ABSTRACT
Mortality of chicken embryos and first-week chickens was reported in a commercial incubator company in Costa Rica. Six 1-day-old Cobb chickens and twenty-four embryonated chicken eggs were examined in the Laboratory of Avian Pathology and the Laboratory of Bacteriology of the National University of Costa Rica. Twelve dead-in-shell embryos showed maceration and were immersed in a putrid, turbid, slightly thick brown liquid. Additionally, the other 12 embryonated eggs had milky yellow-orange content. The livers of those embryos had congestion, haemorrhages and multifocal cream foci of necrosis. Granulocytic infiltration was observed in the bursa of Fabricius, myocardium, liver, lung and kidney. Livers and egg yolks from six embryonated chickens and all 1-day-old chickens were aseptically collected and cultured. In addition, tissues from six better conserved embryos and all 1-day-old chickens were fixed in buffered formalin and embedded in paraffin. Biochemical and molecular tests identified Comamonas testosteroni as the cause of the early, middle and late embryo mortality. As all the eggshells from the sampled embryonated eggs were dirty with soiled a fecal matter, contamination after manipulating the eggs was considered the source of infection. C. testosteroni is an environmental microorganism that has rarely been reported to cause human disease. To our knowledge, this is the first report of C. testosteroni causing mortality in a hatchery. Cleaning and disinfection using ozone were implemented in the hatchery to eliminate the embryo mortality associated with C. testosteroni.
Introduction
Bacterial contamination of the eggshell surface can produce embryo mortality and affect the viability of neonatal chickens. The origin of these bacteria and their possible invasion depend on environmental factors in the housing system, the flock of origin, the moment during the lay period, and the quality of the eggshell (Trudeau et al., Citation2020). Bacteria of four phyla, including Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes, are part of the microbiome found in faecal samples of broiler breeders and in eggshell samples (Trudeau et al., Citation2020). Specifically, potentially pathogenic bacteria have been identified in faeces and eggshells, such as Escherichia coli (Cook et al., Citation2003; Karunarathna et al., Citation2017, Citation2022; Ivan et al., Citation2023), Enterococcus spp. (E. faecalis and E. faecium) (Karunarathna et al., Citation2022), Micrococcus spp. (Cook et al., Citation2003; Trudeau et al., Citation2020), Bacillus cereus (Cruz-Facundo et al., Citation2022), Salmonella spp. (Salmonella Enteritidis) (Gantois et al., Citation2009), Campylobacter jejuni, Pseudomonas aeruginosa, Streptococcus spp., Staphylococcus spp. (S. epidermidis, S. haemolyticus, S. xylosus, S. scuiri, S. aureus), Proteus vulgaris, Klebsiella pneumoniae, Enterobacter spp., Citrobacter freundii (Orajaka & Mohan, Citation1985; Cook et al., Citation2003; Kalita et al., Citation2013; Babaca, Citation2014; Amer et al., Citation2017; Hananeh et al., Citation2021), and Mycoplasma spp (Orajaka & Mohan, Citation1985). In addition to faecal matter, bacteria can emerge from the farm litter, dust, and air (Quarles et al., Citation1970; Gentry & Quarles, Citation1972; Huneau-Salaün et al., Citation2010; Bindari et al., Citation2021). Bacterial contamination can also occur in the hatcher and the vaccination room when in ovo vaccination is applied (Kim & Kim, Citation2010; Karunarathna et al., Citation2017, Citation2022; Projahn et al., Citation2017).
Chicken embryo mortality can occur in early, middle, or late development, although early and late mortality is more common (Hananeh et al., Citation2021). Bacteria such as E.coli, Streptococcus spp., Staphylococcus spp., Pseudomonas spp. (Orajaka & Mohan, Citation1985; Babaca, Citation2014; Hananeh et al., Citation2021), Enterococcus faecalis (Karunarathna et al., Citation2017, Citation2022), Salmonella spp., Citrobacter spp., Proteus spp., Campylobacter spp., Klebsiella spp. (Klebsiella variicola) (Amer et al., Citation2017; Hananeh et al., Citation2021), Bacillus cereus, Proteus vulgaris, Klebsiella pneumoniae (Hananeh et al., Citation2021) and Klebsiella variicola (Shen et al., Citation2021) have been described as cause of embryo mortality and yolk sac infections (Orajaka & Mohan, Citation1985; Babaca, Citation2014; Amer et al., Citation2017; Hananeh et al., Citation2021; Shen et al., Citation2021; Karunarathna et al., Citation2022). E. coli and Enterococcus spp., alone or in association, are the most frequent cause of embryo mortality, producing a significant economic impact on poultry producers (Karunarathna et al., Citation2022). Other causes of embryo mortality include genetic or environmental factors, including mistakes in incubation conditions, nutritional deficiencies, and malformations (Hananeh et al., Citation2021).
