ABSTRACT
The poultry industry has been facing the impact of necrotic enteritis (NE), a disease caused by the bacterium Clostridium perfringens producing the haemolytic toxin NetB. NE severity may vary from mild clinical to prominent enteric signs causing reduced growth rates and affecting feed conversion ratio. NetB production is controlled by the Agr-like quorum-sensing (QS) system, which coordinates virulence gene expression in response to bacterial cell density. In this study, the peptide-containing cell-free spent media (CFSM) from Enterococcus faecium was tested in NE challenged broilers in two battery cage and one floor pen studies. Results showed a significant reduction of NE mortality. Metagenomic sequencing of the jejunum microbiome revealed no impact of the CFSM on the microbial community, and growth of C. perfringens was unaffected by CFSM in vitro. The expression of QS-controlled virulence genes netB, plc and pfoA was found to be significantly repressed by CFSM during the mid-logarithmic stage of C. perfringens growth and this corresponded with a significant decrease in haemolytic activity. Purified fractions of CFSM containing bioactive peptides were found to cause reduced haemolysis. These results showed that bioactive peptides reduce NE mortality in broilers by interfering with the QS system of C. perfringens and reducing bacterial virulence. Furthermore, the microbiome of C. perfringens-challenged broilers is not affected by quorum sensing inhibitor containing CFSM.
Introduction
In recent years, the poultry industry has been facing the impact of bacterial enteric diseases, especially after the ban of antibiotics in Europe, and the increasing restrictions in North America on the use of antimicrobials to promote poultry growth and fight infections (Casewell et al., Citation2003; Wallinga et al., Citation2022). Potential alternatives to prevent or treat these diseases are getting attention to help manage this issue (Aruwa et al., Citation2021).
Clostridium perfringens is a Gram-positive, anaerobic, non-motile bacterium that can be found in the human and animal gastrointestinal microbiome and in many environments, including soil, decaying vegetation, and marine sediments. However, in some conditions, it is the causative agent of histotoxic and enterotoxic illness in humans and animals (Fathima et al., Citation2022). Key to its success as a pathogen, C. perfringens produces a plethora of toxins which cause tissue necrosis, bacteraemia and clostridial myonecrosis known as gas gangrene (Uzal et al., Citation2014; Rood et al., Citation2018). The intestinal disease caused by C. perfringens in chickens is known as necrotic enteritis (NE). Its severity may vary from no apparent clinical signs in mild infections (decreased digestion and absorption of feed, high litter moisture, poor litter quality, and reduced weight gain), to prominent and acute enteric signs (sudden diarrhoea and macroscopic intestinal lesions such as ballooning, redness, abnormal tonus, and mucosal necrosis, and an increase in mortality) in more severe disease (Costa et al., Citation2013; El-Hack et al., Citation2022). Even as a subclinical infection, NE is a critical issue for the broiler industry due to the economic loss and welfare concerns related to reduced growth rates and increased feed conversion ratio (Fancher et al., Citation2020).
The critical role of the intestinal microbiome in health has been frequently described. Resident bacteria of the intestinal tract contribute to a protective barrier that excludes pathogens by competitive niche exclusion and interact with the host by providing beneficial effects such as the production of vitamin, short-chain fatty acids, organic acids, and antimicrobial compounds, stimulation of the immune system, and inhibiting the growth of harmful microbial groups (Yegani & Korver, Citation2008; Shang et al., Citation2018). Conditions like diet changes, stress or health issues of the bird can alter the balance of the normal intestinal microbiome (dysbiosis), allowing pathogens to overgrow through abnormal proliferation and the subsequent disease (McDevitt et al., Citation2006).
Regulation of virulence gene expression in many bacteria relies on quorum-sensing (QS), which is the process by which bacteria regulate their behaviour in response to local cell density (Ohtani et al., Citation2009; Antunes et al., Citation2010). Quorum sensing is the process through which bacteria synthesize and secrete molecules known as autoinducers into the extracellular media and, once a threshold is reached, the cell detects the signal, causing a change in gene expression mediated by specific transcription factors. Two QS systems have been identified in strains of C. perfringens which each use a specific auto inducing molecule: autoinducer-2 (AI-2) is synthesized by the LuxS enzyme, and the auto-inducing peptide (AIP) which is part of the accessory gene-regulator (Agr)-like QS pathway involved in virulence gene regulation (Ohtani et al., Citation2002). The AIP QS system is found predominantly in Gram-positive bacteria and has been studied in detail in Staphylococcus aureus where it comprises two transcripts: RNAII (agrB, agrD, agr,C, agrA) and RNAIII. The agrD propeptide is simultaneously processed into the active AIP and transported from the cell by the transmembrane protein AgrB. Once reaching a threshold concentration, AIP is detected by, and activates, the AgrC-AgrA two component response regulator, which subsequently controls QS-regulated genes through the mRNA effector molecule RNAIII that, in turn, activates many virulence factors (Le & Otto, Citation2015). An Agr-like system has been described in C. perfringens; however, the VirS-VirR two-component response regulator and the small regulatory RNA VR-RNA fulfil the role of AgrC-AgrA and RNAIII, respectively (Shimizu et al., Citation2002; Cheung et al., Citation2009; Yu et al., Citation2017; Li & McClane, Citation2020). The expression of key C. perfringens toxins is controlled by the Agr QS system, while in NE-inducing strains of C. perfringens the expression of NetB is dependent on a functional Agr QS system and VirR to cause NE in vivo (Keyburn et al., Citation2008; Ohtani et al., Citation2009; Cheung et al., Citation2010).
Given that traditional therapies such as antibiotics may lead to the rise of resistant strains, attenuating the mechanisms of bacterial virulence presents an attractive alternate therapeutic strategy. An innovative technology that uses quorum sensing inhibition in cell-free spent media (CFSM) isolated from the fermentation of a probiotic bacterium has been investigated. Therefore, the aim of this study was to evaluate the effect of quorum sensing inhibition CFSM on broiler performance parameters and their intestinal microbiome in three NE clinical trials and in the in vitro growth, virulence gene expression and haemolytic activity of C. perfringens.
Materials and methods
Ethical statement
The clinical component of the present study was divided into three experiments. These experiments were conducted by Southern Poultry Research Group (SPRG). Bird care practices conformed to the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. All birds were euthanized by methods approved by the American Veterinary Medical Association (AVMA).
