli 19-385-M1 were resistant to the same antibiotics as their parental strains. Thus, the in vivo experiments were performed with E. coli 19–381 and the mutants E. coli 19-383-M1 and E. coli 19-385-M1.
Results of the WGS of these three strains are presented in . The E. coli 19-381, 19-383-M1 and 19-385-M1 strains belonged respectively to serotypes O50/O2:H5:K1, O24:H4 and O86:H51, and to ST140, ST117 and ST155. Phylogroups determined by PCR were confirmed by WGS. Based on the various virulence-associated genes screened for using qPCR and the CGE web tool, E. coli 19–381 and 19-383-M1 had a high number of virulence-associated genes (39 each), whereas 29 virulence-associated genes were detected in E. coli 19-385-M1. The first two strains had the ciprofloxacin resistance mutation in the gyrA gene (S83L) and harboured genes encoding resistance to beta-lactams, tetracyclines, sulfonamides, trimethoprim and aminoglycosides. E. coli 19-383-M1 also had genes encoding resistance to macrolides and chloramphenicol. E. coli 19-385-M1 only had resistance genes for beta-lactams and tetracyclines.
Table 4. Characteristics of the three challenge strains selected for use in the pathogenicity experiment.
Pathogenicity experiment
Numbers of bacteria inoculated per chick were 3.2 x107, 3.4 x107 and 2.6 x107 CFU for E. coli 19-381, 19-383-M1 and 19-385-M1, respectively.
Observed mortality and clinical status are given in . The virulence profile for chicks of the three APEC strains was quite different. Mortality in Ross 308 was 0% (R-NI and R-385M groups), 84% (R-383M group) and 100% (R-381 group), all chicks dying as early as the day following inoculation for R-381. The surviving chicks (R-383M) presented a significantly more severe clinical state compared with the control group until day 4 (D4), the difference in score distribution being non-significant thereafter. Regarding the Leghorn chicks, mortality was 0% (L-NI and L-385M), 52% (L-383M) and 100% (L-381), all chicks dying before D4 for L-381. The surviving chicks (L-383M) presented a significantly more severe clinical state compared with the control group until D4, the difference being non-significant thereafter. Thus, the two chicken breeds were susceptible to colibacillosis, although the mortality rate was significantly higher for Ross 308 than for Leghorns for APEC 19-383-M1.
Table 5. Pathogenicity experiment results: subcutaneous inoculation of three APEC strains (19-381, 19-383-M1 and 19-385-M1) to assess their virulence (mortality and clinical status) in day-old chicks for two chicken breeds (Ross 308 and Leghorn).
For the Ross 308 chicks, the average body weight at the end of the study was 173.0 g (25 chicks; standard deviation (SD), 25.5 g) for the R-NI group, 123.5 g (four chicks; SD, 17.2 g) for the R-383M group, and 165.0 g (25 chicks, SD, 23.5 g) for the R-385M group. The average weight was significantly higher in the R-NI and the R-385M groups than in the R-383M group (P = 0.001, ANOVA). The average weights of the R-NI and R-385M groups were not significantly different. Regarding the Leghorn chicks, the average body weight at the end of the study was 92.2 g (25 chicks; SD, 7.2 g) for the L-NI group, 79.4 g (12 chicks; SD, 8.3 g) for the L-383M group, and 89.2 g (24 chicks; SD, 8.5 g) for the L-385M group, respectively. This average weight was significantly higher in the L-NI (P < 0.001) and the L-385M (P = 0.002) groups than in the L-383M group. The average weights of the L-NI and L-385M groups were not significantly different.
Regarding post-mortem findings, no lesions were observed in the control group. When death was sudden, there was generalized congestion. Conversely, when the chicks survived a few days, pericarditis and perihepatitis were observed. Within each group, the numbers of positive liver and spleen samples on the different media tested (MC, MC-Rif and MC-Cip) were not significantly different (P > 0.05). All cultures from the control non-inoculated group were negative. Overall, for the group inoculated with E. coli 19-381, all analysed chicks were positive. For the group inoculated with E. coli 19-383-M1, 19 out of 25 Leghorn and 24 out of 25 Ross 308 chicks were positive (P > 0.05). For the group inoculated with E. coli 19-385-M1, four out of 10 Leghorn and three out of 10 Ross 308 chicks were positive (P > 0.05). The numbers of positive chicks in the inoculated groups were significantly different from each other, with the highest proportion in the 19–381 inoculated group (32 positive chicks out of 32 analyzed), the lowest proportion in the 19-385-M1 group (seven positive chicks out of 20 analyzed), and the 19-383-M1 group showing an intermediate proportion (43 positive chicks out of 50 analyzed). All isolates (153 from MC, 142 from MC-CIP and 85 from MC-Rif) belonged to the expected phylogroups.
