ABSTRACT
To flexibly adjust behaviour to that of other people around us requires some representation of their overt actions, but also of the driving forces behind them, that is, their goals, intentions, and emotions. Socio-affective and -cognitive functions enable such representations via creating vicarious affective states in the observer (empathy) or by accumulating abstract, propositional knowledge of another person’s mental state (Theory of Mind). While the empathic sharing of another’s emotions is implemented by those neural networks that also process first-hand emotion, Theory of Mind activates a widespread network that seems to process information independent of its specific modality or content. Crucially, these two routes can function independently as individual differences in the respective capacities and network activations are unrelated and selective impairments in one or the other function occur in psychopathology. However, they may co-activate and co-operate in complex social situations, determining how prosocially interactive behaviour unfolds.
Introduction
Humans encounter and interact with others, their conspecifics, on a continual basis in a multitude of social relations. Being able to flexibly adjust one’s own behaviour to that of others requires some representation of the other’s actions and, ideally, the motivation behind them, that is, the other’s emotions and thoughts (Adolphs Citation2003; Keysers and Gazzola Citation2009). Social psychological and neuroscience research over the last few years has identified at least two routes to such representation: an affective route that allows us to feel with others (that is, empathy; de Vignemont and Singer Citation2006) and a cognitive route that enables understanding of others’ mental states (that is, Theory of Mind [ToM] or mentalizing; Frith and Frith Citation2005). The present review describes these two routes, including their location within the larger domain of social psychological processes, outlines the neural mechanisms underlying them, and characterizes their interplay during interaction with others. Crucial evidence regarding these questions also comes from alterations of socio-affective and -cognitive functioning in psychopathology and from the relations to actual (pro-)social behaviour.
The social mind
Humans are certainly not the only social species, but the complexity and flexibility of our social interactions are unrivalled. In comparison to chimpanzees, our closest living biological relatives, even children are more ready to co-operate with and rely on their conspecifics (e.g. see van Leeuwen, Call, and Haun Citation2014). The affective and cognitive processes that enable complexity in human social interactions are manifold. Many of them are shared with other species but may vary in the sophistication of their development, such as affiliation, emotion contagion, or some forms of perspective taking or ToM (de Waal Citation2011; Bugnyar, Reber, and Buckner Citation2016). Others have been speculated to be uniquely human, such as aspects of language and the processing of hierarchical structures (Fitch Citation2017).
Theoretical accounts vary widely on which processes are deemed crucial for social interaction (Happe, Cook, and Bird Citation2017). These may range from basic processes like action understanding (Mier et al. Citation2010), social attention, and memory, to higher-order functions such as social inference and attitudes (Fiske and Taylor Citation2013). To give an example based on a developmental perspective, the following compilation of processes has been suggested: affiliation, agent identification, emotion processing, empathy, individuals’ information store, mental state attribution, self-processing, social hierarchy mapping, social policing, and in-group/out-group categorization (Happe and Frith Citation2014). How these processes are related to one another seems even less clear (Happe, Cook, and Bird Citation2017). Across accounts, there is consensus, however, that empathy and ToM are essential elements of higher-level social processing, as they enable access to another person’s inner states.
Socio-affective route
Being with others is one of the major triggers of emotions. These social emotions depend on the situation and on others’ emotions, but to a large extent they depend on the person him/herself. Witnessing another’s misfortune may provoke a shared sadness in one observer and a feeling of schadenfreude in another, a compassionate response, linked with the desire to help, in one or indifference, and no emotion altogether in another. While schadenfreude and compassion are social emotions, they are in a sense complementary to those of the person eliciting them. Other examples are envy, jealousy, guilt and shame, embarrassment, and possibly pride (Feldman Barrett, Lewis, and Haviland-Jones Citation2016). Critically, the emotional state of the interaction partners differs; your anger goes along with my guilt. Empathy, in contrast, denotes the sharing of another’s affect, thus leading to isomorphic emotional states in the experiencer and the observer (de Vignemont and Singer Citation2006). I can share both your grief and your joy. It is therefore not surprising that neuroimaging studies have not revealed a specific network related to empathy. Rather, different networks are involved in sharing different emotions. While another’s pain activates the anterior part of the insular and cingulate cortices (Singer et al. Citation2004; Carr et al. Citation2003), sharing others’ joy activates regions in the ventral striatum (Mobbs et al. Citation2009). The fact that these networks coincide with those that are active during the first-hand experience of the respective emotions has been interpreted as shared networks supporting the sharing of emotions (de Vignemont and Singer Citation2006).