It is worth noting that specific microbial populations on the eggshell surface, for microorganisms acquired during oviposition, could have a detrimental impact on poultry performance and public health (Trudeau et al., Citation2020). Therefore, all poultry farms and hatcheries must have a rigorous sanitation programme to reduce microbial pathogens. This programme includes the treatment of eggs with disinfectants before incubation and cleaning and disinfection of all surfaces which the eggs will contact (Walker et al., Citation2002; Olsen et al., Citation2017). Overlooking these measurements carries the risk of the proliferation of pathogenic bacteria (Olsen et al., Citation2017) and the emergence of pathogens that could cause embryo mortality, egg yolk infection, omphalitis, and other pathologies.
In this article, Comamonas testosteroni is described for the first time as a cause of contamination of chicken embryos. C. testosteroni is a Gram-negative, aerobic environmental bacterium that colonizes different kinds of surfaces and also forms biofilm (Wu et al., Citation2015). C. testosteroni was previously classified as Pseudomonas testosteroni (Tamaoka et al., Citation1987). C. testosteroni is rarely described as a human pathogen. However, it can behave as an aggressive, opportunistic pathogen causing nosocomial and community-acquired infections associated with the contamination of medical devices (Bayhan et al., Citation2013; Duran et al., Citation2015; Farooq et al., Citation2017; Tiwari & Nanda, Citation2019). Besides, this pathogen has acquired increased antibiotic resistance (Duran et al., Citation2015; Tiwari & Nanda, Citation2019). This report aims to describe C. testosteroni as a cause of chicken embryo mortality and show the gross and microscopic findings associated with its colonization in embryonated eggs. The measures to reduce mortality are also discussed.
Materials and methods
Case description
Six 1-day-old Cobb chickens and 24 embryonated eggs were received in the Laboratory of Avian Pathology due to an 11.8% reduction in the hatching percentage in a commercial incubator company located in the town of San Mateo (Alajuela, Costa Rica). The hatchery had 1,068,891 eggs; the percentage of infertility was 7.5%. The percentage of early mortality was 6.7%, middle mortality 1.4% and late mortality was 3.7%. Mortality in 1-day-old chickens was 3.2%.
Evaluation of embryonated eggs and newborn chickens
To collect samples, embryonated eggs were swabbed with 70% alcohol and opened, cutting the air cell and inner membranes, in a gas extraction hood using a Bunsen burner. After opening the shell, all egg contents were put in a sterile Petri dish; samples of the yolk sac, liver, or the complete embryo (if maceration was noted) were selected and placed in a sterile plastic bag. Dead embryos were classified according to their developmental stage, as follows: early mortality was embryos of 0–7 days of incubation that were in decomposition (stage 1–30), middle mortality was embryos of 8–14 days that showed feather tracts (stage 31–41), and late mortality was embryos of 15–21 days which had eyelids and comb, and occupied almost all the space within the egg (stage 42–46) (Bellairs & Osmond Citation2014; Hananeh et al., Citation2021). In 12 chicken embryos, where maceration was noted, the putrid turbid, slightly thick brown liquid surrounding the embryo was collected using a syringe and put in a plastic bag. In the other 12 almost completely developed embryonated eggs, where the celomatic cavity was still open, the yolk sacs and the livers were collected in a sterile plastic bag and sent to the Laboratory of Bacteriology of the National University of Costa Rica. Six neonatal chickens (euthanized by cervical dislocation) and six dead-in-shell chicken embryos that did not have evidence of putrefaction and were better conserved were necropsied, and samples of tissues were collected to fix in buffered formalin.
Sample collection
Tissue samples of the bursa of Fabricius, cartilage, brain, heart, liver, gizzard, eyes, skin, kidney, skeletal muscle and lung of embryonated eggs that were better conserved were fixed in buffered formalin and embedded in paraffin. Additionally, samples of the spleen, bursa of Fabricius, heart, liver, intestine and lung of all culled chickens were collected and evaluated. Tissues were trimmed at 3 µm and stained with haematoxylin-eosin (H&E) and Gram Twort. Photographs were taken using an Olympus trinocular microscope BX53 and a DF73 digital camera using a CellSens Entry CS Photography Program.
Ethical statement
Most of the samples corresponded to dead-in-shell embryonated chicken eggs. The 1-day-old chickens were euthanized following procedures approved by “Programa Nacional de Bienestar Animal” in “Programa para el sacrificio humanitario de aves que representen riesgo sanitario” PN-BA-PG-001.
Culture and identification of the isolates
Egg contents from 12 macerated embryonated eggs and 12 liver and egg yolk samples were sent to the Laboratory of Bacteriology. After collection, samples were directly cultured onto Blood Agar (5% sheep blood) (Becton Dickinson, East Rutherford, NJ, USA), MacConkey agar (Becton Dickinson), and mannitol salt agar (Becton Dickinson) and incubated for 24–48 h under aerobic conditions (5% CO2) at 35°C (Markey et al., Citation2013). Pure isolated colonies were stained with Kopeloff-modified Gram staining (Rodríguez-Cavallini et al., Citation2016). Biochemical identification was performed using the automated Vitek 2 System (bioMérieux, Marcy l'Etoile, France) with the GN ID card to identify Gram-negative bacilli following manufacturer instructions and procedures.
