ABSTRACT
The two most common animal-based indicators used to evaluate leg health in broiler chickens are footpad dermatitis (FPD) and gait scoring, but these indicators are less explored in broiler breeders. This study is the first to investigate FPD and gait scoring in broiler breeders during their lifespan from rearing to end of life. In total, eight flocks were monitored (four Ross and four Hubbard) at five different timepoints, in rearing (5 and 15 weeks of age), during the production period (25 and 45 weeks of age) and at the end of the production period (approximately 60 weeks of age). At each visit, 50 hens and 25 roosters were gait scored (six-point scale) and footpads from another 50 hens and 25 roosters were evaluated (five-point scale) (total n = 3000 breeders, 2000 hens and 1000 roosters). Litter quality and air quality were measured at each visit. The results showed that the overall prevalence of FPD in rearing was low and that it increased towards the end of the production, with a mean FPD score of 2 out of a maximum 4 in the hens, indicating moderate lesions and 1.5 in the roosters. In all houses, the litter was dry and loose. FPD was not related to the litter quality, but to air quality, especially the ammonia concentration (P < 0.001). Overall, the gait score were good, and increased with age in both hens (P < 0.001) and roosters of both hybrids (P < 0.001).
Introduction
Footpad dermatitis (FPD) and gait scores are the two most common animal-based indicators used to evaluate leg health in broiler chickens (Bassler et al., Citation2013). FPD, also called pododermatitis, hyperkeratosis or footpad lesions (De Jong et al., Citation2012), is a type of contact dermatitis that causes inflammation and necrotic lesions on the plantar surface of the footpads, often observed in broiler chickens and turkeys (Ekstrand et al., Citation1998; Shepherd & Fairchild, Citation2010; Opengart et al., Citation2018). The lesions start with discolouration of the skin, eventually followed by hyperkeratosis, severe erosions and necrosis (Kyvsgaard et al., Citation2013). FPD may range from superficial lesions to severe, deep necrotic ulcers on the footpads and toes (Shepherd & Fairchild, Citation2010). The associated pain may negatively affect the gait and reduce the bird’s mobility (Haslam et al., Citation2007; Sirri et al., Citation2007).
FPD in broiler chickens has been investigated since the 1980s (Greene et al., Citation1985). The prevalence varies between different studies, but the overall prevalence of FPD in fast-growing broiler flocks has been found to be high at slaughter age (Haslam et al., Citation2007; Bassler et al., Citation2013). Environmental factors, like moisture and particle size of the litter, stocking density, and ammonia irritation from the bedding, are thought to be important causative factors (Cengiz et al., Citation2011; Kyvsgaard et al., Citation2013; De Jong et al., Citation2014) and, as such, the footpad health reflects the environment in the barn and the farmer’s management. Due to welfare concerns, scoring of FPD is used as an animal-based indicator in the broiler industry in the EU where the feet are monitored postmortem at the abattoir for all broiler chicken flocks slaughtered commercially. The scoring system uses a scale based on lesion severity (Ekstrand et al., Citation1998). High flock scores will elicit sanctions on animal density in future production cycles in Norway, Denmark and Sweden. Scoring of footpad lesions is mandatory for all broiler chicken flocks slaughtered in the EU, but not for the broiler breeders and, therefore, available FPD data from commercial breeder production are scarce. In addition, compared to the literature on FPD in broiler chickens, available studies on FPD in broiler breeders are few, in terms of both prevalence and risk factors, but studies have shown that footpad health declines with the age of the breeders (Kaukonen et al., Citation2016; Thøfner et al., Citation2019). Since the breeders live approximately 55–60 weeks longer than the broiler chickens, it is of utmost importance to determine at which age FPD develops and how the footpad health develops and progresses through the life of the broiler breeders, from rearing to slaughter.
Gait scoring (GS) is a widespread method used to evaluate locomotion and lameness in broiler chickens. The method defines gait on a six-point scale (Kestin et al., Citation1992). GS has been used in broiler chickens for several decades, since it is an easy tool for leg health monitoring on farm. GS provides information about the way the bird moves, but cannot differentiate between the causes of gait abnormalities, which may be of both infectious and non-infectious aetiology (Opengart et al., Citation2018). Fast growth and high body weight are thought to be primary risk factors for poor locomotion (Knowles et al., Citation2008). Several studies indicate that modern fast-growing broiler chickens have a high prevalence of impaired locomotion, especially towards the end of the production period (Knowles et al., Citation2008; Kittelsen et al., Citation2017; Granquist et al., Citation2019). Despite the importance and widespread use of GS in broiler chickens, there are few studies available on GS in broiler breeders. This lack of information is striking, since the breeders have the same genetic growth potential, live longer but are raised to different weight and under different housing conditions and with restricted feeding during parts of their life. To the authors’ knowledge, such a life span analysis has never been conducted in broiler breeders, neither under experimental nor under commercial conditions, and will give important information about broiler breeder health and welfare.
