 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The aim of the present study was to monitor the dynamics and to measure the safety and efficacy of a live, attenuated, thermosensitive Mycoplasma anserisalpingitidis vaccine candidate, namely MA271, in geese breeder flocks under field conditions. Two rearing flocks were vaccinated with MA271 at 4 weeks of age and boosted at 24 weeks of age by cloaca inoculation (1 ml) and eye-dropping (60 µl). The geese then were transported to multi-aged breeding farms. Two breeding flocks served as controls. Colonization of the cloaca by MA271 showed 75% maximum prevalence between 4 and 6 weeks after the first vaccination. Then the prevalence decreased to 25% until the cooler, humid fall months which coincided with the booster vaccination. Boosting raised cloacal colonization to 100%. No clinical signs were observed in the vaccinated birds. After transportation to five multi-aged breeding farms, the wild-type strain appeared as well as MA271 in three flocks. In one flock, the wild-type strain completely displaced MA271, while in one flock only MA271 was detected. Only wild-type strains were detected in the control flocks; however, due to an HPAI outbreak, both flocks were exterminated before the end of the study. Based on the available data, the median percentage of infertile eggs was 3.7-5.1% in the MA271 vaccinated flocks, and 7.7% in the non-vaccinated flock. In conclusion, MA271 can colonize the cloaca of geese under field conditions. MA271 proved to be safe and presumably protects against M. anserisalpingitidis-induced reproduction losses.
Introduction
Mycoplasma anserisalpingitidis (formerly Mycoplasma anserisalpingitis or M. sp. strain 1220) is the most important goose pathogenic mycoplasma, primarily causing reproductive disorders and respiratory disease in the flocks (Volokhov et al., Citation2020). Typical signs of the disease are phallus and cloaca inflammation and/or testicular atrophy, affecting up to 50–100% of sexually active ganders in an infected flock, while salpingitis is the most common lesion in geese (Stipkovits et al., Citation1986; Hinz et al., Citation1994; Stipkovits & Kempf, Citation1996). The production of eggs with abnormal shells rises, the number of fertile eggs drops, and the embryo mortality rates may reach up to 40–60% (Dobos-Kovács et al., Citation2009). The most severe clinical signs and losses generally occur during the first laying period, while, probably due to the developing natural immunity, the clinical manifestation of the disease becomes milder in the following reproduction seasons.
Nowadays, the control of the disease primarily relies on antibiotic therapy, often using metaphylactic antibiotic application that leads to the overuse of drugs and the development of multi-drug resistance in M. anserisalpingitidis in Europe and Asia (Grózner et al., Citation2016; Gyuranecz et al., Citation2020). Moreover, the massive antibiotic administration results in antimicrobial resistance not only in M. anserisalpingitidis but also in other bacteria colonizing the geese, which could pose a zoonotic threat via the food chain or direct human contact. Thus, food safety and human health perspectives associated with the risk of the spread of multi-host bacteria exhibiting antimicrobial resistance highlight the importance of vaccines as a long-term solution in controlling mycoplasmosis in waterfowl.
Currently, no licenced vaccine against M. anserisalpingitidis infection is available, only autogenous vaccines with ambiguous efficacy. Recently, we developed a temperature-sensitive, live, attenuated vaccine candidate, namely clone MA271, using N-methyl-Nʹ-nitro-N-nitrosoguanidine (NTG) mutagenesis (Bekő et al., Citation2022). In small-scale animal experiments, MA271 showed quickly developing and robust colonization capability, which persisted till the end of the 8-week long study period and induced a humoral immune response. Immunization with live vaccines is ideally performed in early rearing time, thus the vaccine strain can colonize the mucous membranes before wild-type strain exposure. The vaccine strain fills the niche and, besides the generalized immune response, it also induces mucosal immunity, which participates in the protection.
M. anserisalpingitidis infection is part of a multifactorial disease of geese where environmental and stress factors are essential to induce clinical manifestation, like phallus inflammation or embryo mortality. The goose is a semi-domesticated animal, which is poorly adapted to modern husbandry technology. The large flock size, the artificial lighting system-induced production cycle, and the barn-based housing are all stress factors triggering the clinical manifestation from sub-clinical M. anserisalpingitidis infection.