Embryo lethality assay
The survival curve of chicken embryos inoculated with C. testosteroni was evaluated following the protocol described by Andersson et al. (Citation2015), with minor modifications. Briefly, groups of 12, 9-day-old specific pathogen-free chicken eggs (LANASEVE, MAG, Heredia, Costa Rica) were inoculated via the allantoid cavity using a dose of 102 or 105 CFU of C. testosteroni or E. coli. The E. coli isolate used was previously obtained from the pericardium of broiler chickens affected with colibacillosis. A negative control group inoculated with saline solution was also included. Both bacteria were preserved in a cryotube and thawed to culture in MacConkey agar and blood agar plates, as previously described (Markey et al., Citation2013). The inoculum was prepared in sterile saline solution.
Unfertile eggs were removed after candling, and those with live embryos were disinfected three times using iodine and alcohol. Eggs were inoculated with a sterile syringe into the allantoic cavity and returned to the hatchery. Eggs were candled 2 h post-inoculation (hpi) and later every 1 day post-inoculation (dpi) until 3 dpi to record their mortality. The embryo mortality rate was calculated as the mean percentage of embryo deaths. All embryonated eggs were evaluated (Supplementary Figure 1), and liver embryo samples were cultured using MacConkey agar and Blood agar plates and incubated overnight at 37°C to reisolate the bacteria. Data were analyzed, and a survival curve was produced using GraphPad Prism (8.0.1 version).
Bacteria isolates were subcultured on blood agar and incubated for 24–48 h at 35°C. Single colonies were suspended in 50 µl of PCR water and heated at 95°C for 20 min. The suspension was centrifuged at 9390× g/5 min. The supernatant was used as a DNA template. The GoTaq Green Master Mix (Promega, Madison, WI, USA) was used to set up the PCR, following the manufacturer’s instructions, using 1.5 µl of DNA and 0.4 µM of the 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′- TACGGYTACCTTGTTACGACTT -3) universal primers (Lane Citation1991). The reaction mixtures were incubated for 5 min at 95°C. Then, 35 cycles were carried out as follows: 30 s at 95°C, 30 s at 55°C and 1 min 30 s at 72°C, with a final extension of 10 min at 72°C. The PCR products were purified with the QIAQuick Gel Extraction Kit (QIAGEN, Germantown, MD, USA). Sequencing was carried out at Macrogen (Macrogen Inc, Seoul, South Korea), following established methodologies using 27F and 1492R primers. The 16S rRNA partial gene sequence alignment and consensus sequence were generated using EMBOSS Cons (EMBOSS EBI web services https://www.ebi.ac.uk/Tools/msa/emboss_cons/#). After alignment, the consensus sequence was compared to nucleotide sequences found in BLAST (http://www.ncbi.nlm.nih.gov/BLAST/).
Results
Evaluation of embryonated eggs and neonatal chickens
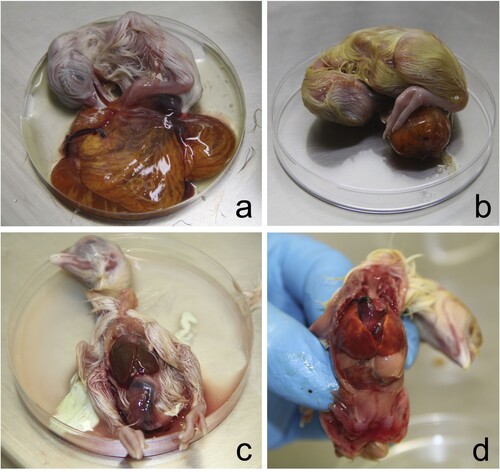
All 1-day-old chickens were huddled and showed ruffled feathers. In necropsy, hepatomegaly with congestion and green-brown discolouration of the yolk sacs was noted (). Faeces and white urates over the eggshell were noted in some of the eggs examined (c). Embryo mortality included 12 chickens, 10 of 2–3 cm, that were macerated or putrefied (early embryo mortality) (d) and two 5–6 cm embryos whose content was putrefied but had no maceration (e). Another 12 were almost completely developed but died, and were classed as late mortality (f). Macerated embryos contained abnormal orange-brown turbid content with white clumps or threads of membranes or egg white and a putrid odour (a,d). Late embryo mortality chickens showed congestion of yolk membranes with orange-brown content (c,f). The coelomic cavity was still open, even after 21 days of incubation. Hepatomegaly, with foci of necrosis, was observed in one chicken, and coalescent multifocal haemorrhages were seen in three chickens (e,f).
Figure 1. One-day-old chickens showed hepatomegaly and brown (a) to green (b) discolouration of the yolk sac.
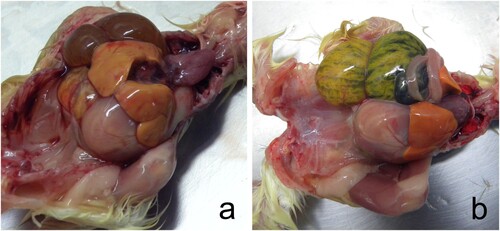
Figure 2. Abnormal egg content consisted of early mortality embryos in different states of decomposition (a, d), middle mortality embryos showing congestion (b, e), and late mortality embryos whose celomatic cavity was open at 21 days of incubation, and the yolk sac was congested (c, d). Orange-brown viscous liquid with a putrid odour was observed in early and middle mortality embryos (a, b, d, e). In late mortality chickens, the coelomic cavity was still open at 21 days of incubation, and the egg yolk was congested with brown content.