The main aim of this study was to deliver descriptive information about the prevalence and severity of FPD and GS in broiler breeders at different time points through their life span, from early rearing to slaughter age. Additional objectives were to assess risk factors for FPD in the broiler breeder and to investigate the potential relationship between FPD and GS.
Materials and methods
Animals and housing
The data were collected from spring 2022 to autumn 2023 in Norwegian broiler breeder flocks. The study population consisted of a total of eight rearing flocks (Ross 308, n = 4 and Hubbard JA757, n = 4) and the eight production flocks (Ross 308, n = 4 and Hubbard JA757, n = 4) they were transferred to. The flocks were visited five times; at 5, 15, 25 and 45 weeks of age (WOA), and finally close to the time of slaughter (range: 55–62 WOA). All flocks were kept in enclosed, heated and environmentally controlled houses. Management practices followed the recommendations from the breeding companies and Norwegian regulations. The main difference between the feed regimes for the two hybrids was that feed was more restricted for the Ross 308 hens during rearing.
The non-beak-trimmed day-old chicks arrived at the rearing farm straight from the hatchery. In the rearing barns, the pullets and cockerels were housed in different compartments in the same barn, separated by netting walls or in separate rooms. The floors of all barns and compartments were covered with fresh wood shavings as litter material. The number of pullets placed ranged from 6997 to 9682, equalling an animal density of 8–10 birds per m2 at 5 WOA. The number of cockerels placed ranged from 660 to 2186, equalling an animal density of 4–12 birds per m2 at 5 WOA. Hours with light per day ranged from 8-13 h, depending on the age and according to the breeder manual. Light intensity during light hours was 4–8 at week 5, and 5–28 at 15 WOA, measured at animal height with a luxometer (Extech LED meter LT40, FLIR Commercial Systems Inc., Nashua, NH, USA). The light programme differed between the breeds; both followed their breeding manuals for their age. All rearing flocks were fed commercial, pelleted feed using a spin-feeder once per day, ranging from 40–50 g/bird at 5 WOA for hens and 60–65 g/bird for the roosters. At 15 WOA, the hens were fed 61–86 g/day and the roosters 85–95 g/day. The roosters were transferred to the production barns at 17 WOA, and the hens at 18 WOA. The average live weight for Hubbard hens at 18 WOA was 1680 g, while the corresponding weight for Ross 308 hens was 1950 g, as expected because of the genetic differences between the hybrids.
All breeder flocks consisted of approximately 7500 placed hens (range: 6980–7566) and 650 placed roosters (range: 600–803), kept in the same house. The barn size varied from 1230 to 1600 m2. Mean animal density was 5.85 birds/m2 (range 5.0–6.6, average for the Ross flocks: 5.25, average for the Hubbard flocks: 6.45) at week 25. All production barns were of the same design; fully insulated, with an identical mechanical ventilation system, no windows, and concrete floor with fresh wood shavings, elevated slats (height: 60 cm) with nest boxes, and round, metal perches on the slats. The elevated slats were approximately 2.4 m in width (range: 1.2–5.0 m) and covered a mean area of 500.2 m2 (range: 144.0–948.0 m2). This constituted a mean 38.3% of the area in the barn (range: 11.6–74.4%). The light regime included 8 h of darkness per day, and lux during light periods varied from 5–30 in different barns at 25 WOA, measured at animal height with a luxometer. All flocks were fed commercial pelleted feed. Rooster feed lines were situated in the litter area and hen feed lines on the slatted area. Food was provided 2–4 times per day, in an amount according to the management guide of the breeding companies, ranging from 115–135 g per day for the hens and from 93–150 g for the roosters. The amount varied with age and with hybrid. Water was provided from 8:30 to 12:30 h and from 15:30 to 16:00 h via drinking nipples. The birds were either culled on-farm or slaughtered at a commercial poultry abattoir at the age of 55–62 weeks.
Sampling methods
Footpad investigation
FPD was investigated at five different times for each flock (5, 10, 25, 45 WOA and prior to slaughter/culling (range: 55–62 WOA)). During each visit, a random selection of 50 hens and 25 roosters per flock were examined. The birds were selected systematically from both the slatted area and from the litter throughout the entire barn at predefined points. The observer scored the bird to the left from the first bird, at the designated points. Assessment of the footpads was based on the presence of visually macroscopic lesions on live birds and scored on a five-point scale () according to the Welfare Quality Assessment Protocol for Poultry (Welfare Quality, Citation2009). The surface area of both footpads was examined after brushing off litter and faecal material with a semi-hard brush. In cases of discrepancy between the footpads, the highest score was recorded for that bird. A score 0 represents no evidence of FPD, scores 1 and 2 represent small to moderate evidence of FPD, while scores 3 and 4 represent evidence of severe FPD, according to the Welfare Quality Assessment Protocol for Poultry (Welfare Quality, Citation2009; see ).