By creating a state in the observer that resembles that of another person, empathy enables access to the other’s inner conditions. Interestingly, such sharing of others’ emotions seems to be spontaneous and happens without the explicit instruction to do so (in healthy individuals, cf. Meffert et al. Citation2013). However, to date, it is unclear whether and, if so, how exactly this information is read out by more controlled, cognitive understanding of mental states, that is, mentalizing or ToM.
Socio-cognitive route
To understand what another person thinks or feels requires more abstract, propositional representations of that state than mere sharing of another’s condition would allow. During human ontogeny, we develop the capacity for such a ‘Theory of Mind’, the ability to infer and reason about others’ perceptions, beliefs, thoughts, and emotions (Frith and Frith Citation2005; see also Perner, Priewasser, and Roessler Citation2018). While elements of ToM develop already earlier in life (Kovács, Téglás, and Endress Citation2010), the critical test whether another person’s beliefs about the world can be represented and named if they deviate from their own beliefs is not passed before the age of four or five years (Wimmer and Perner Citation1983).
The neural network related to ToM largely differs from those brain regions involved in empathy. Across different ToM operationalizations, the temporoparietal junction is consistently activated together with the medial prefrontal cortex (Schurz et al. Citation2014). However, a larger network including the posterior cingulate cortex/precuneus, superior temporal gyrus, and the temporal poles is active for mental state understanding in more realistically complex scenarios (Wolf, Dziobek, and Heekeren Citation2010). Interestingly, each of the different ToM operationalizations activates a different portion of this larger network (see Schneider et al. Citation2014; Saxe and Kanwisher Citation2003; Mitchell, Heatherton, and Macrae Citation2002), suggesting that they differentially tap into the processes constituting full-blown ToM (Schurz and Perner Citation2015).
As emotion sharing is enabled by the neural networks involved in the first-hand emotion experience, the ToM network is also activated for a multitude of other processes. Most prominently, the same regions have reliably been observed during passive control conditions in externally oriented tasks, which led to their denomination as default mode network (Raichle et al. Citation2001) and further specification as crucial for self-generated thought or mind-wandering (Andrews-Hanna, Smallwood, and Spreng Citation2014). Parts of it, in particular, in the medial prefrontal cortex, have also been associated with metacognition, the ability to think about and monitor one’s own cognitive processes (Baird et al. Citation2013). What self-generated thought, metacognition, and ToM seem to share is a focus on internal processes, be they related to own mental states or to those of others.
Disentangling socio-affective and -cognitive functions
Independent capacities
The socio-affective and -cognitive functions described above are conceptually separable and have been investigated mainly in distinct lines of research. But how do they relate to each other? Are strong empathizers also proficient mentalizers? And do the different networks co-activate in complex social settings that require both functions? A recently developed experimental paradigm simulates such complex situations of social understanding and involves participants viewing short autobiographical narrations that vary in emotionality and ToM demands (; Kanske, Böckler, et al. Citation2015). Participants judge the valence of their emotions, probing the empathic sharing of others’ affect, and indicate how much compassion they feel for the narrator. Questions about the narrator’s thoughts probe participants’ ToM capacities. A final rating asks about the confidence in having responded correctly to the previous ToM question, giving an indication of metacognitive accuracy. This study shows that distinct neural networks associated with each of these functions can be isolated within-subject, within-task (for a schematic depiction, see ; Kanske, Böckler, et al. Citation2015; Molenberghs et al. Citation2016). The propensity to empathise with or mentalize on the narrator is also associated with structural brain differences in cortical thickness in these networks (Valk et al. Citation2016, Citation2017). Crucially, the task allows probing whether empathizing and mentalizing are directly related, and thus, whether there is a general social capacity. The answer is clearly no; there is no association on a behavioural or neural level, showing that strong empathizers are not necessarily good mentalizers (Kanske et al. Citation2016).
