ABSTRACT
The increasing global demand for poultry products, together with the growing consumer concerns related to bird health and welfare, pose a significant challenge to the poultry industry. Therefore, the poultry industry is increasingly implementing novel technologies to optimize and enhance bird welfare and productivity. This second part of a bipartite review on omics technologies in poultry health and productivity highlights the implementation of specific diagnostic biomarkers based on omics-research in the poultry industry, as well as the potential integration of multi-omics in future poultry production. A general discussion of the use of multiple omics technologies in poultry research is provided in part 1. To date, approaches focusing on one or more omics type are widely used in poultry research, but the implementation of these omics techniques in poultry production is not expected in the near future. However, great potential lays in the development of diagnostic tests based on disease- or gut health-specific biomarkers, which are identified through omics research. As the cost of omics technologies is rapidly decreasing, implementation of multi-omics measurements in routine poultry monitoring systems might be feasible in the more distant future. Therefore, the opportunities, challenges and requirements to enable the integration of multi-omics-based monitoring of bird health and productivity in future poultry production are discussed.
Introduction
Poultry meat and egg production are characterized by a continuous increase in the size of the operations at all levels of the production chain, from the feed mills over the layer and broiler farms, to the abattoirs and the processing plants. In the production units, larger numbers of highly productive birds allow for an increase the efficacy of the operations. At the same time, however, the evolution of ever-increasing performance holds a higher risk of failures due to metabolic disorders in the highly productive birds and due to the fact that endemic and epidemic infectious diseases may cause more damage. Therefore, the poultry industry is embracing and implementing new technologies. Now, chicken houses can be equipped with cameras registering the movements of the birds, and numerous sensors are available for monitoring the environment, feed and drinking water. Such systems can provide indirect information on the health status of the birds. Direct information, however, still comes from regular inspection of the birds and laboratory analyses of samples taken from the birds by the farm veterinarian. It has become a standard practice to sacrifice some birds for necropsy and scoring of lesions in the intestinal tract, air sacs, breast muscles and skeleton at regular timepoints during the production cycle. Unfortunately, such lesion scoring can detect a disease state only when it is already advanced. Therefore, the industry is looking for methods that allow early detection of disease, even before macroscopic signs of disease are present. The development of these methods is in large part based on systems to monitor chicken-related parameters such as bird behaviour, body temperature or sound, as well as on omics-derived research. The hope is that early detection will allow timely interventions, steering towards better performance. Therefore, this two-part review focusses on the current use and future applications of omics technologies in poultry health and productivity.
A general introduction of the use of multiple omics technologies in poultry research is provided in part 1 of this review, including a discussion on why the integration of multiple omics is essential to advance our understanding of complex biological processes, such as feed efficiency or disease resistance. This second part of the review highlights the implementation of specific diagnostic biomarkers based on omics research, as well as the potential integration of multi-omics in future poultry production.
Implementation of omics-based biomarkers in poultry production
Improving bird performance and disease resistance are key issues in the poultry industry. To date, approaches focusing on one or more omics types are widely used in poultry research, but the implementation of these omics techniques in poultry production is not expected in the near future for a number of reasons. Indeed, today, omics approaches are still too complex, expensive and time-consuming to be implemented directly in commercial poultry production. However, with exponentially advancing technological improvements, the cost of omics technologies is rapidly decreasing and current fourth-generation sequencing approaches already allow real-time, on-site sequencing at relatively low cost (Feng et al., Citation2015; Theuns et al., Citation2018). Additionally, the complexity of the data analysis and interpretation is of major concern. Omics technologies typically yield “big data”, containing information on all molecules (e.g. host and bacterial genes, proteins, and metabolites) present in the sample, which complicates data interpretation. For example, the identification of all microorganisms in a clinical sample results in an enumeration of all microbial taxa present in that sample, which has little to no diagnostic value. Furthermore, this approach might lead to incidental findings of potentially pathogenic microorganisms which pose no clinically significant risk. Without other clinical data and expertise in interpreting those datasets, there is a high risk of overinterpretation and unnecessary treatment of flocks. To overcome the issue of data complexity, many research groups are currently using omics research to identify biomarkers for different pathological facets linked to reduced gut health (e.g. inflammation, gut leakage, bacterial or parasite infection) (Chen et al., Citation2015; Ducatelle et al., Citation2018; Goossens et al., Citation2018; De Meyer et al., Citation2019; Dal Pont et al., Citation2021). These biomarkers can be measured in either the faeces or the blood, and easy and fast diagnostic tests, such as lateral flow assays or ELISAs, have the potential to be readily implemented in the routine health monitoring practices in poultry production. For the majority of these biomarkers, it will, however, not be possible to set a universal threshold value discriminating between healthy and diseased birds, since most of these biomarkers will show a continuum ranging from birds in optimal health to the poorest birds in the flock. Probably, a baseline measurement per farm, house or even production cycle will be necessary.