Figure 1. Scoring of FPD according to the Welfare Quality Assessment Protocol (Welfare Quality, Citation2009). © A Butterworth, University of Bristol.

Gait scoring
Walking ability was investigated by the same observer at four different times for each flock (5, 10, 25 and 45 WOA). During each visit, a random selection of 50 hens and 25 roosters per flock was investigated. The birds were selected at designated points in the barn, from both the slatted area and from the litter, when the trained investigator walked slowly throughout the barn, in order to avoid resampling. The observer scored the bird to the left from the first bird encountered, at the designated points. Walking ability was evaluated using the six-point gait scoring scale as described by Kestin et al. (Citation1992), see . Scoring of individual broilers took between 5 and 30 s. Birds that did not walk away within approximately 30 s were encouraged to walk by slowly walking behind them.
Table 1. Explanation of the gait score criteriaa.
Litter
In all barns, both rearing and production, the bedding consisted of fresh wood shavings, approximately 5–15 cm deep in different areas of the barn. The litter was assessed at six different places. The litter quality was recorded according to the score described in the Welfare Quality Assessment Protocol for Poultry (Welfare Quality, Citation2009) ranging from 0 for dry and flaky litter to 4 for solid litter covered with a crust ().
Table 2. Scoring of litter quality according to the Welfare Quality Assessment Protocol.
At all five visits, carbon dioxide was measured with a CO2 Meter (Extech) and ammonia was measured by Dräger Pac 8000 (©Drägerwerk AG & Co., Lübeck, Germany).
Statistical analysis
Statistical analyses were performed using the software SAS 9.4. The statistical unit was flock by hybrid by age. The eight flocks followed in the study were each visited at five different ages, giving a total of 40 flock visits (20 flock visits for each hybrid). Unfortunately, two of the visits could not be performed due to COVID/illness. Therefore, the final total of flock visits was 38. The data from the individual assessment of FPD and GS were averaged for each sex per flock per age, calculated from the 50 hens and 25 roosters assessed in each flock at each age. The effects of hybrid and age on the FPD and GS were investigated for each sex using the mixed procedure model, which included hybrid, age and their interaction as fixed factors and flock ID as a random factor. The data fit the model assumptions, e.g. normal distribution of the residuals. Post-hoc analyses were performed with the Tukey test (Tukey's honestly significant difference (HSD) test).
The relationships between FPD and GS and the investigated risk factors measured on the farm were assessed using Pearson correlations. The risk factors included in the analyses were: light intensity (Lux), ammonia concentration (ppm), carbon dioxide concentration (ppm), hen mortality (%), density in the production farm (birds per m2), slatted area (% of total floor area) and width of the slats (m). Since the litter was always dry and loose, it did not have enough variability to be included in the data analysis. Due to the low numbers of flocks per hybrid per age, age was not included as a factor in the analyses of correlations. Nevertheless, hybrid was taken into account by running separate correlations for each hybrid.
Results
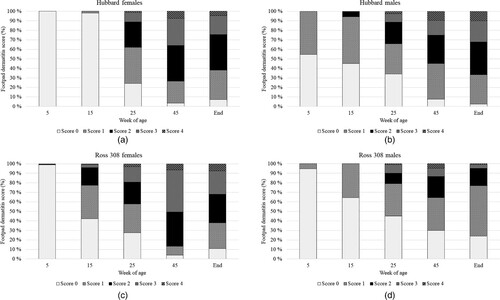
The FPD distributions over age, hybrid and gender are presented in (A–D). Among the female birds, there was an interaction between hybrid and age on their scores for FPD (F4,22 = 2.97; P = 0.04). For the Ross 308 birds, these scores increased steadily with age, with significant differences observed already between weeks 5 and 15 of age (P < 0.05). In comparison, the scores of the Hubbard birds started increasing from 0 only after the second assessment, sometimes between 15 and 25 WOA, and continued to increase thereafter (P < 0.05). Furthermore, there was no significant difference between the hybrids at any of the ages (P > 0.05 after correction for multiple comparisons). For the male birds, there was no interaction between hybrid and age on FPD (F4,23 = 0.86; P = 0.50). However, there was an effect of hybrid (F1,6 = 15.48; P < 0.01), where Hubbard birds had higher FPD scores than Ross 308 birds (LS mean ± SE: Hubbard = 1.26 ± 0.11; Ross 308 = 0.68 ± 0.10). As expected, an effect of age was also found, with the score for FPD increasing with age from week 15 of age (F4,23 = 14.71; P < 0.0001).