Experimental design
For experiments 1 and 2, 660 day-of-hatch Ross x Ross male chickens, obtained from a commercial hatchway (Aviagen Hatchery, Quitman, GA, USA), were selected for each experiment, weighed, and randomly assigned to treatment groups in a total of 60 battery cages adjusted to 10 birds per cage on day 12. Treatment groups were assigned to the cages using a randomized complete block design. The challenged untreated group did not receive any treatment, and the challenged treated group received 12 mg/kg of bodyweight (BW) of Ef30616 CFSM formulation with a blending agent (milk proteins) administered daily in the drinking water from day 13 to day 28. Each cage contained one trough feeder and one trough drinker; each drinker held 2 l of water. Feed and water were available ad libitum. Fresh CFSM solution was prepared daily. Concentration of CFSM in the solution was calculated so that water consumed by the birds in a day would include the correct dose of CFMS based on BW. Water consumption was measured daily by weighing the water remaining before disposal and refilling drinkers. For experiment 3, 600 day-of-hatch Ross × Ross male chickens were selected, weighed, and randomly allocated upon arrival in 12 floor pens of 5 × 5 feet (stocking density of 1.0 feet2 per bird) in a solid-sided barn, with concrete floors, and under ambient humidity. At placement, all pens had fresh pine shavings. Litter was not replaced or altered during this study. Feed and water were available ad libitum. Each pen contained one tube feeder and one Plasson drinker (25 birds to feeder/drinker ratio). The pens were assigned to two treatment groups, with 25 birds per pen. Treatment groups were assigned to pens using a randomized complete block design: a challenged untreated group and a challenged treated group with 12 mg/kg of body weight of CFSM (Ef30616 CFSM with blending agent) administered daily in the drinking water from day 10 to day 19 of the experiment. The amount of test product added every day was calculated from the previous day’s water consumption. Fresh test product solution was mixed and added daily. Water consumption was measured daily from three pens by weighing the water remaining before disposal and refilling drinkers. Based on expected water consumption, the product was diluted so that the bird would ingest the corresponding dose by body weight.
Necrotic enteritis challenges
In experiments 1 and 2 all birds were challenged with coccidia (1500–5000 oocysts of Eimeria maxima) by gavage on day 14 and C. perfringens CP6 strain on days 19 and 20 by gavage using 1.0 ml of a 1.0 × 108 CFU/ml to reach at least 10% mortality. In experiment 3, birds were sprayed with one dose of a commercial coccidia vaccine (COCCIVAC®-B52) that contained live oocysts of Eimeria acervulina, E. maxima, E. maxima MFP, E. mivati, and E. tenella at 1 d of age upon arrival at SPRG. Then on days 13, 14, and 15, the birds were challenged with CP6 at 1.0 ml of a 1.0 × 108 CFU/ml added to 100 ml of water and thoroughly mixed and poured into the feed pan of birds in each challenge pen to be consumed within 30 min (Hofacre et al., Citation1998). All the birds received feed and water ad libitum throughout the trial. In experiment 3, rations consisted of a non-medicated commercial-type broiler starter, grower, and finisher diets compounded according to NRC guidelines and based on corn and soybean meal formulated with no animal by-product. Birds were provided with a lighting programme following the primary breeder’s recommendations.
Measurement of bird performance parameters
BW was recorded by cage on days 0, 14, 22, and 28 and by pen on days 0, 21, 35, and 42 during experiments 1 and 2 and experiment 3, respectively. Bodyweight gain (BWG), feed intake (FI), and feed conversion ratio (FCR) were recorded on days 0–14, 0–22, and 0–28 in experiments 1 and 2 and on days 0–21, 0–35, and 0–42 in experiment 3. Feed added to each pen was weighed at the beginning of each feed formulation change: at day 0 in experiments 1 and 2, and at day 0 (starter feed), 21 (grower feed), and 35 (finisher feed) in experiment 3. Feed remaining in feeders was weighed and disposed of on days 14, 22, and 28 in experiment 1 and 2, while in experiment 3, on day 21 the remaining starter feed was removed, weighed, and replaced with grower feed to day 35 and then, the remaining grower feed was removed, weighed back, and replaced with finisher feed to study termination. Pens were checked daily for mortality. A gross necropsy was performed on all dead or culled birds to determine the probable cause of death.
Lesion scoring and sample collection
On day 22 of experiments 1 and 2 and on day 16 of experiment 3, one bird per cage/pen was humanely euthanized, weighed, and necropsied (n = 60 and n = 24, respectively). Lesion scores were evaluated as previously described by Hofacre et al. (Citation1998) where 0 = Normal; 1 = Slight mucus covering small intestine; 2 = Necrotic small intestine mucosa; 3 = Sloughed and blood small intestine mucosa and contents. Samples of jejunum content and jejunum tissue were taken from the birds selected for lesion scoring. For each scored bird, the jejunum content immediately distal to the lesion score site was squeezed into a tube and flash frozen, then approximately 1 cm of the same section of jejunum was removed and placed into a tube to be flash frozen.
Intestinal microbiome analysis
Metagenomic shotgun sequencing was performed to evaluate the effect of the CFSM treatment in the intestinal microbiome of birds included in experiment 1. Jejunum content samples (n = 15) from the untreated group and from the treated group (n = 15) were selected for microbiome characterization. Frozen samples were sent to CosmosID for genomic DNA extraction, shotgun metagenomic sequencing and bioinformatic analysis, including microbiome alpha and beta diversity and taxonomic relative abundances calculation.
DNA extraction and quantification
Genomic DNA from jejunum content samples was extracted using PowerSoil Pro Kit (Qiagen, Mississauga, Canada) according to manufacturer protocols. DNA was quantified using the GloMax Plate Reader System (Promega, Madison, WI, USA) using the QuantiFluor® dsDNA System (Promega) chemistry.
Library preparation and sequencing
DNA libraries were prepared using the Nextera XT DNA Library Preparation Kit (Illumina, San Diego, CA, USA) and IDT Unique Dual Indexes with total DNA input of 1 ng. Genomic DNA was fragmented using a proportional amount of Illumina Nextera XT fragmentation enzyme. Unique dual indexes were added to each sample followed by 12 cycles of PCR to construct libraries. DNA libraries were purified using AMpure magnetic Beads (Beckman Coulter, Brea, CA, USA) and eluted in QIAGEN EB buffer. DNA libraries were quantified using Qubit 4 fluorometer and Qubit dsDNA HS Assay Kit. Libraries were then sequenced on an Illumina NextSeq2000 at 2 × 150 bp.
Bioinformatics analysis
The bioinformatic system used by CosmosID utilizes a high-performance data-mining k-mer algorithm that rapidly disambiguates millions of short reads into the discrete genomes engendering the sequences. The pipeline has two separable comparators: the first consists of a pre-computation phase for reference databases and the second is a per-sample computation. The inputs to the pre-computation phase are databases of reference genomes, virulence markers, and antimicrobial resistance markers. The output of the pre-computational phase is a phylogeny tree of microbes, together with sets of variable length k-mer fingerprints (biomarkers) uniquely associated with distinct branches and leaves of the tree. The second per-sample computational phase searches the hundreds of millions of short sequences reads, or alternatively contigs from draft de novo assemblies, against the fingerprint sets. This query enables the sensitive yet highly precise detection and taxonomic classification of microbial NGS reads. Taxonomic classification was performed at phylum, class, order, family, genus, and species levels. After the analyses of the sequences, the CosmosID bioinformatic platform calculated the diversity and taxonomic composition of the microbiome of the evaluated samples. Alpha diversity metrics, including Shannon, Chao1, and Simpson, and the taxonomic relative abundances of each sample at phylum, family, and species level were calculated.
Bacterial strains and growth conditions in vitro
C. perfringens strains CP6 and CL-15 were acquired from Southern Poultry Research Group Inc. (Athens, GA, USA) and Microbial Research Inc. (Fort Collins, CO, USA), respectively. C. perfringens was routinely cultured in Brain–Heart infusion broth (ThermoFisher Scientific, Waltham, MA, USA) supplemented with 2 g/l yeast-extract (BHIC), and on tryptic-soy agar supplemented with 5% defibrinated sheep blood (Cedarlane, Burlington, ON, Canada). Bacteria cultures were grown statically at 37˚C in an Anaerobe Systems AS-580 anaerobic chamber (Anaerobe Systems, Morgan Hill, CA, USA) containing an atmosphere of 90% N2, 5% CO2 and 5% H2.