Figure 1. Depiction of events in a trial of the EmpaToM task with an example story and question in the lower row (emotional, ToM condition). Adapted from Kanske, Böckler, et al. (Citation2015).
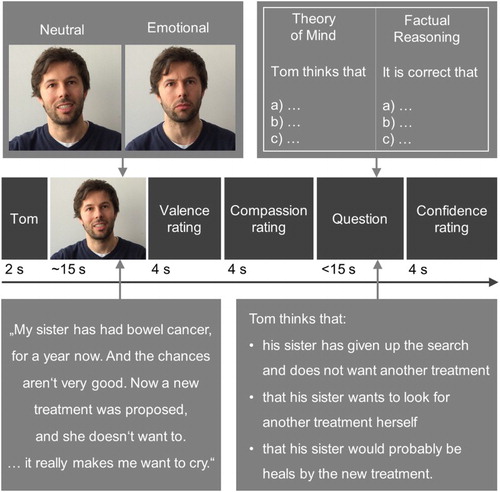
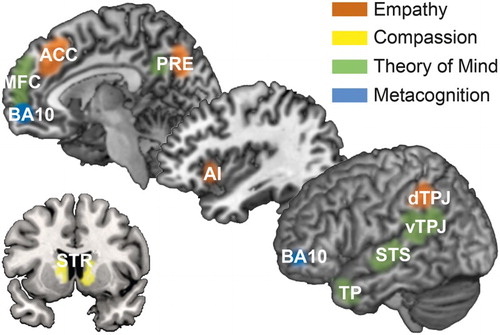
Figure 2. Schematic of the neural networks supporting empathy, compassion, ToM, and metacognition. Anterior cingulate cortex (ACC), anterior insula (AI), Brodmann area 10 (BA10), dorsal/ventral temporoparietal junction (d/vTPJ), medial frontal cortex (MFC), precuneus/posterior cingulate cortex (PRE), striatum (STR), superior temporal sulcus (STS), and temporal poles (TP). Adapted from Kanske, Böckler, and Singer (Citation2017).
If empathy and ToM can function independently, then it should be possible to see selective impairments or changes in either one of them. Indeed, impairments in ToM have been consistently described for patients with autism spectrum disorder (Frith Citation2001) or in ageing (Reiter et al. Citation2017), while empathy seems to be preserved, when controlling for alexithymic deficits (Bird et al. Citation2010). Psychopathic individuals and aggressive criminal offenders, in contrast, show empathy deficits (Meffert et al. Citation2013) without concurrent changes in ToM (Winter et al. Citation2017).
Interactions of empathy and tom
Complex real-life social situations will require the joint, simultaneous, and possibly interactive activation of the different neural networks associated with empathy and ToM. Indirect evidence for such joint activity comes from a meta-analysis on empathy for others’ pain (Lamm, Decety, and Singer Citation2011). Here, the empathy-related network is activated both when we directly see somebody else in pain and when we have to deduce this from an abstract cue symbol. However, in contrast to direct pain presentation, the abstract cues additionally activate the ToM-related network, putatively because the other’s state needs to be inferred from the cues. The affective representation of the other’s pain in the anterior insula would then be secondary and follow that of the ToM-related network.
Co-activation of the two networks is also observed in primary studies that involve participants in complex social tasks. For instance, when observing social exclusion, empathizing may again follow the cognitive understanding of the consequences of the situation for the other, resulting in activation of both networks (Masten, Morelli, and Eisenberger Citation2011). Empathic accuracy studies, in which emotional narrations of others are to be continuously judged regarding the emotional state of the narrator, also jointly activate the empathy- and ToM-related networks (Zaki et al. Citation2009). However, due to the direct accessibility of the other’s emotion, empathic sharing may in this case precede and inform the higher-level cognitive judgment of the other’s affective state.