As described in part 1 of this review, commercial chicks are hatched in a clean environment without contact with the parent birds, and transfer of the microbiota from the hen to the chicks by direct contact does not occur. Therefore, environmental factors have a large effect on microbiome development of the newly hatched chicks, and large variations in the gut microbiome between flocks and production cycles are observed (Stanley et al., Citation2013, Citation2016; Kers et al., Citation2018, Citation2019; Kubasova et al., Citation2019; Rychlik, Citation2020; Bindari & Gerber, Citation2022). Furthermore, the gut microbiome not only regulates the intestinal physiological homeostasis but also has systemic effects that steer bird resistance towards environmental and infectious stressors (Kogut, Citation2019). Altogether, there is a lot of variability in microbiome composition, host molecular signals (such as protein or metabolite abundances), gut physiological parameters (e.g. intestinal villus height, crypt depth, or inflammatory T-cell infiltration) and bird performance between farms and between successive flocks (Kers et al., Citation2018, Citation2019; Van Limbergen et al., Citation2020; Ringenier et al., Citation2021; authors’ unpublished data). When comparing the host or microbiome characteristics of different farms, it seems that these parameters are not necessarily associated with inter-farm or inter-flock differences in bird performance. However, within each flock, various host and microbiome parameters are significantly linked to the occurrence of intestinal disturbances or variations in bird performance. One example is the measurement of faecal ovotransferrin, which can be used as a biomarker for intestinal damage and inflammation (Goossens et al., Citation2018). When comparing the faecal ovotransferrin measurements between different experimental trials, a significant difference in baseline ovotransferrin levels of healthy birds is observed. However, within each trial, the experimental challenge results in a significant deviation from the baseline measurement, which is positively correlated with the macroscopically scored disease severity (Goossens et al., Citation2018). Therefore, longitudinal noninvasive monitoring is important to establish flock-specific baseline values for (gut) health status-specific biomarkers, and to alert the veterinarian or the farmer when deviations from the baseline are detected.
