ABSTRACT
Common enteric viruses affecting commercial broiler flocks include fowl adenovirus (FAdV), chicken parvovirus (ChPV), chicken astrovirus (CAstV), avian reovirus (ARV) and avian rotavirus (AvRV). To investigate their prevalence and to identify single and multiple infections we collected intestinal samples from 49 Austrian broiler flocks during necropsy of dead-on-farm birds twice during a production cycle (7–14 days and 28–35 days). Altogether, up to three consecutive clinically healthy flocks without signs of gastrointestinal disease were sampled from 17 different farms. Samples were analysed using virus isolation and PCR/RT–PCR methods. Virus prevalence was correlated with production data and on-farm biosecurity and management practices. Overall, ARV (75%) was most commonly detected in the flocks, followed by CAstV (61%), ChPV (61%), FAdV (57%) and AvRV (8%). Only in three (6%) flocks were none of the investigated enteric viruses detected. Flock infection profiles were very heterogeneous and included individual detection of the investigated viruses as well as different combinations thereof (up to all five investigated viruses). Even in the absence of clinical diarrhoea and/or macroscopic intestinal lesions, statistical analysis confirmed that the number of viruses detected had a significant economic impact characterised by poor weight gain and increased mortality, particularly due to the presence of FAdV, CAstV and/or ARV. Furthermore, the use of barn-specific clothing and/or footbaths as well as regular vermin control, resulted in lower prevalence of enteric viruses in the flocks studied. This highlights the importance of common biosecurity measures in poultry production to prevent economic losses.
RESEARCH HIGHLIGHTS
Detection timepoints and patterns indicate horizontal introduction of various enteric viruses.
Flock infection profiles were very heterogeneous; no dominating virus profile.
Broiler production was negatively affected by the number of enteric viruses detected.
Common biosecurity measures had a significant negative effect on virus prevalence.
Introduction
Health and function of the intestinal tract is one of the most important factors contributing to successful broiler production (Aruwa et al., Citation2021). Particularly in young broilers, enteric virus infection can result in mild to severe enteritis with diarrhoea leading to uneven growth, poor feed conversion ratio, growth retardation and substantial economic losses (Saif et al., Citation2020). Several enteric viruses have been associated with clinical disease in broilers, commonly referred to as runting-stunting or malabsorption syndrome (RSS/MAS) (Guy, Citation1998). Many studies have investigated the viral component of this enteric disease using conventional diagnostics (e.g. virus isolation, PCR, RT–PCR) and/or advanced molecular techniques (e.g. next generation sequencing, NGS). Among others, the main viruses detected in or isolated from enteric infections in affected broilers include fowl adenovirus (FAdV), chicken parvovirus (ChPV), chicken astrovirus (CAstV), avian reovirus (ARV) and avian rotavirus (AvRV). These viruses have been found in both single and mixed infections with widely varying prevalence and distribution patterns (Decaesstecker et al., Citation1988; Mettifogo et al., Citation2014; La Torre et al., Citation2018; Kim et al., Citation2020; Chen et al., Citation2022). In the field, patterns of viral co-occurrences may explain different disease presentations in coincidence with heterogeneous clinical signs and flock performances.
The viruses mentioned above are especially noticed in young birds underlining the ability of vertical transmission (Saif et al., Citation2020). In addition, horizontal transmission, by direct faecal-oral infection as well as spread through contaminated fomites, plays an important role in the wide distribution of enteric viruses. Virus shedding has been described in experimental settings for the aforementioned viruses (McNulty et al., Citation1983; Jones & Georgiou, Citation1984; Songserm et al., Citation2000; Zsak et al., Citation2013). In modern, intensive broiler production, increasing flock size and close proximity of farms are associated with higher levels of infection pressure and with increased risk of performance losses and even disease outbreaks (Gelaude et al., Citation2014).
In a recent study of the health status of commercial broiler flocks in Austria kept at lower stocking densities and with limited use of antimicrobials, clinical signs of intestinal disease were rarely observed and pathological intestinal lesions were only occasionally observed in the investigated dead-on-farm (DOF) birds, with flocks being considered healthy (Grafl et al., Citation2020). Nevertheless, differences in performance traits (e.g. mortality and live weight at day 28) were observed between flocks and farms.
The purpose of the present study was to assess the prevalence of five selected enteric viruses associated with RSS, namely FAdV, ARV, CAstV, AvRV, and ChPV. A longitudinal study was conducted to identify single and mixed virus infections at the beginning of (between 7–14 days of age), and towards the end of (between 28–35 days of age) the production period. In addition, the prevalence of the investigated virus(es) was correlated with flock data to assess virus impact on poultry performance. Finally, statistical analyses were performed to investigate relationships between virus prevalence and on-farm biosecurity and management measures in order to provide recommendations for improved prevention and control.
Materials and methods
Origin of samples and flock information
During a recent field study, tissue samples (duodenum with ingesta and caecal tonsils) were collected from 49 Ross 308 flocks on 17 individual farms (from 15 farms a total of three consecutive flocks were investigated, from two farms two consecutive flocks were available for study). Flocks were housed at lower stocking densities (25 kg/m2). In addition, environmental enrichment was offered, including natural lighting due to window areas and structural elements in the form of straw bales (Grafl et al., Citation2020). All flocks were examined and sampled at two timepoints: at the beginning (TP1: 1–2 weeks of age) and towards the end of the production period (TP2: 4–5 weeks of age). Per flock and timepoint, routine pathological examination of 8–10 DOF broilers was performed and prevalent pathomorphological changes were recorded. No obvious clinical problems were reported in the 49 investigated flocks, and necropsies of DOF birds revealed only very occasional pathological changes in the intestinal tract.
For virus detection, the intestinal samples described above were collected using sterile, disposable forceps and scalpels. For each flock and timepoint, two pooled samples (I and II) were collected for investigation and stored at −20°C prior to processing.
All investigated flocks were similar in terms of veterinary management; they received coccidiostats as feed additives during the fattening period and were generally vaccinated against infectious bronchitis (Nobilis® IB Ma5, MSD, Vienna, Austria) and infectious bursal disease (Nobilis® Gumboro D78, AviPro® Gumboro Vac or Hipragumboro® CH/80 – CW). Flock performance data comprising weekly (weeks 1–5) and cumulative (days 1–28) mortality rates, live weight at day 28 (weight d28) and condemnation rates were obtained from official records of the processing slaughterhouses.
