ABSTRACT
Since the introduction of West Nile virus (WNV) to North America in 1999, WNV is estimated to have contributed to population-level declines in numerous avian species. However, the potential impacts of this virus on many free-ranging upland game bird species, including the wild turkey (Meleagris gallopavo), which is undergoing regional population declines, remain unknown. Herein, two age groups (∼5 to 6 weeks and ∼15 to 16 weeks post-hatch) of juvenile wild turkeys were subcutaneously inoculated with WNV, sampled daily from 1 to 7 days post-inoculation (dpi), and euthanized on 14 dpi. No clinical signs and minimal gross lesions were attributable to WNV infection. Peak viraemia titres were similar between age groups (<101.7 to 104.6 plaque-forming units [PFU]/ml), but the duration of viraemia was longer in the old group (3–4 days) than in the young group (0–3 days). Intermittent oral and/or cloacal viral shedding from 2 to 7 dpi was detected in both age groups. No infectious virus was detected in the heart, brain, kidney, skeletal muscle, spleen, and feathers from WNV-inoculated turkeys euthanized on 14 dpi. All WNV-inoculated birds seroconverted by 14 dpi, as well as two co-housed sham-inoculated birds. The most consistent microscopic lesions among all WNV-inoculated birds were mild lymphoplasmacytic myocarditis and encephalitis. Minimal immunohistochemical labelling was detected in tissues in addition to scant macrophages within the blood, spleen, and bone marrow. These data suggest WNV is unlikely to pose a significant risk to wild turkey populations, although the possibility remains that WNV may indirectly decrease fitness or predispose wild turkeys to other health stressors.
RESEARCH HIGHLIGHTS
Clinical disease was not observed in wild turkeys experimentally infected with WNV.
Pathology attributed to WNV was mild and included brain and heart inflammation.
Viraemias suggest WNV-infected wild turkeys do not play a role in WNV transmission.
No age-associated differences in WNV clinical disease or pathology were observed.
Introduction
The wild turkey (Meleagris gallopavo) is a culturally and economically important upland game bird in North America that experienced substantial population expansion following intensive restoration efforts in the late twentieth century (Kennamer et al., Citation1992). However, in recent decades, wild turkey populations across the northeastern, southeastern, and midwestern regions of the United States appear to either be in decline or at a slowed rate of increase (Byrne et al., Citation2015; Casalena et al., Citation2015; Eriksen et al., Citation2015). Causes for these perceived declines in turkey abundance and productivity are not well understood but likely are multifaceted, including landscape-level habitat changes, predation, adverse weather, and local density-dependent factors that influence nesting and brood success (Byrne et al., Citation2015; Casalena et al., Citation2015). Pathogens, such as West Nile virus (WNV; family Flaviviridae, genus Flavivirus), may also directly or indirectly contribute to declining wildlife populations, such as through pathogen-attributable mortalities (i.e. direct) or via long-term effects (i.e. indirect). The latter may reduce reproductive success or predispose individuals to secondary causes of mortality, such as predation or inability to forage and acquire adequate nutrition, particularly in poorer quality habitats with less readily available food sources.
North American avian species have demonstrated a wide range of susceptibilities to WNV infection since its introduction to North America (Komar et al., Citation2003; Nemeth & Oesterle, Citation2014; Pérez-Ramírez et al., Citation2014). An early experimental infection trial in which eight 3-week-old domestic turkey poults were subcutaneously inoculated with WNV suggested that domestic turkeys, which are not direct descendants of the eastern wild turkey, were not highly susceptible to developing severe WNV-associated disease (Kennamer et al., Citation1992; Swayne et al., Citation2000). Serosurveys, including small numbers of wild turkeys in the United States, have demonstrated varied, although typically >20%, WNV seroprevalence in this species (Komar et al., Citation2001; Ludwig et al., Citation2002; Beveroth et al., Citation2006; Pedersen et al., Citation2016; MacDonald et al., Citation2022). However, both the experimental infection trial in domestic turkeys and the serosurveys in wild turkeys had small sample sizes, lacked in-depth analysis of pathogenesis (e.g. microscopic lesion distribution), and often did not consider demographic factors such as age in evaluating susceptibility to WNV. Subsequent detections of natural WNV infection in wild turkeys submitted to diagnostic laboratories within the United States suggest that at least a portion of infected wild turkeys may be susceptible to associated disease (Zhang et al., Citation2006; Kunkel et al., Citation2022). Further investigation into the pathogenesis and susceptibility of wild turkeys to infection with WNV is necessary to determine the potential impact of WNV on wild turkey populations and to assist in interpreting field and diagnostic data.
The following study sought to expand on the current understanding of the pathogenesis and susceptibility of wild turkeys to WNV infection. Specific objectives of this study included: (1) assess the susceptibility of wild turkeys to experimental WNV infection and associated clinical signs, (2) characterize WNV-induced viraemia and oropharyngeal and cloacal shedding profiles, viral titres in select tissues, and incidence of seroconversion, (3) characterize gross and microscopic pathobiology following experimental WNV inoculation in wild turkeys, (4) assess potential variation in susceptibility and infection outcome between two age classes of wild turkeys, and (5) assess WNV transmission between co-housed WNV-inoculated and sham-inoculated wild turkeys.
Materials and methods
Birds and facilities
Wild turkey eggs were collected from nests of free-ranging eastern wild turkeys in five different regions of Pennsylvania (Nemeth et al., Citation2021) by Pennsylvania Game Commission personnel. Eggs were ground transported in foam containers or incubators from Pennsylvania to the Poultry Diagnostic Research Center (PDRC) at the University of Georgia, where they were incubated and hatched. Turkeys were co-housed in two, ∼6 m × 4 m × 3 m free-flight enclosures at the PDRC prior to the start of the study. Poultry starter/grower feed (Southern States Traditions Chick Start and Grow Medicated [containing amprolium]; Southern States Cooperative, Richmond, VA, USA) and water were provided ad libitum to the birds throughout the duration of the study. In total, 44 wild turkeys (16 females, 28 males) were randomly separated into two groups for inoculation at different ages. Twenty-four birds were experimentally inoculated at ∼5 to 6 weeks of age (hereafter referred to as the young group), and 20 birds were inoculated at ∼15 to 16 weeks of age (hereafter referred to as the old group). Approximately 2–6 days prior to inoculation, each group of juvenile turkeys was transported from the PDRC to the Animal Health Research Center (AHRC), a biosafety-level 3 facility at the University of Georgia. Blood was collected from each bird on the day of transport to the AHRC and was confirmed to lack anti-WNV antibodies via plaque reduction neutralization test (PRNT; see methods below). Once in the AHRC, the turkeys in the young group were co-housed in one, free-flight, ∼2.5 m × 1 m × 2 m enclosure, while the turkeys in the old group were equally divided between two free-flight, ∼3 m × 1.5 m × 2.5 m enclosures. Rooms were maintained at an approximate temperature of 23.9°C with a 12 hour/day light cycle. Bird care and experimentation were approved by the Institutional Animal Care and Use Committee (University of Georgia; protocol A2018 09-020-Y1-A0) and carried out in accordance with applicable institutional, national, and local guidelines.