Moving forward in the era of precision livestock farming: towards the integration of multi-omics data in poultry production
The increasing global demand for poultry products, together with the growing consumer concerns related to bird health and welfare, pose a significant challenge to the poultry industry. Therefore, the poultry industry is increasingly implementing novel technologies to optimize and enhance bird welfare and productivity, such as precision livestock farming (PLF). The goal of PLF is to support farmers in livestock management by monitoring animal productivity, environmental impacts, as well as health and welfare. The power of PLF is based on automatic acquisition, access, and processing of data from diverse sources to create an automatic management system (Sassi et al., Citation2016). In order to do so, the data are analyzed by machine learning algorithms to generate predictions or risk assessment models. These modelling techniques are essential to interpret data from real-time monitoring devices, in order to develop control systems or to establish risk alerts (Sassi et al., Citation2016). As such, PLF techniques can adopt a farm-specific application that goes beyond a “one size fits all” approach, and as soon as the data deviate from the expected flock curve, the user can be alerted via connected devices (e.g. phones, computers, or tablets). This allows the farmer to detect and react to health issues at an early stage, thereby improving animal welfare and productivity (Sassi et al., Citation2016; Astill et al., Citation2018; Schillings et al., Citation2021). However, up till now, highly trained data specialists are still needed to perform high-level data analysis and accurate risk assessment. Currently, different systems, such as sensors, cameras or microphones, are already employed in the poultry industry to monitor environmental parameters such as air temperature, humidity, ventilation, CO2% and ammonia levels, as well as systems to monitor chicken-related parameters such as feed and water uptake, body temperature and behaviour, amongst others (for reviews on PLF see Sassi et al., Citation2016; Schillings et al., Citation2021). These systems permit real-time monitoring of the production conditions and birds in a relatively simple and efficient manner, at an affordable cost. Moreover, small implantable biosensors can provide detailed, individual, real-time data on various biometric parameters such as body temperature, heart rate or activity, as well as the measurement of specific biomolecules such as glucose, lactate or ATP (Wiedmeyer & DeClue, Citation2008; Koenig et al., Citation2016; Lee et al., Citation2016; Neethirajan, Citation2017; Reynolds et al., Citation2018; Rios et al., Citation2020). The integration of biosensors in PLF systems increases the ability to detect the onset of disease at an early stage, even before clinical signs occur. Even if individual monitoring of all birds is economically unfeasible for the poultry industry, biosensor-derived health data from a fraction of the birds, combined with the aforementioned parameters measured at the flock level, might provide valuable data on the flock health status.
The great potential of PLF relies on early warning, which allows farmers to take action in the initial stages of welfare problems or diseases. However, although these systems can inform the farmer about the health status of the birds, and even have the potential to detect signs of disease (Astill et al., Citation2018; Li et al., Citation2020), they are not specific enough to provide a diagnosis of complex multifactorial diseases such as dysbiosis, necrotic enteritis or bacterial chondronecrosis with osteomyelitis and lameness, all diseases that pose a significant burden on modern poultry industry. Currently, these diseases are diagnosed through routine necropsy of some birds at regular timepoints during the production cycle. As this diagnosis relies on the scoring of macroscopic lesions, only more advanced stages of the disease are detected. In order to minimize loss of bird performance and improve bird welfare, there is an urgent need for methods to detect early stages of the disease, even before clinical signs occur. This is where the implementation of multi-omics approaches has great potential to advance poultry production. Indeed, the pathogenesis of complex, multifactorial diseases involves several cascades of events, that occur at various omics layers (e.g. microbiome, metabolome, host proteome and gene expression), either triggered or not by environmental changes. Given the complex, multifactorial nature of some important diseases challenging the modern poultry industry, together with the above described inter-flock variability in host and microbiome parameters, the development of robust, disease-specific biomarkers is not straightforward, and multi-omics approaches might be needed to identify discriminatory multi-omics signatures linked to each disease.
Previous research in human medicine has highlighted the potential of integrative analysis of longitudinal multi-omics data combined with non-omics clinical data for health assessment and predictions. The first study dates back to 2013, where longitudinal multi-omics monitoring of a single person resulted in the identification of temporal deviations in pathway expression levels that were consistent with disease status and progression (Stanberry et al., Citation2013). This concept was further expanded to a larger patient cohort to derive predictive models for long-term health monitoring that allow early interventions and treatment decisions for glycaemic responses, cardiovascular health, infectious diseases and oncology (Zeevi et al., Citation2015; Kellogg et al., Citation2018; Piening et al., Citation2018; Olivier et al., Citation2019; Schüssler-Fiorenza Rose et al., Citation2019). These studies clearly illustrate the potential for predictive models based on multi-omics and clinical data.
