ABSTRACT
Five novel chicken astrovirus (CAstV) strains, designated ZDF, MHC, WSC, WSW and MHW, were successfully isolated from chickens with gout, and were subjected to full genome sequencing characterization and tested for their pathogenic effects in specific pathogen-free (SPF) chicken embryos and chickens. The complete genomes of the five isolated strains were approximately 7436 nt to 7511 nt in length. Phylogenetic analysis revealed that strains ZDF and MHC were clustered in a clade with strains isolated in China and that the others were clustered with strains from other countries. Based on the amino acids of ORF2, strains MHW and WSW belonged to subgroup Ai, strain WSC belonged to Bii, and strains ZDF and MHC belonged to Bi. The pathogenicity of strains MHW, MHC and WSC, all belonging to different subgroups was studied. The results showed that the mortality of the chicken embryos was 100% when infected with any strain at a dose of more than 103 TCID50, 35% in SPF chickens infected with strain WSC, 25% with MHC and 15% with MHW. The body weights of chickens and embryos infected with 0.2 ml 10 TCID50 were significantly reduced after hatching. SPF chickens infected with any of the strains had similar lesions characterized by urate deposits on the epicardium and kidney, and necrotic spots on the liver. This study identified the three types of genotypic CAstV prevalent in China, with high mortality in embryonated chicken eggs and leading to white chick syndrome, retarded growth and visceral gout in infected chicks.
Introduction
Astroviruses are nonenveloped viruses with a positive-sense, single-stranded RNA genome. At present, the list of animal species susceptible to astrovirus infection has expanded to 22 animal species or families, including domestic, synanthropic and wild animals and avian and mammalian species in terrestrial and aquatic environments (Brussel et al., Citation2020). Astrovirus species infecting mammals are classified into the genus Mamastrovirus, and those infecting avian hosts are classified into the genus Avastrovirus. Officially, three different astrovirus species currently make up the Avastrovirus genus according to the International Committee on Taxonomy of Viruses, namely, Avastrovirus 1, 2 and 3, and these infect turkeys, chickens and ducks, respectively (Smyth, Citation2017). Two astrovirus species, namely, chicken astrovirus (CAstV) and avian nephritis virus (ANV) (including three groups, ANV-1, ANV-2 and ANV-3), infect chickens, and recent research has revealed that CAstV infections are prevalent in broiler chickens (de Wit et al., Citation2011; Kang, El-Gazzar, et al., Citation2012; Ter Veen et al., Citation2017; Xue et al., Citation2017).
Similar to other astroviruses, CAstV is a nonenveloped, single-stranded and positive-sense RNA virus with a genome of approximately 7.5 kb in length, coding for three open reading frames (ORFs) (Kang, Icard, et al., Citation2012). The first two ORFs are ORF 1a and ORF1b. ORF1a, located close to the 5’ terminus of the genome, encodes nonstructural proteins, including a protease, and is followed by ORF1b, which encodes an RNA-dependent RNA polymerase (Smyth et al., Citation2012). The third ORF, ORF2, the most variable region of the genome, is located downstream of ORF1b and before the 3′ untranslated region of the genome and codes for the outer surface of the capsid protein, including the star-like capsid spikes, which interact with the host immune system; hence, variability is inevitable (Smyth, Citation2017). Based on partial ORF1b sequences, CAstVs could be assigned to Group I or II (Smyth et al., Citation2009). Based on the complete ORF2, CAstV strains can be clustered into Groups A and B. The Group A CAstVs comprise three subgroups, Ai, Aii and Aiii, and the B group CAstVs comprise six subgroups, Bi, Bii, Biii, Biv, Bv and Bvi (Kwoka et al., Citation2021; Raji & Omar, Citation2022; Raji et al., Citation2022). Subgroup Biii is associated with visceral gout, and subgroup Biv is associated with white chick syndrome (WCS) (Smyth, Citation2017).
CAstV infections usually occur within the first days or week of life, transmitted horizontally by the faecal-oral route, and some CAstV strains can also be vertically transmitted from naïve in-lay parent birds to embryos; these strains vary widely in pathogenicity and may result in variable clinical manifestations. Kang, Icard, et al. (Citation2012) reported that CAstVs have been linked to “runting-stunting syndrome” and “uneven flock performance”. A runted chick hatches small, while a stunted bird exhibits a failure to grow and often appears to have delayed development. Uneven flock performance occurs when the variance in weights at slaughter is larger than expected, potentially causing carcass processing problems (Smyth et al., Citation2007). Smyth et al.(Citation2013) reported that CAstVs have become associated with hatchery diseases, most notably WCS, and the chicks that hatch have pale plumage, are weak and runted and tend not to survive very long. Bulbule et al. (Citation2013) reported severe kidney disease of young broiler chicks during outbreaks of visceral gout, with the causative agent being identified as a Group B CAstV.
In China, serological investigation of CAstV infection was first reported in 2017 by Xue et al. (Citation2017) and showed that the overall seroprevalence in the birds tested was 60.68% by ELISA, and that the prevalence increased from 34.17% to 74.44% with increasing age. In addition, CAstV-positive birds included layer flocks, layer parent flocks, broiler flocks, broiler parent flocks, and domestic chicken flocks. Nine of the 42 seropositive astrovirus samples were positive for CAstV according to sequence analysis of the ORF1b gene (Xue et al., Citation2020). These data indicated that CAstV infections are very common in China (Xue et al., Citation2017; Xue et al., Citation2020). However, except for Zhao et al. (Citation2020) and Yin et al. (Citation2021) who isolated two strains of CAstV (NJ1701 and GD202013) classified as subgroups Bi and Bii based on phylogenetic analysis of complete ORF2 amino acid sequences, little is known about the variation and pathogenicity of CAstV in China.