Empathy for others’ suffering constitutes a particular case, because it entails an emotionally negative, aversive state in the observer and may, therefore, cause the same consequences as the first-hand negative emotion, such as impaired cognitive functioning (Wessa et al. Citation2013). Using the experimental paradigm described above () allows direct testing of the consequences of sharing negative affect on ToM. On a neural level, mentalizing-related activity in the temporoparietal junction is indeed inhibited by empathy-related activation in the anterior insula, when the video content is emotionally negative (Kanske et al. Citation2016). On a behavioural level, this inhibition comes with impairments in ToM performance. This pattern of network interaction resembles that of the task-control or salience network and the default mode network in situations of high external task demands (Menon and Uddin Citation2010; Trautwein, Singer, and Kanske Citation2016; Bonhage et al. Citation2016). The adaptive function may be to prioritize the processing of those aspects of a situation that require immediate reaction, such as others’ emotion. The pattern is, however, also reminiscent of increased emotional distraction in psychopathology (Kanske et al. Citation2013; Kanske, Schonfelder, et al. Citation2015) and the hypothesis of stress-impaired mentalization, particularly in borderline personality disorder (Fonagy and Luyten Citation2009; Mier et al. Citation2013). Impaired emotion regulation and the resulting ‘empathic distress’ (Singer and Klimecki Citation2014) may, therefore, lead to a chronified ToM inhibition in psychopathology.
Consequences for social behaviour
Understanding what the emotional consequences of our actions for another person are and sharing these emotions may enable more flexible and prosocial behaviour. Several studies have verified the role of empathy and ToM for prosociality (Masten, Morelli, and Eisenberger Citation2011; Klimecki et al. Citation2016; Hare et al. Citation2010). Specifically, costly helping of others in need is predicted by both activation in the anterior insula and the temporoparietal junction (Tusche et al. Citation2016). The weight that empathy for or cognitive understanding of the other in need had in the social decision to help was selectively reflected by these regions’ activation patterns, respectively. The same held true when empathy and ToM were assessed independently of the specific helping situation, suggesting that stable, individual propensities to engage one or the other of these processes determine social behaviour.
Considering the complex context in which social interactions take place in real life, a number of other important factors play a role. A direct, static relation of, for instance, empathy to prosocial behaviour seems to be an oversimplification. A particularly important factor is group membership. The spontaneous empathic response is reduced for members of another group, which goes along with decreased helping (Hein et al. Citation2010). Moreover, the suffering of out-group members may even elicit pleasurable emotions such as schadenfreude (Cikara et al. Citation2014). Because of the putative relation to increased in-group empathy, these negative outcomes have been discussed as ‘dark sides of empathy’ (Breithaupt Citation2017, Citation2018).
However, there are studies showing that empathy cannot only be trained but even improved in inter-group settings. Pharmacologically, the administration of oxytocin, a peptide hormone, increased sensitivity to the pain of Palestinians in Jewish Israeli participants (Shamay-Tsoory et al. Citation2013). Also, the repeated experience of being helped by an out-group member increases empathy for members of other groups (Hein et al. Citation2016). Another approach has been to train individuals’ capacities to cultivate positive emotions of compassion and concern for others in need, which also increases prosocial behaviour (Leiberg, Klimecki, and Singer Citation2011; Klimecki et al. Citation2014).
As research proceeds in answering how we represent not only the overt, behavioural but also the covert, mental states of others, it becomes evident that the challenges lying ahead are to (1) study the different underlying mechanisms in concert, ideally in truly interactive settings, (2) investigate their developmental origins to illuminate the causes of impairments, and (3) probe their malleability via training.
Acknowledgements
I wish to thank Tania Singer, Anne Böckler-Raettig, Fynn-Mathis Trautwein, Katrin Preckel, and Matthias Schurz for the insightful discussions on social affective and cognitive processes that have greatly stimulated the writing of this article.
Disclosure statement
No potential conflict of interest was reported by the author.
Notes on contributor
Philipp Kanske is a neuroscientist and psychotherapist whose research focuses on emotion–cognition interactions such as emotional attention, emotion regulation, empathy and mentalizing. Using neuroimaging methods, he also studies these functions in psychopathology to further our understanding of impairments and find starting points for intervention. He is a graduate of the University of Oregon (MSc), Technische Universität Dresden (Dipl.-Psych.), University of Leipzig (PhD) and Heidelberg University (Habilitation).