Despite the great potential of multi-omics approaches in early disease detection, implementation of omics in clinical practice is non-existing in livestock production or veterinary medicine. It is also still in its infancy in human medicine, with only anecdotal usage in human oncology (John et al., Citation2020; D’Adamo et al., Citation2021). Hurdles to be taken before the implementation of these approaches are related to integration of these large omics datasets in an effective, practical way, and even more importantly, to the lack of large, publicly available, multi-omics datasets. Multi-omics approaches yield large datasets with significant heterogeneity, making it impossible to analyze these datasets with traditional statistical techniques. Over the last decade, artificial intelligence and machine learning techniques have become promising methods in multi-omics data analysis and have significantly improved the ability to monitor “big datasets” from multiple sources (D’Adamo et al., Citation2021; Kang et al., Citation2022). However, before machine learning models can be used in a clinical setting, these algorithms need to be trained and validated by using large amounts of data, after which these models can be used to perform risk assessments and disease diagnoses based on new data (Wu et al., Citation2021). To date, the necessary datasets to train these models are not available.
To advance poultry health and productivity by implementation of multi-omics approaches in PLF, research should focus on the characterization of integrated multi-omics disease signatures. In a first step, experimental models can be used to generate well-organized, structured datasets that link multi-omics measurements to specific diseases. In order to translate these multi-omics signatures to the practical conditions in the field, further research should focus on the longitudinal monitoring of birds in commercial poultry houses. In such longitudinal monitoring, each flock serves as its own control, thereby solving the problem of inter-flock variability. Data derived from continuous, real-time monitoring of environmental conditions should be supplemented with multi-omics measurements at specific pre-defined timepoints. In order to obtain a holistic profile of the bird’s health status, these multi-omics measurements should target both the host and its microbiome. Whenever the real-time monitoring system detects an increased risk of disease onset (e.g. drop in water or feed intake), daily multi-omics sampling should be performed, combined with thorough veterinary examination to diagnose diseases. Such longitudinal datasets that combine environmental measurements, multi-omics data and detailed veterinary diagnoses, are essential to identify integrated signatures specific for complex diseases. Furthermore, the longitudinal sampling enables study of the transition between health and disease. These datasets can be used to train and validate decision models to be implemented in current PLF technologies. The resulting monitoring approaches should alert the farmer, not only on onset of disease, but also provide a disease diagnosis, which allows targeted timely interventions by the farmer or the veterinarian (). However, to date, the development of such monitoring systems is still hampered by both the cost of the analyses and the lack of reference databases to train the decision models.
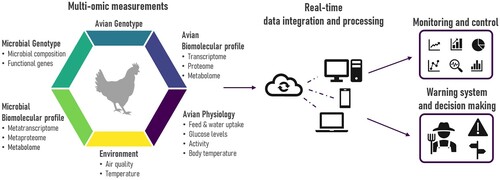
Figure 1. Overview of future integration of multi-omics measurements in precision livestock farming (PLF) technologies. Omics measures can target both the avian host and its microbiome. The type of omics analysis can target various biological layers, such as the (meta-)genome, (meta-)transcriptome, (meta-)proteome or the metabolome. These multi-omics measurements should be complemented with continuous monitoring of both avian physiological parameters and environmental measurements. All these data are integrated and processed in real-time using machine learning techniques and can be accessed from a mobile device such as a smartphone, tablet or computer. As such, the farmer can monitor the flock’s status and will be alerted when an increased risk of disease is detected. Furthermore, by using longitudinal monitoring, each flock serves as its own control, thereby solving the problem of inter-flock variability.
Conclusion
Roughly every decade a new set of revolutionary research tools is discovered and further developed into techniques that give completely new insights in major research questions. Some of these research tools further evolve into routine analytical techniques that are widely implemented in the daily routine of medical and veterinary diagnostics. Others remain research tools. Omics technologies are not expected to be implemented directly in poultry production or veterinary diagnostics in the very short-term because they do not fulfil all of the criteria that need to be met to make the move to the routine labs. These criteria include, amongst others, speed, accuracy, specificity, low cost, easy scaling-up and a clear cut-off (an ideal cut-off would mean that there is no overlap between the healthy and the diseased population). As research tools, however, omics technologies are already proving to be extremely valuable, and this value will only grow in years to come. Their future contribution to the advancement of poultry health and productivity lies not only in the new insights in poultry physiology and pathology, which are directly derived from the implementation of these tools, but also in the discovery of new biomarkers for specific clinically and economically important physiological and pathological conditions in the birds. The latter strategy is already delivering its first successes, including biomarkers for intestinal health based on, for example, host proteins that can be detected in faeces (Goossens et al., Citation2018; De Meyer et al., Citation2019; Giannuzzi et al., Citation2021). One may speculate that further future developments may be expected from the integration of longitudinal multi-omics monitoring into the sensor-based machine learning strategies of precision livestock farming.