The rearing period in goose husbandry generally lasts until 23–28 weeks of age when the birds are moved to the production farm where they are kept in production for 3–4 years. The normal production cycle of geese is associated with the seasons and in Hungary starts in January or February and lasts till June. In meat-type breeds, an artificial climate and lighting programme-based production technology, the so-called climate production cycle is also applied. This provides the possibility of all-year-round production in closed stables but also represents extra stress for the animals. Most of the time the climate production cycle starts in March and finishes in September.
Testing the efficacy of vaccine candidate MA271 under farm conditions was necessary due to the long rearing period of geese and because environmental and stress factors are needed for the clinical manifestation of M. anserisalpingitidis infection. The aim of the present study was to monitor the dynamics of MA271 in geese rearing and breeder flocks under field conditions and to measure the safety and protective effect by comparing the production of vaccinated and control flocks based on the percentage of infertile eggs.
Materials and methods
Flock histories
Meet-type geese breeder flocks were involved in the study. Animal experiments were approved and permissions were issued under reference numbers 5300/1819-1/2021 and 5300/1819-3/2021 by the National Food Chain Safety Office, Hungary. Two rearing flocks were vaccinated with MA271. Both flocks were raised at separate farms near the village of Viss, Hungary. The first flock (Flock V1R) was established with 9127, day-old Lipitsch breed goslings on the 21st of April, 2021. The second flock (Flock V2R) was established with 8010, day-old Golden Goose breed animals on the 4th of June, 2021. Flocks V1R and V2R were vaccinated (see details below) at 4 weeks of age on the 19th of May and on the 5th of July, respectively. A booster vaccination was applied on the 5th of October and the 23rd of November, respectively. Flock V1R was moved to two breeding farms, Szilaspogony (Flock V1B1, flock size: 3346) and Tótújfalu (Flock V1B2, flock size: 2456), while 1628 remaining male geese formed a reserve stock at Cered-Gyepűsmajor (Flock V1B3) on the 20th of October. Flocks V1B1 and V1B2 lay in the normal cycle from February 2022 till the end of June. Rearing Flock V2R was separated into two breeding flocks: Derekegyház (Flock V2B1, flock size: 3384) and Hajdúböszörmény-Dedőhát (Flock V2B2, flock size: 3184) on the 20th of December and lay in a climate-driven cycle from March to July, 2022. At the rearing farms, the studied flocks were housed on their own, while the breeding farms were multi-age farms where other older birds in their second and third reproductive cycles were housed as well next to the studied flocks. The reserve gander flock (V1B3) was housed on its own on the farm.
A rearing flock with 7857 Golden Goose breed goslings was settled at Tiszaeszlár, Hungary on the 12th of April, 2021 and served as a control flock (Flock CR). This flock was separated into normal (Flock CB1, flock size: 3205) and climate-driven cycle (Flock CB2, flock size: 3197) breeding farms in Szentes in November 2021 and started to lay in February and end of March 2023, respectively. Two- and 3-year-old birds were housed on both breeding farms as well as next to the 1-year-old flocks used as controls in the present study. Unfortunately, both control farms had to be eradicated due to a highly pathogenic avian influenza (HPAI) outbreak on the 6th of May, 2023. Flock histories are visualized in .
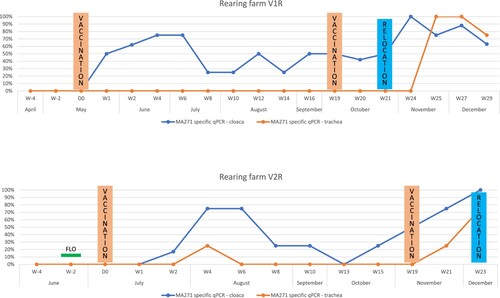
Figure 1. Colonisation dynamics of vaccine candidate MA271 on the tracheal and cloacal mucosa of geese vaccinated by eye-dropping and cloaca inoculation at the two rearing farms in 2021. The orange/solid (trachea) and blue/dashed (cloaca) lines show the percentage of tested birds (20-60 individuals pooled by five) found to be positive with MA271 specific TaqMan real-time PCR. The dates of primer and booster vaccinations, as well as the time of relocation to breeding farms, are also marked. A black line labeled FLO represents the time of florfenicol medication of rearing flock V2R to treat E. coli infection.