Information concerning daily hygiene, along with general biosecurity and health management data of the respective farms and flocks, was collected by the University’s Institute of Animal Welfare Science by a comprehensive questionnaire (). Information was collected from farmers on a voluntary basis.
Table 1. Frequency of occurrence of biosecurity and hygiene measures from the investigated flocks.
Detection and classification of fowl adenoviruses (FAdVs) and avian reoviruses (ARV)
In a first step, samples were screened in cell culture. For this, tissue homogenates (20%) were prepared in antibiotic (1 mg/ml streptomycin and 100,000 IU/ml penicillin) in phosphate buffered saline (PBS; Gibco™, ThermoFisher Scientific, Vienna, Austria); samples were subjected to three freeze–thaw cycles followed by centrifugation and filter sterilization using a 0.2 μm syringe filter (VWR, Vienna, Austria). Chicken embryo liver cells were prepared from 14-day-old SPF chicken embryos (VALO Biomedia GmbH, Osterholz-Scharmbeck, Germany) according to the protocol of Sellers & Schat (Citation2016). Nearly confluent chicken embryo liver cells were inoculated with the sample material and cells were incubated at 37.8°C in 5% CO2 for 5 days or until a cytopathic effect (CPE) was observed. A sample was considered negative for FAdVs and ARVs if no CPE was observed over three passages in cell culture. From cell cultures with CPE, 200 μl of clarified supernatant was used for RNA and DNA extraction using the IndiSpin Pathogen Kit (Indical Bioscience, Leipzig, Germany).
For the detection of FAdVs, a conventional PCR amplifying the loop-1 region of the hexon gene, as previously described by Meulemans et al. (Citation2001), was used. Samples were further investigated using a nested PCR amplifying a partial sequence of the adenoviral DNA polymerase gene according to Wellehan et al. (Citation2004). Two conventional PCR methods were used to detect ARVs. The primary method used was a RT–PCR as described by Kant et al. (Citation2003), which amplifies a partial sequence of the S1 segment of the ARV σC gene. Negative samples were additionally processed using a RT–PCR method amplifying the S4 gene segment of the σ NS protein as described previously (Pantin-Jackwood et al., Citation2008). The primers used for the PCRs are listed in Online Supplementary Table 1.
Amplification products were analysed by agarose gel electrophoresis, fragments of the correct length were excised from the gel and purified using the QIAquick gel extraction kit (Qiagen, Vienna, Austria) Sequencing services were provided by LGC Genomics GmbH (Berlin, Germany). Sequence assembly and analysis and amino acid alignments were performed using Accelrys Gene Software, V.2.5 (Accelrys, San Diego, CA, USA). Phylogenetic analyses were performed based on hexon and/or polymerase gene sequences by comparing the obtained nucleotide sequences with sequences of FAdV reference strains classified into species A to E as described by Schachner et al. (Citation2016). Molecular characterization of ARV strains into clusters 1–6 was based on amino acid alignment of the σC protein (Kant et al., Citation2003; Lu et al., Citation2015). Briefly, for phylogenetic analysis, deduced amino acid sequences of σC protein (266 amino acids) from investigated samples were aligned with sequences from the NCBI database that included representatives of all six clusters, including a region of σC protein from amino acid 28–293. The analysis was performed in MegAlignPro module of DNASTAR Lasergene software v17 (DNASTAR; Madison, WI, USA), using Neighbor-Joining analysis with default settings. The final tree was rooted at the midpoint branch.
Detection of chicken astroviruses (CAstV), chicken parvovirus (ChPV) and avian rotaviruses (AvRV)
For the detection of CAstV, ChPV and AvRV, pools of duodenum with ingesta and caecal tonsils were homogenized using a TissueLyser (Qiagen) according to the manufacturer’s instructions. Samples were clarified by centrifugation and supernatants were used for RNA and DNA extraction using the IndiSpin Pathogen Kit (Indical Bioscience). For the detection of CAstV and AvRV, protocols and virus-specific primers described by Day et al. (Citation2007) were used (Online Supplementary Table 1). The same samples were investigated for ChPV using a PCR based upon NS gene-specific primers and a protocol published previously (Zsak et al., Citation2009) (Online Supplementary Table 1). PCR products were separated by standard agarose gel electrophoresis and visualized under ultraviolet light (Biorad Universal Hood II; Bio-Rad Laboratories, Hercules, CA, USA). Fragment sizes were determined by reference to a 100-bp ladder (Invitrogen, ThermoFisher Scientific, Waltham, MA, USA).
Statistical analysis
Statistical analyses were carried out in R (version 4.2.3, R Core Team, Citation2022), using an alpha level of 0.05 for all statistical tests. At the flock level, we compared prevalence of each virus at both sampling timepoints by applying chi-square tests of independence.
To investigate the effect of number of virus detections across both timepoints on performance at the flock level, a linear mixed model (LMM) using a Gaussian error structure was applied separately to each performance variable. Models included the total count of virus detections (range: 0–7) as a continuous predictor, as well as farm and flock nested within farm as random intercept effects. To further investigate this relationship between performance and the presence of individual viruses, we fitted two additional LMMs with Gaussian error structure that included the flock status for each virus (FAdV, ARV, CAstV, ChPV and AvRV) as predictors. These models serve to identify the importance of each virus on flock performance. By including each virus in the same model we controlled for their confounding effects, in order to obtain unbiased estimates. As random intercept effects, farm and flock nested in farm were included. Because mortality of week 5 comprised only 15 observations, mortality data were only analysed until d28.
To investigate the effect of selected hygiene practices and biosecurity management on enteric virus load we applied a generalized linear mixed model (GLMM) with Poisson error structure and log link function. In the full model, we included the number of virus infections across both timepoints (range: 0–7) as a response variable. We included the following factors with levels yes and no as test predictors: foot bath with disinfection; barn-specific shoes and coat; storage of carcasses; regular fly and vermin control; storage of manure <500 m from barn; duration of downtime over 14d; routine disinfection of waterline before new flock (). Notably, foot bath with disinfection and barn-specific clothing were highly collinear. Therefore, separate models were additionally analysed. Farm and flock nested in farm were included as random intercept effects. To further investigate how biosecurity measurements affect the presence of individual enteric viruses, we fitted GLMMs with binomial error structure and logit link function. In each model we included individual virus presence (with levels no / yes) as a response. Because the data did not allow us to include all above-mentioned variables in these models, we decided to only include foot bath with disinfection, barn-specific clothing and regular fly and vermin control as predictors. As random intercept effects farm and flock nested in farm were included. As only four farms tested positive for AvRV we were unable to include this virus in the analysis.