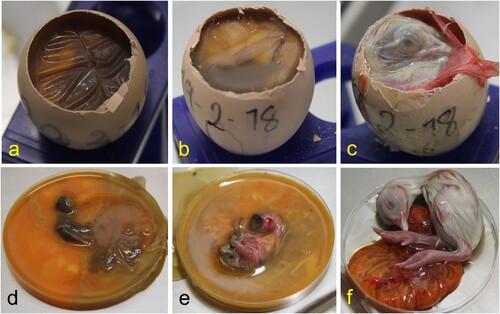
Figure 3. Chicken embryos with late mortality showed brown-orange discolouration of the yolk sac (a, b). The same chicken embryos showing hepatomegaly with congestion (c, d) and multifocal white-spotted foci of necrosis (c) and multifocal coalescent ecchymotic haemorrhages (d).
Tissues from six embryonated eggs were evaluated histologically. Heterophilic perivascular inflammation and heamorrhages were observed in the integument, bursa of Fabricius, pericardium, brain and lung (). Lymphoid depletion was found in the spleen and the bursa of Fabricius. Calcification was observed in the kidney. Aggregates of Gram-negative bacilli bacteria were seen in the brain, liver, gizzard, eye, pericardium, and over the skin surface. In addition, scattered colonies of bacteria and cell debris were observed covering the integument and in the brain ventricles, heart, eye, liver parenchyma, and in the parietal peritoneum in the coelomic cavity (). Slight granulocytic infiltration in the bursa of Fabricius was the only lesion observed in neonatal chickens.
Figure 4. Histological sections of the late mortality embryos showing congestion and interstitial granulocytic infiltration in the bursa of Fabricius (a), kidney (b), liver (c), and the lung (d) (haematoxylin & eosin).
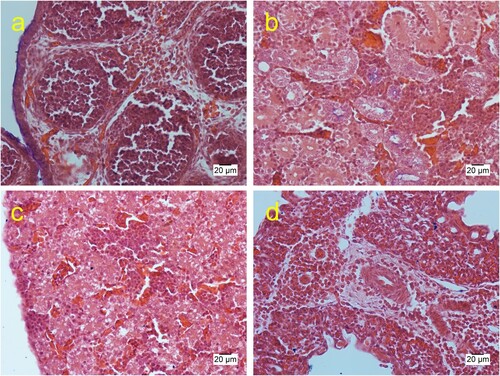
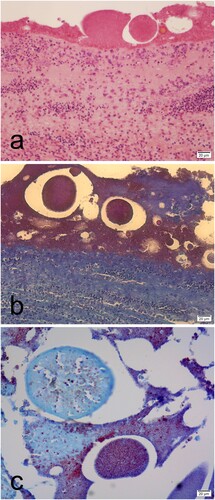
Figure 5. Histological sections of the embryo showing granular deposits around the integument embryo that stain eosinophilic with haematoxylin & eosin (a) and granular dark red with Gram Twort (b, c). Granular deposits with macrophage infiltrations were observed in the integument (a). Detail of the integument showed a feather surrounded by granular deposits stained dark red with Gram Twort (c).
Culture and identification of the isolates
After incubation, pure small colonies (1–2 mm in diameter) were obtained from the 12 macerated embryonated eggs and 12 liver and egg yolk samples cultured in blood agar and MacConkey agar. No growth was observed in mannitol salt agar. Growth was observed at 24 h, but colonies were better noted after 48 h. Colonies were nonpigmented, translucent, slimy, shiny, and creamy in appearance. In addition, colonies were non-haemolytic on blood agar and non-lactose-fermenting MacConkey agar. Gram staining showed typical Gram-negative bacilli. Biochemical identification with Vitek 2 system indicated C. testosteroni. 16S rRNA gene sequencing was also performed to confirm biochemical identification. BLAST analyses of the consensus sequence showed 98% coverage and 99.10% identity to the Comamonas genus. When the sequence was specifically compared to C. testosteroni sequences available in the NCBI database, the coverage was 98% and the identity 96.16% (online Supplementary Figure 2). These 16S rRNA sequencing results show that these isolates belong to the genus Comamonas. However, due to limitations of highly similar sequences in 16S rRNA, a more resolutive technique (such as whole genome sequencing) could be used to confirm the species by a molecular method.
Embryo lethality assay
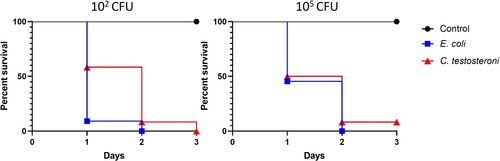
Embryo mortality rate results for E. coli and C. testosteroni are shown in . Both bacteria caused mortality at 1-day post-inoculation. C. testosteroni, compared E. coli, caused less mortality with 102 CFU, but mortality was similar when inoculated with a dose of 105 CFU. Additionally, survival was recorded with both doses of C. testosteroni at 3 dpi. Pure cultures were reisolated for each E. coli and C. testosteroni group. No mortality was observed in the control group. Lesions observed in the embryos were similar between E. coli and C. testosteroni, and consisted of oedema, congestion, and haemorrhages (online Supplementary Figure 1).