Virus inoculum
The WNV strain used for inoculation was isolated from a pooled heart and kidney sample collected in September 2018 from a WNV-infected wild ruffed grouse (Bonasa umbellus) from Bradford County, Pennsylvania. The viral isolate was passaged once and titrated by plaque assay (see methods below) on Vero cells. The virus preparation was diluted in bovine albumin-1 (BA-1) medium (minimum essential medium [MEM] supplemented with 1.5% bovine serum albumin fraction V [7.5%] solution, 2.2 g/l sodium bicarbonate, 10,000 units/ml penicillin, 10 mg/ml streptomycin, 25 µg/ml amphotericin B) immediately prior to inoculation.
Antemortem experimental protocol
On the day of inoculation (i.e. 0 days post-inoculation [dpi]), each age group was randomly subdivided into WNV-inoculated and sham-inoculated (i.e. contact control) treatment groups. In the young group, 12 birds were separated into the WNV-inoculated treatment group, and 12 birds were separated into the sham-inoculated treatment group. In the old group, 13 birds were separated into the WNV-inoculated treatment group, and seven birds were separated into the sham-inoculated treatment group. On 0 dpi, turkeys were subcutaneously inoculated with 0.1 ml BA-1 medium containing 106 plaque-forming units (PFU) WNV or with 0.1 ml BA-1 medium over the left pectoral muscle. All birds were monitored at least twice daily for the duration of the study for clinical signs, such as decreased food and/or water intake, lethargy, ruffled feathers, torticollis, paresis, ataxia, and recumbency. Turkeys that developed clinical signs due to cage-related injuries and all other birds that survived to the predetermined study endpoint (14 dpi) were euthanized via intrajugular sodium pentobarbital overdose. Body weights were recorded on 0, 7, and 14 dpi on a digital scale rounded to the nearest 1 g for the young group and to the nearest 100 g for the old group. Oropharyngeal and cloacal swabs and a blood sample (not to exceed 1% of the body weight of the turkey) were collected daily from each bird from 1 through 7 dpi and on 14 dpi for those birds that survived to the end of the study.
Blood samples were placed into serum separator tubes immediately after collection and allowed to coagulate at ambient temperature for 3–5 hours prior to being centrifuged at 10,000 × g for 4 min. Swabs were placed into 1.0 ml BA-1 medium, agitated in a mixer mill (2 min for 5 cycles/s; Retsch MM 300, Haan, Germany) and subsequently centrifuged at 10,000 × g for 10 min. Serum and swabs were then stored at −80°C until testing.
Postmortem experimental protocol and pathology
All turkeys underwent complete necropsies within 1 hour of euthanasia, and all gross findings were documented. Approximately 0.5 cm3 samples of heart, brain, kidney, spleen, and skeletal muscle and about three breast feathers were collected and placed into 1.0 ml BA-1 media, homogenized in a mixer mill (2 min for 5 cycles/s) and then centrifuged at 10,000 × g for 10 min. Tissue samples were stored at −80°C until testing. Representative samples of heart, cerebrum, midbrain, cerebellum, brainstem, cervical spinal cord, kidney, spleen, liver, lung, gonad, eye, femoral bone marrow, cloacal bursa, oesophagus, trachea, thyroid gland, proventricular-ventricular junction, duodenum, pancreas, jejunum, large intestine and caeca, quadriceps and pectoral skeletal muscle (not corresponding to inoculation site), and skin (not corresponding to inoculation site) were collected and placed into 10% neutral buffered formalin for ∼72 to 96 h and then moved to 70% ethanol for histopathologic examination and immunohistochemistry.
Formalin-fixed tissues were routinely trimmed, embedded in paraffin, sectioned (4 µm), and stained with haematoxylin and eosin. Duplicate sections of select tissues from birds in the young group and from a bird in the old group that was euthanized on 6 dpi were sectioned and processed for WNV immunohistochemistry (IHC) as previously described in Kunkel et al. (Citation2021). Tissues from birds that were representative of more severe microscopic lesions were selected for IHC. A blinded, board-certified veterinary pathologist (NMN) screened all histologic tissues for microscopic lesions and immunoreactivity. Microscopic lesions were subjectively scored based on the percentage of tissue involvement as follows: no lesions observed; minimum if ≤1% of the tissue section was affected; mild with >1% to <10% tissue section affected; moderate with 10% to 25% tissue section affected; and severe with >25% tissue section affected. Immunoreactivity for anti-WNV antibodies was scored as for the histopathological scoring categories, based on the percentage of tissue that contained immunoreactive cells.
Virus isolation, virus titration, and serology
Serum samples collected from 1 to 7 dpi from all sham-inoculated (i.e. contact control) turkeys were screened for circulating WNV by virus isolation, as described in Kunkel et al. (Citation2021). Viral titres of serum collected from 1 to 7 dpi from all WNV-inoculated birds and those sham-inoculated birds that became infected were determined by standard plaque assay, as previously described (Kunkel et al., Citation2021). Similarly, oropharyngeal and cloacal swabs collected from 1 to 7 dpi and homogenized tissues collected at the time of euthanasia from all WNV-inoculated and sham-inoculated turkeys underwent standard plaque assay in duplicate to determine viral titres (Kunkel et al., Citation2021), which was also used to determine the viral titre of the inoculum. Minimum titres of WNV detection were 101.7 PFU/ml serum, 100.7 PFU/swab, and 100.7 PFU/0.5 cm3 tissue homogenate. Viral RNA was extracted from tissue homogenates of a sham-inoculated bird in the young group that became infected and tested for WNV RNA as described in Roy et al. (Citation2022), with a cycle threshold (Ct) value of ≤35 considered positive.
Sera collected prior to inoculation and at the time of euthanasia were screened for anti-WNV antibodies at a 1:10 dilution by plaque reduction neutralization test (PRNT; Beaty et al., Citation1995), with details provided in Kunkel et al. (Citation2021). Pre-inoculation sera that neutralized ≤70% of WNV PFU compared to control wells were considered negative for anti-WNV antibodies, while post-inoculation sera that neutralized ≥80% of WNV PFU underwent serial 2-fold dilutions starting at 1:10 to determine antibody titres, which were recorded as the reciprocal of the highest serum dilution that neutralized ≥90% of WNV PFU (PRNT90; Kunkel et al., Citation2021).
Statistical analyses
Peak viraemia and oropharyngeal and cloacal swab titres and number of days of detectable viraemia and oropharyngeal and cloacal shedding were compared between the two age groups using the Mann–Whitney test in R version 4.0.2 (R core team, Citation2020). The significance level was set at P < 0.05. One WNV-inoculated bird that was euthanized on 2 dpi in the old group was excluded from statistical comparisons due to missing data points but was included in descriptive statistics of detectable virus titre ranges when virus was detected before euthanasia.