In this study, five strains of CAstV were isolated from broiler chicken flocks, Ross breed broilers and “Yellow” chickens (a breed of broilers) in China. The molecular characterization of the strains by complete genome sequencing and the pathogenicity of the isolates for specific pathogen-free (SPF) chicken embryos and chickens were conducted. These results will provide a foundation for understanding CAstV in China.
Materials and methods
Detection of chicken astrovirus in clinical samples
Five clinical samples from a broiler chicken flock, Ross breed broilers and “Yellow” chickens in Shandong and Guangdong provinces of China were negative for common viruses, such as influenza virus, Newcastle disease virus, infectious bronchitis virus, infectious bursal disease virus, and fowl adenovirus, by PCR for the conserved genes of viruses (primers in ). Total RNA was extracted from these samples using TRIzol reagent (Invitrogen, Shanghai, China). Reverse transcription PCR for detecting CAstV was conducted according to the method of Smyth et al. (Citation2009) (primers in ). Reverse transcription PCR was performed as follows: reverse transcription at 50°C for 30 min and initial PCR activation at 95°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 60 s, DNA extension at 72°C for 60 s, and a final extension step at 72°C for 10 min.
Table 1. Primer pairs for detection of common viruses in the study.
Isolation of chicken astrovirus
Positive homogenates were freeze-thawed three times and centrifuged at 6000× g for 30 min. Aliquots (0.2 ml) of the supernatants were filtered through a 0.22 μm syringe-driven filter before adsorption onto the chicken hepatocellular carcinoma cell line (LMH) (ATCC®CRL -2117TM) obtained from the American Type Culture Collection (Manassas, VA, USA). Following a 1 h adsorption period at 37°C, the inoculum was removed and replaced with MEM containing 10% foetal bovine serum (the normal LMH cell culture medium). After incubation for 2 days, the cells were freeze-thawed three times, and the supernatants were harvested for plaque purification. Plaque purification was performed as reported by Baxendale and Mebatsion (Citation2004).
Purified virus-infected LMH cells were serially passaged five times, their cytopathic effects were observed, and a quantitative real-time PCR assay (qRT–PCR) was performed for the detection and quantification of CAstV based on the ORF1b gene according to the method of Wang et al. (Citation2016). A pair of primers used to amplify a 510-bp product from the conserved region of CAstVs was selected from the report of Smyth et al. (Citation2009). After PCR amplification, the generated product was inserted into a pMD19-T vector (TaKaRa, Dalian, China) and sequenced to verify the correct target amplification. To determine the sensitivity of the assay, plasmid DNA was quantified, and a curve was generated based on ten serial dilutions with a base of 10, diluted from 1 × 108 to 1 × 103 copies/μl. The reaction was performed using a 5100 real-time PCR system (Thermo Fisher, Waltham, MA, USA) in fast mode using the following steps: 95°C for 2 min, 40 cycles of 95°C for 30 s, 60°C for 30 s and 72°C for 30 s. The absolute quantification of CAstV was performed based on the standard curve, which was generated and presented as viral gene copies per µl.
The isolated strains were named CAstV/ZDF/chicken/20180910 (ZDF), CAstV/MHC/chicken/20191125 (MHC), CAstV/WSC/chicken/20191117(WSC), CAstV/WSW/chicken/20191117 (WSW) and CAstV/MHW/chicken/20191125 (MHW), and viral titre (culture infective doses, TCID50) assays were conducted according to the report of Smither et al. (Citation2013).
Complete genome sequencing and phylogenetic analyses
Total RNA was extracted from infected LMH cells with TRIzol reagent (Invitrogen) and treated with RNase free DNase I (Invitrogen) according to the manufacturer’s instructions. Overlapping PCR fragments were amplified across the viral genome with primers selected from the report of Zhao et al. (Citation2020). Then, the PCR products were cloned into the pMD18-T vector according to the manufacturer’s protocol and sequenced by Sangon Biotech (Shanghai, China). The whole viral genomes were assembled, and ORFs were predicted by Lasergene 7 and guided using the genomes of CAstV in NCBI. The full-length genome sequences derived in this study were deposited in GenBank with accession numbers OL874466 (ZDF), OL874467 (MHC), OL874468 (MHW), OL874469 (WSC) and OL874470 (WSW). Transmembrane domains were predicted using TMHMM version 2.0 (http://www.cbs.dtu.dk/services/TMHMM/). The nucleotide and deduced amino acid sequences of ORF1a, ORF1b and ORF2 were aligned using the Clustal W method, and phylogenetic trees were generated using the neighbour-joining method and 1,000 bootstrap replicates in MEGA 7.0. For phylogenetic analysis, representative sequences of CAstV in different subgroups (Smyth, Citation2017; Palomino-Tapia et al., Citation2020) and some sequences of astrovirus in GenBank were used ().