Discussion
Embryonated egg contamination and early post-hatching mortality were reported in a commercial farm in Costa Rica. The bacteriological examination of all dead-in-shell embryos, but not in 1-day-old chickens, confirmed that embryo mortality was caused for C. testosteroni. Comamonas spp. is a natural inhabitant of the soil, wastewater (including effluents discharged by poultry industries), and water of ponds and rivers. Additionally, Comamonas spp. have been found in the nipple drinking system of commercial layer houses (Wan et al., Citation2021). Furthermore, Comamonas spp. have been reported as part of the animal intestinal microbiome, including chickens (Ryan et al., Citation2022; Zhang et al., Citation2022). In fact, in studies to evaluate its effects on poultry health and growth performance, Comamonas spp. was prevalent in chickens with low bodyweight (Zhang et al., Citation2022). In the present case, the possible source of contamination was not investigated at the site, although all farms used chlorinated water to avoid bacterial overgrowth.
As mentioned, an extensive list of bacteria is associated with egg contamination and embryo mortality. Most of these are Gram-positive bacteria that also are part of the typical chicken microbiome (Zhang et al., Citation2022). Besides, bacteria such as E. coli, Enterococcus spp. (E. faecalis and E. faecium) are also considered part of the eggshell microbiota (Landman et al., Citation1999). Commonly, E. coli and Enterococcus spp. are also related to gut disease and low performance in poultry (Zhang et al., Citation2022). E. coli represents the most important differential diagnosis, causing yolk sac infections, omphalitis and early mortality in chickens (Babaca, Citation2014; Rezaee et al., Citation2021; Karunarathna et al., Citation2022) while E. faecalis has been demonstrated in cases of arthritis after exposure through the egg albumen (Karunarathna et al., Citation2022). Besides, E. coli causes congenital abnormalities in the neck and beak of unhatched chicks (Hananeh et al., Citation2021). On the contrary, congenital abnormalities were not observed in this case. Similarly, bacteria such as Klebsiella spp., Streptococcus spp., Staphylococcus spp., and P. aeruginosa are frequently described as a cause of yolk sac infections and embryo mortality (Olsen et al., Citation2012; Amer et al., Citation2017; Hananeh et al., Citation2021). However, none of these bacteria were detected in the egg contents, yolk sacs and livers examined in this case. Nonetheless, we did not exclude the possibility that those bacteria could have been present over the egg surface, as the eggshell was not examined in this case. Nevertheless, during the evaluation of the present case, eggs were sprayed and swabbed with alcohol to avoid contamination during sample collection.
An embryo mortality assay confirmed that C. testosteroni could be pathogenic to the chicken embryo. Our results show that C. testosteroni caused mortality from 1 dpi. Survival was recorded at 3 dpi in one embryo inoculated with either dose of C. testosteroni. No previous studies assessed the chicken embryo mortality caused by C. testosteroni. On the other hand, the E. coli isolate used caused high mortality at 102 and 105 CFU from 1 dpi. Similarly, pathogenic E. coli isolates (APEC), assessed through an embryo mortality assay, usually showed high mortality early after infection with macroscopic lesions similar to that observed in this assay (Wooley et al., Citation2000; Rezaee et al., Citation2021).
Egg contamination can occur through penetration of the eggshell exposed to gut contents or faeces during oviposition, known as horizontal transmission. This contamination occurs because when the egg is laid, its temperature is warmer than the environment, causing the contraction of its contents and creating a negative pressure in the egg (Lock et al., Citation1992; Bruce & Drysdale, Citation1994). This temperature and inner pressure change allow environmental bacteria to invade and cause embryo mortality (Berrang et al., Citation1999). In addition, vertical transmission occurs by direct contamination of the egg before oviposition, when the reproductive tract is infected, and the yolk, albumen, eggshell membranes, and embryo are in contact with an infectious agent (Gantois et al., Citation2009). However, in this case, the breeding hens did not show a reduction in the production of eggs, the embryos did not have developmental anomalies, and many embryos did achieve full development. The only finding in 1-day-old chickens was slight granulocytic infiltration in the bursa of Fabricius. Many bacteria can be present in the reproductive tract without causing evident disease in the breeder hen, embryo, or chicks (Lee et al., Citation2019; Wen et al., Citation2021). Therefore, a detailed study of the hens’ microbiota within the reproductive, urinary, and intestinal tracts would be necessary to exclude the presence of C. testosteroni in this case and its vertical transmission.
Considering that the eggshells were dirty before spraying with alcohol, we suggest that the primary source of the bacteria was probably the poor management of the nest, inappropriate egg collection and selection, or deficient hatchery cleaning measures. Other studies have found that contamination in the hatchery after hatching the chicks was the main transmission route of bacteria such as E. coli (Projahn et al., Citation2017).