Disclosure statement
E. Goossens, R. Ducatelle and F. Van Immerseel are listed as coinventors on a patent describing the use of acute-phase proteins as marker for intestinal barrier failure: “In vitro method to detect intestinal barrier failure in animals” (International Publication Number [WO2019166531A1]). E. Goossens, R. Ducatelle and F. Van Immerseel are listed as coinventors on the patent “Intestinal and fecal biomarkers for intestinal health of poultry” (International Publication Number [WO2019206585A1]). F. Van Immerseel is listed as a coinventor on the patent “Intestinal biomarkers for gut health in domesticated birds” (International Publication Number [WO2020205841A1]).
Additional information
Funding
References
- Astill, J., Fraser, E., Dara, R. & Sharif, S. (2018). Detecting and predicting emerging disease in poultry with the implementation of new technologies and big data: a focus on avian influenza virus. Frontiers in Veterinary Science, 5, 263.
- Bindari, Y.R. & Gerber, P.F. (2022). Centennial review: factors affecting the chicken gastrointestinal microbial composition and their association with gut health and productive performance. Poultry Science, 101, 101612.
- Chen, J., Tellez, G., Richards, J.D. & Escobar, J. (2015). Identification of potential biomarkers for gut barrier failure in broiler chickens. Frontiers in Veterinary Science, 2, 14.
- D’Adamo, G.L., Widdop, J.T. & Giles, E.M. (2021). The future is now? Clinical and translational aspects of “Omics” technologies. Immunology and Cell Biology, 99, 168–176.
- Dal Pont, G.C., Belote, B.L., Lee, A., Bortoluzzi, C., Eyng, C., Sevastiyanova, M., Khadem, A., Santin, E., Farnell, Y.Z., Gougoulias, C. & Kogut, M.H. (2021). Novel models for chronic intestinal inflammation in chickens: intestinal inflammation pattern and biomarkers. Frontiers in Immunology, 12, 1–15.
- De Meyer, F., Eeckhaut, V., Ducatelle, R., Dhaenens, M., Daled, S., Dedeurwaerder, A., De Gussem, M., Haesebrouck, F., Deforce, D. & Van Immerseel, F. (2019). Host intestinal biomarker identification in a gut leakage model in broilers. Veterinary Research, 50, 1–14.
- Ducatelle, R., Goossens, E., De Meyer, F., Eeckhaut, V., Antonissen, G., Haesebrouck, F. & Van Immerseel, F. (2018). Biomarkers for monitoring intestinal health in poultry, present status and future perspectives. Veterinary Research, 49, 1–9.
- Feng, Y., Zhang, Y., Ying, C., Wang, D. & Du, C. (2015). Nanopore-based fourth-generation DNA sequencing technology. Genomics, Proteomics & Bioinformatics, 13, 4–16.
- Giannuzzi, D., Biolatti, B., Longato, E., Divari, S., Starvaggi Cucuzza, L., Pregel, P., Scaglione, F.E., Rinaldi, A., Chiesa, L.M. & Cannizzo, F.T. (2021). Application of RNA-sequencing to identify biomarkers in broiler chickens prophylactic administered with antimicrobial agents. Animal, 15, 100113.
- Goossens, E., Debyser, G., Callens, C., De Gussem, M., Dedeurwaerder, A., Devreese, B., Haesebrouck, F., Flügel, M., Pelzer, S., Thiemann, F., Ducatelle, R. & Van Immerseel, F. (2018). Elevated faecal ovotransferrin concentrations are indicative for intestinal barrier failure in broiler chickens. Veterinary Research, 49, 1–8.