Figure 2. (A) FPD scores in Hubbard hens at different timepoints. (B) FPD scores in Hubbard roosters at different timepoints. (C) FPD scores in Ross 308 hens at different timepoints. (D) FPD scores in Ross 308 roosters at different timepoints.
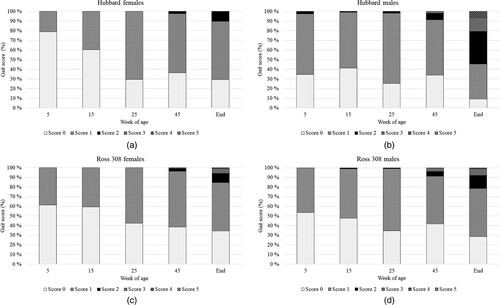
The GS distributions over age, hybrid and gender are presented in (A–D). Regarding the GS, there was no effect of hybrid on the score of the female birds (LS mean ± SE: Hubbard = 0.55 ± 0.04; Ross = 0.58 ± 0.04; F1,6 = 0.29; P = 0.61). The GS did, nevertheless, increase with age as expected (F4,22 = 15.45; P < 0.0001). For the males, however, there was an observed effect of hybrid, where the Hubbard birds had a higher overall GS (LS means ± SE: 0.91 ± 0.06) compared to the Ross birds (LS means ± SE: 0.67 ± 0.06; F1,4 = 7.96; P = 0.04). Across both hybrids, male GS remained relatively stable until 45 WOA and increased thereafter (F4,21 = 10.73; P < 0.0001).
Figure 3. (A) Gait scores in Hubbard hens at different time points. (B) Gait scores in Hubbard roosters at different timepoints. (C) Gait scores in Ross hens at different timepoints. (D) Gait scores in Ross roosters at different timepoints.
Including all ages, there was a strong positive correlation between the scores of FPD between male and female birds in each flock (Pearson correlation coefficient: 0.71; n = 38; P = < 0.0001) and between FPD and GS of female birds (Pearson correlation coefficient: 0.64; n = 38; P = < 0.0001). For the male birds, also within flocks, there was a medium positive correlation between FPD and GS (Pearson correlation coefficient: 0.59; n = 38; P = < 0.0001). In addition, there was a medium positive correlation between female GS and male GS (Pearson correlation coefficient: 0.56; n = 38; P = 0.0002).
Descriptive statistics for the risk factors for FPD assessed on farm in Ross 308 and Hubbard flocks are presented in and , respectively. Likewise, the correlations between the scores for FPD and GS and the on-farm risk factors are presented in and for Ross 308 and Hubbard birds, respectively.
Table 3. Descriptive statistics for the Ross 308 flocks.
Table 4. Descriptive statistics for the Hubbard flocks.
Table 5. Pearson correlation coefficient results for the Ross 308 flocks.
Table 6. Person correlation coefficient results for the Hubbard flocks.
Light intensity measured in lux was medium to strongly positively correlated to the scores of both hybrids and sexes. The only exceptions were the lack of correlation between light intensity and FPD of Ross hens and GS of Hubbard roosters. Litter quality was loose and dry in the production barns, all scores 0, and there was no correlation between litter quality and FPD. Likewise, in the rearing facilities, the litter was dry and loose (score 0) in the pullet compartments at both 5 and 15 WOA. However, in the cockerel departments, the litter was moist at both 5 and 15 WOA, with a medium score of 1.5. Air quality assessed in the concentrations of ammonia and carbon dioxide had medium to very strong positive correlations with FPD scores of Ross 308 birds of both genders. The scores of Hubbard birds, on the other hand, seemed less affected by CO2, but still showed medium to strong positive correlations with ammonia. Hen mortality did not correlate with the scores for FPD or gait of the hens of either hybrid. Stocking density, the number of birds per m2 in the production farm, showed medium to strong negative correlations with the gait of hens of both hybrids and the FPD scores of Ross hens. Finally, there was no observed correlation between the percentage area of the slats or the width of the slats on the leg health of male or female birds of either hybrid.