Microbial contamination of the egg, and embryo mortality, produces significant losses to poultry farms; consequently, good hatchery practices are essential to avoid embryo death or early chicken mortality (Agabou, Citation2009). Biosecurity in the hatchery was fundamental to solving the problem, starting with incubating only clean and dry eggs. In this case, a sanitation programme was also performed, thoroughly cleaning all surfaces (including the incubator) using a high-foam flushing detergent and ozone at 10 ppm for 30 min to reduce new infections. Studies about the use of ozone for disinfection of eggshell of hatching eggs demonstrated a reduction of the microbial counts (Braun et al., Citation2011; Melo et al., Citation2019); however, variable effects on the hatchability have been described (Melo et al., Citation2019; Wlazlo et al., Citation2020). For example, Wlazlo et al. (Citation2020) indicated that using ozone reduced hatching and increased mortality. Other hatchery disinfectants including ultraviolet light, hydrogen peroxide, and peracetic acid, are all effective, according to the literature (Melo et al., Citation2019, Citation2020; Wlazlo et al., Citation2020; Cassar et al., Citation2021). Additionally, there is an extensive range of disinfectants that have been studied with different results (Scott et al., Citation1993). In this case, ozone effectively reduced embryo mortality, and the clinical veterinarian has not described new outbreaks, adverse effects in the hatching results, or detrimental effects on the quality of 1-day-old chickens.
Supplemental Material
Download JPEG Image (1.5 MB)Supplemental Material
Download MS Word (144 KB)Acknowledgements
The authors thank Ricardo Mora-Cartin, DMV, PhD, for his helpful suggestions. We would like to thank Universidad Nacional, Costa Rica for providing financial support to publish the article.
Disclosure statement
The authors declare that this study was conducted in absence of any commercial or financial relationship that could represent a potential conflict of interest.
References
- Agabou, A. (2009). Air-borne bacterial contaminations in two broiler hatcheries in the North-East of Algeria. Veterinary World, 2, 49–50.
- Amer, M., Elbayoumi, K., Girh, Z., Mekky, H. & Rabie, N. (2017). A study on bacterial contamination of dead in shell chicken embryos and culled one day chicks. International Journal of Pharmaceutical and Phytopharmacological Research, 7, 5–11.
- Andersson, C., Gripenland, J. & Johansson, J. (2015). Using the chicken embryo to assess virulence of Listeria monocytogenes and to model other microbial infections. Nature Protocols, 10, 1155–1164.
- Babaca, Z. (2014). Epidemiological and bacteriological studies on dead-in-shell embryos. Journal of Veterinary Science and Technology, 5, 170.
- Bayhan, Gİ, Tanır, G., Karaman, I. & Ozkan, S. (2013). Comamonas testosteroni: an unusual bacteria associated with acute appendicitis. Balkan Medical Journal, 30, 447–448.
- Bellairs, R. & Osmond, M. (2014). Appendix II – Normal table of Hamburger and Hamilton (1951; 1992). In R. Bellairs & M. Osmond (Eds.), Atlas of Chick Development (pp. 603–621). London: Academic Press.
- Berrang, M.E., Cox, N.A., Frank, J.F. & Buhr, R.J. (1999). Bacterial penetration of the eggshell and shell membranes of the chicken hatching egg: a review. Journal of Applied Poultry Research, 8, 499–504.
- Bindari, Y.R., Moore, R.J., Van, T.T.H., Hilliar, M., Wu, S.-B., Walkden-Brown, S.W. & Gerber, P.F. (2021). Microbial communities of poultry house dust, excreta and litter are partially representative of microbiota of chicken caecum and ileum. PLoS One, 16, e0255633.
- Braun, P.G., Fernandez, N. & Fuhrmann, H. (2011). Investigations on the effect of ozone as a disinfectant of egg surfaces. Ozone: Science & Engineering, 33, 374–378.
- Bruce, J. & Drysdale, E.M. (1994). Trans-shell transmission. In R.G. Board & R. Fuller (Eds.), Microbiology of the Avian Egg, 1st edn (pp. 63–91). Boston, MA: Springer. https://doi.org/10.1007/978-1-4615-3060-2_4.
- Cassar, J.R., Bright, L.M., Patterson, P.H., Mills, E.W. & Demirci, A. (2021). The efficacy of pulsed ultraviolet light processing for table and hatching eggs. Poultry Science, 100, 100923.
- Cook, M.I., Beissinger, S.R., Toranzos, G.A., Rodriguez, R.A. & Arendt, W.J. (2003). Trans–shell infection by pathogenic micro–organisms reduces the shelf life of non–incubated bird’s eggs: a constraint on the onset of incubation? Proceedings of the Royal Society of London. Series B: Biological Sciences, 270, 2233–2240.
- Cruz-Facundo, I.M., Adame-Gómez, R., Vences-Velázquez, A., Rodríguez-Bataz, E., Muñoz-Barrios, S., Pérez-Oláis, J.H. & Ramírez-Peralta, A. (2022). Bacillus cereus in eggshell: enterotoxigenic profiles and biofilm production. Brazilian Journal of Poultry Science, 24, 1–10.