Passive immunization experiment
No clinical signs were observed in the vaccinated or the control hens. Moreover, egg production and fertility of both groups were similar. For this experiment, the dose of E. coli (in CFU in 0.1 ml volume) that was administered per chick was 1.2 × 107 and 3.2 × 107 for strains 19–381 and 19-383-M1, respectively.
Results are presented in . No mortality nor clinical signs occurred in the control groups (negative control and vaccine control). No significant difference was observed, either in mortality (90% versus 95%) or in the clinical status over time, between the non-vaccinated and vaccinated chicks that were challenged with the APEC 19–381 strain. Conversely, for chicks that were challenged with the APEC 19-383-M1 strain, the mortality rate was significantly lower in the vaccinated group (40%) than in the non-vaccinated group (80%), as shown in (P < 0.05). However, no significant differences were observed between groups for their clinical status over time.
Figure 2. Mortality rate over time after challenge with the E. coli 19-383-M1 strain in non-vaccinated (NVC) and vaccinated (VC) chicks.
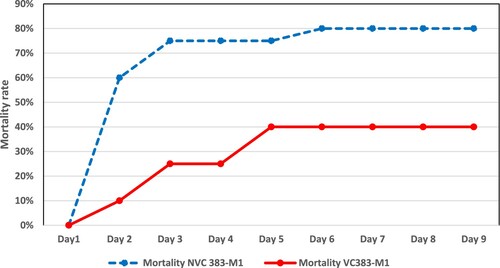
Table 6. Evaluation of the protection of day-old chicks against an E. coli homologous challenge via the passive immunization conferred by their autogenous vaccinated mother hens. The autogenous vaccine, inactivated and adjuvanted, was prepared from E. coli strains 19–381 and 19-383-M1.
For the comparisons that were carried out (vaccinated vs non-vaccinated groups that were challenged with either E. coli strain 19–381 or 19-383-M1), no significant differences were noted between the different groups regarding body weight at day 9. Regarding post-mortem findings, the results obtained during the first experiment were confirmed: generalized congestion in case of rapid death or pericarditis and/or perihepatitis for chicks that survived several days.
Similar to the first trial, results obtained on the three media were not significantly different (P > 0.05). Overall, most samples from challenged chicks, vaccinated or not, were found to be positive by culture. All birds collected up to and on day 6 were positive. Thereafter, only two birds of the NVC381 and one bird from the VC383 group were negative. All tested isolates (46 from the MC media, 40 from the MC-CIP media and 23 from the MC-Rif media) belonged to the expected phylogroup.
Discussion
The genes and mutations detected in the genomes of the three strains were consistent with the susceptibility phenotypes, i.e. the mutation S83L in the gyrA gene of E. coli 19–381 and 19–383 leading to quinolone resistance, and the presence of the following resistance genes: blaTEM-1B for resistance to ampicillin (19-381), tet(A) or tet(B) for resistance to tetracycline (the three strains), sul1 or sul2 for resistance to sulfamethoxazole (the three strains), dfrA1 for resistance to trimethoprim (19-381 and 19-383) and catA1 for resistance to chloramphenicol (19-383). E. coli 19–381 and 19–383 also had strA, strB or aadA1, which encode resistance to streptomycin, but this antibiotic was not tested.