- John, A., Qin, B., Kalari, K.R., Wang, L. & Yu, J. (2020). Patient-specific multi-omics models and the application in personalized combination therapy. Future Oncology, 16, 1737–1750.
- Kang, M., Ko, E. & Mersha, T.B. (2022). A roadmap for multi-omics data integration using deep learning. Briefings in Bioinformatics, 23, 1–8.
- Kellogg, R.A., Dunn, J. & Snyder, M.P. (2018). Personal omics for precision health. Circulation Research, 122, 1169–1171.
- Kers, J.G., Velkers, F.C., Fischer, E.A.J., Hermes, G.D.A., Lamot, D.M., Stegeman, J.A. & Smidt, H. (2019). Take care of the environment: housing conditions affect the interplay of nutritional interventions and intestinal microbiota in broiler chickens. Animal Microbiome, 1, 1–14.
- Kers, J.G., Velkers, F.C., Fischer, E.A.J., Hermes, G.D.A., Stegeman, J.A. & Smidt, H. (2018). Host and environmental factors affecting the intestinal microbiota in chickens. Frontiers in Microbiology, 9, 1–14.
- Koenig, A., Hoenig, M.E. & Jimenez, D.A. (2016). Effect of sensor location in dogs on performance of an interstitial glucose monitor. American Journal of Veterinary Research, 77, 805–817.
- Kogut, M.H. (2019). The effect of microbiome modulation on the intestinal health of poultry. Animal Feed Science and Technology, 250, 32–40.
- Kubasova, T., Kollarcikova, M., Crhanova, M., Karasova, D., Cejkova, D., Sebkova, A., Matiasovicova, J., Faldynova, M., Pokorna, A., Cizek, A. & Rychlik, I. (2019). Contact with adult hen affects development of caecal microbiota in newly hatched chicks. PLoS One, 14, e0212446.
- Lee, Y., Bok, J.D., Lee, H.J., Lee, H.G., Kim, D., Lee, I., Kang, S.K. & Choi, Y.J. (2016). Body temperature monitoring using subcutaneously implanted thermo-loggers from Holstein steers. Asian-Australasian Journal of Animal Sciences, 29, 299.
- Li, N., Ren, Z., Li, D. & Zeng, L. (2020). Review: automated techniques for monitoring the behaviour and welfare of broilers and laying hens: towards the goal of precision livestock farming. Animal, 14, 617–625.
- Neethirajan, S. (2017). Recent advances in wearable sensors for animal health management. Sensing and Bio-Sensing Research, 12, 15–29.
- Olivier, M., Asmis, R., Hawkins, G.A., Howard, T.D. & Cox, L.A. (2019). The need for multi-omics biomarker signatures in precision medicine. International Journal of Molecular Sciences, 20, 19.
- Piening, B.D., Zhou, W., Contrepois, K., Röst, H., Gu Urban, G.J., Mishra, T., Hanson, B.M., Bautista, E.J., Leopold, S., Yeh, C.Y., Spakowicz, D., Banerjee, I., Chen, C., Kukurba, K., Perelman, D., Craig, C., Colbert, E., Salins, D., Rego, S., Lee, S., Zhang, C., Wheeler, J., Sailani, M.R., Liang, L., Abbott, C., Gerstein, M., Mardinoglu, A., Smith, U., Rubin, D.L., Pitteri, S., Sodergren, E., McLaughlin, T.L., Weinstock, G.M., & Snyder, M.P. (2018). Integrative personal omics profiles during periods of weight gain and loss. Cell Systems, 6, 157–170.e8.
- Reynolds, J., Ahmmed, P. & Bozkurt, A. (2018). Preliminary evaluation of an injectable sensor for subcutaneous photoplethysmography in animals. 2018 IEEE Biomedical Circuits and Systems Conference, BioCAS 2018 - Proceedings.
- Ringenier, M., Caekebeke, N., De Meyer, F., Van Limbergen, T., Eeckhaut, V., Ducatelle, R., Van Immerseel, F. & Dewulf, J. (2021). A field study on correlations between macroscopic gut health scoring, histological measurements and performance parameters in broilers. Avian Pathology, 50, 500–506.