Table 2. Sequences of chicken astrovirus in GenBank used in the study for phylogenetic analysis.
Infection of SPF egg embryos
According to the results of the genome sequencing analyses, three strains (MHC, MHW and WSC) from the different subgroups were then tested in animal experiments.
A total of 152 7-day-old SPF chicken embryos (Merial Vital, Beijing, China) were randomly divided into four groups. In total, 144 embryos in three test groups (48 embryos in each group) were inoculated with virus stocks of MHC, MHW and WSC, and eight embryos in the control group were inoculated with 0.2 ml cell culture medium. Forty-eight embryos in each test group received 0.2 ml 106 TCID50, 105 TCID50, 104 TCID50, 103 TCID50, 102 TCID50 or 10 TCID50 virus via the allantoic route, and each dose of virus was tested in eight embryos. The eggs were then incubated at 37°C in an egg incubator, and their viability was observed daily. Mortality in the different groups was calculated, and the body weights of the chicks were measured after hatching.
Infection of SPF chickens
Eighty 1-day-old SPF White Leghorn chicks (Merial Vital) were randomly allotted to four groups. Inoculation was performed in three groups via intramuscular leg injection using 500 μl of inoculum containing 105 TCID50 of strains MHC, MHW and WSC. Chicks in the control group were inoculated with the same amount of cell culture medium. The chicks were housed in high-efficiency particulate air-filtered negative-pressure isolators and provided irradiated food and acidified water ad libitum. The number of deaths in every group was calculated every day until 15 days after infection.
Two hundred 1-day-old SPF White Leghorn chicks (Merial Vital) were randomly allotted into four groups and treated and raised as described above. The body weights of the chicks were measured before inoculation and every 3 days after inoculation. Six birds from each group were randomly euthanized by intravenous injection of sodium pentobarbitone and autopsied every 3 days after inoculation until 15 days after infection.
Statistical analysis
The experiments were performed three times independently, and the results are shown as the means ± standard deviations (SD). The significance of the variability between the control group and test groups was analysed using Student’s t-test in GraphPad software (version 5.0). P values ≤ 0.05 or ≤0.01 were considered significant and extremely significant, respectively.
Results
Isolation of chicken astrovirus strains
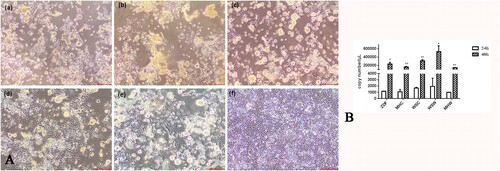
The fragments were amplified by the primers for CAstV from the five field samples, and the CAstV sequence was confirmed by sequencing. A homogenate of these five samples was inoculated into LMH cells. The infected cells became rounded in shape and began to detach and form clusters at 48 hours post-infection (hpi) (A). The viral copy number of CAstV in the inoculated LMH cells was significantly increased from 24 hpi to 48 hpi as shown by qRT–PCR (B). The five isolated strains of CAstV were confirmed by their cytopathic effects and molecular techniques.
Figure 1. Isolation and identification of CAstV. (A) LMH cells at 48 h post-infection with CAstV strains; infected cells were round and detached from the bottom, and uninfected cells maintained shape (400×). (a) strain ZDF; (b) strain MHC; (c) strain WSC; (d) strain WSW; (e) strain MHW; (f) uninfected. (B) Viral copy number of CAstV in LMH cells at 24 hpi and 48 hpi (*P ≤ 0.05, **P ≤ 0.01).
Genome sequence analysis of chicken astrovirus strains
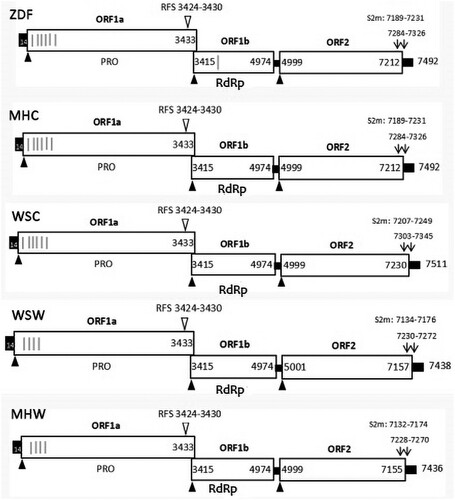
All of the sequenced fragments of the five strains MHC, MHW, ZDF, WSC and WSW were successfully amplified, and the full-length genomes were assembled. The five genomes consisted of 7492, 7492, 7511, 7438 and 7436 nt in length, including ORF1a, ORF1b and ORF2, which are typical astrovirus genome structures. The nucleotide position of the start site of the ribosomal frameshift signal (RFS) sequence was located at the same position in all of the strains, and four to seven transmembrane domains were predicted in the different strains. The stem–loop-II motif was at the position of 304 nt (WSW, WSC and MHW) or 303 nt (ZDF and MHC) from the 3′UTR of the genomes. The genome structures are shown in .
Figure 2. Predicted genome organization of the three strains. Three ORFs with their locations are shown and translation start sites are indicated by solid black triangles. The nucleotide position of the start site of the ribosomal frameshift signal (RFS) sequence is indicated by inverted open triangles. Transmembrane helical domain in ORFs are represented by grey lines. The locations of stem-loop-II-motif (s2m) are shown and start sites are indicated by black arrows.