E. coli 19–381 is a O50/O2:K1:H5, B2, ST140 isolate. Indeed E. coli strains belonging to this ST have been reported among the most prevalent isolated from avian colibacillosis and may be involved in human diseases. Mehat et al. (Citation2021) reported that the O1 and O2 serotypes, which, with O78, represent 80% of APEC isolates, belong to a lineage including ST-95, ST140 and ST428/ST429 strains. Zhu Ge et al. (Citation2014) studied the APEC isolate IMT5155 (O2:K1:H5; ST140), isolated from a diseased chicken in Germany in 2000, and showed that it shared close relationship with ST95 APEC O1:K1 and human ExPEC O18:K1 strains. E. coli 19-383-M1 belongs to serotype O24:H4, phylogroup G. Indeed, phylogroup G is composed of one main ST complex, STc117, a poultry-associated lineage with extensive resistance to antibiotics (Clermont et al., Citation2019). E. coli 19-383M1 carries most of the virulence genes frequently present in strains of STc117 (Clermont et al., Citation2019). ST117 APEC were previously implicated in large outbreaks of colibacillosis in both parent stock and broilers in Nordic countries (Ronco et al., Citation2017). Thus, the two strains selected to prepare the autogenous vaccine are clearly important poultry pathogens. E. coli 19–381 and 19–383 were highly virulent for day-old chicks, because they induced a high percentage of mortality within a few days. Both strains contain a high number of virulence-associated genes. In particular, they have five (19-381) or four (19-383) of the predictors of pathogenicity (iss, iutA, hlyF, iroN and ompT) proposed by Johnson et al. (Citation2008) and, according to this scheme, they would be classified as virulent. Regarding the E. coli 19–385 strain, in our experimental conditions, it was not virulent for day-old chicks. Interestingly, this strain contains the plasmid virulence-associated genes hlyF, iroN, iss and ompT, and would have been classified as virulent according to the scheme of Johnson et al. (Citation2008), whereas, according to the diagnostic strategy proposed by Schouler et al. (Citation2012), E. coli 19–381 would be classified as virulent while E. coli 19–383 and E. coli 19-385 would not.
Regardless of the chicken strain used in the first experiment, challenge with APEC 19–381 resulted in 100% mortality 4 days post-inoculation at the latest. Mortality during the second experiment was at least 90%. This APEC strain probably has a lower lethal dose than the 19-383-M1 strain. For the E. coli strains that were pathogenic, mortality rate or pattern was different for the Ross 308 and for the Leghorn breeds. Thus, significantly higher mortality was observed in the R-383M (Ross 308 chicks infected with 19-383-M1 strain) group (84%) than in the L-383M (Leghorn chicks infected with 19-383-M1 strain) group (52%). Regarding the APEC 19–381 strain, although the mortality rate was 100% for both chicken breeds, all R-381 chicks (Ross 308, infected with 19–381 strain) died before D1, whereas mortality was slightly delayed for the L-381 chicks (Leghorn, infected with 19–381 strain), all of them dying before D4. This difference in susceptibility of the two chicken breeds is in line with other studies (Yunis et al., Citation2000; Ask et al., Citation2006) and may be due to their different growth rates. Likewise, several studies have demonstrated an inverse relationship between growth rate and resistance to colibacillosis (Yunis et al., Citation2000; Citation2002); the average daily weight gain was 13 and 5 g/day in the Ross 308 R-NI (non-infected) and Leghorn L-NI (non-infected) groups, respectively.
The mortality induced by the APEC 19-383-M1 strain in Leghorn chicks was different between the two experiments (52% mortality in experiment 1 vs 80% in experiment 2). This difference may be related to breeder age. The layers were 45 weeks old in experiment 1 versus 22–24 weeks old in experiment 2. Mortality during the first week of life is higher in chicks from young hens, thus indicating a greater fragility of these chicks (Pedroso et al., Citation2005; Peebles et al., Citation2005). In the present study, we successfully demonstrated the partial protection of chicks through the vaccination of breeder hens. Our results show that APEC 19-383-M1 caused 40% mortality in chicks from hens that were vaccinated, compared with 80% for chicks from hens that were non-vaccinated. This protection against homologous APEC strains is in line with the two passive immunization studies that have been published to date for chickens (Rosenberger et al., Citation1985; Heller et al., Citation1990). According to these authors, the passive immunization process is linked to the level of maternally-derived antibodies following hen vaccination. In addition, there is a correlation between the hen’s antibody titre and percentage of survival of her progeny.