Models were fitted using the functions lmer (for LMMs) and glmer (for GLMMs) from the package lme4 (version 1.1-31; Bates et al., Citation2015). Prior to fitting Gaussian models, we inspected whether the distribution of each response was roughly symmetrical. This led us to log-transform a number of responses to ease model fit. After fitting our models, we confirmed that model assumptions were not violated. For the LMMs we visually inspected QQ-plots and plots of residuals vs. fitted values to confirm normally distributed and homogeneous residuals. The Poisson models were checked for over-dispersion, which showed over-dispersion was not an issue; in fact, models were underdispersed. Finally, for all models we confirmed (1) that the “Best Linear Unbiased Predictors” (BLUPS) were approximately normally distributed (Harrison et al., Citation2018) and (2) model stability by comparing model estimates of the full model to estimates of models in which levels of random effects were excluded one at a time (Nieuwenhuis et al., Citation2012).
Results
Detection and distribution of enteric viruses
Virus growth characterized by CPE in cell culture was detected in 56 (57.1%) and 67 (68.4%) samples at TP1 and TP2, respectively. All samples positive in cell culture were investigated for the presence of FAdVs and ARVs. Results are detailed in .
Table 2. Detection and distribution of investigated enteric viruses in individual sample pools. Results of PCR for fowl adenovirus (FAdV), avian reovirus (ARV), chicken astrovirus (CAstV), chicken parvovirus (ChPV) and avian rotavirus (AvRV) in the investigated flocks (A-C) at the beginning of the production period (between 7–14 days of age – TP1) and at the end of the production period (between 28–35 days of age – TP2).
Using conventional hexon and nested PCR, FAdVs were detected in 50 (25.5%) of the individual sample pools tested. They were detected significantly more often towards the end of the production period (χ2: 36.757, df = 1, P < 0.001), with six (6.1%) and 44 (44.9%) of the samples positive at TP1 and TP2, respectively, representing the most prevalent virus detected in older birds. Sequencing results showed that FAdV-B (62%) was the most frequently detected species, followed by FAdV-E (24%), FAdV-A (8%) and FAdV-D (6%). FAdV-C was not detected in any of the samples.
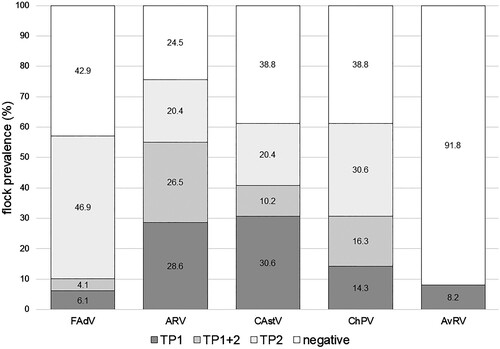
In total, FAdVs were not detected in 21 (42.9%) flocks (). However, FAdVs were present in five (10.2%) and 25 (51.0%) of the 49 flocks examined at TP1 and TP2, respectively. In general, FAdV detection (presence/absence and also prevalent species) within a flock differed between TP1 and TP2. Furthermore, in six flocks different FAdV species were identified from the two samples collected at a single TP.
Figure 1. Chronological variation in the detection of enteric viruses in flocks. Detection of fowl adenovirus (FAdV), avian reovirus (ARV), chicken astrovirus (CAstV), chicken parvovirus (ChPV) and avian rotavirus (AvRV) in 49 broiler flocks investigated at the beginning (TP1) and towards the end (TP2) of production. Virus infection in a flock / at a timepoint was recognized when at least one of two investigated sample pools per TP showed a positive PCR result.
Overall, ARVs were the most frequently detected enteric viruses in the present study. They were detected in 52 (53.1%) and 42 (42.8%) individual samples at TP1 and TP2, respectively (χ2: 1.656, df = 1, P = 0.198). While 14 samples were positive only by ARV S4 PCR and further identification was not possible, 80 field isolates were classified into different clusters based on alignments of the amino acid sequences of the σC gene (Kant et al., Citation2003; Lu et al., Citation2015) (Online Supplementary Figure 1). The investigated strains were most frequently identified as ARV cluster 4 (39.3%), followed by cluster 1 (21.3%), cluster 2 (18.1%) and cluster 3 (10.6%). No strains representing clusters 5 or 6 were detected.
On the flock level, 12 (24.5%) flocks were ARV negative (). ARVs were found in 27 (55.1%) and 23 (46.9%) of the 49 flocks investigated at TP1 and TP2, respectively. ARV detection at TP1 did not correlate with detection at TP2. In seven flocks different ARV clusters were detected at TP1 and TP2, respectively. In addition, in two flocks, isolates from different ARV clusters were detected at the same timepoint in the two pooled samples investigated.
In the current study, ChPVs and CAstVs were the second and third most frequently detected viruses, respectively. ChPVs were detected significantly more often towards the end of production (χ2: 8.247, df = 1, P = 0.004) with 23 (23.5%) and 43 (43.9%) of the samples positive at TP1 and TP2, respectively. CAstVs were detected at similar rates at TP1 and TP2, in 34 (34.7%) and 29 (29.6%) of the samples, respectively (χ2: 0.374, df = 1, P = 0.541). In contrast, AvRVs were detected in only five (5.1%) samples at TP1 and in none of the samples at TP2. Results are detailed in .
Altogether, both CAstV and ChPV were detected in 30 (61.2%) of the investigated flocks and AvRV in four (8.2%) flocks (). Within flocks, there was no correlation between the respective virus detections at TP1 and TP2.