Phylogenetic analysis of isolated chicken astrovirus
The phylogenetic analysis was based on the complete nucleotide sequences of 26 chicken astrovirus reference strains from GenBank. ANV and CAstV strains were grouped into different clades. The nucleotide sequences of the five strains were compared with other full-length genomes of CAstV, and the results showed that the five isolates were clustered into two branches. Strains ZDF and MHC have the highest similarity (99.2% and 98.4%) to the recently published Chinese CAstV strain NJ170 and more than 95.5% similarity with other strains from China (CAstV/HBLP, CZ180 and CAstV/GDYHTJ). The similarity between the ZDF and MHC strains was 98.2%. Strains ZDF and MHC shared 71.4% to 77.6% similarity with MHW, WSW and WSC. Strain MHW has the highest similarity to the strains WSW (99.4%) and Poland-G059-2014 (81.9%). Strain WSC has the highest similarity to strain GA-2011-USA-2016 (88.2%) (A).
Figure 3. Phylogenetic tree of nucleotide sequences and amino acid sequences of isolated strains (solid circles) and selected representative astroviruses using neighbor-joining method with 1,000 bootstraps. Phylogenetic analyses based on the nucleotide sequences of the complete genome (A), amino acid sequences of ORF1a (B), ORF1b (C) and ORF2 (D).
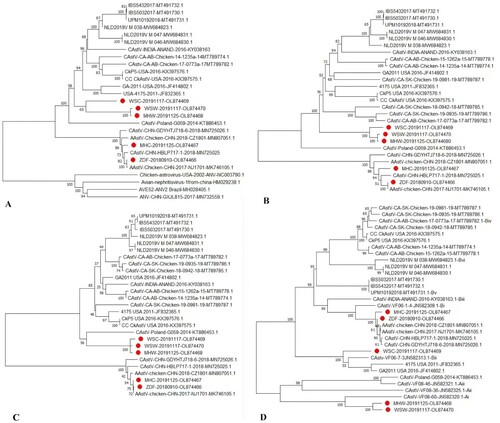
The similarity of the amino acid sequences of the three viral proteins was compared after prediction from the nucleotide sequences. The results showed that the five isolates were clustered into different branches of CAstV strains according to the analysis of ORF1a and ORF1b. Strains ZDF and MHC were clustered in the clade with four strains isolated in China (GDYHTJ, HBLP, CZ180 and NJ170) with similarity at levels of 97.2-100%. Strains MHW, WSW and WSC were clustered in the clade with strains from the USA and the Canada, with similarity at levels of 90.1-90.5% (ORF1a) and 87.6–88.9% (ORF1b) (B,C).
According to the reports of Smyth (Citation2017) and Raji and Omar (Citation2022), the representative strains belonging to different subgroups were referenced. Based on the analysis of the amino acid of ORF2, the five strains isolated in this study were assigned into Groups A and B, in which strain MHW and WSW were subgrouped in Ai with the highest similarity of 86% to the strain VF08-60. Strain WSC was subgrouped in Bii with the highest similarity of 86.5% to strain VF06-7-3. Strains ZDF and MHC were subgrouped in Bi together with four strains isolated in China (GDYHTJ, HBLP, CZ180 and NJ170). Strain ZDF had the highest similarity of 98% to strain NJ1701 and MHC 98.2% to strain GDYHTJ. Among the five isolated strains, the strains ZDF and MHC had homology of approximately 47.5% to MHW and WSW and 74% to WSC, which had homology of approximately 48.2% to MHW and WSW (D).
Infection experiment in SPF chicken embryos
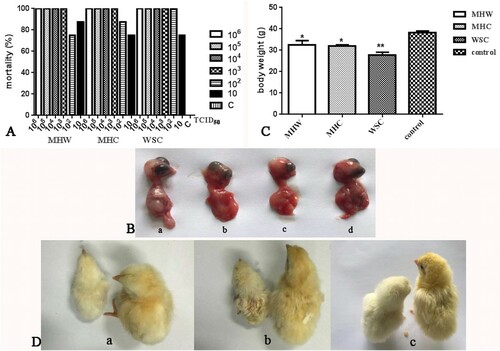
The infection study of the CAstV strains belonging to different subgroups was conducted after the virus titres were calculated, and the TCID50 values of the strains MHC, MHW and WSC were 1 × 10−7.88/100 μl, 1 × 10−6.6/100 μl and 1 × 10−6.33/100 μl, respectively. When different doses were used to infect chicken embryos via the allantoic route, the results showed that the mortality of the chicken embryos in the test groups was 100% for all that received more than 103 TCID50 of any strain, and the chicken embryos infected with the WSC strain at 102 TCID50 all died. The hatch rate in the groups infected with strains MHC and MHW at 102 TCID50 or 10 TCID50 was no more than 25%, while the hatch rate of the embryos in the control group was 100% (A). Growth depression and haemorrhage were observed in the dead virus-infected embryos (B). The chicks that hatched out in the test groups were smaller than those in the control group, and their entire body was covered with white fluff (D). The body weights of the chicks after hatching in different groups infected with 10 TCID50 strains were significantly reduced compared with those in the control group (P = 0.0475, 0.0101 or 0.0097 respectively) (C). All of the chicks that hatched died within the first 4 days.