Preparation of peptide containing CFSM
Cell-free spent medium (CFSM) containing bioactive peptides was prepared as follows. A culture of Enterococcus faecium NCIMB 30616 (Ef30616) was inoculated into 20l of complex medium (milk proteins and lactose, proprietary blend) and grown at 37°C with agitation in a Sartorius Biostat C-DCU 30L fermenter (Sartorius, Oakville, Ontario, Canada). At the end of fermentation, cells were heat-killed in the fermenter at 80°C for 30 min before separation of the cell pellet from the supernatant by centrifugation (17,696× g, 10 min, 4°C, Avanti J-25I) to obtain the CFSM. The obtained CFSM was spray-dried with a Yamato Pulvin Mini GB210 (Yamato Scientific, Santa Clara, CA, USA), and the dried powder was stored at 4°C until needed.
Clostridium challenge with CFSM
Culture medium containing resuspended CFSM was first prepared as follows. The powdered CFSM was weighed on an analytical balance and resuspended in BHIC medium to a final concentration of 50 mg/ml. The pH was adjusted to 7.0 ± 0.2 followed by centrifugation at 3217× g for 15 min to pellet insoluble material (Eppendorf, Hamburg, Germany). The clarified supernatant was sterilized with a 0.22 µm-pore PES filter and left under anaerobic conditions overnight to remove any dissolved oxygen. Single colony isolates of C. perfringens were inoculated into BHIC and grown anaerobically overnight at 37°C. One ml of each overnight culture was centrifuged at 5000× g for 5 min and the supernatant discarded. Cells were then resuspended in 1 ml PBS and adjusted to a final concentration of OD600 = 1.0 ± 0.1 (corresponding to ∼1.0 × 107 CFU/ml). Normalized cultures were diluted 1:100 into BHIC alone (untreated) or BHIC supplemented with Ef30616 CFSM.
Effect of CFSM on C. perfringens growth
C. perfringens growth was measured by preparing cultures in BHIC, or BHIC with CFSM, as described above. Bacterial growth was monitored by measuring cell turbidity at OD600 nm using a Biowave CO8000 spectrophotometer (VWR, Mississauga, ON, Canada) or by preforming serial dilutions followed by plating onto TSA + SB to determine CFU/ml.
RNA extraction and cDNA synthesis
Isolation of RNA from C. perfringens was performed from cultures grown in BHIC alone or with CFSM as described above. At specific times, 0.5–5 ml of culture was transferred to a tube containing two culture volumes of RNAprotect Bacterial Reagent (Qiagen), vortexed for 30 s and incubated at room temperature for 10 min, followed by centrifugation at 5000× g for 10 min. The supernatant was discarded, and cell pellets were stored at −80°C until further use. Total bacterial RNA was extracted using the RNeasy Mini Kit (Qiagen) following the manufacturer's recommendations for Gram-positive bacteria with enzymatic lysis. Briefly, pellets were thawed and resuspended in 100 µl TE (10 mM Tris, 1 mM EDTA, pH 8.0) buffer with 5 mg/ml lysozyme, 0.5 mg/ml proteinase K and incubated at 37°C for 30 min. Afterwards, 350 µl of buffer RLT was added and vortexed followed by addition of 250 µl of 100% ethanol and mixed by pipetting. The lysate was then transferred to a RNeasy Mini spin column and centrifuged at 13,000× g for 1 min. The flow-through was discarded and the spin column was washed with 350 µl of buffer RW1 followed by centrifugation. An on-column DNase I digest was performed by adding 27 U DNase I dissolved in 80 µl buffer RDD directly to the membrane and incubating at room temperature for 15 min. After incubation, 350 µl buffer RWI was added to the column and centrifuged, discarding the flow-through. Two wash steps were done by adding 500 µl buffer RPE and centrifuging, discarding the flow-through. The spin column was then transferred to a clean collection tube and centrifuged for 2 min to dry the membrane. RNA was eluted with 50 µl of RNase-free water into a new, RNase-free 1.5 ml microfuge tube. RNA purity and quantity was measured using a Nanodrop 2000, and RNA integrity checked on a 2% agarose gel. Only samples with a 260/280 nm ratio of 2.0–2.2 and displayed no degradation on the gel were used for cDNA synthesis. RNA was stored at −80°C until further use.
Total cDNA synthesis was performed using the BioRad iScript gDNA clear cDNA synthesis kit (Bio-Rad, Mississauga, Ontario, Canada). Contaminating genomic DNA was removed by treating 500 ng of total RNA with iScript DNase in a total volume of 16 µl and incubated at 25°C for 15 min, followed by DNase I heat inactivation at 75°C for 5 min. Reverse transcription was performed by adding 4 µl of iScript Reverse Transcription Supermix directly to each reaction tube or 4 µl iScript No-RT Control Supermix for no reverse-transcriptase controls. Reactions were run on a Bio-Rad iCycler iQ thermocycler with the following parameters: 25°C, 5 min; 46°C, 20 min; 95°C, 1 min; 4°C hold. Following cDNA synthesis, samples were diluted 10-fold in RNase-free water to be used immediately or stored at −20°C.
Real-time quantitative PCR
Real-time qPCR assays were set up in 96-well PCR plates as follows. Five µl of diluted cDNA was used in a 20 µl qPCR reaction containing 10 µl of 2× SsoAdvanced Universal SYBR® Green Supermix (Bio-Rad) and 0.6 µl of each 10 µM forward and reverse primer (Table S1). Amplification was performed on an Applied Biosystems QuantStudio3 thermocycler running the following reaction parameters: 95°C for 2 min, followed by 40 cycles of 95°C for 15 s, 60°C for 15 s, 72°C for 30 s, and a final elongation step of 72°C for 2 min. A dissociation curve from 60°C to 95°C was performed at the end of each run to ensure product specificity. Each PCR plate included an inter-plate calibrator (IPC) run in sextuplicate which functions as a normalizing standard to compare expression across multiple qPCR plates and runs. Results were exported to the ThermoFisher Cloud connect server to calculate normalized Ct values. The relative quantification of mRNA expression was normalized to the level of the housekeeping genes ftsZ and recA. The relative fold-change in gene expression was calculated by the comparative threshold cycle (2-ΔΔCt) method (Livak & Schmittgen, Citation2001).
Size-exclusion chromatography fractionation of CFSM
Dried CFSM was resuspended in Milli-Q water (Milli-Q IQ 7000, MilliporeSigma, Burlington, MA, USA) to a concentration of 50 mg/ml and filter sterilized through a 0.22 µm PES filter. Fractionation of the material by size-exclusion chromatography (SEC) was performed using an ÄKTAexplorer 100 System with an XK 26/100 column packed with Superdex 30 Prep Grade resin (packed bed height: 90 cm, diameter: 26 mm, average particle size: 34 μm) (Cytiva Life Sciences, Marlborough, MA, USA), equipped with Frac-950 Fraction Collector. Samples were fractionated isocratically at a flow rate of 3 ml/min, at room temperature. Absorbance (280 and 214 nm) and pH were monitored during runs. Collected eluates were pooled into four fractions, frozen at −80°C, freeze-dried, then stored at 4°C until use.