Flock infection profiles
None of the enteric viruses tested were detected in 12 (24.5%) of the flocks at TP1 or in six (12.2%) of the flocks at TP2. Flock infection profiles comprised individual detection of the investigated agents (FAdV, ARV, CAstV, ChPV and/or AvRV) as well as different combinations thereof, resulting in up to 15 different profiles per TP. At TP1, a single virus was detected in 13 (26.5%) flocks. Combinations of two different viruses were noted in 15 (30.6%) flocks and mixed infections with three or four viruses were detected in eight (16.0%) and in one (2.0%) of the investigated flocks, respectively. At TP2, 13 (26.5%) flocks showed evidence of a single virus infection, 17 (34.7%) flocks showed various combinations of two enteric viruses and 13 (26.5%) flocks showed co-infections with three viruses.
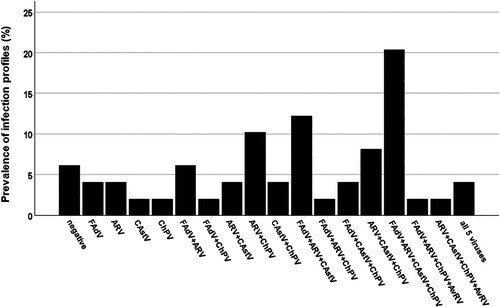
Altogether, in only three flocks (6.1%) were none of the enteric viruses under investigation detected. Considering the timepoint(s) of detection, the vast majority of flock infection profiles were unique (Online Supplementary Table 2).
Infection profiles, independent of the time of detection, resulted in 18 profiles (). They comprised single virus detection of FAdV, ARV, CAstV or ChPV in six (12.2%) flocks as well as varying combinations of the investigated agents. Mixed infections with both two and three viruses were both found in 13 (26.5%) flocks, followed by detection of four different viruses in 12 (24.5%) flocks and infections with all five viruses investigated in two (4.1%) flocks. The most common “cumulative” flock infection profile was the detection of FAdV, ARV, CAstV and ChPV in 10 (20.4%) flocks, followed by FAdV, ARV and CAstV in six (12.2%) flocks and ARV and ChPV in five (10.2%) flocks.
Enteric virus detection and flock performance
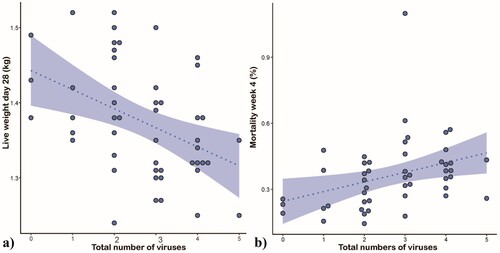
No virus profile was directly correlated to obvious clinical or pathological signs in the investigated broiler flocks. The applied LMM revealed a significant effect of the total number of viral detections in a flock on live weight on d28 as well as on mortality in the 4th week of life ( (a and b); Online Supplementary Table 3).
Figure 3. Effect of the number of enteric viruses detected on flock performance. Scatterplots displaying total number of enteric viruses in relation to (a) bodyweight at 28 days of life and (b) mortality in week 4. The dashed line shows the fitted model, and the shaded area represents the 95% confidence interval.
Performance data with regards to individual virus prevalence in a flock is summarized in . Detection of FAdV in a flock showed a significant negative impact on weight on d28 and on mortality days 1–28 (Online Supplementary Table 4). The presence of ARV in a flock was associated with a lower bodyweight at d28 and higher mortality; however, a LMM showed no statistical significance. CAstV detection in a flock correlated with significantly increased mortality. No statistically significant production losses were associated with the detection of ChPV and AvRV in flocks. Detection of investigated enteric virus(es) had no significant effect on slaughterhouse condemnations.
Table 3. Performance data with regards to individual virus prevalence in a flock.
Enteric virus detection and practiced hygiene / biosecurity management
All farms had a physical, step-over hygiene barrier between the “clean” and “dirty” parts of the working area. Furthermore, hand cleaning facilities were available on all premises.
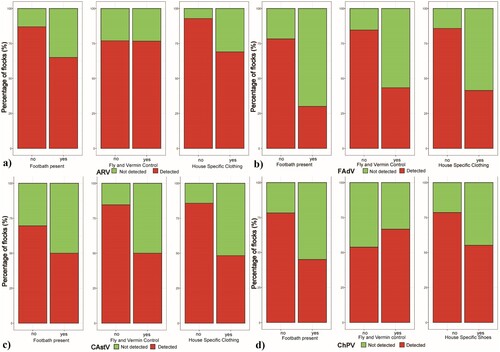
Separate models indicate an effect of the presence of foot bath with disinfection and the use of barn-specific clothing (foot dip bath: 2.15 ± 1.42; no foot dip bath: 4.22 ± 1.38 enteric viruses detected; barn-specific clothing yes: 2.62 ± 1.57; no: 4.57 ± 1.28; Online Supplementary Table 5), the full GLMM showed a significantly decreased total number of virus detections in broiler houses which had a foot bath with disinfection (χ2: 5.53, df = 1, P = 0.019; Online Supplementary Table 5). Further, hygiene measures, biosecurity management and general cleaning and disinfection practices between flocks (using common commercial disinfections based on combinations of aldehydes and quaternary ammonium or on peroxygen compounds) showed no significant effects on number of virus detections in the investigated flocks: storage of carcasses (on farm: 3.06 ± 1.66; off farm: 3.40 ± 1.80) or manure (< 500 m from broiler house: 2.78 ± 1.86; > 500 m from broiler house: 3.60 ± 1.58), regular vermin control (yes: 2.63 ± 1.52, no: 4.05 ± 1.68), duration of downtime between flocks (< 14d: 3.54 ± 1.81; > 14d: 3.13 ± 1.72), routine disinfection of waterline between flocks (yes: 3.03 ± 1.82; no: 3.91 ± 1.30) or temperature of cleaning water (hot: 3.24 ± 1.76; cold: 3.40 ± 1.67). Statistical analysis showed an impact of selected common biosecurity and hygiene measures on the detection of individual viruses ((a–d), Online Supplementary Table 6). The full GLLM showed significant changes as follows: FAdV prevalence was decreased by the presence of foot bath containing disinfection (χ2: 5.43, df = 1, P = 0.020); and by regular fly and vermin control measures (χ2: 4.16, df = 1, P = 0.041). The prevalence of CAstV in flocks was significantly decreased by fly and vermin control measures (χ2: 4.15, df = 1, P = 0.042) and showed decreased numbers due to the use of barn-specific clothing (χ2: 3.46, df = 1, P = 0.063).