Figure 4. Mortality and growth depression of SPF chicken embryos infected with CAstV strains. (A) Mortality of the chicken embryos infected with strains at different titres. (B) Haemorrhage and growth depression in dead embryos at 4 days post-infection with CAstV strains: (a) uninfected; (b) strain MHW; (c) strain MHC; (d) strain WSC. (C) Body weights of chickens infected with CAstV strains before hatching. (D) Growth depression and covering with white fluff in chicken (left) after hatching following infection with CAstV at the embryo stage, and the chicken on the right was uninfected. (a) strain MHW; (b) strain MHC; (c) strain WSC.
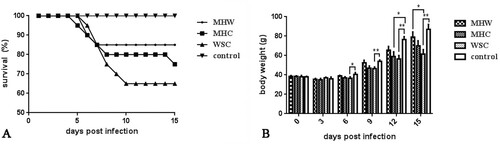
SPF chicks infected with the various strains tended to die between 5 and 10 dpi. At 15 dpi, the survival rate was 65% in the WSC-infected group, 75% in the MHC-infected group and 85% in the MHW-infected group, whereas there was no mortality in the control group (A). The body weights of chicks in the infected groups were lower than those in the control group 6 days after infection. This was especially true for the WSC-infected group, where the difference in their weights reached the level of statistical significance (P = 0.0267, 0.0100, 0.0080 or 0.0022 at 6-15 dpi, respectively). Although the body weights in the MHC- and MHW-infected groups were lower than those in the control group after infection for 6 days, the difference was significant only in the MHC-infected group at 12 dpi (P = 0.0381) (B).
Figure 5. Mortality and growth depression at different times post-infection in SPF chickens infected with strains MHW, MHC and WSC. (A) Survival rate of SPF chickens infected with different CAstV strains. (B) Body weights of SPF chickens infected with different CAstV strains.
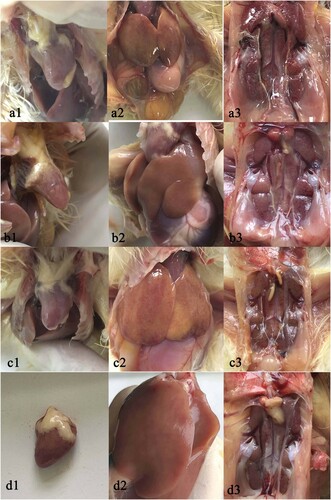
At necropsy, infection with the different strains all resulted in similar lesions in the chickens. Urate was deposited on the epicardium and had a white colour, there were multiple pale necrotic spots on the liver, and swelling and haemorrhage in the kidney, with obvious white urate in the ureter. In addition, steatosis and haemorrhage were observed in the enlarged liver of chickens infected with strains MHW and WSC ().
Figure 6. Gross lesions in organs of SPF chickens infected with different CAstV strains: (a) strain MHW; (b) strain MHC; (c) strain WSC. (d) uninfected. a1, heart, white urate deposit on the epicardium. a2, liver, steatosis haemorrhage with pinpoint necrotic areas. a3, kidney, swollen haemorrhagic kidney with obvious white urate in the ureter. b1, heart, white urate deposited on the epicardium. b2, liver, pinpoint necrotic areas. b3, kidney, swelling and with obvious white urate in the ureter. c1, heart, white urate deposit on the epicardium. c2, liver, mild steatosis, haemorrhage and necrotic spots. c3, kidney, mild haemorrhage with obvious white urate deposits in the ureter. d1, d2 and d3, heart, liver and kidney in uninfected chicken.
Discussion
Astroviruses have been isolated from humans and numerous mammalian and avian species with asymptomatic or enteric disease and a range of other clinical signs (Zhang et al., Citation2018; Lulla & Firth, Citation2020). There is evidence for a high degree of cross-species transmission of avian astroviruses between farmed poultry species and other avian and mammalian species, suggesting that avian strains may be readily transmitted to humans under prolonged close contact (Donato & Vijaykrishna, Citation2017). In recent years, goose astrovirus has caused great damage to the goose industry (Zhang et al., Citation2018, Citation2021), but the prevalence of CAstV in China as a primary pathogen is still a matter of debate, and its pathogenic characteristics have not been confirmed due to limited reports of epidemiological investigations of CAstV in China (Xue et al., Citation2017) and the relatively poor growth of CAstV in cell culture (Smyth et al., Citation2012). In this study, five strains of CAstV were successfully isolated by culturing in LMH cells and provided the basis for the infection experiments in SPF chicken embryos and chickens.
To better understand the genetic characteristics of CAstV currently circulating in China, five full-length genomes of the viral isolates were amplified and assembled. The sequences of the strains were 7436 nt to 7511 nt in length and had the typical characteristics of astrovirus, including a 5’-untranslated region (UTR), three open reading frames (ORFs), a 3’-UTR, and a poly-A tail (Pantin-Jackwood et al., Citation2011). The RFS and s2 m in the strains were consistent with strains previously isolated in China (Xue et al., Citation2020; Zhao et al., Citation2020). The genome structure and length of strains ZDF and MHC were the same as those of the recently published Chinese CAstV strain NJ1701 (Zhao et al., Citation2020). The five strains isolated in this study belong to the CAstV group and were classified into two different clades of CAstV based on the phylogenetic analysis results of the complete nucleotide sequences and amino acid sequences of the viral proteins ORF1a and ORF1b. Strains ZDF and MHC belong to the Chinese CAstV branch, and strains WSC, WSW and MHW belong to another branch, which includes strains isolated from the USA, India, Poland, Canada, Malaysia and Netherlands. These results suggested that WSC, WSW and MHW are new emerging genotypic strains in China.