Results
Clinical observations
None of the WNV-inoculated or sham-inoculated (i.e. contact control) birds developed clinical signs attributable to infection with WNV throughout the duration of the experimental study. Two WNV-inoculated turkeys in the old group exhibited cage-related injuries (i.e. trauma to the tail, bilateral ataxia to paresis, head tremors, and mentally dull) and were euthanized on 2 and 6 dpi, respectively. All other birds in both groups survived to the predetermined study endpoint (i.e. 14 dpi). All birds in the young group gained weight throughout the duration of the study. Four WNV-inoculated turkeys in the old group gained between 4% and 50% of their pre-inoculation weights by the study endpoint, while the remaining WNV-inoculated and sham-inoculated turkeys in the old group lost up to 33% of their pre-inoculation weights by the endpoint of the study.
Viraemia, viral shedding profiles, and tissue tropism
The majority of WNV-inoculated birds in the young group (11/12) and all inoculated birds in the old group (13/13) developed detectable viraemia titres within the first 4 dpi (; Supplementary Table 1). Peak viraemia titres for inoculated turkeys in the young group (median 103.2 PFU/ml; range <101.7 to 104.6 PFU/ml) and the old group (median 103.6 PFU/ml; range 102.7 to 104.4 PFU/ml) were similar (Mann–Whitney test, P = 0.18). Number of days of detectable viraemia was longer in the old group (median 3 days; range 3–4 days) than in the young group (median 2.5 days; range 0–3 days; Mann–Whitney test, P = 0.002).
Figure 1. Experimental West Nile virus (WNV) mean (± standard error of the mean) viraemia profiles for all WNV-inoculated birds in the two age groups and the viraemia profile of the infected sham-inoculated bird in the old group. None of the individual birds developed daily viraemia titres above the solid black line, which represents the peak viraemia titre (105 PFU/ml) deemed sufficient to infect Culex pipiens mosquitoes (Turell et al., Citation2000; Komar et al., Citation2003).
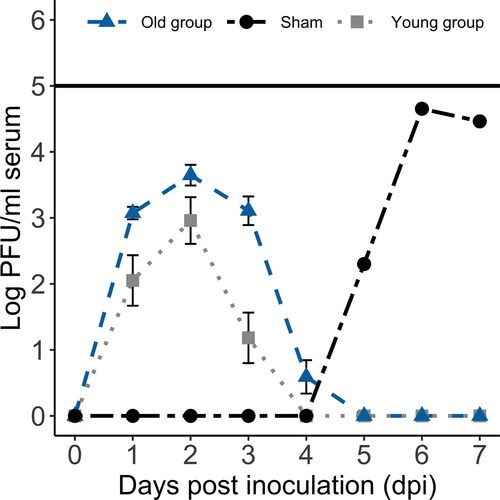
Virus was isolated from serum collected on 7 dpi from one sham-inoculated (i.e. contact control) bird in the old group. Infectious viral titres were detectable by standard plaque assay on sera collected from 5 to 7 dpi (peak 104.7 PFU/ml) from the sham-inoculated bird from the old group that had positive virus isolation results (). Virus was not isolated from the serum of any additional sham-inoculated birds. However, one additional sham-inoculated turkey in the young group developed detectable anti-WNV antibody titres by 14 dpi (see results below), and WNV RNA was detected in the spleen (Ct value 34.3) of this sham-inoculated bird (young group). In total, two sham-inoculated wild turkeys (one from each age group) became infected following inoculation of their same-age cohorts.
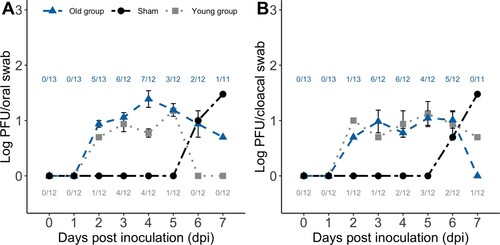
Intermittent shedding of infectious virus occurred in similar proportions of WNV-inoculated birds in the young (10/12) and old groups (10/13). Oropharyngeal shedding occurred in seven WNV-inoculated birds in the young group and in eight WNV-inoculated birds in the old group ((A)). The number of days of detectable oropharyngeal shedding was similar between WNV-inoculated birds in the young (median 1 day; range 0–2 days) and old groups (median 1.5 days; range 0–6 days; Mann–Whitney test, P = 0.22). Peak oropharyngeal viral titres in all inoculated birds in the young group (median 100.7 PFU/ml; range <100.7 to 101.2 PFU/ml) and old group (median 101.0 PFU/ml; range <100.7 to 102.0 PFU/ml) were similar (Mann–Whitney test, P = 0.21). Seven WNV-inoculated birds in the young group and eight WNV-inoculated birds in the old group had detectable cloacal shedding ((B)). The number of days of detectable cloacal shedding between WNV-inoculated birds in the young (median 1 day; range 0–2 days) and old groups (median 2 days; range 0–5 days) was similar (Mann–Whitney test, P = 0.15). Peak cloacal shedding titres in all inoculated turkeys were similar between the young group (median 100.7 PFU/ml; range <100.7 to 101.5 PFU/ml) and old group (median 100.8 PFU/ml; range <100.7 to 101.9 PFU/ml; Mann–Whitney test, P = 0.32). Infectious virus was detected in oropharyngeal and cloacal swabs of one sham-inoculated bird that developed viraemia in the old group on 6 dpi (101.0 PFU/ml orally and 100.7 PFU/ml cloacally) and 7 dpi (101.5 PFU/ml orally and 101.5 PFU/ml cloacally; (A,B)). Infectious virus was not detected in any oropharyngeal and cloacal swabs of the additional sham-inoculated birds.
Figure 2. Experimental West Nile virus (WNV) mean (± standard error of the mean) oropharyngeal (A) and cloacal (B) viral shedding profiles for those WNV-inoculated birds in the two age groups that shed virus each day and the oropharyngeal (A) and cloacal (B) viral shedding profiles for the infected sham-inoculated bird in the old group. Numbers correspond to the ratio of WNV-inoculated birds in the young group (bottom) and old group (top) with detectable virus shed each day (and thus included in the mean and standard error of the mean calculations for each day).
Infectious WNV was not detected in tissue homogenates of heart, kidney, brain, spleen, skeletal muscle, and three pooled breast feathers from any of the WNV-inoculated or sham-inoculated birds that survived to the 14 dpi study endpoint. Low titres of virus were detected in the heart (102.2 PFU/0.5 cm3), kidney (102.7 PFU/0.5 cm3), spleen (102.4 PFU/0.5 cm3), and pooled breast feathers (102.1 PFU/0.5 cm3) of the WNV-inoculated bird in the old group that was euthanized on 2 dpi and in the heart (101.3 PFU/0.5 cm3) of the WNV-inoculated bird in the old group that was euthanized on 6 dpi.