Vaccination
Vaccine material was prepared under GMP conditions. Eight ml of masterseed culture of MA271 was inoculated into 4000 ml mycoplasma broth medium (Thermo Fisher Scientific Inc./Oxoid Inc./, Waltham, MA, USA) and incubated at 33°C until acidic colour change (2 days), aliquoted into 40 ml units, and stored at −70°C until use. The titre of the frozen-thawed material was 1 × 108 CCU/ml (colour-changing units) determined by the broth microdilution method (Hannan, Citation2000). The frozen vaccine was transported to the rearing farms on dry ice, thawed, and diluted 1:10 in sterile phosphate-buffered saline immediately before administration. Thus, the applied vaccine contained 1 × 107 CCU/ml ts+ M. anserisalpingitidis MA271 live cells, which was confirmed with the broth microdilution method at each time.
Each bird was inoculated using approximately 60 µl single eye-drop and intracloacally with 1 ml vaccine during the first immunization and the booster vaccination. Eye-dropping vials and mass-injectors with blunt tip syringes were used to vaccinate 4000–5000 geese per day, so the vaccination of each flock lasted for 2 days at each time.
The antimicrobial susceptibility (minimal inhibitory concentration [MIC]) of MA271 was examined on custom-made 96-well Micronaut-S plates (MERLIN Diagnostika GmbH., Berlin, Germany) following the guidelines of Hannan (Citation2000). The plates contained lyophilized dilutions of 10 antibiotics, a growth control, and a sterility control. Antimicrobial agents used were the following: enrofloxacin, spectinomycin, oxytetracycline, erythromycin, spiramycin, tilmicosin, tylosin, lincomycin, florfenicol, and tiamulin.
Sample collection and testing
The vaccinated flocks were monitored and sampled from 1-day-old till the end of their first laying season. Cloaca and trachea swab samples were collected for PCR and isolation at an average of 2-week intervals during the rearing and a monthly interval during the laying periods. The detailed data on sampling dates and sample numbers are listed in online supplementary Tables S1 and S2, and . For PCR, the swab samples were pooled by five and DNA extraction was performed with the ReliaPrep gDNA Tissue Miniprep System (Promega Inc., Madison, WI, USA) according to the manufacturer’s instructions. Samples collected before vaccination were tested with an M. anserisalpingitidis-specific real-time PCR assay as described previously (Bekő et al., Citation2022).
Table 1. Presence of vaccine candidate MA271 and wild-type M. anserisalpingitidis strains on the tracheal and cloacal mucosa of vaccinated and control breeding geese during laying season in 2022: 20 animals were sampled in each flock on each occasion and samples were pooled in groups of five for DIVA qPCR testing. Laying started in February and March in the normal cycle farms and in the climate cycle breeding farms, respectively. Control flocks were exterminated because of a highly pathogenic avian influenza outbreak in April.