Haemolysis Assays
The effects of bulk CFSM on C. perfringens haemolytic activity were first investigated by measuring the zone of haemolysis surrounding the bacterial colony when grown on blood agar. Two µl of a normalized C. perfringens culture was spotted onto TSA plates supplemented with 5% v/v sterile defibrinated sheep blood (Cedarlane, Burlington, Ontario, Canada) or TSA + SB containing 25 mg/ml CFSM. The cells were allowed to absorb into the media for 1 h before inverting plates and incubating overnight at 37°C under anaerobic conditions. After 16–24 h growth, the clarified zone of haemolysis was measured using a set of callipers. All tests were done with three biological replicates.
The effect of CFSM on C. perfringens haemolytic activity was also measured using the doubling dilution assay described in Riddler et al. (Citation2021). CP6 cultures were grown as described above in BHIC alone, BHIC with CFSM at 50 mg/ml or BHIC with SEC fractions 0–3 resuspended to 50 mg/ml. Cultures were assayed for haemolysis after 5- or 24-hours growth as follows. One ml of each culture was pelleted by centrifugation and supernatant was sterilized with a 0.22 µm-pore PES filter. Samples were serially diluted two-fold in 1 × PBS across the columns of a 96-well microtitre plate (ranging from 2 to 2048-fold supernatant dilution) in a total volume of 300 µl. Defibrinated sheep red blood cells (RBCs) (Cedarlane) were prepared by mixing 1 ml RBCs with 12 ml cold 1 × PBS before centrifuging at 1450× g for 10 min and discarding the supernatant. This was repeated for a total of two washes, or until the PBS was clear and no longer contained any haemoglobin, before resuspending in 12 ml 1 × PBS to a final concentration of ∼8 × 108 RBC/ml. Washed RBCs (100 µl) were added to each well of a new 96-well plate followed by 100 µl of the diluted sample. Samples mixed by pipetting and plates were incubated at 37°C without agitation for 1 h. PBS-only controls (0% haemolysis) and 1% Triton X-100 in PBS controls (100% haemolysis) were included. After incubation, the density of intact RBCs was measured at OD650 nm using a BioTek Synergy HTX microplate reader (Agilent, Santa Clara, CA, USA).
The supernatant dilution resulting in 50% haemolysis (HA50) was determined by plotting the OD650 against the logarithm of the supernatant dilution and fitting a four-parameter logistic regression, constraining the bottom to 0. All experiments were performed in biological triplicate with technical duplicates.
Statistical analysis
The impact of the CFSM inclusion on the performance parameters of birds from three clinical experiments, as well as microbiome metrics from the first experiment in the NE challenge, were assessed using two-way ANOVA models after examining the distributions of the residuals for normality and homoscedasticity. Parameters like net mortality, feed intake (FI), adjusted feed conversion ratio (FCR), as well as Shannon and Simpson diversity indices and the relative abundance of the Firmicutes phylum, were transformed using the Box–Cox power transformation analysis (Box & Cox, Citation1964). The residuals were re-evaluated after transformation. For each performance parameter, alpha diversity metric, and taxonomic level, a separate two-way ANOVA model was built. Differences in bacterial growth were analysed using a two-tailed t-test on the generation times calculated from the linear portion of exponential growth for each condition. Differences between haemolysis spot tests were analysed using an unpaired t-test. The HA50 values for T5 and T24 with bulk CFSM were compared by unpaired t-test, and HA50 values for SEC fractions F0-3 with one-way ANOVA with Dunnett’s multiple comparisons. Gene expression results were analysed using a repeat-measures two-way ANOVA with Sidak post-hoc multiple comparisons. Clinical results were statistically analysed with the Stata 16.1 software, while in vitro results were analysed with GraphPad Prism 9 (version 9.5.0) for macOS.
Results
Ef30616 CFSM in-water administration reduces broilers net mortality percentage
The results of mortality, lesion scores, BWG, feed intake (FI), and adjusted feed conversion ratio (FCR) by treatment of the first clinical experiment are shown in . The treated group had lower net mortality (6.6%) compared with the untreated group (16.3%) (P < 0.05). At the same time, the treated group showed an NE mean lesion score numerically lower than the untreated control (P = 0.09). Prior to the challenge that began on day 14, the BWG and FI of the treated group, were 0.02 kg (P = 0.06) and 0.15 kg/cage (P = 0.04) higher than the untreated group, respectively. However, at the peak of the challenge (on day 22) and at the study termination (on day 28), there were no significant differences on BWG and FI between the groups. On day 28, both groups had a similar adjusted FCR (P = 0.89) with a numerically lower non-adjusted FCR in the treated group (1.670) compared to the untreated group (1.775) (P = 0.05).
Table 1. Effect of in-water CFSM treatment on broiler performance parameters, mortality, and lesion score (Experiment 1).
The means of the performance parameters of experiment 2, evaluated by treatment in the present study, are summarized in . A significant difference (P < 0.05) was observed in the net mortality of the two groups, where the treated group had 13.9% less mortality compared with the untreated group. The NE lesion scores were not significantly different between groups (P = 0.23). The BWG throughout all the study was similar for the two groups, whereas, on day 14, the FI of the treated group, was similar to the untreated group, (P = 0.11). However, at the peak of the challenge (on day 22) and at the study termination (on day 28), the treated group had an increased FI of 0.62 and 1.56 kg/cage, respectively, compared with the untreated group (P < 0.05). In the case of the adjusted FCR, on day 22 of the study, both groups had a marginal significant difference (P = 0.06), and on days 14 and 28, the adjusted FCR of the two groups were not different. As observed with the first study, the non-adjusted FCR was numerically reduced at both 22 and 28 days, from 2.21 to 1.87 (P = 0.17) and 2.09 to 1.81 (P = 0.24).
Table 2. Effect of in-water CFSM treatment on broiler performance parameters, mortality, and lesion score (Experiment 2).
For experiment 3, where the birds were housed in floor pens, the means of the performance parameters are summarized in . A numerical difference (P = 0.12) was observed in the net mortality where the treated group had 2.7% less mortality than the untreated group, resulting in a 67% reduction (from 4% down to 1.33%). The NE lesion scores of both groups were not different (P = 0.53). The BWG throughout the experiment had no significant differences for the two groups. The FI of the treated group on day 42, at the peak of the challenge, was 4.39 kg/pen higher than the untreated group (P = 0.08). In the case of day 21 adjusted FCR and non-adjusted FCR, the treated group had 0.05 (P = 0.16) and 0.09 (P = 0.06) units less than the untreated group.
Table 3. Effect of in-water CFSM treatment on broiler performance parameters, mortality, and lesion score (Experiment 3).