Serology
The pre-inoculation sera of all WNV-inoculated and sham-inoculated (i.e. contact control) birds in both groups had <60% neutralization (i.e. seronegative) on the day of transfer to the AHRC. Anti-WNV antibodies were detected in the sera collected on 14 dpi from all WNV-inoculated birds in both groups and in the two sham-inoculated birds that became infected with WNV during the study. The WNV-inoculated bird (old group) that was euthanized on 6 dpi seroconverted and had a serum PRNT90 titre of 320 at the time of euthanasia. Serum neutralization titres on 14 dpi often were higher in WNV-inoculated birds in the old group (range 1280–5120) than in the young group (range 40–2560). Serum PRNT90 titres in the two sham-inoculated turkeys that became infected (i.e. seroconverted) in the young and old groups were 320 and 1280, respectively.
Gross and microscopic pathology
All birds in both age groups were in fair nutritional condition with abundant gastrointestinal contents at the time of euthanasia. Gross lesions attributable to infection with WNV were minimal to absent in all birds. Mild petechiation scattered throughout the epicardium and endocardium of the heart of the WNV-inoculated bird in the old group that was euthanized on 6 dpi was the only gross lesion attributable to WNV in all birds.
Microscopic lesions attributable to WNV infection among both groups were most consistent in the heart and central nervous system (; Supplementary Table 2). The majority of WNV-inoculated birds in both age groups (8/12 and 13/13 in the young and old groups, respectively) and the infected sham-inoculated bird in the old group that developed a detectable viraemia had multifocal (occasionally perivascular) lymphoplasmacytic, histiocytic myocarditis ((A)), most frequently in the left free wall and septum, although the endocardium, epicardium, and right free wall occasionally were also involved. In addition to the inflammation within the heart, underlying cardiomyocyte degeneration, necrosis, mineralization with adjacent rare multinucleated giant cells, and/or fibrosis also were evident in six of the eight WNV-inoculated birds in the young group and in five of the 13 birds in the old group with microscopic cardiac lesions, including in the WNV-inoculated bird in the old group that was euthanized on 6 dpi. Lymphoplasmacytic, perivascular encephalitis and loosely aggregated microglial cell nodules within the grey matter, and less frequently the white matter, of the cerebrum, midbrain ((B)), cerebellum, and brainstem were evident in the majority of WNV-inoculated birds in both age groups. Cerebellar lymphoplasmacytic infiltrates and aggregates of microglial cells were most frequently in the molecular layer and white matter and often extended into the adjacent brainstem. Few lymphocytes and plasma cells were adjacent to blood vessels within the meninges overlying the cerebrum, midbrain, and/or cerebellum in few birds (1/12 and 2/13 in the young and old age groups, respectively). Lesions, similar to those described in the brain, were also evident in the cervical spinal cord of approximately half WNV-inoculated birds in both age groups. Lesions in the cervical spinal cord often were minimal, including perivascular cuffing of 1–2 cell layers and more consistently involved the grey matter. No lesions were observed in the central nervous system of the WNV-inoculated bird that was euthanized on 6 dpi nor in the two sham-inoculated birds that became infected during the study.
Figure 3. Haematoxylin and eosin (H&E) microscopic lesions and WNV immunohistochemistry (IHC) of experimental WNV-inoculated wild turkeys. (A) Heart; lymphoplasmacytic, histiocytic myocarditis overlay few degenerated cardiomyocytes (old group, 14 dpi, 20×, H&E). (B) Midbrain; lymphoplasmacytic perivascular encephalitis with microgliosis (old group, 14 dpi, 20×, H&E). (C) Caecum; lymphoplasmacytic typhlitis and ganglioneuritis (myenteric plexus) (old group, 14 dpi, 20×, H&E). (D) Proventriculus; serosal lymphoplasmacytic perivascular proventriculitis (old group, 14 dpi, 20×, H&E). (E) Ocular pecten-optic nerve; lymphoplasmacytic pectenitis and neuritis (old group, 14 dpi, 20×, H&E). (F) Testis; lymphoplasmacytic orchitis (old group, 14 dpi, 10×, H&E). (G) Ventriculus; WNV antigen within the cytoplasm of macrophages and lymphocytes in the lamina propria (young group, 14 dpi, 40×, IHC). (H) Lung; few macrophages and lymphocytes in bronchus-associated lymphoid tissue with intracytoplasmic WNV antigen (young group, 14 dpi, 40×, IHC).
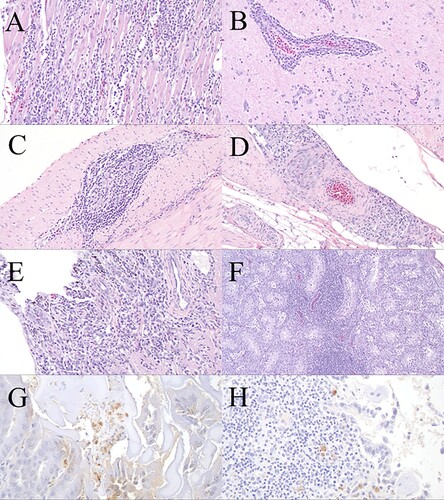
Table 1. Summary of distribution of microscopic lesions and West Nile virus (WNV)-specific immunohistochemical labelling (ratio with microscopic lesions and immunohistochemical labelling and percentage of tissue affected) in tissues of experimentally-inoculated wild turkeys and two sham-inoculated turkeys that became infected.
Inflammatory cell aggregates, similar to those described in the heart and central nervous system, occasionally were detected in other tissues. Approximately half of the WNV-inoculated birds in the old group and a few WNV-inoculated birds in the young group had small numbers of loose aggregates of lymphocytes, plasma cells, and rare histiocytes scattered throughout the renal and/or adrenal gland interstitium. Similar inflammatory infiltrates and rare eosinophils were also present in the interstitium of the adrenal gland in the infected sham-inoculated bird in the old group.
Few small aggregates of lymphocytes and rare plasma cells and histiocytes were either scattered in the pancreatic parenchyma, adjacent to blood vessels or nerve ganglia within the pancreatic parenchyma, or in the adjacent connective tissue in a small number of WNV-inoculated birds in both age groups and in the infected sham-inoculated bird in the old group. Minimal to mild lymphoplasmacytic orchitis was observed in a small number of WNV-inoculated birds in the young group, in the majority of WNV-inoculated birds in the old group ((F)), and in the sham-inoculated turkey in the old group which seroconverted. Minimal to low numbers of lymphocytes and plasma cells and rare histiocytes variably surrounded and infiltrated the myenteric plexus and blood vessels within the serosal muscular layer and serosal adipose of the gastrointestinal tract of approximately half of the WNV-inoculated birds in both age groups ((C)) and in the duodenum and jejunum of the infected sham-inoculated turkey in the old group. These lesions extended into the proventriculus ((D)) and/or ventriculus (and occasionally involved the proventricular-ventricular junction) in a small number (3/12 and 3/13 turkeys in the young and old groups, respectively) of WNV-inoculated birds. Small numbers of lymphocytes and plasma cells infiltrated the pecten and the junction of the pecten and optic nerve and rarely surrounded blood vessels in this region in three WNV-inoculated birds in the old group ((E)).