Discussion
The main aim of this study was to deliver descriptive information about the prevalence and severity of FPD and GS in broiler breeders, both hens and roosters, at different timepoints through their lifespan, from early rearing to slaughter. Based on previous broiler breeder research, we hypothesized that the incidence and severity of FPD would increase with age (Kaukonen et al., Citation2016; Thøfner et al., Citation2019; van den Oever et al., Citation2020). Our results showed that the prevalence of FPD was low in rearing (age 5 and 15 weeks) for hens of both hybrids, but slightly higher for the Ross hens compared to the Hubbard hens. Only mild lesions were observed in rearing. The scores in both hybrids increased steadily from 25 WOA, which is somewhat earlier than the reported findings (Kaukonen et al., Citation2016; Thøfner et al., Citation2019). From week 25 to the end of the production, the FPD results were similar for hens of both hybrids. At the last visit (55–62 WOA), the mean FPD score for the hens was 2, which is slightly lower than what was reported by Kaukonen et al. (Citation2016). The prevalence of FPD in roosters in rearing was slightly higher compared to the observed lesions in hens at the same age. Thereafter, the FPD in the roosters increased steadily from week 15 of age. Hubbard roosters had slightly higher FPD scores than Ross 308 roosters. During the last visit (55–62 WOA), the Ross roosters had better footpad scores than the hens at similar ages. To the authors’ knowledge, no published papers have investigated the development of FPD throughout the life of the roosters, thus no comparison with previous results can be made. Overall, the prevalence of FPD was surprisingly similar and generally low in all eight flocks, with few variations between birds of the same age in different flocks. Kaukonen et al. (Citation2016) found that 0–5.5% of birds had severe FPD at 19, 24 and 36 WOA, after which it significantly increased to 25% at the age of 48 weeks and increased further toward 64% at 60 WOA. In comparison, at weeks 55–62 in our study, 29.1% of the breeder hens and 17.6% of the roosters had severe FPD (i.e. a score 3 or 4). These are lower numbers compared to Kaukonen’s study, but still high enough to indicate that FPD is an important health and welfare problem at the end of lay for the broiler breeders, especially since severe lesions are associated with inflammation, infection and pain (Martland, Citation1984; Gentle, Citation2011; Sinclair et al., Citation2015; Weber Wyneken et al., Citation2015; Thøfner et al., Citation2019).
In addition to age, bodyweight is a known risk factor for FPD in broiler chickens (Shepherd & Fairchild, Citation2010), where light broilers are found to have significantly lower FPD and GS than heavier broilers (Opengart et al., Citation2018). Wolanski et al. (Citation2004) assessed the foot condition of 62–week-old broiler breeder roosters and found high bodyweight to have a negative effect on the footpad condition. This is in contrast to our results where no difference between hens of the two hybrids was observed, even though the Hubbard hens are lighter and smaller compared to the Ross 308 breeder hens (Hubbard, Citation2015; Ross, Citation2018). This finding is supported by results from other studies, finding footpad scores to be evenly distributed among different bodyweight classes in 62-week-old breeder hens (Renema et al., Citation2007). Furthermore, there was a hybrid difference in the roosters, even though roosters of the two hybrids are quite similar in size and weight (Hubbard, Citation2015; Ross, Citation2018). Due to feed restriction, the breeders grow slower than broiler chickens, perhaps making bodyweight less important in the aetiology of FPD. Unfortunately, we do not have individual weights for the birds assessed for FPD and GS. This should be investigated in future studies.
In the present study, at 55–62 WOA, 29.1% of the hens and 17.6% of the roosters had severe FPD (i.e. a score 3 or 4). These differences between hens and roosters are in line with results from Kapell et al. (Citation2012), who found that hens showed higher prevalence of FPD than roosters. Nagaraj et al. (Citation2007) have suggested that higher prevalence of FPD in hens is related to a lower content of collagen and protein in the skin of the hens compared to roosters, which may predispose them to skin injuries (Nagaraj et al., Citation2007). However, this is in contrast to other studies, where higher prevalence of FPD has been observed in roosters (Greene et al., Citation1985). This shows that the effect of gender on FPD in broiler breeders is still unclear and should be investigated further.