Ef30616 CFSM in-water administration does not affect intestinal microbiome of broilers
The microbiome analysis of the present study was conducted on the intestinal content from the first clinical experiment. Alpha diversity metrics evaluated were Shannon, Chao1, and Simpson indices. The means for the untreated group for these measures were 2.22, 0.73, and 11.2, respectively. The treated group showed means for Shannon of 2.3, Simpson of 0.8, and Chao1 of 12.9, being not statistically different relative to the untreated group (P > 0.05 for all measures) (). For taxonomy composition, at the phylum level, both groups shared the most predominant phyla (Firmicutes, Proteobacteria, and Actinobacteria), with relative abundances of 90.6%, 9%, and 0.2%, respectively, for the untreated group, and 88%, 9.7%, and 1%, respectively for the treated group. No statistical differences were observed at the phylum level (P > 0.05). At the family level, Lactobacillaceae and Clostridiaceae were the most prevalent in both groups, with a relative abundances of 75% and 7%, respectively, for the untreated group and 79% and 5%, respectively, for the treated group. No statistical differences were observed at the family level (P > 0.05). At the species level, Lactobacillus sp. and C. perfringens were the most prevalent in both groups, with relative abundances of 20% and 19%, respectively, for the untreated group and 23% and 11%, respectively for the treated group. No significant differences were observed at the species level (P > 0.05) ().
Table 4. Effect of in-water CFSM treatment on broiler intestinal microbiome alpha diversity and relative abundances.
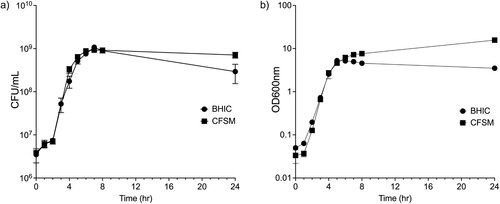
Ef30616 CFSM does not affect C. perfringens growth
The growth of C. perfringens CP6 was unaffected when treated with CFSM, indicating that CFSM is neither bacteriostatic nor bactericidal. CFSM-treated cells had a doubling time of 28.9 ± 1.0 min compared to 33.8 ± 1.2 min for untreated cells, which was a significant decrease when measured by optical density at 600 nm (P = 0.02) ((a)). However, it was observed that the CFSM-treated medium became opaque over the course of the growth curve, and it was therefore necessary to directly enumerate viable C. perfringens via serial dilution plating. When growth was tracked by CFU/ml, the difference in doubling time was not significantly different between CFSM treated (27.7 ± 0.9 min) and untreated cells (29.6 ± 0.2 min) (P 2= 0.11) ((b)).
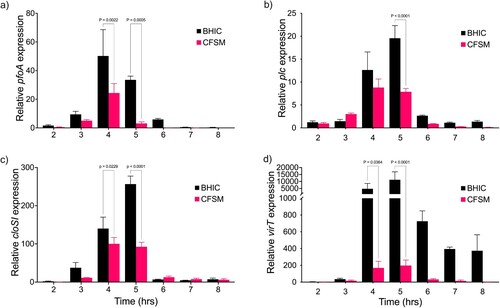
Ef30616 CFSM represses QS-regulated virulence genes
RT-qPCR results showed that in untreated CP6, transcription of pfoA, plc, cloSI, and virT began to increase after 3 h and reach maximal expression between 4 h and 5 h, corresponding with the late logarithmic stage of growth ((a–d)). By 6 h cells had reached stationary phase and transcription decreased to pre-induction levels for pfoA, plc, and cloSI. When CP6 was treated with CFSM the expression of each virulence gene was significantly repressed in late log phase, pfoA and plc were repressed 10.7 and 2.5-fold, respectively, relative to the untreated control ((a,b)), while cloSI was repressed 2.77-fold ((c)). To support that virulence gene expression was being repressed by CFSM interfering with the Agr-QS pathway, we evaluated expression of virT, whose expression is dependent on VirR mediated activation in response to AIP induced QS-activation. In accordance with the repression of other QS-controlled genes, virT expression was repressed 57.1-fold relative to the untreated cultures ((d)).
Figure 2. QS-activated virulence genes are repressed by CFSM. Time-course of CP6 toxin gene expression as measured by RT-qPCR for (a) pfoA, (b) plc (c) cloSI and (d) virT grown in BHIC alone or treated with CFSM. Expression was normalized to the reference genes ftsZ and recA, and the fold change is relative to the untreated culture at 2 h. Data are the mean and standard deviation of three biological replicates and technical duplicates. Two-way analysis of variance (ANOVA) with Sidak pairwise multiple comparison test was used to evaluate significance.
The pore-forming toxin gene netB is repressed by Ef30616 CFSM
The expression of netB was measured by RT-qPCR at timepoints corresponding to mid-log (2 h), late-log (4 h), and stationary phase (24 h) in C. perfringens CL-15 (). Strain CL-15 was chosen for its consistent expression of netB in vitro, and two sets of RT-qPCR primers which bind at separate locations along the netB coding sequence were used to independently validate transcript abundance. The results showed that netB was upregulated at the late-logarithmic phase of growth, with a return to pre-activation levels after 24 h ((a,b)). When CL-15 cultures were treated with Ef30616 CFSM, the expression of netB was significantly reduced 2.6 and 1.6-fold for netB1 and netB3 primers, respectively. These results further demonstrate that CFSM interferes with the AIP-mediated QS system of virulence gene activation in C. perfringens.
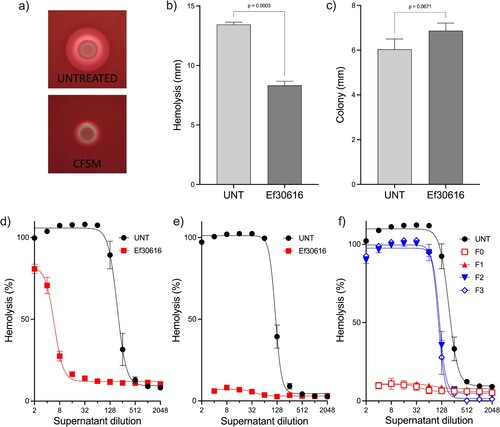
C. perfringens haemolytic activity is reduced by Ef306161 CFSM
When C. perfringens was grown on blood agar containing 25 mg/ml CFSM, the characteristic zone of haemolysis was found to be significantly reduced ((a,b)), despite there being no impact on colony diameter ((c)). Haemolytic activity was therefore measured using a doubling dilution assay to quantify the effect of CFSM against CP6 in broth culture. The results show that in late log-phase the HA50 of untreated C. perfringens significantly decreased 97.7% from 189.5-fold to 4.2-fold (P < 0.0001) when treated with CFSM, and by 24 h haemolytic activity was no longer detectable in the CFSM treated cultures ((d,e)). SEC was used to separate bulk CFSM into four fractions to determine if peptides were responsible for the observed impact on haemolysis. Based on compositional analysis, fraction 1 (F1) was found to predominantly contain bioactive peptides. Both fractions F0 and F1 completely abolished C. perfringens haemolytic activity, while fractions F2 and F3 reduced the untreated HA50 from 190.4–113.5 (P = 0.008) and 107.3 (P = 0.005), respectively. Neither the media or CFSM and SEC controls had any impact on background haemolysis. These results demonstrate that the reduced transcriptional expression of haemolytic toxin genes translates into a reduced haemolytic phenotype for C. perfringens CP6.
Figure 3. Expression of the netB pore-forming toxin is repressed by CFSM. RT-qPCR was performed using primers (a) netB1 and (b) netB3 on mid-log (2 h), late-log (4 h) and stationary phase (24 h) cultures of CL-15 grown in BHIC alone or treated with CFSM. Expression was normalized to the reference genes ftsZ and recA, and the fold change is relative to the untreated culture at 2 h. Data are the mean and standard deviation of three biological replicates and technical duplicates. Two-way analysis of variance (ANOVA) with Sidak pairwise multiple comparison test was used to evaluate significance.