Samples collected post-vaccination were examined with an MA271 clone-specific DIVA (differentiating infected from vaccinated animals) PCR system. Protein translocase subunit SecY (secY) and PTS system, N-acetylglucosamine-specific components (IIA, IIB, IIC) (Nag) were suitable for the design of primers and probes. The sequences of the designed primers and probes were the following: MA271-specific assay: secY-Vacc-F 5ʹ- CCTCAAGCAATTTTCTTCAT -3ʹ, secY-Vacc-R 5ʹ- ATTTTCATTCATTCCGTTT -3ʹ, and secY-Vacc-P 5ʹ- HEX- ACTTGAAGCTCCTCCAGCAGT -BHQ-1-3ʹ. The size of the amplicon is 93 bps. Wild-type-specific assay: Nag-WT-F 5ʹ- CACCAAGAAACAAYCTRG -3ʹ, Nag-WT-R 5ʹ- TARCGGAAAARCYAGAAA -3ʹ and Nag-WT-P 5ʹ- 6-FAM- CACCAGAAACTTTAGACAA -BHQ-1-3ʹ. The secY-Vacc-F and Nag-WT-P were equipped with locked nucleic acids (underlined nucleotides) to enhance target specificity and oligo stability. The size of the amplicon is 123 bp. The PCR master mixes consisted of 6 µl 2 qPCRBIO Probe Mix No-ROX (PCR Biosystems Ltd., London, UK), 0.5 µl of each primer (10 µM), 0.25 µl probe and 2 µl DNA in the final volume of 12 µl. Thermocycling parameters were 95°C for 2 min, followed by 45 cycles at 95°C for 5 s, 58°C (MA271-specific assay)/60°C (WT-specific assay) for 20 s. The PCR assay was optimized on a Bio-Rad CFX96 Touch Real-Time PCR Detection System (Bio-Rad Inc., Hercules, CA, USA) with the Bio-Rad CFX Maestro software version 1.1 (Bio-Rad Inc.). The specificities of the assays were analyzed by testing-type strains of M. anatis (NCTC 10156), M. anseris (ATCC 49234), M. cloacale (NCTC 10199), M. columbinasale (ATCC 33549), M. columbinum (ATCC 29257), M. columborale (ATCC 29258), M. gallinaceum (ATCC 33550), M. gallinarum (ATCC 19708), M. gallisepticum (ATCC 19610), M. gallopavonis (ATCC 33551), M. imitans (ATCC 51306), M. iners (ATCC 19705), M. iowae (ATCC 33552), M. meleagridis (NCTC 10153), M. pullorum (ATCC 33553), M. synoviae (NCTC 10124), and Acholeplasma laidlawii (NCTC 10116). In addition, 93 M. anserisalpingitidis, as well as M. anatis, M. anseris, and M. cloacale field isolates (10 of each) were also included. To determine the sensitivity of the assay, tenfold dilutions of M. anserisalpingitidis ATCC BAA-2147-type strain and MA271 clone were used in the range of 106–100 template copy number/μl. To evaluate the performance of the designed assays in mixed infections, the DNA of the target genotype (MA271 or wild-type) at the dilution of the detection limit of the assay was mixed in a ratio of 1:1 with the DNA of the non-target genotype (wild-type or MA271) at various template copy numbers/μl values (106–100) in all combinations. Specificity testing demonstrated no cross-amplification with any other examined Mycoplasma/Acholeplasma strain. According to the sensitivity testing, the minimum genetic equivalents detectable in the samples, including mixed infections, were 101 (MA271-specific assay) and 102 (WT-specific assay).
For isolation, cloaca swab samples were pooled by two. The medium used for the isolation and propagation of the organism consisted of Mycoplasma broth medium (pH 7.8) (Thermo Fisher Scientific Inc./Oxoid Inc.) supplemented with 0.5% (w/v) sodium pyruvate, 0.5% (w/v) glucose, 0.005% (w/v) phenol red and 0.15% (w/v) L-arginine hydrochloride, and Oxoid Mycoplasma Supplement G (Thermo Fisher Scientific Inc./Oxoid Inc.). Pooled swab samples were washed in 2 ml broth, filtered through a 0.65 µm pore size syringe filter (Minisart® NML with surfactant-free cellulose acetate (SFCA), Sartorius GmbH, Goettingen, Germany), and incubated at 33°C. After acidic colour change, the cultures were inoculated onto solid Mycoplasma medium (passage 1) (Thermo Fisher Scientific Inc./Oxoid Inc.) supplemented with 0.15% (w/v) L-arginine hydrochloride and were incubated at 33°C with 5% CO2 until visible colonies appeared (4 days). Well-separated colonies were picked, filter-cloned, and identified by species-specific PCRs as described previously (Bekő et al., Citation2022) and MA271-specific DIVA assays as detailed above.