References
- Adolphs, R. 2003. “Cognitive Neuroscience of Human Social Behaviour.” Nature Reviews Neuroscience 4 (3): 165–178. doi:10.1038/nrn1056.
- Andrews-Hanna, J. R., J. Smallwood, and R. N. Spreng. 2014. “The Default Network and Self-Generated Thought: Component Processes, Dynamic Control, and Clinical Relevance.” Annals of the New York Academy of Sciences 1316: 29–52. doi:10.1111/nyas.12360.
- Baird, B., J. Smallwood, K. J. Gorgolewski, and D. S. Margulies. 2013. “Medial and Lateral Networks in Anterior Prefrontal Cortex Support Metacognitive Ability for Memory and Perception.” Journal of Neuroscience 33 (42): 16657–16665. doi:10.1523/JNEUROSCI.0786-13.2013.
- Bird, G., G. Silani, R. Brindley, S. White, U. Frith, and T. Singer. 2010. “Empathic Brain Responses in Insula are Modulated by Levels of Alexithymia But Not Autism.” Brain 133 (Pt 5): 1515–1525. doi:10.1093/brain/awq060.
- Bonhage, C., F. Weber, C. Exner, and P. Kanske. 2016. “Thinking About Thinking: Neural Mechanisms and Effects on Memory.” NeuroImage 127: 203–214. doi:10.1016/j.neuroimage.2015.11.067.
- Breithaupt, F. 2017. Die dunklen Seiten der Empathie. Berlin: Suhrkamp.
- Breithaupt, F. 2018. “The Bad Things We Do Because of Empathy.” Interdisciplinary Science Reviews. doi:10.1080/03080188.2018.1450928.
- Bugnyar, T., S. A. Reber, and C. Buckner. 2016. “Ravens Attribute Visual Access to Unseen Competitors.” Nature Communications 7: 10506. doi:10.1038/ncomms10506.
- Carr, L., M. Iacoboni, M. C. Dubeau, J. C. Mazziotta, and G. L. Lenzi. 2003. “Neural Mechanisms of Empathy in Humans: A Relay From Neural Systems for Imitation to Limbic Areas.” Proceedings of the National Academy of Sciences of the USA 100 (9): 5497–5502. doi:10.1073/pnas.0935845100.
- Cikara, M., E. Bruneau, J. J. Van Bavel, and R. Saxe. 2014. “Their Pain Gives Us Pleasure: How Intergroup Dynamics Shape Empathic Failures and Counter-Empathic Responses.” Journal of Experimental Social Psychology 55: 110–125. doi:10.1016/j.jesp.2014.06.007.
- de Vignemont, F., and T. Singer. 2006. “The Empathic Brain: How, When and Why?” Trends in Cognitive Sciences 10 (10): 435–441. doi:10.1016/j.tics.2006.08.008.
- de Waal, F. B. M. 2011. “What is an Animal Emotion?” Year in Cognitive Neuroscience 1224: 191–206. doi:10.1111/j.1749-6632.2010.05912.x.
- Feldman Barrett, L., M. Lewis, and J. M. Haviland-Jones. 2016. Handbook of Emotions. New York: Guilford Press.
- Fiske, S. T., and S. E. Taylor. 2013. Social Cognition: From Brains to Culture. London: Sage.
- Fitch, W. T. 2017. “Empirical Approaches to the Study of Language Evolution.” Psychonomic Bulletin & Review 24 (1): 3–33. doi:10.3758/s13423-017-1236-5.
- Fonagy, P., and P. Luyten. 2009. “A Developmental, Mentalization-Based Approach to the Understanding and Treatment of Borderline Personality Disorder.” Development and Psychopathology 21 (4): 1355–1381. doi:10.1017/S0954579409990198.
- Frith, U. 2001. “Mind Blindness and the Brain in Autism.” Neuron 32 (6): 969–979. doi: 10.1016/S0896-6273(01)00552-9
- Frith, C., and U. Frith. 2005. “Theory of Mind.” Current Biology 15 (17): R644–R645. doi:10.1016/j.cub.2005.08.041.