- Duran, A., Okur, F., Sahin, V., Uyar, I., Abacilar, A., Akpinar, M., Alayunt, E. & Ates, M. (2015). Comamonas testosteroni endocarditis in Turkey: a case report and review of the literature. International Medical Journal of Sifa University, 2, 44.
- Farooq, S., Farooq, R. & Nahvi, N. (2017). Comamonas testosteroni: is it still a rare human pathogen? Case Reports in Gastroenterology, 11, 42–47.
- Gantois, I., Ducatelle, R., Pasmans, F., Haesebrouck, F., Gast, R., Humphrey, T.J. & Van Immerseel, F. (2009). Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiology Reviews, 33, 718–738.
- Gentry, R. & Quarles, C. (1972). The measurement of bacterial contamination on egg shells. Poultry Science, 51, 930–933.
- Hananeh, W.M., Al-Natour, M.Q., Alaboudi, A.R., Abo-Shehada, M.N. & Bani Ismail, Z.A. (2021). Congenital abnormalities in dead-in-shell chicks associated with mixed bacterial infections. Heliyon, 7, e06272.
- Huneau-Salaün, A., Michel, V., Huonnic, D., Balaine, L. & Le Bouquin, S. (2010). Factors influencing bacterial eggshell contamination in conventional cages, furnished cages and free-range systems for laying hens under commercial conditions. British Poultry Science, 51, 163–169.
- Ivan, R., Karasova, D. & Crhanova, M. (2023). Microbiota of chickens and their environment in commercial production. Avian Diseases, 67, 1–9.
- Kalita, N., Pathak, N., Ahmed, M. & Saikia, G.K. (2013). Various causes related to dead-in-shell embryos of crossbred (PB-2 x Indigenous) chicken egg. Veterinary World, 6, 774–777.
- Karunarathna, R., Ahmed, K.A., Goonewardene, K., Gunawardana, T., Kurukulasuriya, S., Liu, M., Gupta, A., Popowich, S., Ayalew, L., Chow- Lockerbie, B., Willson, P., Ngeleka, M. & Gomis, S. (2022). Exposure of embryonating eggs to Enterococcus faecalis and Escherichia coli potentiates E. coli pathogenicity and increases mortality of neonatal chickens. Poultry Science, 101, 101983.
- Karunarathna, R., Popowich, S., Wawryk, M., Chow-Lockerbie, B., Ahmed, K.A., Yu, C., Liu, M., Goonewardene, K., Gunawardana, T., Kurukulasuriya, S., Gupta, A., Willson, P., Ambrose, N., Ngeleka, M. & Gomis, S. (2017). Increased incidence of enterococcal infection in nonviable broiler chicken embryos in Western Canadian hatcheries as detected by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Avian Diseases, 61, 472–480.
- Kim, J.H. & Kim, K.S. (2010). Hatchery hygiene evaluation by microbiological examination of hatchery samples. Poultry Science, 89, 1389–1398.
- Landman, W.J.M., Mekkes, D.R., Chamanza, R., Doornenbal, P. & Gruys, E. (1999). Arthropathic and amyloidogenic Enterococcus faecalis infections in brown layers: a study on infection routes. Avian Pathology, 28, 545–557.
- Lane, D. J. (1991). 16S/23S rRNA sequencing. In E. Stackebrandt & M. Goodfellow (Eds.), Nucleic Acid Techniques in Bacterial Systematic (pp. 115–175). New York: Wiley.
- Lee, S., La, T.-M., Lee, H.-J., Choi, I.-S., Song, C.-S., Park, S.-Y., Lee, J.-B. & Lee, S.-W. (2019). Characterization of microbial communities in the chicken oviduct and the origin of chicken embryo gut microbiota. Scientific Reports, 9, 6838.
- Lock, J.L., Dolman, J. & Board, R.G. (1992). Observations on the mode of bacterial infection of hens’ eggs. FEMS Microbiology Letters, 100, 71–73.
- Markey, B., Leonard, F., Archambault, M., Cullinane, A. & Maguire, D. (2013). Clinical veterinary microbiology. In R. Edwards & C. Hewat (Eds.), Clinical Veterinary Microbiology, 2nd edn (p. 920). Edinburgh: Mosby Elsevier.
- Melo, E.F., Clímaco, W.L.S., Triginelli, M.V., Vaz, D.P., de Souza, M.R., Baião, N.C., Pompeu, M.A. & Lara, L.J.C. (2019). An evaluation of alternative methods for sanitizing hatching eggs. Poultry Science, 98, 2466–2473.
- Melo, E.F., McElreath, J.S., Wilson, J.L., Lara, L.J.C., Cox, N.A. & Jordan, B.J. (2020). Effects of a dry hydrogen peroxide disinfection system used in an egg cooler on hatchability and chick quality. Poultry Science, 99, 5487–5490.
- Olsen, R.H., Frantzen, C., Christensen, H. & Bisgaard, M. (2012). An investigation on first-week mortality in layers. Avian Diseases, 56, 51–57.
- Olsen, R., Kudirkiene, E., Thøfner, I., Pors, S., Karlskov-Mortensen, P., Li, L., Papasolomontos, S., Angastiniotou, C. & Christensen, J. (2017). Impact of egg disinfection of hatching eggs on the eggshell microbiome and bacterial load. Poultry Science, 96, 3901–3911.