Data analysis
The number of laid eggs and the number of infertile eggs were recorded weekly from each studied flock and the percentage of infertile eggs was calculated (online supplementary Table S3). Statistical analyses were accomplished with the R programme (R Core Team, Citation2021). To examine the effect of the vaccination the ratio of infertile eggs was compared between the vaccinated flocks and the control flock in the first 8 weeks of egg production (before the control flock was eradicated). First, a Shapiro–Wilk normality test was performed to test the normal distribution of the data. As this test showed no normal distribution (P = 0.65), first a Kruskal–Wallis non-parametric ANOVA test was carried out to determine whether the difference among the medians of the three study groups was statistically significant. If the results of the Kruskal–Wallis test were significant, a Dunn’s test was performed to determine exactly which groups were different by making pairwise comparisons between each group. Since multiple groups were considered at the same time, P-values were adjusted for multiple comparisons by the Bonferroni method.
Results
We could not detect M. anserisalpingitidis in any of the flocks between 1 day and 4 weeks of age when the birds were vaccinated (, online supplementary Table S1 and S2). Flock V2R had Escherichia coli infection at 2 weeks of age and was treated with florfenicol (the result of MIC testing of MA271 is presented in online supplementary Table S4). Following vaccination, the prevalence of cloaca colonisation of MA271 increased to 75% till the 4th week post-vaccination. Six weeks after vaccination the cloaca prevalence rate decreased to 25–50% and stayed at this level throughout the summer months. It started to rise again during autumn following the booster vaccination around 24 weeks of age of the goslings. After the booster vaccination, the cloaca prevalence of MA271 reached 100%. MA271 was undetectable in the trachea until the booster vaccination except in the V2R flock at 4 weeks following the first immunisation. The re-isolation of MA271 was successful in 93.75% and 36.36% of the collected cloaca swab samples in flocks V1R and V2R, respectively. The DIVA PCRs did not show the presence of wild-type strains in any of the vaccinated rearing flocks.
Four weeks after the booster vaccination the birds were moved to multi-aged breeding farms where they were exposed to wild-type M. anserisalpingitidis strains (). The detectable rate of the bacteria in the trachea decreased again over time, while the mycoplasma population in the cloaca stabilized at 100% prevalence as the laying cycle started. Wild-type strains appeared beside MA271 in three flocks (V1B1, V1B2, and V2B2). In one flock (V2B1) the wild-type strain completely displaced MA271, while in the reserve gander flock (V1B3) only MA271 was detected. Only wild-type strains were detected in the control flocks; however, due to the HPAI outbreak both flocks were exterminated at the beginning of the laying period.
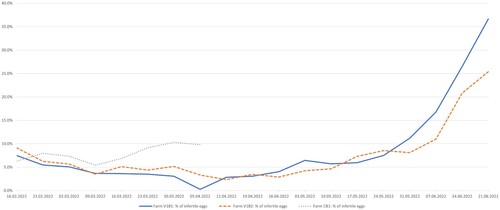
Clinical signs of mycoplasmosis were not observed in the vaccinated animals during rearing. Occasionally phallus inflammation was recorded in ganders in vaccinated breeding flock V2B2, while no signs of disease were recorded in the other vaccinated populations. Phallus and cloaca inflammations were recorded in approximately 30% of the animals in the control flocks according to the observations of the owners. As both control farms were exterminated because of HPAI on the 6th of May candling data were available only from the first two laying weeks of the climate-driven cycle farm CB2 which was insufficient for further analysis. The normal production cycle farm was in production since February; thus, altogether, candling data of 8 weeks were available (, online supplementary Table S3). By analysing the candling data, the median percentage of weekly identified infertile eggs was 3.7 and 5.1% in vaccinated flocks V1B1 and V1B2, respectively, while 7.7% in the non-vaccinated control flock CB1. Statistical analyses showed significant differences among the three examined groups (P = 0.004), with a significantly lower ratio of infertile eggs in flock V1B1 compared to control flock CB1 (P = 0.004). The difference between flock V1B2 and CB1 was notable, but not significant (P = 0.059) (online supplementary Table S3).