- Rios, H.V., Waquil, P.D., de Carvalho, P.S. & Norton, T. (2020). How are information technologies addressing broiler welfare? A systematic review based on the welfare quality® assessment. Sustainability, 12, 1413.
- Rychlik, I. (2020). Composition and function of chicken gut microbiota. Animals, 10, 103.
- Sassi, N.B., Averós, X. & Estevez, I. (2016). Technology and poultry welfare. Animals, 6, 62.
- Schillings, J., Bennett, R. & Rose, D.C. (2021). Exploring the potential of precision livestock farming technologies to help address farm animal welfare. Frontiers in Animal Science, 2, 13.
- Schüssler-Fiorenza Rose, S.M., Contrepois, K., Moneghetti, K.J., Zhou, W., Mishra, T., Mataraso, S., Dagan-Rosenfeld, O., Ganz, A.B., Dunn, J., Hornburg, D., Rego, S., Perelman, D., Ahadi, S., Sailani, M.R., Zhou, Y., Leopold, S.R., Chen, J., Ashland, M., Christle, J.W., Avina, M., Limcaoco, P., Ruiz, C., Tan, M., Butte, A.J., Weinstock, G.M., Slavich, G.M., Sodergren, E., McLaughlin, T.L., Haddad, F. & Snyder, M.P. (2019). A longitudinal big data approach for precision health. Nature Medicine, 25, 792–804.
- Stanberry, L., Mias, G., Haynes, W., Higdon, R., Snyder, M. & Kolker, E. (2013). Integrative analysis of longitudinal metabolomics data from a personal multi-omics profile. Metabolites, 3, 741–760.
- Stanley, D., Geier, M.S., Hughes, R.J., Denman, S.E. & Moore, R.J. (2013). Highly variable microbiota development in the chicken gastrointestinal tract. PLoS One, 8, 1–7.
- Stanley, D., Hughes, R.J., Geier, M.S. & Moore, R.J. (2016). Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: challenges presented for the identification of performance enhancing probiotic bacteria. Frontiers in Microbiology, 7, 187.
- Theuns, S., Vanmechelen, B., Bernaert, Q., Deboutte, W., Vandenhole, M., Beller, L., Matthijnssens, J., Maes, P. & Nauwynck, H.J. (2018). Nanopore sequencing as a revolutionary diagnostic tool for porcine viral enteric disease complexes identifies porcine kobuvirus as an important enteric virus. Scientific Reports, 8, 1–13.
- Van Limbergen, T., Sarrazin, S., Chantziaras, I., Dewulf, J., Ducatelle, R., Kyriazakis, I., McMullin, P., Méndez, J., Niemi, J.K., Papasolomontos, S., Szeleszczuk, P., Van Erum, J. & Maes, D. (2020). Risk factors for poor health and performance in European broiler production systems. BMC Veterinary Research, 16, 1–13.
- Wiedmeyer, C.E. & DeClue, A.E. (2008). Continuous glucose monitoring in dogs and cats. Journal of Veterinary Internal Medicine, 22, 2–8.
- Wu, W.T., Li, Y.J., Feng, A.Z., Li, L., Huang, T., Xu, A.D. & Lyu, J. (2021). Data mining in clinical big data: the frequently used databases, steps, and methodological models. Military Medical Research, 8, 1–12.
- Zeevi, D., Korem, T., Zmora, N., Israeli, D., Rothschild, D., Weinberger, A., Ben-Yacov, O., Lador, D., Avnit-Sagi, T., Lotan-Pompan, M., Suez, J., Mahdi, J.A., Matot, E., Malka, G., Kosower, N., Rein, M., Zilberman-Schapira, G., Dohnalová, L., Pevsner-Fischer, M., Bikovsky, R., Halpern, Z., Elinav, E. & Segal, E. (2015). Personalized nutrition by prediction of glycemic responses. Cell, 163, 1079–1094.