Due to the diversity of APEC strains and the need for an acceptable level of protection as early as hatch time, no satisfactory commercial vaccine is currently available. Under these circumstances, recent publications have highlighted new knowledge on APEC colonization and the usefulness of autogenous vaccines. Today, most scientists agree that E. coli colonizing day-old chicks may originate from their mother hens (Poulsen et al., Citation2017). These studies estimate horizontal spreading of E. coli in the hatcher to be 95% in comparison to the 5% of genuine vertical transfer. On the other hand, a recent paper (Lozica et al., Citation2021) investigated the effect of autogenous E. coli vaccines on the prevalence of 84 virulence-associated genes in E. coli isolated from four and five consecutive flocks at two broiler breeder farms. Results indicate that continuous application of autogenous vaccines led to lower genetic diversity of E. coli housekeeping genes, even if no such effect was observed for the diversity of virulence genes. The successful use of autogenous vaccines, including through passive immunization, will require a rational and judicious choice of the included APEC strains by characterization of the strains and determination of their pathogenicity using modern methods (machine learning, etc.), to establish a link between clinical outbreaks and other factors, including management (Christensen et al., Citation2021).
Conclusion
In this study, for our experimental trials, we selected three representative strains (19-381, 19-383-M1 and 19-385-M1) among several hundred APEC strains that cause colibacillosis disease. We first performed pathogenicity experiments to develop colibacillosis in in vivo models. Two APEC strains (19-381 and 19-383-M1) were found to be highly virulent for day-old chicks, whereas the 19-385-M1 strain induced no mortality or morbidity. We then developed a bivalent autogenous vaccine (19-381 and 19-383-M1) and carried out a passive immunization trial. We demonstrated partial protection of chicks when challenged with the 19-383-M1 strain. Further work is needed to assess the effect of the APEC challenge dose inoculated, hen age, the persistence and mechanisms of protection by passive immunization, the number of APEC strains to use in the autogenous vaccine, the judicious choice of adjuvants, and the heterologous protection of the vaccine made from strain 19-383-M1.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgements
The authors are grateful to the veterinarians and farmers who participated in the study, the Finalab, Labocea, and Resalab laboratories for isolating the strains and the staff of Anses facilities for taking care the study animals.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ask, B., van der Waaij, E.H., Stegeman, J.A. & van Arendonk, J.A. (2006). Genetic variation among broiler genotypes in susceptibility to colibacillosis. Poultry Science, 85, 415–421.
- Bolger, A.M., Lohse, M. & Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics, 30, 2114–2120.
- Chen, Y., Ye, W., Zhang, Y. & Xu, Y. (2015). High speed BLASTN: an accelerated MegaBLAST search tool. Nucleic Acids Research, 43, 7762–7768.
- Chrétien, L., Boutant, J., Lyazrhi, F. & Galliard, N. (2021). Retrospective assessment of Escherichia coli vaccination in broiler turkeys under field conditions in 37 farms from brittany (France). Avian Diseases, 65, 659–662.
- Christensen, H., Bachmeier, J. & Bisgaard, M. (2021). New strategies to prevent and control avian pathogenic Escherichia coli (APEC). Avian Pathology, 50(5), 370–381.
- Clermont, O., Bonacorsi, S. & Bingen, E. (2000). Rapid and simple determination of the Escherichia coli phylogenetic group. Applied and Environmental Microbiology, 66, 4555–4558.
- Clermont, O., Ojas, V.A.D., Vangchhia, B., Condamine, B., Dion, S., Bridier-Nahmias, A., Denamur, E. & Gordon, D. (2019). Characterization and rapid identification of phylogroup G in Escherichia coli, a lineage with high virulence and antibiotic resistance potential. Environmental Microbiology, 21, 3107–3117.
- Delannoy, S., Schouler, C., Souillard, R., Yousfi, L., Le Devendec, L., Lucas, C., Bougeard, S., Keita, A., Fach, P., Galliot, P., Balaine, L., Puterflam, J. & Kempf, I. (2021). Diversity of Escherichia coli strains isolated from day-old broiler chicks, their environment and colibacillosis lesions in 80 flocks in France. Veterinary Microbiology, 252, 108923.
- Eboigbodin, K.E. & Biggs, C.A. (2008). Characterization of the extracellular polymeric substances produced by Escherichia coli using infrared spectroscopic, proteomic, and aggregation studies. Biomacromolecules, 9, 686–695.