Rare small lymphoid nodules were scattered throughout many tissues in most WNV-inoculated and sham-inoculated turkeys in both age groups, variably including the spleen, kidney, bone marrow, adrenal gland, lung, liver, pancreas, gastrointestinal tract, and testis (interpreted as normal). The cloacal bursa in all birds in both age groups demonstrated regression deemed age appropriate.
Incidental gross and microscopic findings included focal granulomas within the right lung of one WNV-inoculated turkey (young group), within a caudal air sac of one WNV-inoculated bird (young group), adhered to and within the liver of one WNV-inoculated bird (old group), adhered to the tracheal serosa of one WNV-inoculated bird (old group), and adhered to the duodenal serosa of one WNV-inoculated bird (old group). Multifocal granulomas expanded the conjunctiva of one sham-inoculated bird in the old group, and additional focal granulomas were adhered to the connective tissue adjacent to the proximal ventriculus and caecum of one WNV-inoculated bird (old group) and were in the coelomic cavity of one WNV-inoculated bird (old group) and three sham-inoculated turkeys (two in the young group, one in the old group). Foreign material, including two screws in ventricular lumens and one stick partially embedded within the koilin layer of the ventriculus, were present in three WNV-inoculated turkeys in the young group at the time of necropsy. The caecal mucosa focally was expanded by degenerated heterophils in one sham-inoculated bird (young group). Lymphocytes, plasma cells, and rare heterophils and oedema were scattered within the adipose adjacent to the proventriculus and were adhered to the proventricular serosa in the WNV-inoculated bird in the old group that was euthanized on 2 dpi. There was a cataract in the lens of one WNV-inoculated bird (young group) and mild hepatocellular lipid in one sham-inoculated turkey in the young and old groups. Scant mineralized debris was in renal tubular lumens in two WNV-inoculated birds and one sham-inoculated bird in the old group. Six WNV-inoculated birds (two in the young group, four in the old group) and three sham-inoculated turkeys in the old group had minimal to mild lymphoplasmacytic myositis, which rarely was perivascular and overlaid few degenerated myocytes. Two additional WNV-inoculated birds (old group) and one sham-inoculated bird (old group) had few degenerated to necrotic myocytes without associated inflammatory cells (all interpreted as perimortem exertional capture myopathy). There was also a small focus of haemorrhage intermixed with the affected myocytes in the skeletal muscle along the left hind limb of the sham-inoculated bird (old group). Clinically, this bird was mildly lame prior to euthanasia, and the associated skeletal muscle lesions were attributed to minor trauma to that limb. No significant WNV-attributable or incidental lesions were detected in spleen, bone marrow, cloacal bursa, ovary, oesophagus, thyroid gland, and thymus of any of the turkeys. No lesions were observed in tissues from any of the sham-inoculated turkeys, unless previously explicitly described.
Immunohistochemistry
The majority of WNV immunoreactivity in examined tissues in all birds was minimal, but a few birds had moderate to strong immunolabelling of small numbers of cells within select tissues (). There was rare, moderate to strong, granular immunohistochemical labelling of the cytoplasm of individual macrophages circulating within blood vessels of the heart in two WNV-inoculated birds in the young group. Similar immunoreactivity was rarely observed in the cytoplasm of few cardiomyocytes, which were overlaid by inflammatory cells, of one WNV-inoculated bird in the young group and in the WNV-inoculated bird (old group) that was euthanized on 6 dpi. The cytoplasm of a few macrophages and lymphocytes in the splenic red pulp of all seven examined WNV-inoculated turkeys in the young group exhibited mild to strong immunoreactivity. Moderate to strong, granular immunohistochemical labelling was evident in the cytoplasm of a few renal tubular epithelial cells in two WNV-inoculated birds (young group) and in rare circulating macrophages in the kidney in four WNV-inoculated birds (young group). The cytoplasm of rare haematopoietic cells (primarily macrophages) within the bone marrow of most examined WNV-inoculated turkeys, including the bird that was euthanized on 6 dpi, exhibited mild to strong immunoreactivity. A few circulating macrophages within the subepithelial space of the cloacal bursa in one WNV-inoculated bird (young group) exhibited moderate, intracytoplasmic, granular immunohistochemical labelling. The cytoplasm of occasional macrophages and lymphocytes within blood adjacent to the adrenal gland exhibited mild to moderate immunoreactivity in a few WNV-inoculated turkeys in the young group. Moderate to strong immunohistochemical labelling was evident in the cytoplasm of circulating macrophages within the lungs of all examined birds, including the turkey that was euthanized on 6 dpi, and additionally within the cytoplasm of a few macrophages and lymphocytes in bronchus-associated lymphoid tissue of one bird (young group; (H)). There was moderate to strong immunoreactivity in the cytoplasm of a few macrophages and lymphocytes within the lamina propria of the ventriculus in one WNV-inoculated turkey in the young group ((G)). No immunohistochemical labelling was evident in the spleen, kidney, cloacal bursa, central nervous system, and ovary of the turkey euthanized on 6 dpi.
Discussion
The wild turkey is one of the most culturally and economically important upland game birds in North America, largely due to its classification as the second most popular hunted big game species after white-tailed deer (Odocoileus virginianus) throughout much of its range (United States Department of Interior et al., Citation2018). Although a prior experimental challenge study in domestic turkeys suggested low susceptibility to infection with WNV, natural WNV has been diagnosed in free-ranging wild turkeys (Zhang et al., Citation2006; Kunkel et al., Citation2022). Such findings suggest that a more in-depth analysis of the susceptibility of wild turkeys to WNV infection and associated disease manifestation is warranted, particularly in the face of declining wild turkey populations (Byrne et al., Citation2015; Casalena et al., Citation2015; Eriksen et al., Citation2015). The present study aimed to expand upon our current understanding of the pathogenesis and susceptibility of wild turkeys to infection with WNV. Our results support the general notion that wild turkeys are not highly susceptible to the development of overt disease following experimental infection with WNV. No clinical signs attributable to infection with WNV were observed in any of the experimentally infected wild turkeys, and no gross lesions associated with WNV infection were observed in birds evaluated at the 14 dpi study termination. However, microscopic lesions attributable to WNV infection were documented in multiple tissues of many wild turkeys in this study. The clinical signs of the two birds euthanized at 2 and 6 dpi were attributed to cage-related (traumatic) injuries, supported by the lack of moderate to severe WNV-associated microscopic lesions and immunohistochemical labelling and comparable laboratory results with the WNV-infected birds that survived to 14 dpi.