Figure 2. Percentage of infertile eggs. Data of vaccinated flocks V1B1 and V1B2 are compared with those of non-vaccinated control breeding flock CB1 from laying season 2022. Data are shown based on the date when the eggs were put into the incubator. The data of flock CB1 are incomplete as the flock was exterminated because of a high pathogenic avian influenza outbreak.
Discussion
M. anserisalpingitidis is the most important goose pathogenic Mycoplasma species globally, primarily causing venereal and occasionally respiratory infection in geese farms (Volokhov et al., Citation2020). It is a poorly studied agent and our knowledge about the pathogenesis and epizootiology of the disease is limited. Although an inactivated vaccine was patented in 2016 (US Citation20160082094 A1) to prevent Mycoplasma infection in waterfowl, so far, no licenced vaccine is commercially available.
Our patented MA271 clone is a promising vaccine candidate based on the in vitro and in vivo characteristics described before (Bekő et al., Citation2022). Evaluating the efficacy of MA271 under farm conditions is necessary because of the long rearing period, long reproduction cycle, and special housing condition requirements of geese (open pastures), and also due to the predisposing factors (stress, crowd, mating season, etc.) needed for the clinical manifestation of the disease.
After vaccination, the colonization of the cloaca was rapid by MA271 although it did not reach a 100% prevalence and colonization was somewhat slower than what was observed during the previous small-scale animal house experiment (Bekő et al., Citation2022). The colonization was slower in flock V2R than in flock V1R, which could be explained by the florfenicol treatment against E. coli infection. However, administration of the antibiotic was 2 weeks before vaccination and the growth of MA271 could be inhibited at as low as 1 µg/ml MIC with florfenicol (online supplementary Table S4). Another explanation might be the difference in the dates of vaccinations. Flock V2R was vaccinated only in July when the summer heat and UV radiation could have hampered the survival of MA271 in the faecal material and bedding on the open meadow, thus the re-infection of the birds via the oro-nasal route or cloacal drinking from the environment was also hindered. By the time a balance between mucosal immunity and bacterial colonization was assumed to be established (6 weeks post-vaccination), the colonisation percentage dropped to around 25% and stayed at this prevalence throughout the summer months, probably due to the above-mentioned climatic reasons. The prevalence of MA271 in the cloaca increased to 100% in flock V1R after the booster vaccination which coincided with the arrival of the cooler, rainy autumn weather. In flock V2R the elevation of MA271 prevalence in the cloaca also started with the arrival of the cooler, humid season 1 month before the booster vaccination. This strengthens the hypothesis that the weather conditions have an effect on the survival of M. anserisalpingitidis in the environment and thus the colonization of the geese. The maturation of the birds might be another factor which helps M. anserisalpingitidis to colonize the cloaca and the genital organs. The lack of colonisation of the trachea supports the previous assumption that M. anserisalpingitidis has primary enterotropic and venereal adaptations. But the heat exposure of the birds during summer on the open grasslands, inhaling the dry and warm air, might also hamper the respiratory colonization with a thermosensitive strain, like MA271. The rapid increase of the MA271 population in the trachea observed in the samples collected in the wet and foggy months (starting from November) supports this hypothesis.
Following the booster vaccination, the geese were moved to infected, multi-age farms, where, within a short time, the birds were colonized with the local wild-type strain as well as MA271. Flock V1B3 was kept alone on a farm, thus wild-type strain infection source was ruled out. Unfortunately, in breeding flock V2B1 the wild-type strain completely displaced MA271. In conclusion, MA271 is not able to prevent the colonisation of geese with wild-type M. anserisalpingitidis strains. But based on the available and analysed production data from breeding farms V1B1 and V1B2, MA271 provides some protection for the vaccinated birds as the percentage of infertile eggs was lower in these vaccinated flocks than in the non-vaccinated control flock. The low incidence of ganders with phallus inflammation in the vaccinated breeding flocks, compared to the non-vaccinated flocks, according to the observation of the owner, further supports this hypothesis. Furthermore, it is known that geese suffer from the most severe lesions caused by M. anserisalpingitidis infection during their first laying season, when they are naïve, while milder clinical problems are observed during the following (2nd, 3rd, and 4th cycle) laying periods, which presumes the development of some sort of protection. It seems that vaccination with MA271 during the rearing season can provide immunization of the birds even from their first laying period. As clinical signs of mycoplasmosis were not observed in the vaccinated birds, either during rearing or laying, the safety of MA271 can be assumed.