High moisture content in the bedding material may lead to the attachment of litter, and ammonia irritation to the feet (Kyvsgaard et al., Citation2013). Therefore, litter quality, specifically litter moisture and ammonia content, is listed as the major causative environmental factor for FPD (Martland, Citation1984; Mayne, Citation2005; van den Oever et al., Citation2020). The relationship between FPD and litter quality has been well established in studies from both broiler chickens and broiler breeders (Kaukonen et al., Citation2016; van den Oever et al., Citation2020). Overall, the litter quality in our study was good, with dry and loose litter scoring 0. However, during rearing the litter in all the cockerel compartments was moister (average score 1.5) than in the pullet compartments. This may explain why the FPD score in the cockerel flocks was higher than in the pullets. However, during the production phase, the litter was always dry and loose in all areas investigated, at all visits. This indicates that there are other factors besides litter quality that may affect footpad health in the broiler breeder barns. It is obvious that the litter quality is important for birds that spend most of their lives in direct contact with litter material, such as broiler chickens. However, in the breeder production barns that are common in Europe, the nest boxes and the feeder and water lines for the hens are placed on elevated slats. Providing slats has been suggested to benefit foot cleanliness and health (Brake, Citation1998), but it could also be speculated that these barn adaptations make the hens spend less time in the litter area, thus reducing contact with the litter. Consequently, the hens will defecate more on the slats, and faeces on the slats will not be absorbed by the litter. Hence, the faeces will remain on the slats until they dry out or until a bird steps on them, soiling the footpad. If the bird continues to stay on the slatted area, the faeces will stay attached to the footpad and dry there, making the footpad embedded in solid faecal material with high ammonia content. This will in turn lead to FPD. Kaukonen et al. (Citation2016) found a larger slatted area was related to poorer footpad conditions. In our study, we found no correlation between the percentage area of the slats or the width of the slats on the FPD of male or female birds of either hybrid, which is in accordance with an experimental study where no effects of slats on FPD were observed (van den Oever et al., Citation2021). Due to the adjusted water and feed line and the nest boxes, the slatted area is more attractive for the hens than the roosters. In addition, mating activity takes place in the litter area. Excessive and aggressive mating can be a problem in the broiler breeder production, making hens stay away from the litter. The feed and water line for the rooters are located in the litter area, making this more attractive for them and they spend more time there than the hens do. It could therefore be speculated that this can be the cause for fewer roosters with lesion scores 3 or 4 at the end of the production period, compared to the results in the hens. This warrants further studies.
The ammonia level in several of the barns was very high; so high that it may be aversive to the birds. These concentrations were measured during the winter with very low outside temperatures, resulting in reduced ventilation to maintain temperature inside the barn. The concentration of ammonia and carbon dioxide measured in the air had medium to very strong positive correlations with FPD scores of Ross 308 birds of both genders. The scores of Hubbard birds, on the other hand, seemed less affected by carbon dioxide, but still showed medium to strong positive correlations with ammonia. This is in line with previous published papers, listing ammonia as one the major causative environmental factors for FPD (Martland, Citation1984; Dawkins et al., Citation2004; Mayne, Citation2005; van den Oever et al., Citation2020). Further studies should focus on keeping good air quality and low ammonia levels in the winter season.
Lameness and impaired gait are major welfare issues in broiler chickens (Bessei, Citation2006) with estimated prevalences between 14% and 30% (Knowles et al., Citation2008; Kittelsen et al., Citation2017; Granquist et al., Citation2019). Birds with scores ≥ 3 are considered to have an impaired gait since this affects manoeuvrability, speed and acceleration and is likely associated with pain (Kestin et al., Citation1992; McGeown et al., Citation1999). Overall, the GS were low in our study, in both hens and roosters of both hybrids. Gait problems have not been investigated to the same degree in broiler breeders as in broiler chickens, but a study by van den Oever et al. (Citation2020) found impaired gait in broiler breeders to be rare, with severe gait problems in only 2.7% of the hens. The GS increased with age in our study, which was also found by van den Oever et al., (Citation2020). Across both hybrids, male GS remained relatively stable until 45 WOA and increased thereafter. For the roosters, but not the hens, there was an observed effect of hybrid, where the Hubbard birds had a higher GS. But it must be emphasized that the GS, even at the highest ages, were low compared to the prevalence reported in much younger broiler chickens. The causation of FPD is multifactorial, comprised of infectious, developmental and degenerative diseases, for impaired gait (Bradshaw et al., Citation2002; Wideman, Citation2016). However, rapid growth rate and high bodyweight are considered the main underlying causes (Kestin et al., Citation1992; Bessei, Citation2006; Knowles et al., Citation2008). Due to restricted feeding, the breeders grow slower than the broiler chickens, which may explain the positive results in both hybrids in our study, even though the Hubbard hens are lighter than the Ross 308 hens. Severe FPD may lead to lameness in broiler chickens (Greene et al., Citation1985). There was a positive correlation between footpad and GS for both hens and roosters, which is in line with Opengart et al. (Citation2018) who found significantly greater odds of GS worsening as FPD worsened. This can be seen in our material as well, even though the GS and FPD scores overall were low.
The study found a correlation between light intensity and both FPD and GS. We hypothesized that more light would give more active birds and therefore better footpad health and GS. However, to our surprise, the statistics showed that brighter light led to worse FPD and GS. We do not know the rationale for this finding. This is the first-ever lifecycle study of broiler breeder FPD and GS, and light intensity as a risk factor should be investigated further.
All footpad evaluations were performed on the farm after manually brushing of dirt and litter. Evaluation and classification of FPD are easier to perform on the slaughter line after cleaning, compared to on farm (Martrenchar et al., Citation2002). This may have affected the results, since it is more difficult to evaluate footpads before cleaning. Another important weakness of the study that must be considered is the relatively small sample size. Only eight flocks were included, four from each hybrid. Therefore, further studies should be performed to confirm the results found here. This is particularly necessary regarding the correlations between FPD/GS and the environmental risk factors assessed. It was not possible to perform these analyses by age, due to the low sample size (i.e. maximum four, but sometimes only three flocks visited per hybrid per age). For this reason, age is a confounding effect in these correlations that must be investigated further in future studies.