- Furet, J.P., Firmesse, O., Gourmelon, M., Bridonneau, C., Tap, J., Mondot, S., Doré, J. & Corthier, G. (2009). Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. FEMS Microbiology Ecology, 68, 351–362.
- Galal, H.M., Tawfek, A.M., Abdrabou, M.I., Hessain, A.M., Alhaaji, J.H., Kabli, S.A., Elbehiry, A., Alwarhi, W. & Moussa, I.M. (2018). Recent approaches for control of E. coli and respiratory complex in Middle East. Saudi Journal of Biological Sciences, 25, 1302–1307.
- Ghunaim, H., Abu-Madi, M.A. & Kariyawasam, S. (2014). Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: potentials and limitations. Veterinary Microbiology, 172, 13–22.
- Greenacre, M.J. (1984). Theory and applications of correspondence analysis. London: Academic Press. 364.
- Guabiraba, R. & Schouler, C. (2015). Avian colibacillosis: still many black holes. FEMS Microbiology Letters, 362, 1–8.
- Heller, E.D., Leitner, H., Drabkin, N. & Melamed, D. (1990). Passive immunisation of chicks against Escherichia coli. Avian Pathology, 19, 345–354.
- Johnson, T.J., Wannemuehler, Y., Doetkott, C., Johnson, S.J., Rosenberger, S.C. & Nolan, L.K. (2008). Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. Journal of Clinical Microbiology, 46, 3987–3996.
- Kathayat, D., Lokesh, D., Ranjit, S. & Rajashekara, G. (2021). Avian pathogenic Escherichia coli (APEC): an overview of virulence and pathogenesis factors, zoonotic potential, and control strategies. Pathogens (basel, Switzerland), 10, 467.
- Kemmett, K., Williams, N.J., Chaloner, G., Humphrey, S., Wigley, P. & Humphrey, T. (2014). The contribution of systemic Escherichia coli infection to the early mortalities of commercial broiler chickens. Avian Pathology, 43, 37–42.
- Koutsianos, D., Gantelet, H., Franzo, G., Lecoupeur, M., Thibault, E., Cecchinato, M. & Koutoulis, K.C. (2020). An assessment of the level of protection against colibacillosis conferred by several autogenous and/or commercial vaccination programs in conventional pullets upon experimental challenge. Veterinary Science, 7, 80.
- Kromann, S., Olsen, R.H., Bojesen, A.M., Jensen, H.E. & Thøfner, I. (2021). Protective potential of an autogenous vaccine in an aerogenous model of Escherichia coli infection in broiler breeders. Vaccines, 9(1233), 1–12.
- Landman, W.J.M. & van Eck, J.H.H. (2017). The efficacy of inactivated Escherichia coli autogenous vaccines against the E. coli peritonitis syndrome in layers. Avian Pathology, 46, 658–665.
- Lê, S., Josse, J. & Husson, F. (2008). Factominer: an R package for multivariate analysis. Journal of Statistical Software, 25, 1–18.
- Liu, C.M., Stegger, M., Aziz, M., Johnson, T.J., Waits, K., Nordstrom, L., Gauld, L., Weaver, B., Rolland, D., Statham, S., Horwinski, J., Sariya, S., Davis, G.S., Sokurenko, E., Keim, P., Johnson, J.R. & Price, L.B. (2018). Escherichia coli ST131-H22 as a foodborne uropathogen. mBio, 9, e00470–e00418.
- Lloyd, S.P. (1982). Least squares quantization in PCM. IEEE Transactions on Information Theory, 28, 129–137.
- Lozica, L., Repar, J. & Gottstein, Z. (2021). Longitudinal study on the effect of autogenous vaccine application on the sequence type and virulence profiles of Escherichia coli in broiler breeder flocks. Veterinary Microbiology, 259, 109159.
- MacQueen, J.B. (1967). Some methods for classification and analysis of multivariate observations. In L.M. Le Cam & J. Neyman (Eds.), Proceedings of the fifth Berkeley symposium on mathematical statistics and probability (pp. 281–297). Los Angeles: University of California Press.
- Mehat, J.W., Van Vliet, A.H.M. & La Ragione, R.M. (2021). The avian pathogenic Escherichia coli (APEC) pathotype is comprised of multiple distinct, independent genotypes. Avian Pathology, 50, 402–416.