- Orajaka, L.J. & Mohan, K. (1985). Aerobic bacterial flora from dead-in-shell chicken embryos from Nigeria. Avian Diseases, 29, 583–589.
- Projahn, M., Daehre, K., Roesler, U. & Friese, A. (2017). Extended-spectrum-beta-lactamase- and plasmid-encoded cephamycinase-producing enterobacteria in the broiler hatchery as a potential mode of pseudo-vertical transmission. Applied and Environmental Microbiology, 83, e02364–16.
- Quarles, C., Gentry, R. & Bressler, G. (1970). Bacterial contamination in poultry houses and its relationship to egg hatchability. Poultry Science, 49, 60–66.
- Rezaee, M.S., Liebhart, D., Hess, C., Hess, M. & Paudel, S. (2021). Bacterial infection in chicken embryos and consequences of yolk sac constitution for embryo survival. Veterinary Pathology, 58, 71–79.
- Rodríguez-Cavallini, E.R., Gamboa-Coronado, M. del M., López-Ureña, D., Quesada-Gómez, C. & Rodríguez-Sánchez, C. (2016). In Bacteriología general: Principios y prácticas de laboratorio, 2nd edn. San José: Editorial Universidad de Costa Rica.
- Ryan, M.P., Sevjahova, L., Gorman, R. & White, S. (2022). The emergence of the genus Comamonas as important opportunistic pathogens. Pathogens, 11, 1032.
- Scott, T.A., Swetnam, C. & Kinsman, R. (1993). Screening sanitizing agents and methods of application for hatching eggs III. Effect of concentration and exposure time on embryo viability. Journal of Applied Poultry Research, 2, 12–18.
- Shen, X., Yin, L., Ma, H., Pan, X., Zhang, D., Zhao, R., Dai, Y., Hou, H. & Hu, X. (2021). Comprehensive genomic analysis and characterization of a new ST 174 type Klebsiella variicola strain isolated from chicken embryos. Infection, Genetics and Evolution, 90, 104768.
- Tamaoka, J., Duk-Mo, H. & Komagata, K. (1987). Reclassification of Pseudomonas acidovorans den Dooren de Jong 1926 and Pseudomonas testosteroni Marcus and Talalay 1956 as Comamonas acidovorans comb. Nov. And Comamonas testosteroni comb. Nov., with an emended description of the genus Comamonas. International Journal of Systematic and Evolutionary microbiology, 37, 52–59.
- Tiwari, S. & Nanda, M. (2019). Bacteremia caused by Comamonas testosteroni an unusual pathogen. Journal of Laboratory Physicians, 11, 087–090.
- Trudeau, S., Thibodeau, A., Côté, J.C., Gaucher, M.L. & Fravalo, P. (2020). Contribution of the broiler breeders’ fecal microbiota to the establishment of the eggshell microbiota. Frontiers in microbiology, 11, 666.
- Walker, S.E., Sander, J.E., Cheng, I.-H. & Wooley, R.E. (2002). The in vitro efficacy of a quaternary ammonia disinfectant and/or ethylenediaminetetraacetic acid-tris against commercial broiler hatchery isolates of pseudomonas aeruginosa. Avian Diseases, 46, 826–830.
- Wan, Y., Ma, R., Chai, L., Du, Q., Yang, R., Qi, R., Liu, W., Li, J., Li, Y. & Zhan, K. (2021). Determination of bacterial abundance and communities in the nipple drinking system of cascading cage layer houses. Scientific Reports, 11, 19169.
- Wen, C., Li, Q., Lan, F., Li, X., Li, G., Yan, Y., Wu, G., Yang, N. & Sun, C. (2021). Microbiota continuum along the chicken oviduct and its association with host genetics and egg formation. Poultry Science, 100, 101104.
- Wlazlo, L., Drabik, K., Al-Shammari, K.I.A., Batkowska, J., Nowakowicz-Debek, B. & Gryzińska, M. (2020). Use of reactive oxygen species (ozone, hydrogen peroxide) for disinfection of hatching eggs. Poultry Science, 99, 2478–2484.
- Wooley, R.E., Gibbs, P.S., Brown, T.P. & Maurer, J.J. (2000). Chicken embryo lethality assay for determining the virulence of avian Escherichia coli isolates. Avian Diseases, 44, 318–324.
- Wu, Y., Arumugam, K., Tay, M.Q.X., Seshan, H., Mohanty, A. & Cao, B. (2015). Comparative genome analysis reveals genetic adaptation to versatile environmental conditions and importance of biofilm lifestyle in Comamonas testosteroni. Applied Microbiology and Biotechnology, 99, 3519–3532.
- Zhang, X., Akhtar, M., Chen, Y., Ma, Z., Liang, Y., Shi, D., Cheng, R., Cui, L., Hu, Y., Nafady, A.A., Ansari, A.R., Abdel-Kafy, E.-S.M. & Liu, H. (2022). Chicken jejunal microbiota improves growth performance by mitigating intestinal inflammation. Microbiome, 10, 107.