ORF2 codes for the capsid protein, the most variable region of the genome. To date, two groups of CAstV have been identified, A and B, and the hypervariability of ORF2 has added many subgroups within both groups (Xue et al., Citation2017; Raji & Omar, Citation2022). According to the reference strains in different subgroups (Smyth, Citation2017; Raji & Omar, Citation2022), the five strains isolated in the study were assigned to different groups and subgroups. Strains MHW and WSW were placed in subgroup Ai, strain WSC in Bii and strains ZDF and MHC in Bi together with four strains previously isolated in China. The strains assigned to the A and B groups shared intergroup amino acid identities of 47.4–48.2%, which is higher than the 38-40% reported by Smyth et al. (Citation2012). The strains in subgroups Bi and Bii displayed intersubgroup identities ranging from 74.6–77.4%, which is lower than the 84–85% reported by Smyth et al. (Citation2012). It has been reported that the strains GDYHTJ, HBLP and NJ170 isolated in China have very high homology (Zhao et al., Citation2020). Six strains isolated in China in subgroup Bi (two strains isolated in this study and four strains reported previously) displayed intrasubgroup identities of 97.7–99.1%, consistent with the report of Smyth et al. (Citation2012). Astrovirus is a dynamic virome with the spatial and temporal changes of rapidly evolving RNA viruses (Smyth, Citation2017). The capsid protein is where the most hypervariable regions associated with antigenicity are located, and analysis of the amino acid sequence of capsid protein is important for the study of the circulation of the virus and the identification by genotyping of astrovirus in chickens. This is the first report of many genotypes of CAstV strains in China and suggests that circulating CAstV strains in China are diverse.
Transmission of CAstV can be horizontal through the faecal-oral route; some can also be vertically transmitted from naïve in-lay parent birds to the embryos, and the chicks may hatch, shedding high levels of CastV (Smyth, Citation2017). As early as 1984, vertical transmission of CAstV was suggested by Spackman et al. (Citation1984) in chicks with hatchability problems and runting. In this study, three virus strains caused growth depression in embryos and hatchability loss. The mortality of embryos in every group infected with more than 103 TCID50 was approximately 100% and was 75–87.5% even when the chicken embryos were inoculated with only 10 TCID50. It has previously been reported that the hatchability loss by CAstV ranged between 5 and 16% when chicks were infected with Biv (Palomino-Tapia et al., Citation2020) or 63.3% when infected with Bi (Zhao et al., Citation2020), but the dose of virus inoculated was not mentioned. It has been confirmed that progenitor flocks naïve to CAstV are challenged during production and experience a variable decrease in hatchability (Smyth, Citation2017). The results of this study proved that the strains in subgroups Ai, Bi and Bii isolated in China have a serious effect on hatchability.
Vertical transmission of CAstV was also reported in relation to WCS (Smyth et al., Citation2009), and, more recently, it was shown that WCS could be reproduced by inoculating embryos (Sajewicz-Krukowska et al., Citation2016). In this study, the 1-day-old chickens hatched out in the infected groups were small, weak and sick, and the whole body of the chick was covered with white fluff; none lived for more than 4 days, which is similar to the report of Naranjo Nuñez et al. (Citation2020). This condition in chickens is called WCS and it occurs by vertical transmission, principally affecting broiler chickens, and was reproduced by inoculating the virus into embryonated chicken eggs. Outbreaks of WCS have been reported in Canada, Brazil and in some countries in Europe (Smyth et al., Citation2013) and were regarded as associated with CAstV from subgroups Bii (Long et al., Citation2018), Bvi (Smyth, Citation2017) and Aiii (Sajewicz-Krukowska et al., Citation2016). In this study, the isolated strains were assigned to subgroups Ai, Bi and Bii, and the results showed that the virus related to WCS has high genetic variability. This is the first report that CAstV strains in subgroups Ai, Bi and Bii isolated in China can cause WCS.
CAstV has been associated with a spectrum of diseases ranging from subclinical infection in seemingly healthy adult birds to heavy flock losses. Runting-stunting syndrome (RSS) is the main clinical sign of CAstV infection in chickens, characterized by poor weight gain, lower feed conversion, and mortality, resulting in economic losses (Baxendale & Mebatsion, Citation2004). Challenge experiments of day-old SPF chicks with isolated CAstV strains have resulted in different mortality and varying degrees of growth suppression, which is typical of the wide-ranging pathogenicities of viruses with RNA genomes (McNeilly et al., Citation1994; Smyth, Citation2017). Infection experiments on CAstV in SPF chickens have not been reported for strains from China (Xue et al., Citation2017, Citation2020; Zhao et al., Citation2020). Among the three isolates, the mortality and poor weight gain effect on SPF chickens of the strain MHW (in group Ai) was relatively mild, WSC (in Group Bii) was the most serious and MHC (in Group Bi) was moderate. This result was consistent with the report of Smyth et al. (Citation2007) that lesions in chickens infected with strain B were more serious than those in Group A. This effect was also reflected in the body weight of the embryos.