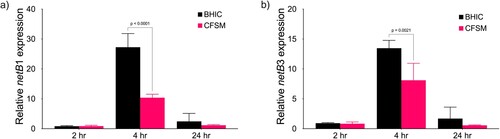
Figure 4. Haemolysis is reduced by CFSM and fractions containing bioactive peptides. (a) CP6 haemolysis on TSA-SB plates (Untreated) or with CFSM. Diameter of (b) haemolysis zone and (c) colony size for CP6 grown on TSA with sheep blood. Quantification of CP6 supernatant haemolytic activity against sheep red blood cells treated with bulk CFSM at (d) 5 h (e) 24 h, or (f) SEC fractions F0-3 at 24 h. All data are the mean and standard deviation of three biological replicates.
Discussion
In recent years, the reduction in use of antibiotics as a therapeutic and preventive treatment in poultry production has increased the prevalence of enteric diseases, including NE. Mortality caused by acute NE has been observed primarily in models where birds are infected with Eimeria before administration of the C. perfringens challenge. The induction of this disease usually requires previous intestinal damage, particularly the sloughing of intestinal epithelial cells and leakage of plasma protein into the intestinal lumen, which provides a growth substrate for the proliferation of C. perfringens, worsening the damage of the intestinal epithelium (Dahiya et al., Citation2006).
Given the concern for human health issues associated with the preventative use of antibiotics, non-anti-microbial alternative solutions are the focus of many studies; however, there are no reports about the use of CFSM QS inhibitors as one of these alternatives applied to poultry. Rossi et al. (Citation2020) described some botanical components and nature-identical compounds as a non-antibiotic strategy to improve performance in poultry. Specifically, the antibiofilm and the anti-QS activities of carvacrol and thymol, components extracted from plants like oregano (Rossi et al., Citation2020). These compounds act by permeabilizing the bacterial membrane causing a collapse of the proton motive force, depleting ATP pools, and inhibiting motility of pathogens, while simultaneously playing an important anti-inflammatory and antioxidant role in protecting the integrity of the intestinal mucosa. Studies that included essential oils extracted from plants as an alternative to antibiotics have described reductions in mortality during NE challenge experiments in broilers, showing 7% less mortality in the treated group when compared with the non-treated group (Coles et al., Citation2021). In the present study, results of the clinical work demonstrated significant reductions (ranging from 10% to 16%) in mortality of the CFMS-treated group compared with the challenged controls. Performance metrics further demonstrated improvements in non-adjusted FCR. Although there was no significant difference in adjusted FCR, more extensive field studies may better assess this metric and yield favourable results.
Diversity and taxonomic composition of the intestinal microbiome from birds of experiment 1 of the present study were measured on jejunum content samples. No differences were found in microbiome metrics when the treatment groups were compared; therefore, CFSM treatment may not affect the richness, evenness, and taxonomical abundance of the jejunum microbiome in C. perfringens-infected birds. Several predisposing factors are necessary for a NE infection, including intestinal dysbiosis. Based on the literature, in a NE infection the relative abundance of C. perfringens can increase to 58–70% compared to 0.02% in healthy chickens (Fu et al., Citation2022). In healthy chickens, the gut immune system and the gut microbiome are separated by a mucus layer that allows constant interaction and balance, protecting the tissues against pathogens and foreign substances (Carrasco et al., Citation2019).
In the present study, C. perfringens was the second most prevalent species in both treated and untreated groups which may confirm the absence of any effect of the CFSM treatment on the relative abundance at the species level. Several studies have investigated the effect of conventional NE treatments, such as antibiotics, on the intestinal microbiome diversity and composition. Fasina et al. (Citation2016) compared the effect of the bacitracin methylene disalicylate antibiotic treatment on the relative abundance of the jejunal microbiome at phylum, family, and genus level in chickens challenged with C. perfringens, versus a no medicated group (Fasina et al., Citation2016). The results of the mentioned study agree with the present study, where the most prevalent families were Lactobacillaceae, Clostridiaceae, and Enterobacteriaceae in both treatment groups. However, they reported in the unmedicated treatment a level of Lactobacillaceae of 16% versus 84% in the medicated treatment at 1 d post-challenge, while in the present study, the levels of Lactobacillaceae were 75% and 79% in the untreated and treated groups, respectively. At the same time, Clostridiaceae relative abundance in the unmedicated group was 68% versus 5.4% in the medicated group, while, in the present study, this family had a relative abundance of 7% and 5% in both groups.
This study demonstrated that CFSM from Ef306161 reduces NE-induced mortality of poultry while having no impact on the intestinal microbiome. The effects of CFSM against several C. perfringens QS-controlled virulence factors in vitro demonstrate that CFSM attenuates the expression of several haemolytic toxins while having no impact on overall bacterial fitness. Treatment of CP6 with CFSM significantly reduced expression of the QS-activated genes plc, pfoA and cloSI, accompanied by an abolition of CP6 induced haemolysis of sheep red blood cells. This decrease in virulence is proposed to be mediated by small bioactive peptides, as demonstrated by the effects of SEC fraction F1 against CP6 haemolytic activity.
Expression of C. perfringens toxins, such as the alpha-toxin phospholipase C, perfringolysin O and NetB, is controlled by the Agr QS system (Ohtani et al., Citation2009; Vidal et al., Citation2009). Transcription of pfoA and cloSI is controlled through direct binding of the promoters by activated VirR, while plc is activated by VR-RNA (Cheung & Rood, Citation2000; Shimizu et al., Citation2002; Okumura et al., Citation2008). However, there may be an overlap in the regulation of pfoA by both VirR and VR-RNA, and additional environmental factors are involved in the narrow temporal expression of plc including acidification of the extracellular environment (Ohtani & Shimizu, Citation2015; Adachi et al., Citation2018). Some S. aureus virulence factors, such as alpha-haemolysin, increase expression during growth once activated by Agr (Dunman et al., Citation2001), whereas C. perfringens relies on tight temporal control of expression to synthesize toxins during a narrow period of growth (Adachi et al., Citation2018; Li & McClane, Citation2020). This control of toxin expression aligns with the pathogenesis of C. perfringens in poultry, where the bacteria rapidly proliferate in response to changes in diet and synthesize secreted toxins during this burst in growth, leading to disease (Yang et al., Citation2019). Even slight delays in the expression of AgrA in S. aureus have been shown to abrogate alpha-haemolysin production (Traber & Novick, Citation2006). This study shows that toxin gene expression of the bacterium is reduced by treatment with CFSM without altering the growth of the cell, implicating an effect on the AIP QS system.
The pore-forming toxin NetB is the primary agent responsible for C. perfringens-induced necrotic enteritis in poultry (Keyburn et al., Citation2008). The netB gene is encoded in a conserved 40-kb region of a conjugative plasmid and its transcription is directly activated in response to AIP QS through VirR binding at two distinct elements within the netB promoter. Furthermore, C. perfringens requires a functional Agr system to elicit NetB-induced NE in vivo (Cheung et al., Citation2010; Yu et al., Citation2017). The CFSM treatment reduced netB expression at the mid-log phase, like pfoA and plc being under similar tight temporal control with expression beginning during the mid-logarithmic growth stage with NetB toxin accumulating in the medium from mid-log onward.