- Happe, F., J. L. Cook, and G. Bird. 2017. “The Structure of Social Cognition: In(ter)Dependence of Sociocognitive Processes.” Annual Review of Psychology 68: 243–267. doi:10.1146/annurev-psych-010416-044046.
- Happe, F., and U. Frith. 2014. “Annual Research Review: Towards a Developmental Neuroscience of Atypical Social Cognition.” Journal of Child Psychology and Psychiatry 55 (6): 553–577. doi:10.1111/jcpp.12162.
- Hare, T. A., C. F. Camerer, D. T. Knoepfle, J. P. O'Doherty, and A. Rangel. 2010. “Value Computations in Ventral Medial Prefrontal Cortex During Charitable Decision Making Incorporate Input from Regions Involved in Social Cognition.” The Journal of Neuroscience 30 (2): 583–590. doi:10.1523/JNEUROSCI.4089-09.2010.
- Hein, G., J. B. Engelmann, M. C. Vollberg, and P. N. Tobler. 2016. “How Learning Shapes the Empathic Brain.” Proceedings of the National Academy of Sciences of the United States of America 113 (1): 80–85. doi:10.1073/pnas.1514539112.
- Hein, G., G. Silani, K. Preuschoff, C. D. Batson, and T. Singer. 2010. “Neural Responses to Ingroup and Outgroup Members’ Suffering Predict Individual Differences in Costly Helping.” Neuron 68 (1): 149–160. doi: 10.1016/j.neuron.2010.09.003
- Kanske, P., A. Böckler, and T. Singer. 2017. “Models, Mechanisms and Moderators Dissociating Empathy and Theory of Mind.” Current Topics in Behavioral Neurosciences 30: 193–206. doi:10.1007/7854_2015_412.
- Kanske, P., A. Böckler, F. M. Trautwein, F. H. Parianen Lesemann, and T. Singer. 2016. “Are Strong Empathizers Better Mentalizers? Evidence for Independence and Interaction Between the Routes of Social Cognition.” Social Cognitive and Affective Neuroscience 11: 1383–1392. doi:10.1093/scan/nsw052.
- Kanske, P., A. Böckler, F. M. Trautwein, and T. Singer. 2015. “Dissecting the Social Brain: Introducing the EmpaToM to Reveal Distinct Neural Networks and Brain–Behavior Relations for Empathy and Theory of Mind.” NeuroImage 122: 6–19. doi:0.1016/j.neuroimage.2015.07.082 doi: 10.1016/j.neuroimage.2015.07.082
- Kanske, P., J. Heissler, S. Schonfelder, J. Forneck, and M. Wessa. 2013. “Neural Correlates of Emotional Distractibility in Bipolar Disorder Patients, Unaffected Relatives, and Individuals with Hypomanic Personality.” The American Journal of Psychiatry 170 (12): 1487–1496. doi:10.1176/appi.ajp.2013.12081044.
- Kanske, P., S. Schonfelder, J. Forneck, and M. Wessa. 2015. “Impaired Regulation of Emotion: Neural Correlates of Reappraisal and Distraction in Bipolar Disorder and Unaffected Relatives.” Translational Psychiatry 5: e497. doi:10.1038/tp.2014.137.
- Keysers, C., and V. Gazzola. 2009. “Expanding the Mirror: Vicarious Activity for Actions, Emotions, and Sensations.” Current Opinion in Neurobiology 19 (6): 666–671. doi: 10.1016/j.conb.2009.10.006
- Klimecki, O. M., S. Leiberg, M. Ricard, and T. Singer. 2014. “Differential Pattern of Functional Brain Plasticity After Compassion and Empathy Training.” Social Cognitive and Affective Neuroscience 9 (6): 873–879. doi:10.1093/scan/nst060.
- Klimecki, O. M., S. V. Mayer, A. Jusyte, J. Scheeff, and M. Schonenberg. 2016. “Empathy Promotes Altruistic Behavior in Economic Interactions.” Scientific Reports 6: 31961. doi:10.1038/srep31961.