Discussion
Worldwide, enteric viruses (FAdV, ARV, ChPV, CAstV, and AvRV) have been associated with acute clinical diseases (e.g RSS, MAS), characterized by digestive problems (diarrhoea), stunted or uneven growth, and increased mortality, with a significant negative impact on the economic performance of commercial broiler chickens (Rebel et al., Citation2006; Mettifogo et al., Citation2014; La Torre et al., Citation2018; Kim et al., Citation2020; Chen et al., Citation2022). Available studies have documented a wide variation in virus prevalence and distribution in affected poultry flocks. Direct comparisons between studies are difficult as species and age of investigated birds, flock location and husbandry, sampling schemes and testing methods influence the virus profiles detected. Besides, the same viruses have also been detected in apparently healthy birds, leading to confusion about the aetiological role and pathogenicity of these viruses (Devaney et al., Citation2016; Lobani et al., Citation2016; Kubacki et al., Citation2022). It can be perceived that more advanced diagnostic techniques (NGS, RT-qPCR, etc) create more updated knowledge on the prevalence, diversity and biology of different enteric viruses in broilers (Liebhart et al., Citation2023). Lima et al. (Citation2019) suggested that some intestinal viruses are endemic in commercial broiler farms and part of the normal gut virome.
Our results showed that almost all flocks were positive throughout the fattening period for one or more of the enteric viruses investigated in the current study. We found a high diversity of prevalence profiles, with no particular enteric virus profile dominating, as well as changing profiles by testing the same flocks twice throughout broiler production, indicating substantial variation in infection dynamics. However, this high prevalence and diversity of enteric viruses, even in clinically healthy birds, is in line with previous studies from other countries (Ter Veen et al., Citation2017; Kubacki et al., Citation2022).
Of the viruses studied, ARVs were the most frequently detected. In the detected isolates, a great heterogeneity of the σC gene sequences was observed, with isolates from ARV clusters 1–4 being present (data not shown). In general, ARVs appear to be widespread in the poultry industry and are frequently detected in the intestinal tract (Kovács et al., Citation2022). As isolates belonging to the same genotypic cluster have been shown to differ in their pathogenicity, a direct relationship between the phylogenetic classification of the σC gene and the observed clinical signs has not been consistently reported (Egaña-Labrin et al., Citation2021). Although the causal link between ARVs and arthritis/tenosynovitis is well established (Walker et al., Citation1972), a causative role in enteric diseases is less clear. For instance, the prevalence of ARVs in poultry with enteritis has been documented to range from 0% (La Torre et al., Citation2018; Chen et al., Citation2022) to 100% (Ter Veen et al., Citation2017). Furthermore, in experimental settings ARV isolates showed little effect on weight gain of commercial birds, despite replication in the intestinal epithelium and the development of lesions in the small intestine (Songserm et al., Citation2003; Spackman et al., Citation2010). In the present study, we did not see clinical signs of enteritis. Nonetheless, production losses (e.g. lower weight) were noted in ARV-infected flocks compared with negative flocks. Therefore, while ARVs alone may not cause clinical enteritis, it can be hypothesized that their presence may contribute to the severity of clinical signs and production losses. As previously described in commercial poultry flocks (Kovács et al., Citation2022), the present study documented that different strains (belonging to different clusters) are circulating within commercial flocks at the same time. Such mixed infections can lead to genetic re-assortment, resulting in the emergence of new strain variants that may pose a risk for disease outbreaks (Liu et al., Citation2003). In addition, shifts between genetic clusters were observed between the timepoints studied. Altogether, these findings suggest high ARV infection pressure in local broiler populations, with substantial risk of horizontal transmission. Previously, Islam et al. (Citation2020) documented that birds raised with higher standards of hygiene had lower ARV prevalence than those reared in poorer conditions. However, there was no direct evidence of this in the current flocks investigated, possibly because of the generally high prevalence in all the flocks studied.
In our study, FAdV were detected in 57.1% of the flocks and phylogenetic analysis of the isolated strains revealed the prevalence of all FAdV species except FAdV-C. Overall, FAdV species related to well-characterized broiler diseases (e.g FAdV-D and -E: inclusion body hepatitis, or FAdV-A: adenoviral gizzard erosion (Schachner et al., Citation2018)) comprised only one-third of the detected isolates. The majority of the detected isolates were allocated to FAdV-B, which historically has been detected in healthy birds and those with unspecified pathologies, but no primary disease has been associated with the strains (Marek et al., Citation2010; Kaján et al., Citation2013). Given that multiple strains of different serotypes/species have been found in the same flock or even the same bird (Niu et al., Citation2018; Kaján et al., Citation2022), it is not surprising that we detected the presence of different FAdV species in a flock at one timepoint as well as at the different investigation timepoints. Despite a lack of clinical signs in the investigated flocks, significantly decreased bodyweight and higher overall mortality were noted in flocks which harboured FAdV. Experimentally, digestive and metabolic dysfunction due to oral FAdV-8b (FAdV-E) infection of 3-week-old broilers has been described with consequences on performance (Matos et al., Citation2018). While this has not been described in relation to FAdV-B so far, recent studies have reported the emergence of variant FAdV-B strains, the exact pathological role of which remains to be investigated (Kaján et al., Citation2022). FAdV prevalence was significantly higher in older broilers. Virus replication in the gut is reported to coincide with long-lasting shedding (Cook, Citation1983; Jones & Georgiou, Citation1984). Consequently, horizontal, faecal-oral transmission plays an important role in the wide distribution of FAdV within and between flocks. Previously, poultry workers have been identified as an important vector for transmitting and spreading FAdV during outbreaks (Akhtar et al., Citation1992). In line with this, common biosecurity measures, in particular the presence of foot dip baths, resulted in significantly lower FAdV prevalence in the examined flocks. While FAdV DNA has been detected in darkling beetles (Achari et al., Citation2014), there is little information on FAdV transmission and carrier status of common pests in poultry houses. Nonetheless, the implementation of regular fly and vermin control measures showed a significant reduction in the prevalence of FAdV in flocks. Overall, we conclude that general deficiencies in both daily hygiene practices as well as general farm biosecurity measures will have an impact on the horizontal introduction of FAdV. As this may lead to significant economic losses, prudent hygiene and biosecurity decisions play a major role in maintaining bird health and in optimizing broiler productivity and profitability.