The present study results show that CFSM reduced toxin gene expression which correlates with a reduced total haemolytic potential against sheep RBCs in vitro. The major haemolytic toxins phospholipase C (PLC) and perfringolysin O (PFO) act synergistically to contribute to the development of gas gangrene and necrohaemorrhagic enteritis via direct enzymatic hydrolysis of phospholipids (PLC) and pore formation in the membrane (PFO) (Awad et al., Citation1995, Citation2001; Tilley et al., Citation2005) causing cytolysis. NetB also induces cytolysis through oligomerization into large pore-forming toxins following binding of cholesterol in the target cell membrane (Savva et al., Citation2013). Although the direct measure of each individual cytolytic toxin was not performed, the total reduction in haemolysis may be associated with decreased toxin production after the observed reduction in gene expression. PFO and NetB, both of which genes are significantly repressed, are the two toxins which likely contribute to the observed haemolysis in vitro, since PLC causes lysis through a hot-cold phenomenon (Yan et al., Citation2013). While not directly correlated in this study, CFSM reducing overall C. perfringens haemolysis in vitro may explain the reduced mortality in clinical settings as the severity of clinical C. perfringens virulence is correlated with the in vitro NetB titre (Hustá et al., Citation2021).
As demonstrated by the SEC F1 fraction which inhibited C. perfringens haemolytic activity, peptides are proposed to mediate the anti-virulence effects of Ef30616 CFSM. Singh et al. (Citation2015) undertook a study to design quorum-quenching (QQ) peptide antagonists to inhibit the Agr QS-system of C. perfringens and found that the AIP analogue Z-AIPCp-F4A/T5S was effective at reducing pfoA gene expression at sub-micromolar concentrations (Singh et al., Citation2015). Furthermore, a synthetic 6-R AIP antagonist was found to have QQ activity against multiple C. perfringens toxinotypes, demonstrating the broad-spectrum application of such molecules (Ma et al., Citation2015). The CFSM from Ef30616 fermentation is hypothesized to contain many hundreds if not thousands of unique peptides all with varying levels of QQ activity, which could function by blocking recognition of the AIP signal, or by competing with AIP for VirS binding. Further research to understand the mode of action of CFSM is currently underway.
The results of the present study demonstrated in the NE challenge experiments, CFSM treatment significantly reduced the mortality of the chickens and showed numerical improvements in non-adjusted feed conversion and feed intake, while the jejunal microbiome diversity and taxonomic composition were not affected by the treatment in diseased birds. The objective of the study was to determine the effects of the treatment on challenged birds with disrupted microbiome so the non-challenged treated group was not included in these studies. Further work is underway to address the effect of the product on normal microbiome composition. The expression of key QS-activated virulence genes was repressed by CFSM during the period of C. perfringens growth when it would have maximal pathogenic impact in vivo, including the NetB encoding gene which was significantly repressed by CFSM. Overall haemolytic activity C. perfringens is dramatically reduced by CFSM in mid-logarithmic phase of growth and abolished by stationary phase. Bioactive peptides resulting from probiotic fermentation are thought to be responsible for the observed reduction in virulence through inhibition of the Agr-like QS system of C. perfringens.
Supplemental Material
Download MS Word (21.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adachi, K., Ohtani, K., Kawano, M., Singh, R.P., Yousuf, B., Sonomoto, K., Shimizu, T. & Nakayama, J. (2018). Metabolic dependent and independent pH-drop shuts down VirSR quorum sensing in Clostridium perfringens. Journal of Bioscience and Bioengineering, 125, 525–531.
- Antunes, L.C.M., Ferreira, R.B.R., Buckner, M.M.C. & Finlay, B.B. (2010). Quorum sensing in bacterial virulence. Microbiology, 156, 2271–2282.
- Aruwa, C.E., Pillay, C., Nyaga, M.M. & Sabiu, S. (2021). Poultry gut health – microbiome functions, environmental impacts, microbiome engineering and advancements in characterization technologies. Journal of Animal Science and Biotechnology, 12, 119.
- Awad, M.M., Bryant, A.E., Stevens, D.L. & Rood, J.I. (1995). Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Molecular Microbiology, 15, 191–202.
- Awad, M.M., Ellemor, D.M., Boyd, R.L., Emmins, J.J. & Rood, J.I. (2001). Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infection and Immunity, 69, 7904–7910.
- Box, G.E.P. & Cox, D.R. (1964). An analysis of transformations. Journal of the Royal Statistical Society: Series B (Methodological), 26, 211–243.
- Carrasco, J.M.D., Casanova, N.A. & Miyakawa, M.E.F. (2019). Microbiota, gut health and chicken productivity: what is the connection? Microorganisms, 7, 374.
- Casewell, M., Friis, C., Marco, E., McMullin, P. & Phillips, I. (2003). The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. Journal of Antimicrobial Chemotherapy, 52, 159–161.
- Cheung, J.K., Awad, M.M., McGowan, S. & Rood, J.I. (2009). Functional analysis of the VirSR phosphorelay from Clostridium perfringens. PLoS One, 4, e5849.
- Cheung, J.K., Keyburn, A.L., Carter, G.P., Lanckriet, A.L., Immerseel, F.V., Moore, R.J. & Rood, J.I. (2010). The VirSR two-component signal transduction system regulates NetB toxin production in Clostridium perfringens. Infection and Immunity, 78, 3064–3072.
- Cheung, J.K. & Rood, J.I. (2000). The VirR response regulator from Clostridium perfringens binds independently to two imperfect direct repeats located upstream of the pfoA promoter. Journal of Bacteriology, 182, 57–66.
- Coles, M.E., Forga, A.J., Señas-Cuesta, R., Graham, B.D., Selby, C.M., Uribe, ÁJ, Martínez, B.C., Angel-Isaza, J.A., Vuong, C.N., Hernandez-Velasco, X., Hargis, B.M. & Tellez-Isaias, G. (2021). Assessment of Lippia origanoides essential oils in Salmonella typhimurium, Eimeria maxima, and Clostridium perfringens challenge model to induce necrotic enteritis in broiler chickens. Animals, 11, 1111.
- Costa, S.P.F.d., Mot, D., Bokori-Brown, M., Savva, C.G., Basak, A.K., Immerseel, F.V. & Titball, R.W. (2013). Protection against avian necrotic enteritis after immunisation with NetB genetic or formaldehyde toxoids. Vaccine, 31, 4003–4008.
- Dahiya, J.P., Wilkie, D.C., Kessel, A.G.V. & Drew, M.D. (2006). Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Animal Feed Science and Technology, 129, 60–88.
- Dunman, P.M., Murphy, E., Haney, S., Palacios, D., Tucker-Kellogg, G., Wu, S., Brown, E.L., Zagursky, R.J., Shlaes, D. & Projan, S.J. (2001). Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. Journal of Bacteriology, 183, 7341–7353.
- El-Hack, M.E.A., El-Saadony, M.T., Elbestawy, A.R., El-Shall, N.A., Saad, A.M., Salem, H.M., El-Tahan, A.M., Khafaga, A.F., Taha, A.E., AbuQamar, S.F. & El-Tarabily, K.A. (2022). Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives – a comprehensive review. Poultry Science, 101, 101590.
- Fancher, C.A., Zhang, L., Kiess, A.S., Adhikari, P.A., Dinh, T.T.N. & Sukumaran, A.T. (2020). Avian pathogenic Escherichia coli and Clostridium perfringens: challenges in no antibiotics ever broiler production and potential solutions. Microorganisms, 8, 1533.