- Mellata, M. (2013). Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathogens and Disease, 10, 916–932.
- Mombarg, M., Bouzoubaa, K., Andrews, S., Vanimisetti, H.B., Rodenberg, J. & Karaca, K. (2014). Safety and efficacy of an aroA-deleted live vaccine against avian colibacillosis in a multicentre field trial in broilers in Morocco. Avian Pathology, 43, 276–281.
- Moulin-Schouleur, M., Reperant, M., Laurent, S., Bree, A., Mignon-Grasteau, S., Germon, P., Rasschaert, D. & Schouler, C. (2007). Extraintestinal pathogenic Escherichia coli strains of avian and human origin: link between phylogenetic relationships and common virulence patterns. Journal of Clinical Microbiology, 45, 3366–3376.
- NF U47-107. (2012). Animal health analysis methods - Guidelines for conducting antibiograms using the diffusion method in an agar medium. (https://www.boutique.afnor.org/en-gb/standard/nf-u47107/animal-health-analysis-methods-guidelines-for-conducting-antibiograms-using/fa170310/40286#AreasStoreProductsSummaryView).
- Pedroso, A.A., Andrade, M.A., Cafe, M.B., Leandro, N.S., Menten, J.F. & Stringhini, J.H. (2005). Fertility and hatchability of eggs laid in the pullet-to-breeder transition period and in the initial production period. Animal Reproduction Sciences, 90, 355–364.
- Peebles, E.D., Keirs, R.W., Bennett, L.W., Cummings, T.S., Whitmarsh, S.K. & Gerard, P.D. (2005). Relationships among prehatch and posthatch physiological parameters in early nutrient restricted broilers hatched from eggs laid by young breeder hens. Poultry Science, 84, 454–461.
- Poulsen, L.L., Thofner, I., Bisgaard, M., Christensen, J.P., Olsen, R.H. & Christensen, H. (2017). Longitudinal study of transmission of Escherichia coli from broiler breeders to broilers. Veterinary Microbiology, 207, 13–18.
- Prjibelski, A., Antipov, D., Meleshko, D., Lapidus, A. & Korobeynikov, A. (2020). Using SPAdes de novo assembler. Current Protocols in Bioinformatics, 70, e102.
- Ronco, T., Stegger, M., Olsen, R.H., Sekse, C., Nordstoga, A.B., Pohjanvirta, T., Lilje, B., Lyhs, U., Andersen, P.S. & Pedersen, K. (2017). Spread of avian pathogenic Escherichia coli ST117 O78:H4 in Nordic broiler production. BMC Genomics, 18, 13.
- Rosenberger, J.K., Fries, P.A. & Cloud, S.S. (1985). In vitro and in vivo characterization of avian Escherichia coli. III. immunization. Avian Diseases, 29, 1108–1117.
- Schouler, C., Schaeffer, B., Bree, A., Mora, A., Dahbi, G., Biet, F., Oswald, E., Mainil, J., Blanco, J. & Moulin-Schouleur, M. (2012). Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. Journal of Clinical Microbiology, 50, 1673–1678.
- Sokal, R.P. & Sneath, P.H.A. (1963). Principles of numerical taxonomy. San Francisco, CA: W.H. Freeman, 359.
- Yunis, R., Ben-David, A., Heller, E.D. & Cahaner, A. (2000). Immunocompetence and viability under commercial conditions of broiler groups differing in growth rate and in antibody response to Escherichia coli vaccine. Poultry Science, 79, 810–816.
- Yunis, R., Ben-David, A., Heller, E.D. & Cahaner, A. (2002). Antibody responses and morbidity following infection with infectious bronchitis virus and challenge with Escherichia coli, in lines divergently selected on antibody response. Poultry Sciences, 81, 149–159.
- Zhu Ge, X., Jiang, J., Pan, Z., Hu, L., Wang, S., Wang, H., Leung, F.C., Dai, J. & Fan, H. (2014). Comparative genomic analysis shows that avian pathogenic Escherichia coli isolate IMT5155 (O2:K1:H5; ST complex 95, ST140) shares close relationship with ST95 APEC O1:K1 and human ExPEC O18:K1 strains. PLoS One, 9, 1–16.