Furthermore, a high proportion of the investigated flocks harboured ChPV and/or CAstV. High prevalences of these viruses have been documented frequently in commercial poultry with enteritis (Ter Veen et al., Citation2017; La Torre et al., Citation2018; Chen et al., Citation2022). Likewise, studies have documented the virus presence predominantly in flocks without signs of enteric disease (Lobani et al., Citation2016; Zhang et al., Citation2020; Kubacki et al., Citation2022). Although there is no direct correlation between their presence and enteric disease in the field, experimental infection studies have documented cystic enteropathy, particularly in the jejunum, with negative effects on the weight gain (Kang et al., Citation2018; Nuñez et al., Citation2020). In the present study, there was no correlation between the individual presence of ChPV and CAstV and weight retardation in flocks. However, production losses were documented by higher mortality rates in flocks with CAstV. Previously, vertically acquired CAstV infections have been correlated with reduced hatchability and early mortality in broilers (Smyth, Citation2017). Similarly, ChPV has been documented in very young birds, as early as 5 days of age (Zsak et al., Citation2009). In the present study, CAstV and ChPV were detected in more than 30% of the flocks examined at TP1, confirming that infection occurs early in life. Considering the efficient and long-lasting cloacal shedding of these viruses and the fact that they are considerably resistant to disinfection (Zsak et al., Citation2013; Smyth, Citation2017), transmission via the faecal-oral route from infected broilers to naïve individuals must play an important role in the dissemination within and between flocks. In practice, therefore, hygiene and biosecurity should reduce the risk of infection and potentially associated losses in production. In the present study, no significant effect of hygiene and biosecurity parameters was noted on the detection of ChPV. However, the flock prevalence of CAstV was significantly reduced by daily personal hygiene measures and by common biosecurity practices. Similarly, Zhang et al. (Citation2020) documented higher infection rates in “open houses” compared to “closed houses” due to inadequate hygiene measures.
Lastly, in the present study, we detected AvRV in only 8% of the investigated flocks, indicating that this virus is not widespread in clinically healthy, local broiler flocks. Furthermore, while all the other enteric viruses investigated were found in birds at both stages of production, AvRV was only detected in young broilers. Previous detection of the virus in newly hatched birds has suggested that transmission of AvRV may occur in or on the eggs (Pantin-Jackwood et al., Citation2007). While infection in young birds can lead to enteritis and diarrhoea, chicks may remain clinically healthy due to protection by maternally-derived antibodies (Dhama et al., Citation2015). It is unclear whether this was the case or whether the strains detected were simply non-pathogenic, but in the current study there was no effect on flock performance.
Altogether, the present study demonstrates a high diversity of enteric viruses in Austrian broiler flocks with a high prevalence of sequential and/or concurrent co-infections, even in the absence of commonly associated clinical signs and lesions. The virus profiles detected were very heterogeneous and no virus profile showed a significant correlation with documented performance shortfalls. However, a negative impact on flock health and performance, characterized by reduced bodyweight and increased mortality, was associated with a larger enteric virus burden. Thus, it appears that an increased presence of enteric viruses may have an additive, negative effect on the health and resilience of birds in commercial settings. This is in line with recent NGS analyses documenting a higher abundance of different enteric viruses in broiler flocks with reduced bodyweight compared to healthy flocks (Kubacki et al., Citation2022). Although the results do not shed light on aetiological questions or pathogenetic mechanisms, they confirm the economic importance of enteric viruses. Horizontal entry and spread of the investigated viruses, namely FAdV, ARV and ChPV, was confirmed by increased virus burden throughout the production period and detection of shifts in virus species (FAdV) / clusters (ARV) between the two detection timepoints. As flock management in compliance with basic hygiene and biosecurity standards (foot dip baths, barn-specific protective clothing, and regular fly and vermin control) served to reduce the total virus burden and/or individual virus prevalence, this confirms that hygiene and biosecurity regulations play a major role in maintaining bird health and in optimizing broiler productivity and profitability.
Supplementary Figure 1.docx
Download MS Word (650.8 KB)Supplementary Figure 1.tiff
Download TIFF Image (11.3 MB)Supplementary tables 1 to 6 plus Supplementary Figure Legend.docx
Download MS Word (40.1 KB)Acknowledgements
The authors would like to thank their colleagues at the Institute for Animal Welfare Science (Dr Knut Niebuhr† and Dr Fehim Smajlhodžić) for their excellent cooperation on this project, as well as all involved poultry veterinarians and farmers for their contributions. The authors would also like to thank Irina Prokofieva and Evelyn Berger for their assistance with laboratory work. Finally, we thank REWE International AG for funding the project.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Achari, R., Islam, A.F., Renz, K., Hunt, P., Arzey, E. & Walkden-Brown, S.W. (2014, 11–12 September). Monitoring of fowl adenoviruses using environmental samples. World Veterinary Poultry Association (WVPA). WVPA Asia Meeting 2014, Bangkok, Thailand.
- Akhtar, S., Zahid, S. & Khan, M.I. (1992). Risk factors associated with hydropericardium syndrome in broiler flocks. The Veterinary Record, 131, 481–484.
- Aruwa, C.E., Pillay, C., Nyaga, M.M. & Sabiu, S. (2021). Poultry gut health - microbiome functions, environmental impacts, microbiome engineering and advancements in characterization technologies. Journal of Animal Science and Biotechnology, 12, 119.
- Bates, D., Mächler, M., Bolker, B. & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48.
- Chen, L., Chen, L., Wang, X., Huo, S. & Li, Y. (2022). Detection and molecular characterization of enteric viruses in poultry flocks in Hebei province, China. Animals, 12, 2873.
- Cook, J.K. (1983). Fowl adenoviruses: studies on aspects of the pathogenicity of six strains for 1-day-old chicks. Avian Pathology, 12, 35–43.
- Day, J.M., Spackman, E. & Pantin-Jackwood, M. (2007). A multiplex RT-PCR test for the differential identification of turkey astrovirus type 1, of turkey astrovirus type 2, chicken astrovirus, avian nephritis virus, and avian rotavirus. Avian Diseases Digest, 2, e13.