While CAstV is predominantly an enteric virus associated with malabsorption and growth problems, it is also known to infect organs outside of the enteric tract, including the liver and kidneys (Smyth, Citation2017). In this study, gross lesions in the affected chicks were consistent, with focal to multifocal plaques on the liver surface, visceral gout in the epicardium, and swelling and haemorrhage in the kidney. This is similar to the report of Long et al. (Citation2018), who observed that affected chicks had characteristic gross and histologic liver lesions. Severe kidney disease of young broiler chicks with visceral gout was observed in SPF chickens infected with viruses isolated in India and the Middle East, and the viruses were identified as group B and subgroup Biii CAstV (Bulbule et al., Citation2013; Smyth, Citation2017). In this study, infection with strains MHW, MHC and WSC all caused lesions in the kidney and visceral gout in SPF chickens, suggesting that not only strains in subgroups Bi and Bii but also subgroup Ai are associated with kidney disease and visceral gout.
In conclusion, five strains of CAstV were successfully isolated in China and assigned to subgroups Ai, Bi and Bii. The strains all had very high lethality and led to WCS after infection of embryonated chicken eggs, and visceral gout after infection of SPF chicks, highlighting the need for investigation of the prevalence of CAstV in China and its impact on the chicken industry.
Ethics statement
This study was approved by the Institutional Animal Care and Use Ethics Committee of Qingdao Agricultural University.
Conflict of interest statement
The authors declared no potential conflict of interests with respect to the research, authorship and publication of this article.
Additional information
Funding
References
- Baxendale, W. & Mebatsion, T. (2004). The isolation and characterisation of astroviruses from chickens. Avian Pathology, 33, 364–370.
- Brussel, K., Wang, X., Shi, M., Carrai, M., Li, J., Martella, V., Beatty, J., Holmes, E. & Barrs, V. (2020). Identification of novel astroviruses in the gastrointestinal tract of domestic cats. Viruses, 12, 1301.
- Bulbule, N., Mandakhalikar, K., Kapgate, S., Deshmukh, V., Schat, K. & Chawak, V. (2013). Role of chicken astrovirus as a causative agent of gout in commercial broilers in India. Avian Pathology, 42, 464–473.
- Cavanagh, D., Mawditt, K., Britton, P. & Naylor, C.J. (1999). Longitudinal field studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions. Avian Pathology, 28, 593–605.
- de Wit, J.J., Dam, G.B., de Laar, J.M., Biermann, Y., Verstegen, I., Edens, F. & Schrier, C.C. (2011). Detection and characterization of a new astrovirus in chicken and turkeys with enteric and locomotion disorders. Avian Pathology, 40, 453–461.
- Donato, C. & Vijaykrishna, D. (2017). The broad host range and genetic diversity of mammalian and avian astroviruses. Viruses, 9, 102.
- Kang, K., El-Gazzar, M., Sellers, H., Dorea, F., Willliams, S., Kim, T., Collett, S. & Mundt, V. (2012). Investigation into the aetiology of runting and stunting syndrome in chickens. Avian Pathology, 41, 41–50.
- Kang, K., Icard, A., Linnemann, E., Sellers, H. & Mundt, V. (2012). Determination of the full length sequence of a chicken astrovirus suggests a different replication mechanism. Virus Genes, 44, 45–50.
- Kwoka, K., de Rooij, M., Messink, A., Wouters, I., Smit, L., Heederik, D., Koopmans, M. & Phan, M. (2021). Comparative viral metagenomics from chicken feces and farm dust in the Netherlands. bioRxiv, 3, 434704.
- Lee, M.S., Chang, P.C., Shien, J.H., Cheng, M.C. & Shieh, H.K. (2001). Identification and subtyping of avian influenza viruses by reverse transcription-PCR. Journal of Virological Methods, 97, 13–22.
- Liu, H.J., Lee, L.H., Shih, W.L., Lin, M.Y. & Liao, M.H. (2003). Detection of infectious bronchitis virus by multiplex polymerase chain reaction and sequence analysis. Journal of Virological Methods, 109, 31–37.
- Long, K., Ouckama, R., Weisz, A., Brash, M. & Ojkíc, D. (2018). White chick syndrome associated with chicken astrovirus in Ontario, Canada. Avian Diseases, 62, 247–258.
- Lulla, V. & Firth, A. (2020). A hidden gene in astroviruses encodes a viroporin. Nature Communicaitons, 11, 4070.
- McNeilly, F., Connor, T.J., Calvert, V.M., Smyth, J.A., Curran, W.L., Morley, A.J., Thompson, D., Singh, S., McFerran, J.B., Adair, B.M. & McNulty, S. (1994). Studies on a new enterovirus-like virus isolated from chickens. Avian Pathology, 23, 313–327.
- Meulemans, G., Boschamans, M., Berg, T.P. & Decaesstecker, M. (2001). Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses. Avian Pathology, 30, 655–660.
- Naranjo Nuñez, L., Santander-Parra, S., Kyriakidis, N., Astolfi-Ferreira, C., Buim, M. & Dela, T. (2020). Molecular characterization and determination of relative cytokine expression in naturally infected day-old chicks with chicken astrovirus associated to white chick syndrome. Animals, 10, 1195.