In conclusion, vaccine candidate MA271 showed good colonizing ability under farm conditions as well although, seemingly the climate has an effect on its environmental survival and host colonisation. It is hypothesized that the maturity of the birds might also enhance the colonization. Clinical signs of mycoplasmosis were not observed in the vaccinated animals which supports the attenuated nature of MA271. Our results showed that vaccination with MA271 does not protect geese from wild-type M. anserisalpingitidis colonization, but, according to the available data, it decreases the percentage of clinical presentation of the disease (like the percentage of infertile eggs). But we have to mention that further efficacy studies are required to prove the effectiveness of MA271. However, conducting such experiments is challenging as it would require large flock sizes, which can be handled only under farm conditions which are affected by various unexpected events (such as changes in farm management or avian influenza outbreaks).
Supplemental Material
Download MS Excel (27.3 KB)Disclosure statement
No potential conflict of interest was reported by the author.
Data availability statement
All data are available in the manuscript and the supplementary materials.
Additional information
Funding
References
- Bekő, K., Grózner, D., Mitter, A., Udvari, L., Földi, D., Wehmann, E., Kovács, ÁB, Domán, M., Bali, K., Bányai, K., Gyuris, É, Thuma, Á, Kreizinger, Z. & Gyuranecz, M. (2022). Development and evaluation of temperature-sensitive Mycoplasma anserisalpingitidis clones as vaccine candidates. Avian Pathology, 51, 535–549.
- Dobos-Kovács, M., Varga, Z., Czifra, G. & Stipkovits, L. (2009). Salpingitis in geese associated with Mycoplasma sp. strain 1220. Avian Pathology, 38, 239–243.
- Grózner, D., Kreizinger, Z., Sulyok, K.M., Rónai, Z., Hrivnák, V., Turcsányi, I., Jánosi, S. & Gyuranecz, M. (2016). Antibiotic susceptibility profiles of Mycoplasma sp. 1220 strains isolated from geese in Hungary. BMC Veterinary Research, 12, 170.
- Gyuranecz, M., Mitter, A., Kovács, ÁB, Grózner, D., Kreizinger, Z., Bali, K., Bányai, K. & Morrow, C.J. (2020). Isolation of Mycoplasma anserisalpingitidis from swan goose (Anser cygnoides) in China. BMC Veterinary Research, 16, 178.
- Hannan, P.C.T. (2000). Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. Veterinary Research, 31, 373–395.
- Hinz, K.H., Pfützner, H. & Behr, K.P. (1994). Isolation of mycoplasmas from clinically healthy adult breeding geese in Germany. Journal of Veterinary Medicine Series B, 41, 145–147.
- R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. 2022.
- Stipkovits, L. & Kempf, I. (1996). Mycoplasmoses in poultry. Revue Scientifique et Technique, 15, 1495–1525.
- Stipkovits, L., Varga, Z., Czifra, G. & Dobos-Kovacs, M. (1986). Occurrence of mycoplasmas in geese affected with inflammation of the cloaca and phallus. Avian Pathology, 15, 289–299.
- US 20160082094 A1: Szathmary, S., Stipkovits, L. (2016). Vaccine to prevent mycoplasmal infections in waterfowl.
- Volokhov, D.V., Grózner, D., Gyuranecz, M., Ferguson-Noel, N., Gao, Y., Bradbury, J.M., Whittaker, P., Chizhikov, V.E., Szathmary, S. & Stipkovits, L. (2020). Mycoplasma anserisalpingitidis sp. nov., isolated from European domestic geese (Anser anser domesticus) with reproductive pathology. International Journal of Systematic and Evolutionary Microbiology, 70, 2369–2381.