Microscopic lesions attributable to WNV most often were observed in the heart and brain of wild turkeys in both age groups ((A,B)), which is consistent with previously documented lesions associated with infection with WNV in numerous avian species, including some galliforms and a wild turkey with a natural WNV infection (Senne et al., Citation2000; Zhang et al., Citation2006; reviewed in Gamino & Höfle, Citation2013; Gamino et al., Citation2016; Nemeth et al., Citation2017). The two WNV-inoculated birds that were euthanized prior to the study endpoint (i.e. 2 and 6 dpi) and the two infected sham-inoculated birds (presumably infected via cage-mate transmission at a later time-point than those that were needle-inoculated) lacked microscopic lesions in the central nervous system, suggesting that central nervous system lesions may be a more chronic disease sequela to infection with WNV. However, three of four of these birds had myocarditis of varying severity. Similarly, lymphocytic myocarditis was also observed on 8 dpi in a domestic turkey experimentally infected with WNV that lacked brain lesions (this bird likely died due to a bacterial septicaemia based on microscopic lesions; Swayne et al., Citation2000). This more chronic manifestation of encephalitis has been suggested based on the microscopic lesion and immunohistochemical labelling patterns in other experimentally infected bird species, including ruffed grouse and domestic chickens (Senne et al., Citation2000; reviewed in Pérez-Ramírez et al., Citation2014; Nemeth et al., Citation2017).
Approximately half of the WNV-inoculated turkeys in both age groups in our study had inflammatory infiltrates surrounding the myenteric ganglia of the gastrointestinal tract ((C,D)), and few birds had minimal to mild lesions, mostly inflammation, in the kidney, pancreas, testis ((F)), and the ocular pecten ((E)). Although not severe, the inflammation within the ocular pecten is noteworthy because this lesion only has been documented in a few avian species, such as experimentally infected red-legged partridges (Alectoris rufa) and naturally infected red-tailed hawks (Buteo jamaicensis; Gamino et al., Citation2014; Wünschmann et al., Citation2017). However, the rarity of this observation may in part be due to lack of microscopic examination of eyes in many diagnostic and experimentally-induced cases. The absence of more widespread immunohistochemical labelling in examined tissues except in cells (primarily macrophages) circulating in the blood of a few tissues, including the spleen and bone marrow, likely suggests that in most cases, the virus was effectively cleared from these tissues by the time of euthanasia. The lack of detectable infectious virus in tissues collected from all birds that survived to the study endpoint provides further support for viral clearance. The remaining WNV immunolabelling in circulating cells likely represents viral tropism for macrophages and the role of macrophages in infection control (Stone et al., Citation2019).
Although the severity of microscopic lesions observed in wild turkeys in our study was considered unlikely to directly contribute to morbidity, the possibility remains that these lesions may predispose free-ranging wild turkeys to secondary infections, predation, or other morbidity/mortality causes. We cannot rule out the possibility that WNV infection in the WNV-inoculated birds may have predisposed some of these birds to the development of some of the observed incidental findings, albeit we believe this less likely, as incidental pathological findings were detected in both the WNV- and sham-inoculated birds in our study. Further, we cannot rule out the possibility that more chronic tissue damage in organs, such as the testes, heart, or brain, could have potential long-term effects in infected birds, such as reduced reproductive success or foraging effort leading to decreased fitness. The inclusion of negative control birds that were separately housed from the WNV- and sham-inoculated turkeys may have assisted in pathological comparison and interpretation; however, the number of wild turkey eggs available for this study limited our ability to include such controls.
Viraemia and viral shedding titres in wild turkeys in our study were relatively low and consistent with other avian species that are considered less susceptible to severe disease associated with WNV infection, such as the domestic chicken, northern bobwhite quail (Colinus virginianus), and ring-necked pheasant (Phasianus colchicus; Senne et al., Citation2000; Komar et al., Citation2003; Kunkel et al., Citation2021), as well as those reported for domestic turkeys (Swayne et al., Citation2000). Although the median duration of detectable viraemia was slightly higher in the older group of turkeys in the current study, the duration of viraemia for both age groups is consistent with previous experimentally infected avian species, often lasting 1 to 4 or 5 days, particularly in less susceptible species (Komar et al., Citation2003). The reason for the slightly longer viraemia duration in the older group of birds in our study is unknown but may be related to varied immune responses or elevated stress levels due to prolonged captivity. Additionally, oropharyngeal and cloacal shedding of the infectious virus among WNV-inoculated wild turkeys was intermittent and generally 1- to 2-fold lower than viraemia titres. Although the titres of infectious virus shed among these WNV-inoculated wild turkeys generally are not considered likely to contribute to significant bird-to-bird transmission of WNV in free-ranging birds, two sham-inoculated birds in the current study became infected with WNV and seroconverted by the study endpoint, presumably via ingestion of infectious bodily secretions via water or food shared with WNV-inoculated turkeys. Cage-mate transmission of WNV has been observed in other avian experimental trials, including among domestic chickens, domestic goslings (Anser anser domesticus), American crows (Corvus brachyrhynchos), blue jays (Cyanocitta cristata), black-billed magpies (Pica hudsonia), and ring-billed gulls (Larus delawarensis; Langevin et al., Citation2001; Swayne et al., Citation2001; Komar et al., Citation2003). However, to the best of our knowledge, this is the first documented occurrence of bird-to-bird transmission among wild turkeys. The frequency of bird-to-bird transmission in the wild is unknown but is unlikely to be ecologically significant among wild turkeys given the low viral titres in the oropharyngeal and cloacal swabs and the much lower densities of wild turkeys across natural landscapes and thus less prolonged close contact and sharing of resources. However, it is possible that this transmission route may occur in the wild during communal feeding and drinking in close contact where turkeys defaecate.
Previous studies demonstrate that WNV viraemia titres of at least 105 PFU/ml in vertebrate hosts are needed to successfully infect feeding Culex pipiens mosquitoes and thus perpetuate the transmission cycle (Turell et al., Citation2000; Komar et al., Citation2003). Culex pipiens mosquitoes often are considered one of the more important vector species for WNV, particularly in the eastern United States (Kilpatrick et al., Citation2007). Similar to experimentally infected domestic turkeys (Swayne et al., Citation2000), none of the wild turkeys in the current study reached this minimum titre. Therefore, if natural infections lead to similar viraemia profiles, the wild turkey would not be considered a competent WNV-amplifying host species.