In conclusion, the results indicate that moderate and severe FPD are rare in the pullet period but increase with age in both Ross and Hubbard breeders. The prevalence of moderate to severe FPD was higher in the hens compared to roosters at the end of the production period and was strongly related to ammonia concentration in the house. Overall, lameness, as assessed by GS, was low in both hens and roosters. Further studies are needed for both FPD and GS, to investigate causative factors.
Supplemental Material
Download TIFF Image (40.1 KB)Supplemental Material
Download TIFF Image (40.7 KB)Acknowledgements
The authors wish to thank the farmers that participated in the study; for granting us repeated access to the farms and for allowing us to spend time in the barns and to examine the birds.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bassler, A.W., Arnould, C., Butterworth, A., Colin, L., De Jong, I.C., Ferrante, V., Ferrari, P., Haslam, S., Wemelsfelder, F. & Blokhuis, H.J. (2013). Potential risk factors associated with contact dermatitis, lameness, negative emotional state, and fear of humans in broiler chicken flocks. Poultry Science, 92, 2811–2826.
- Bessei, W. (2006). Welfare of broilers: a review. World’s Poultry Science Journal, 62, 455–466.
- Bradshaw, R., Kirkden, R.D. & Broom, M.B. (2002). A review of the aetiology and pathology of leg weakness in broilers in relation to their welfare. Avian and Poultry Biology Reviews, 13, 45–104.
- Brake, J. (1998). Equipment design for breeding flocks. Poultry Science, 77, 1833–1841.
- Cengiz, Ö, Hess, J.B. & Bilgili, S.F. (2011). Effect of bedding type and transient wetness on footpad dermatitis in broiler chickens. Journal of Applied Poultry Research, 20, 554–560.
- Dawkins, M.S., Donnelly, C.A. & Jones, T.A. (2004). Chicken welfare is influenced more by housing conditions than by stocking density. Nature, 427, 342–344.
- De Jong, I.C., Gunnink, H. & Van Harn, J. (2014). Wet litter not only induces footpad dermatitis but also reduces overall welfare, technical performance, and carcass yield in broiler chickens. Journal of Applied Poultry Research, 23, 51–58.
- De Jong, I.C., Van Harn, J., Gunnink, H., Lourens, A. & Van Riel, J.W. (2012). Measuring foot-pad lesions in commercial broiler houses. Some aspects of methodology. Animal Welfare, 21, 325–330.
- Ekstrand, C., Carpenter, T.E., Andersson, I. & Algers, B. (1998). Prevalence and control of foot-pad dermatitis in broilers in Sweden. British Poultry Science, 39, 318–324.
- Gentle, M.J. (2011). Pain issues in poultry. Applied Animal Behaviour Science, 135, 252–258.
- Granquist, E.G., Vasdal, G., De Jong, I.C. & Moe, R.O. (2019). Lameness and its relationship with health and production measures in broiler chickens. Animal, 13, 2365–2372.
- Greene, J.A., Mccracken, R.M. & Evans, R.T. (1985). A contact dermatitis of broilers - clinical and pathological findings. Avian Pathology, 14, 23–38.
- Haslam, S.M., Knowles, T.G., Brown, S.N., Wilkins, L.J., Kestin, S.C., Warriss, P.D. & Nicol, C.J. (2007). Factors affecting the prevalence of foot pad dermatitis, hock burn and breast burn in broiler chicken. British Poultry Science, 48, 264–275.
- Hubbard. (2015). Breeders management manual.
- Kapell, D.N.R.G., Hill, W.G., Neeteson, A.M., McAdam, J., Koerhuis, A.N.M. & Avendaño, S. (2012). Genetic parameters of foot-pad dermatitis and body weight in purebred broiler lines in 2 contrasting environments. Poultry Science, 91, 565–574.
- Kaukonen, E., Norring, M. & Valros, A. (2016). Effect of litter quality on foot pad dermatitis, hock burns and breast blisters in broiler breeders during the production period. Avian Pathology, 45, 667–673.
- Kestin, S.C., Knowles, T.G., Tinch, A.E. & Gregory, N.G. (1992). Prevalence of leg weakness in broiler chickens and its relationship with genotype. The Veterinary Record, 131, 190–194.
- Kittelsen, K.E., David, B., Moe, R.O., Poulsen, H.D., Young, J.F. & Granquist, E.G. (2017). Associations among gait score, production data, abattoir registrations, and postmortem tibia measurements in broiler chickens. Poultry Science, 96, 1033–1040.