- Palomino-Tapia, V., Mitevski, D., Inglis, T., Vander, M., Martin, E., Brash, M., Provost, C. & Gagnon, C. (2020). Chicken astrovirus (CAstV) molecular studies reveal evidence of multiple past recombination events in sequences originated from clinical samples of white chick syndrome (WCS) in Western Canada. Viruses, 12, 1096.
- Pantin-Jackwood, M., Strother, K., Mundt, E., Zsak, L., Day, J. & Spackman, E. (2011). Molecular characterization of avian astroviruses. Archives of Virology, 156, 235–244.
- Raji, A., Ideris, A., Bejo, M. & Omar, A. (2022). Molecular characterization and pathogenicity of novel Malaysian chicken astrovirus isolates. Avian Pathology, 51, 51–65.
- Raji, A. & Omar, A. (2022). An insight into the molecular characteristics and associated pathology of chicken astroviruses. Viruses, 14, 722.
- Sajewicz-Krukowska, J. & Domanska-Blicharz, K. (2016). Nearly full-length genome sequence of a novel astrovirus isolated from chickens with ‘white chicks’ condition. Archives of Virology, 161, 2581–2587.
- Sajewicz-Krukowska, J., Pać, K., Lisowska, A., Pikuła, A., Minta, Z., Króliczewska, B. & Domanska-Blicharz, K. (2016). Astrovirus-induced “white chicks” condition – field observation, virus detection and preliminary characterization. Avian Pathology, 45, 2–12.
- Smither, S., Lear-Rooney, C., Biggins, J., Pettitt, J., Lever, M. & Olinger, G. (2013). Comparison of the plaque assay and 50% tissue culture infectious dose assay as methods for measuring filovirus infectivity. Journal of Virological Methods, 193, 565–571.
- Smyth, V. (2017). A review of the strain diversity and pathogenesis of chicken astrovirus. Viruses, 9, 29.
- Smyth, J., Connor, T., McNeilly, F., Moffet, D., Calvert, V. & McNulty, V. (2007). Studies on the pathogenicity of enterovirus-like viruses in chickens. Avian Pathology, 36, 119–126.
- Smyth, V., Jewhurst, H., Adair, B. & Todd, V. (2009). Detection of chicken astrovirus by reverse transcriptase-polymerase chain reaction. Avian Pathology, 38, 293–299.
- Smyth, V., Todd, D., Trudgett, J., Lee, A. & Welsh, V. (2012). Capsid protein sequence diversity of chicken astrovirus. Avian Pathology, 41, 151–159.
- Smyth, V., Trudgett, J., Wylie, M., Jewhurst, H., Conway, B., Welsh, M., Kaukonen, E. & Perko-Mäkelä, P. (2013). Chicken astrovirus detected in hatchability problems associated with ‘white chicks’. Veterinary Record, 173, 403–404.
- Spackman, D., Gough, R., Collins, M. & Lanning, D. (1984). Isolation of an enterovirus-like agent from the meconium of dead-in-shell chicken embryos. Veterinary Record, 114, 216–218.
- Ter Veen, C., de Bruijn, N.D., Dijkman, R. & de Wit, J.J. (2017). Prevalence of histopathological intestinal lesions and enteric pathogens in Dutch commercial broilers with time. Avian Pathology, 46, 95–105.
- Wang, J., Cao, Z., Guo, X., Zhang, Y., Wang, D., Xu, S. & Yin, Y. (2016). Cytokine expression in three chicken host systems infected with H9N2 influenza viruses with different pathogenicities. Avian Pathology, 45, 630–639.
- Xue, J., Han, T., Xu, M., Zhao, J. & Zhang, G. (2017). The first serological investigation of chicken astrovirus infection in China. Biologicals, 47, 22–24.
- Xue, J., Han, T., Zhao, Y., Yang, H. & Zhang, G. (2020). Complete genome sequence and phylogenetic analysis of novel avastroviruses circulating in China from 2016 to 2018. Virus Research, 3, 278.
- Yi, J. & Liu, C. (2011). Detecting Newcastle disease virus in combination of RT-PCR with red blood cell absorption. Virology Journal, 8, 202.
- Yin, L., Zhou, Q., Mai, K., Huang, J., Yan, Z., Wei, X., Shen, H., Li, Q., Chen, L. & Zhou, Q. (2021). Isolation and characterization of a novel chicken astrovirus in China. Poultry Science, 100, 101363.
- Zhang, Q., Cao, Y., Wang, J., Fu, G., Sun, M., Zhang, L., Meng, L., Cui, G., Huang, Y., Hu, X. & Su, J. (2018). Isolation and characterization of an astrovirus causing fatal visceral gout in domestic goslings. Emerging Microbes & Infections, 7, 1–11.
- Zhang, X., Deng, T., Song, Y., Liu, J., Jiang, Z., Peng, Z., Guo, Y., Yang, L., Qiao, H., Xia, Y., Li, X., Wang, Z. & Bian, C. (2021). Identification and genomic characterization of emerging goose astrovirus in central China, 2020. Transboundary and Emerging Diseases, 69, 1046–1055.
- Zhao, W., Wu, Z., Yao, Y., Qin, A. & Qian, K. (2020). The isolation and molecular characterization of an astrovirus from “Yellow” chickens, China. Frontiers in Veterinary Science, 7, 581862.