We also assessed for variation in susceptibility to WNV infection and infection outcome among two age groups (∼5- to 6-week-old and ∼15- to 16-week-old groups) of wild turkeys. Significant differences in the various infection outcomes evaluated between the two age groups were limited to the duration of detectable viraemia; however, we had limited power to detect statistically significant differences between the two age groups due to our small sample size. Further, we were unable to evaluate potential differences in immunohistochemical labelling as this evaluation was limited to a subset of WNV-inoculated birds. Interpretation of body weights over time was challenging due to varied growth rates and stress levels associated with prolonged and varied periods of captivity between the two age groups. There were no differences among peak viraemia and oropharyngeal and cloacal shedding titres and no apparent differences among clinical signs, gross and microscopic pathology, and survivorship between the two age groups. The significance of the longer duration of viraemia in the older group of wild turkeys in our study is unknown but may reflect elevated stress levels from prolonged time in captivity (i.e. longer than that of the younger group), variation in immune system response associated with age, or a combination of these or unrecognized factors (Nemeth et al., Citation2009). Although the duration of viraemia frequently was not compared among age groups, previous experimental and natural infection studies involving domestic chickens, domestic geese, domestic chukar partridges, and domestic Impeyan pheasants (Lophophorus impeyanus) reported higher viraemia titres and more severe WNV-associated disease in juveniles (Austin et al., Citation2004; Wünschmann & Ziegler, Citation2006; Nemeth & Bowen, Citation2007).
Experimental infection trials provide useful information regarding species susceptibility to WNV and pathogenesis but often are unable to replicate field conditions or natural transmission routes, which may alter disease development and final infection outcome. For example, needle inoculation does not elicit the same local immune response as mosquito inoculation, but has led to similar infection outcomes (e.g. viraemia profiles) as mosquito inoculations and allows for more accurate inoculation dosage and delivery and reduces bird handling time (Styer et al., Citation2006, Holicki et al., Citation2020). In addition, stress responses to repeated close human presence and handling may alter physiologic and immune activity (Nemeth et al., Citation2009). Many factors may alter the course of infection outcome in the wild, including virus strain, viral infectious dose, route of delivery, physiologic stressors such as predator abundance, avian taxonomy and behaviour, age, concurrent infections, and environmental stressors such as resource availability, climatic conditions, and habitat quality. The young life stage of wild turkeys used in this study broadly corresponds temporally to the age of juvenile wild turkeys during peak WNV transmission in much of the geographic range of wild turkeys in the eastern United States. Although our results suggest that wild turkeys are not highly susceptible to morbidity and mortality due to experimental infection with WNV, continued diagnostic evaluation of natural deaths in wild turkeys and more widespread WNV serologic surveys would contribute to a more holistic understanding of the potential role WNV may play in wild turkey populations. Field studies, such as those that focus on survivorship and/or demographic parameters in wild turkeys, should incorporate WNV diagnostic testing (e.g. assessment of serostatus, active infection, and natural mortality investigations) to provide valuable insight into the potential subclinical effects of WNV on reproductive success, fitness, predisposition to secondary infections and morbidity/mortality factors and, ultimately, population-level declines.
Supplemental Material
Download MS Word (71 KB)Acknowledgements
The authors thank Justin Brown (Pennsylvania State University) and Lisa Williams (Pennsylvania Game Commission) for their expertise and Michelle Campbell, Annie Vizurraga, and Alex Dhom (Southeastern Cooperative Wildlife Disease Study, University of Georgia) for assistance with sampling efforts. Pennsylvania Game Commission staff and Pennsylvania hunters and landowners assisted in locating and collecting wild turkey eggs. Brent Lovern (University of Georgia) provided invaluable husbandry assistance at the PDRC. They thank the Athens Veterinary Diagnostic Histopathology Laboratory staff for histopathology and immunohistochemistry processing and the AHRC staff for bird care assistance. The authors thank all the member state wildlife management agencies of the Southeastern Cooperative Wildlife Disease Study for support provided by the Federal Aid to Wildlife Restoration Act (50 Stat. 917), and the United States Fish and Wildlife Service and the United States Geological Survey Ecosystems Mission Area for the continued support of the Southeastern Cooperative Wildlife Disease Study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Austin, R.J., Whiting, T.L., Anderson, R.A. & Drebot, M.A. (2004). An outbreak of West Nile virus-associated disease in domestic geese (Anser anser domesticus) upon initial introduction to a geographic region, with evidence of bird to bird transmission. Canadian Veterinary Journal, 45, 117–123.
- Beaty, B., Calisher, C. & Shope, R. (1995). Arboviruses. In E.H. Lennette, D.A. Lennette & E.T. Lennette (Eds.), Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections 7th edn (pp. 204–205). Washington, DC: American Public Health Association.
- Beveroth, T.A., Ward, M.P., Lampman, R.L., Ringia, A.M. & Novak, R.J. (2006). Changes in seroprevalence of West Nile virus across Illinois in free-ranging birds from 2001 through 2004. American Journal of Tropical Medicine and Hygiene, 74, 174–179.
- Byrne, M.E., Chamberlain, M.J. & Collier, B.A. (2015). Potential density dependence in wild turkey productivity in the southeastern United States. In Proceedings of the 11th National Wild Turkey Symposium (pp. 329–351).
- Casalena, M.J., Schiavone, M.V., Bowling, A.C., Gregg, I.D. & Brown, J. (2015). Understanding the new normal: wild turkeys in a changing northeastern landscape. In Proceedings of the 11th National Wild Turkey Symposium (pp. 45–57).
- Eriksen, R.E., Hughes, T.W., Brown, T.A., Akridge, M.D., Scott, K.B. & Penner, C.S. (2015). Status and distribution of wild turkeys in the United States: 2014 status. Proceedings of the 11th National Wild Turkey Symposium (pp. 7–18).
- Gamino, V., Escribano-Romero, E., Blázquez, A.B., Gutiérrez-Guzmán, A.V., Martín-Acebes, M.A., Saiz, J.C. & Höfle, U. (2016). Experimental North American West Nile virus infection in the red-legged partridge (Alectoris rufa). Veterinary Pathology, 53, 585–593.
- Gamino, V., Escribano-Romero, E., Gutiérrez-Guzmán, A.V., Blázquez, A.B., Saiz, J.C. & Höfle, U. (2014). Oculopathologic findings in flavivirus-infected gallinaceous birds. Veterinary Pathology, 51, 1113–1116.
- Gamino, V. & Höfle, U. (2013). Pathology and tissue tropism of natural West Nile virus infection in birds: a review. Veterinary Research, 44, 39.
- Holicki, C.M., Michel, F., Vasić, A., Fast, C., Eiden, M., Raileanu, C., Kampen, H., Werner, D., Groschup, M.H. & Ziegler, U. (2020). Pathogenicity of West Nile virus lineage 1 to German poultry. Vaccines, 8, 507.
- Kennamer, J.E., Kennamer, M.C. & Brenneman, R. (1992). History. In J.G. Dickson (Ed.), The Wild Turkey: Biology and Management (pp. 6–17). Mechanicsburg, PA: Stackpole.