- Fasina, Y.O., Newman, M.M., Stough, J.M. & Liles, M.R. (2016). Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poultry Science, 95, 247–260.
- Fathima, S., Hakeem, W.G.A., Shanmugasundaram, R. & Selvaraj, R.K. (2022). Necrotic enteritis in broiler chickens: a review on the pathogen, pathogenesis, and prevention. Microorganisms, 10, 1958.
- Fu, Y., Alenezi, T. & Sun, X. (2022). Clostridium perfringens-induced necrotic diseases: an overview. Immuno, 2, 387–407.
- Hofacre, C.L., Froyman, R., George, B., Goodwin, M.A. & Brown, J. (1998). Use of Aviguard, Virginiamycin, or Bacitracin MD against Clostridium perfringens-associated necrotizing enteritis. The Journal of Applied Poultry Research, 7, 412–418.
- Hustá, M., Ducatelle, R., Immerseel, F.V. & Goossens, E. (2021). A rapid and simple assay correlates in vitro NetB activity with Clostridium perfringens pathogenicity in chickens. Microorganisms, 9, 1708.
- Keyburn, A.L., Boyce, J.D., Vaz, P., Bannam, T.L., Ford, M.E., Parker, D., Rubbo, A.D., Rood, J.I. & Moore, R.J. (2008). Netb, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathogens, 4, e26.
- Le, K.Y. & Otto, M. (2015). Quorum-sensing regulation in Staphylococci – an overview. Frontiers in Microbiology, 6, 1174.
- Li, J. & McClane, B.A. (2020). Evidence that VirS is a receptor for the signaling peptide of the Clostridium perfringens Agr-like quorum sensing system. MBio, 11, e02219–20.
- Livak, K.J. & Schmittgen, T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods, 25, 402–408.
- Ma, M., Li, J. & McClane, B.A. (2015). Structure-function analysis of peptide signaling in the Clostridium perfringens Agr-like quorum sensing system. Journal of Bacteriology, 197, 1807–1818.
- McDevitt, R.M., Brooker, J.D., Acamovic, T. & Sparks, N.H.C. (2006). Necrotic enteritis: a continuing challenge for the poultry industry. World’s Poultry Science Journal, 62, 221–247.
- Ohtani, K., Hayashi, H. & Shimizu, T. (2002). The luxS gene is involved in cell–cell signalling for toxin production in Clostridium perfringens. Molecular Microbiology, 44, 171–179.
- Ohtani, K. & Shimizu, T. (2015). Regulation of toxin gene expression in Clostridium perfringens. Research in Microbiology, 166, 280–289.
- Ohtani, K., Yuan, Y., Hassan, S., Wang, R., Wang, Y. & Shimizu, T. (2009). Virulence gene regulation by the agr system in Clostridium perfringens. Journal of Bacteriology, 191, 3919–3927.
- Okumura, K., Ohtani, K., Hayashi, H. & Shimizu, T. (2008). Characterization of genes regulated directly by the VirR/VirS system in Clostridium perfringens. Journal of Bacteriology, 190, 7719–7727.
- Riddler, M.J., Daly, S.M., Hall, P.R. & Bose, J.L. (2021). Quantitative hemolysis assays. Methods in Molecular Biology, 2341, 25–30.
- Rood, J.I., Adams, V., Lacey, J., Lyras, D., McClane, B.A., Melville, S.B., Moore, R.J., Popoff, M.R., Sarker, M.R., Songer, J.G., Uzal, F.A. & Immerseel, F.V. (2018). Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe, 53, 5–10.
- Rossi, B., Toschi, A., Piva, A. & Grilli, E. (2020). Single components of botanicals and nature-identical compounds as a non-antibiotic strategy to ameliorate health status and improve performance in poultry and pigs. Nutrition Research Reviews, 33, 218–234.
- Savva, C.G., Costa, S.P.F.d., Bokori-Brown, M., Naylor, C.E., Cole, A.R., Moss, D.S., Titball, R.W. & Basak, A.K. (2013). Molecular architecture and functional analysis of NetB, a pore-forming toxin from Clostridium perfringens. Journal of Biological Chemistry, 288, 3512–3522.
- Shang, Y., Kumar, S., Oakley, B. & Kim, W.K. (2018). Chicken gut microbiota: importance and detection technology. Frontiers in Veterinary Science, 5, 254.
- Shimizu, T., Yaguchi, H., Ohtani, K., Banu, S. & Hayashi, H. (2002). Clostridial VirR/VirS regulon involves a regulatory RNA molecule for expression of toxins. Molecular Microbiology, 43, 257–265.
- Singh, R.P., Okubo, K., Ohtani, K., Adachi, K., Sonomoto, K. & Nakayama, J. (2015). Rationale design of quorum-quenching peptides that target the VirSR system of Clostridium perfringens. FEMS Microbiology Letters, 362, doi.org/10.1093/femsle/fnv188
- Tilley, S.J., Orlova, E.V., Gilbert, R.J.C., Andrew, P.W. & Saibil, H.R. (2005). Structural basis of pore formation by the bacterial toxin pneumolysin. Cell, 121, 247–256.
- Traber, K. & Novick, R. (2006). A slipped-mispairing mutation in AgrA of laboratory strains and clinical isolates results in delayed activation of agr and failure to translate δ- and α-haemolysins. Molecular Microbiology, 59, 1519–1530.
- Uzal, F.A., Freedman, J.C., Shrestha, A., Theoret, J.R., Garcia, J., Awad, M.M., Adams, V., Moore, R.J., Rood, J.I. & McClane, B.A. (2014). Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiology, 9, 361–377.
- Vidal, J.E., Chen, J., Li, J. & McClane, B.A. (2009). Use of an EZ-Tn5-based random mutagenesis system to identify a novel toxin regulatory locus in Clostridium perfringens Strain 13. PLoS ONE, 4, e6232.
- Wallinga, D., Smit, L.A.M., Davis, M.F., Casey, J.A. & Nachman, K.E. (2022). A review of the effectiveness of current US policies on antimicrobial use in meat and poultry production. Current Environmental Health Reports, 9, 339–354.
- Yan, X.-X., Porter, C.J., Hardy, S.P., Steer, D., Smith, A.I., Quinsey, N.S., Hughes, V., Cheung, J.K., Keyburn, A.L., Kaldhusdal, M., Moore, R.J., Bannam, T.L., Whisstock, J.C. & Rood, J.I. (2013). Structural and functional analysis of the pore-forming toxin NetB from Clostridium perfringens. MBio, 4, e00019–13.
- Yang, W.-Y., Lee, Y., Lu, H., Chou, C.-H. & Wang, C. (2019). Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS One, 14, e0205784.
- Yegani, M. & Korver, D.R. (2008). Factors affecting intestinal health in poultry. Poultry Science, 87, 2052–2063.
- Yu, Q., Lepp, D., Gohari, I.M., Wu, T., Zhou, H., Yin, X., Yu, H., Prescott, J.F., Nie, S.-P., Xie, M.-Y. & Gong, J. (2017). The Agr-like quorum sensing system is required for pathogenesis of necrotic enteritis caused by Clostridium perfringens in poultry. Infection and Immunity, 85, e00975-16.