- Decaesstecker, M., Charlier, G. & Meulemans, G. (1988). Epidemiological study of enteric viruses in broiler chickens: comparison of tissue culture and direct electron microscopy. Avian Pathology, 17, 477–486.
- Devaney, R., Trudgett, J., Trudgett, A., Meharg, C. & Smyth, V. (2016). A metagenomic comparison of endemic viruses from broiler chickens with runting-stunting syndrome and from normal birds. Avian Pathology, 45, 616–629.
- Dhama, K., Saminathan, M., Karthik, K., Tiwari, R., Shabbir, M.Z., Kumar, N., Malik, Y.S. & Singh, R.K. (2015). Avian rotavirus enteritis - an updated review. The Veterinary Quarterly, 35, 142–158.
- Egaña-Labrin, S., Jerry, C., Roh, H.J., Da Silva, A.P., Corsiglia, C., Crossley, B., Rejmanek, D. & Gallardo, R.A. (2021). Avian reoviruses of the same genotype induce different pathology in chickens. Avian Diseases, 65, 530–540.
- Gelaude, P., Schlepers, M., Verlinden, M., Laanen, M. & Dewulf, J. (2014). Biocheck.ugent: a quantitative tool to measure biosecurity at broiler farms and the relationship with technical performances and antimicrobial use. Poultry Science, 93, 2740–2751.
- Grafl, B., Gaußmann, B., Sulejmanovic, T., Hess, C. & Hess, M. (2020). Risks and disease aetiologies of compromised performance in commercial broilers kept at lower stocking density and limited antimicrobial use. Avian Pathology, 49, 621–630.
- Guy, J.S. (1998). Virus infection of the gastrointestinal tract of poultry. Poultry Science, 77, 1166–1175.
- Harrison, X.A., Donaldson, L., Correa-Cano, M.E., Evans, J., Fisher, D.N., Goodwin, C.E.D., Robinson, B.S., Hodgson, D.J. & Inger, R. (2018). A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ, 6, e4794.
- Islam, M.S., Sabuj, A.A.M., Haque, Z.F., Pondit, A., Hossain, M.G. & Saha, S. (2020). Seroprevalence and risk factors of avian reovirus in backyard chickens in different areas of Mymensingh district in Bangladesh. Journal of Advanced Veterinary and Animal Research, 7, 546–553.
- Jones, R.C. & Georgiou, K. (1984). Experimental infection of chickens with adenoviruses isolated from tenosynovitis. Avian Pathology, 13, 13–23.
- Kaján, G.L., Kecskeméti, S., Harrach, B. & Benkő, M. (2013). Molecular typing of fowl adenoviruses, isolated in Hungary recently, reveals high diversity. Veterinary Microbiology, 167, 357–363.
- Kaján, G.L., Schachner, A., Gellért, Á & Hess, M. (2022). Species fowl aviadenovirus B consists of a single serotype despite genetic distance of FAdV-5 isolates. Viruses, 14, 248.
- Kang, K.-I., Linnemann, E., Icard, A.H., Durairaj, V., Mundt, E. & Sellers, H.S. (2018). Chicken astrovirus as an aetiological agent of runting-stunting syndrome in broiler chickens. Journal of General Virology, 99, 512–524.
- Kant, A., Balk, F., Born, L., van Roozelaar, D., Heijmans, J., Gielkens, A. & ter Huurne, A. (2003). Classification of Dutch and German avian reoviruses by sequencing the sigma C protein. Veterinary Research, 34, 203–212.
- Kim, H.-R., Kwon, Y.-K., Jang, I. & Bae, Y.-C. (2020). Viral metagenomic analysis of chickens with runting-stunting syndrome in the Republic of Korea. Virology Journal, 17, 53.
- Kovács, E., Varga-Kugler, R., Mató, T., Homonnay, Z., Tatár-Kis, T., Farkas, S., Kiss, I., Bányai, K. & Palya, V. (2022). Identification of the main genetic clusters of avian reoviruses from a global strain collection. Frontiers in Veterinary Science, 9, 1094761.
- Kubacki, J., Qi, W. & Fraefel, C. (2022). Differential viral genome diversity of healthy and RSS-affected broiler flocks. Microorganisms, 10, 1092.
- La Torre, D.I.d., Nuñez, L.F., Astolfi-Ferreira, C.S. & Piantino Ferreira, A.J. (2018). Enteric virus diversity examined by molecular methods in Brazilian poultry flocks. Veterinary Sciences, 5, 38.
- Liebhart, D., Bilic, I., Grafl, B., Hess, C. & Hess, M. (2023). Diagnosing infectious diseases in poultry requires a holistic approach: a review. Poultry, 2023, 252–280.
- Lima, D.A., Cibulski, S.P., Tochetto, C., Varela, A.P.M., Finkler, F., Teixeira, T.F., Loiko, M.R., Cerva, C., Junqueira, D.M., Mayer, F.Q. & Roehe, P.M. (2019). The intestinal virome of malabsorption syndrome-affected and unaffected broilers through shotgun metagenomics. Virus Research, 261, 9–20.
- Liu, H.J., Lee, L.H., Hsu, H.W., Kuo, L.C. & Liao, M.H. (2003). Molecular evolution of avian reovirus: evidence for genetic diversity and reassortment of the S-class genome segments and multiple cocirculating lineages. Virology, 314, 336–349.
- Lobani, A.M., Gharaibeh, S.M. & Al-Majali, A.M. (2016). Relationship between different enteric viral infections and the occurrence of diarrhea in broiler flocks in Jordan. Poultry Science, 95, 1257–1261.
- Lu, H., Tang, Y., Dunn, P.A., Wallner-Pendleton, E.A., Lin, L. & Knoll, E.A. (2015). Isolation and molecular characterization of newly emerging avian reovirus variants and novel strains in Pennsylvania, USA, 2011-2014. Scientific Reports, 5, 14727.
- Marek, A., Günes, A., Schulz, E. & Hess, M. (2010). Classification of fowl adenoviruses by use of phylogenetic analysis and high-resolution melting-curve analysis of the hexon L1 gene region. Journal of Virological Methods, 170, 147–154.