- Kilpatrick, A.M., LaDeau, S.L. & Marra, P.P. (2007). Ecology of West Nile virus transmission and its impact on birds in the Western Hemisphere. The Auk, 124, 1121–1136.
- Komar, N., Langevin, S., Hinten, S., Nemeth, N., Edwards, E., Hettler, D., Davis, B., Bowen, R. & Bunning, M. (2003). Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerging Infectious Diseases, 9, 311–322.
- Komar, N., Panella, N.A., Burns, J.E., Dusza, S.W., Mascarenhas, T.M. & Talbot, T.O. (2001). Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerging Infectious Diseases, 7, 621–625.
- Kunkel, M.R., Mead, D.G., Berghaus, R.D., Adcock, K.G., Ruder, M.G. & Nemeth, N.M. (2021). Experimental West Nile virus infection in northern bobwhite quail (Colinus virginianus). Avian Diseases, 65, 522–528.
- Kunkel, M.R., Mead, D.G., Ruder, M.G. & Nemeth, N.M. (2022). Our current understanding of West Nile virus in upland game birds. Wildlife Society Bulletin, 46, e1269.
- Langevin, S.A., Bunning, M., Davis, B. & Komar, N. (2001). Experimental infection of chickens as candidate sentinels for West Nile virus. Emerging Infectious Diseases, 7, 726–729.
- Ludwig, G.V., Calle, P.P., Mangiafico, J.A., Raphael, B.L., Danner, D.K., Hile, J.A., Clippinger, T.L., Smith, J.F., Cook, R.A. & McNamara, T. (2002). An outbreak of West Nile virus in a New York City captive wildlife population. American Journal of Tropical Medicine and Hygiene, 67, 67–75.
- MacDonald, A.M., Johnson, J.B., Casalena, M.J., Nemeth, N.M., Kunkel, M., Blake, M. & Brown, J.D. (2022). Active and passive disease surveillance in wild turkeys (Meleagris gallopavo) from 2008 to 2018 in Pennsylvania, USA. Wildlife Society Bulletin, 46, e1289.
- Nemeth, N.M., Bosco-Lauth, A.M., Williams, L.M., Bowen, R.A. & Brown, J.D. (2017). West Nile virus infection in ruffed grouse (Bonasa umbellus): experimental infection and protective effects of vaccination. Veterinary Pathology, 54, 901–911.
- Nemeth, N.M. & Bowen, R.A. (2007). Dynamics of passive immunity to West Nile virus in domestic chickens (Gallus gallus domesticus). American Journal of Tropical Medicine and Hygiene, 76, 310–317.
- Nemeth, N.M. & Oesterle, P.T. (2014). West Nile virus from an avian conservation perspective. International Zoo Yearbook, 48, 101–115.
- Nemeth, N.M., Oesterle, P.T. & Bowen, R.A. (2009). Humoral immunity to West Nile virus is long-lasting and protective in the house sparrow (Passer domesticus). American Journal of Tropical Medicine and Hygiene, 80, 864–869.
- Nemeth, N.M., Williams, L.M., Bosco-Lauth, A.M., Oesterle, P.T., Helwig, M., Bowen, R.A. & Brown, J.D. (2021). West Nile virus infection in ruffed grouse (Bonasa umbellus) in Pennsylvania, USA: a multi-year comparison of statewide serosurveys and vector indices. Journal of Wildlife Diseases, 57, 51–59.
- Pedersen, K., Marks, D.R., Wang, E., Eastwood, G., Weaver, S.C., Goldstein, S.M., Sinnett, D.R. & De Liberto, T.J. (2016). Widespread detection of antibodies to eastern equine encephalitis, West Nile, St. Louis encephalitis, and Turlock viruses in various species of wild birds from across the United States. American Journal of Tropical Medicine and Hygiene, 95, 206–211.
- Pérez-Ramírez, E., Llorente, F. & Jiménez-Clavero, MÁ. (2014). Experimental infections of wild birds with West Nile virus. Viruses, 6, 752–781.
- R Core Team. (2020). R: a language and environment for statistical computing, version 4.0.2. Vienna: R Foundation for Statistical Computing.
- Roy, C.L., Carstensen, M., LaSharr, K., Humpal, C., Dick, T., Kunkel, M. & Nemeth, N.M. (2022). West Nile virus exposure and infection among hunter-harvested ruffed grouse cohorts in a stable population. Journal of Wildlife Diseases, 58, 30–39.
- Senne, D.A., Pedersen, J.C., Hutto, D.L., Taylor, W.D., Schmitt, B.J. & Panigrahy, B. (2000). Pathogenicity of West Nile virus in chickens. Avian Diseases, 44, 642–649.
- Stone, A.E.L., Green, R., Wilkins, C., Hemann, E.A. & Gale, M. (2019). RIG-I-like receptors direct inflammatory macrophage polarization against West Nile virus infection. Nature Communications, 10, 3649.
- Styer, L.M., Bernard, K.A. & Kramer, L.D. (2006). Enhanced early West Nile virus infection in young chickens infected by mosquito bite: effect of viral dose. American Journal of Tropical Medicine and Hygiene, 75, 337–345.
- Swayne, D.E., Beck, J.R., Smith, C.S., Shieh, W.J. & Zaki, S.R. (2001). Fatal encephalitis and myocarditis in young domestic geese (Anser anser domesticus) caused by West Nile virus. Emerging Infectious Diseases, 7, 751–753.
- Swayne, D.E., Beck, J.R. & Zaki, S. (2000). Pathogenicity of West Nile virus for turkeys. Avian Diseases, 44, 932–937.
- Turell, M.J., O’Guinn, M. & Oliver, J. (2000). Potential for New York mosquitoes to transmit West Nile virus. American Journal of Tropical Medicine and Hygiene, 62, 413–414.
- United States Department of the Interior, United States Fish and Wildlife Service, United States Department of Commerce, & United States Census Bureau. (2018). 2016 National Survey of Fishing, Hunting, and Wildlife-Associated Recreation.
- Wünschmann, A., Armién, A.G., Khatri, M., Martinez, L.C., Willette, M., Glaser, A., Alvarez, J. & Redig, P. (2017). Ocular lesions in red-tailed hawks (Buteo jamaicensis) with naturally acquired West Nile disease. Veterinary Pathology, 54, 277–287.
- Wünschmann, A. & Ziegler, A. (2006). West Nile virus-associated mortality events in domestic chukar partridges (Alectoris chukar) and domestic Impeyan pheasants (Lophophorus impeyanus). Avian Diseases, 50, 456–459.
- Zhang, Z., Wilson, F., Read, R., Pace, L. & Zhang, S. (2006). Detection and characterization of naturally acquired West Nile virus infection in a female wild turkey. Journal of Veterinary Diagnostic Investigation, 18, 204–208.