- Knowles, T.G., Kestin, S.C., Haslam, S.M., Brown, S.N., Green, L.E., Butterworth, A., Pope, S.J., Pfeiffer, D. & Nicol, C.J. (2008). Leg disorders in broiler chickens: prevalence, risk factors and prevention. PLoS One, 3, 1–5.
- Kyvsgaard, N.C., Jensen, H.B., Ambrosen, T. & Toft, N. (2013). Temporal changes and risk factors for foot-pad dermatitis in Danish broilers. Poultry Science, 92, 26–32.
- Martland, M.F. (1984). Wet litter as a cause of plantar pododermatitis, leading to foot ulceration and lameness in fattening turkeys. Avian Pathology, 13, 241–252.
- Martrenchar, A., Boilletot, E., Huonnic, D. & Pol, F. (2002). Risk factors for foot-pad dermatitis in chicken and turkey broilers in France. Preventive Veterinary Medicine, 52, 213–226.
- Mayne, R.K. (2005). A review of the aetiology and possible causative factors of foot pad dermatitis in growing turkeys and broilers. World’s Poultry Science Journal, 61, 256–267.
- McGeown, D., Danbury, T.C., Waterman-Pearson, A.E. & Kestin, S.C. (1999). Effect of carprofen on lameness in broiler chickens. Veterinary Record, 144, 668–671.
- Nagaraj, M., Wilson, C.A.P., Hess, J.B. & Bilgili, S.F. (2007). Effect of high-protein and all-vegetable diets on the incidence and severity of pododermatitis in broiler chickens. Journal of Applied Poultry Research, 16, 304–312.
- Opengart, K., Bilgili, S.F., Warren, G.L., Baker, K.T., Moore, J.D. & Dougherty, S. (2018). Incidence, severity, and relationship of broiler footpad lesions and gait scores of market-age broilers raised under commercial conditions in the southeastern United States. Journal of Applied Poultry Research, 27, 424–432.
- Renema, R.A., Robinson, F.E., Beliveau, R.M., Davis, H.C. & Lindquist, E.A. (2007). Relationships of body weight, feathering, and footpad condition with reproductive and carcass morphology of end-of-season commercial broiler breeder hens. Journal of Applied Poultry Research, 16, 27–38.
- Ross. (2018). Broiler management handbook. Aviagen Ross Management Guide, 1–147.
- Shepherd, E.M. & Fairchild, B.D. (2010). Footpad dermatitis in poultry. Poultry Science, 89, 2043–2051.
- Sinclair, A., Weber Wyneken, C., Veldkamp, T., Vinco, L.J. & Hocking, P.M. (2015). Behavioural assessment of pain in commercial turkeys (Meleagris gallopavo) with foot pad dermatitis. British Poultry Science, 56, 511–521.
- Sirri, F., Minelli, G., Folegatti, E., Lolli, S. & Meluzzi, A. (2007). Foot dermatitis and productive traits in broiler chickens kept with different stocking densities, litter types and light regimen. Italian Journal of Animal Science, 6, 734–736.
- Thøfner, I.C.N., Poulsen, L.L., Bisgaard, M., Christensen, H., Olsen, R.H. & Christensen, J.P. (2019). Correlation between footpad lesions and systemic bacterial infections in broiler breeders. Veterinary Research, 50, 1–5.
- van den Oever, A.C.M., Bolhuis, J.E., van de Ven, L.J.F., Kemp, B. & Rodenburg, T.B. (2020). High levels of contact dermatitis and decreased mobility in broiler breeders, but neither have a relationship with floor eggs. Poultry Science, 99, 3355–3362.
- van den Oever, A.C.M., Candelotto, L., Kemp, B., Rodenburg, T.B., Bolhuis, J.E., Graat, E.A.M., van de Ven, L.J.F., Guggisberg, D. & Toscano, M.J. (2021). Influence of a raised slatted area in front of the nest on leg health, mating behaviour and floor eggs in broiler breeders. Animal, 15, 100–109.
- Weber Wyneken, C., Sinclair, A., Veldkamp, T., Vinco, L.J. & Hocking, P.M. (2015). Footpad dermatitis and pain assessment in turkey poults using analgesia and objective gait analysis. British Poultry Science, 56, 522–530.
- Welfare Quality. (2009). Welfare quality assessment protocol for poultry. Welfare Quality® Consortium. Nederland.
- Wideman, R.F. (2016). Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poultry Science, 95, 325–344.
- Wolanski, N.J., Renema, R.A., Robinson, F.E., & Wilson, J.L. (2004). End-of-season carcass and reproductive traits in original and replacement male broiler breeders. Journal of Applied Poultry Research, 13, 451–460.