- Matos, M., Dublecz, K., Grafl, B., Liebhart, D. & Hess, M. (2018). Pancreatitis is an important feature of broilers suffering from inclusion body hepatitis leading to dysmetabolic conditions with consequences for zootechnical performance. Avian Diseases, 62, 57–64.
- McNulty, M.S., Allan, G.M. & McCracken, R.M. (1983). Experimental infection of chickens with rotaviruses: clinical and virological findings. Avian Pathology, 12, 45–54.
- Mettifogo, E., Nuñez, L.F.N., Chacón, J.L., Santander Parra, S.H., Astolfi-Ferreira, C.S., Jerez, J.A., Jones, R.C. & Piantino Ferreira, A.J. (2014). Emergence of enteric viruses in production chickens is a concern for avian health. The Scientific World Journal, 2014, 450423.
- Meulemans, G., Boschmans, M., Berg, T.P. & Decaesstecker, M. (2001). Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses. Avian Pathology, 30, 655–660.
- Nieuwenhuis, R., te Grotenhuis, M. & Pelzer, B. (2012). Influence.ME: tools for detecting influential data in mixed effects models. R Journal, 4, 38–47.
- Niu, Y., Sun, Q., Zhang, G., Sun, W., Liu, X., Xiao, Y., Shang, Y. & Liu, S. (2018). Epidemiological investigation of outbreaks of fowl adenovirus infections in commercial chickens in China. Transboundary and Emerging Diseases, 65, e121–e126.
- Nuñez, L.F.N., Santander-Parra, S.H., La Torre, D.I.d., Sá, L.R.M.d., Buim, M.R., Astolfi-Ferreira, C.S. & Piantino Ferreira, A.J. (2020). Molecular characterization and pathogenicity of chicken parvovirus (ChPV) in specific pathogen-free chicks infected experimentally. Pathogens, 9, 606.
- Pantin-Jackwood, M.J., Day, J.M., Jackwood, M.W. & Spackman, E. (2008). Enteric viruses detected by molecular methods in commercial chicken and turkey flocks in the United States between 2005 and 2006. Avian Diseases, 52, 235–244.
- Pantin-Jackwood, M.J., Spackman, E., Day, J.M. & Rives, D. (2007). Periodic monitoring of commercial turkeys for enteric viruses indicates continuous presence of astrovirus and rotavirus on the farms. Avian Diseases, 51, 674–680.
- R Core Team. (2022). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
- Rebel, J., Balk, F.R.M., Post, J., van Hemert, S., Zekarias, B. & Stockhofe, N. (2006). Malabsorption syndrome in broilers. World’s Poultry Science Journal, 62, 17–30.
- Saif, Y.M., Guy, J.S., Day, J.M., Cattoli, G. & Hayhow, C.S. (2020). Viral enteric infections. In D.E. Swayne (Ed.), Diseases of poultry 14th edn (pp. 401–445). Hoboken, NJ: Wiley-Blackwell.
- Schachner, A., Marek, A., Grafl, B. & Hess, M. (2016). Detailed molecular analyses of the hexon loop-1 and fibers of fowl aviadenoviruses reveal new insights into the antigenic relationship and confirm that specific genotypes are involved in field outbreaks of inclusion body hepatitis. Veterinary Microbiology, 186, 13–20.
- Schachner, A., Matos, M., Grafl, B. & Hess, M. (2018). Fowl adenovirus-induced diseases and strategies for their control - a review on the current global situation. Avian Pathology, 47, 111–126.
- Sellers, H.S. & Schat, K.A. (2016). Cell culture methods. In S.M. Williams, L. Dufour-Zavala, M.W. Jackwood, M.D. Lee, B. Lupiani, W.M. Reed, E. Spackman & P.R. Woolcock (Eds.), A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens: Cell-Culture Methods 6th edn (pp. 327–338). Madison, WI: Omnipress Inc.
- Smyth, V.J. (2017). A review of the strain diversity and pathogenesis of chicken astrovirus. Viruses, 9, 29.
- Songserm, T., Pol, J.M.A., van Roozelaar, D., Kok, G.L., Wagenaar, F. & ter Huurne, A. (2000). A comparative study of the pathogenesis of malabsorption syndrome in broilers. Avian Diseases, 44, 556–567.
- Songserm, T., van Roozelaar, D., Kant, A., Pol, J., Pijpers, A. & ter Huurne, A. (2003). Enteropathogenicity of Dutch and German avian reoviruses in SPF White Leghorn chickens and broilers. Veterinary Research, 34, 285–295.
- Spackman, E., Day, J.M. & Pantin-Jackwood, M.J. (2010). Astrovirus, reovirus, and rotavirus concomitant infection causes decreased weight gain in broad-breasted white poults. Avian Diseases, 54, 16–21.
- Ter Veen, C., de Bruijn, N.D., Dijkman, R. & de Wit, J.J. (2017). Prevalence of histopathological intestinal lesions and enteric pathogens in Dutch commercial broilers with time. Avian Pathology, 46, 95–105.
- Walker, E., Friedman, M.H. & Olson, N.O. (1972). Electron microscopic study of an avian reovirus that causes arthritis. Journal of Ultrastructure Research, 41, 67–79.
- Wellehan, J.F.X., Johnson, A.J., Harrach, B., Benkö, M., Pessier, A.P., Johnson, C.M., Garner, M.M., Childress, A. & Jacobson, E.R. (2004). Detection and analysis of six lizard adenoviruses by consensus primer PCR provides further evidence of a reptilian origin for the atadenoviruses. Journal of Virology, 78, 13366–13369.
- Zhang, Y., Feng, B., Xie, Z., Deng, X., Zhang, M., Xie, Z., Xie, L., Fan, Q., Luo, S., Zeng, T., Huang, J. & Wang, S. (2020). Epidemiological surveillance of parvoviruses in commercial chicken and turkey farms in Guangxi, Southern China, during 2014-2019. Frontiers in Veterinary Science, 7, 561371.
- Zsak, L., Cha, R.M. & Day, J.M. (2013). Chicken parvovirus-induced runting-stunting syndrome in young broilers. Avian Diseases, 57, 123–127.
- Zsak, L., Strother, K.O. & Day, J.M. (2009). Development of a polymerase chain reaction procedure for detection of chicken and turkey parvoviruses. Avian Diseases, 53, 83–88.