- Kovács, ÁM, E. Téglás, and A. D. Endress. 2010. “The Social Sense: Susceptibility to Others’ Beliefs in Human Infants and Adults.” Science 330 (6012): 1830–1834. doi:10.1126/science.1190792.
- Lamm, C., J. Decety, and T. Singer. 2011. “Meta-analytic Evidence for Common and Distinct Neural Networks Associated with Directly Experienced Pain and Empathy for Pain.” NeuroImage 54 (3): 2492–2502. doi:10.1016/j.neuroimage.2010.10.014.
- Leiberg, S., O. Klimecki, and T. Singer. 2011. “Short-term Compassion Training Increases Prosocial Behavior in a Newly Developed Prosocial Game.” PLoS One 6 (3): e17798. doi:10.1371/journal.pone.0017798.
- Masten, C. L., S. A. Morelli, and N. I. Eisenberger. 2011. “An fMRI Investigation of Empathy for “Social Pain” and Subsequent Prosocial Behavior.” NeuroImage 55 (1): 381–388. doi:10.1016/j.neuroimage.2010.11.060.
- Meffert, H., V. Gazzola, J. A. den Boer, A. A. J. Bartels, and C. Keysers. 2013. “Reduced Spontaneous But Relatively Normal Deliberate Vicarious Representations in Psychopathy.” Brain 136 (8): 2550–2562. doi:10.1093/brain/awt190.
- Menon, V., and L. Q. Uddin. 2010. “Saliency, Switching, Attention and Control: A Network Model of Insula Function.” Brain Structure and Function 214 (5–6): 655–667. doi:10.1007/s00429-010-0262-0.
- Mier, D., S. Lis, C. Esslinger, C. Sauer, M. Hagenhoff, J. Ulferts, B. Gallhofer, and P. Kirsch. 2013. “Neuronal Correlates of Social Cognition in Borderline Personality Disorder.” Social Cognitive and Affective Neuroscience 8 (5): 531–537. doi: 10.1093/scan/nss028
- Mier, D., S. Lis, K. Neuthe, C. Sauer, C. Esslinger, B. Gallhofer, and P. Kirsch. 2010. “The Involvement of Emotion Recognition in Affective Theory of Mind.” Psychophysiology 47 (6): 1028–1039. doi:10.1111/j.1469-8986.2010.01031.x.
- Mitchell, J. P., T. F. Heatherton, and C. N. Macrae. 2002. “Distinct Neural Systems Subserve Person and Object Knowledge.” Proceedings of the National Academy of Sciences of the USA 99 (23): 15238–15243. doi:10.1073/pnas.232395699.
- Mobbs, D., R. Yu, M. Meyer, L. Passamonti, B. Seymour, A. J. Calder, S. Schweizer, C. D. Frith, and T. Dalgleish. 2009. “A Key Role for Similarity in Vicarious Reward.” Science 324 (5929): 900. doi:10.1126/science.1170539.
- Molenberghs, P., F. M. Trautwein, A. Bockler, T. Singer, and P. Kanske. 2016. “Neural Correlates of Metacognitive Ability and of Feeling Confident: A Large-Scale fMRI Study.” Social Cognitive and Affective Neuroscience 11 (12): 1942–1951. doi:10.1093/scan/nsw093.
- Perner, J., B. Priewasser, and J. Roessler. 2018. “The Practical Other: Teleology and Its Development.” Interdisciplinary Science Reviews. doi:10.1080/03080188.2018.1453246.
- Raichle, M. E., A. M. MacLeod, A. Z. Snyder, W. J. Powers, D. A. Gusnard, and G. L. Shulman. 2001. “A Default Mode of Brain Function.” Proceedings of the National Academy of Sciences of the USA 98 (2): 676–682. doi:10.1073/pnas.98.2.676.
- Reiter, A. M. F., P. Kanske, B. Eppinger, and S. C. Li. 2017. “The Aging of the Social Mind: Differential Effects on Components of Social Understanding.” Scientific Reports 7 (1): 11046. doi:10.1038/s41598-017-10669-4.
- Saxe, R., and N. Kanwisher. 2003. “People Thinking About Thinking People: The Role of the Temporo-Parietal Junction in “Theory of Mind”.” NeuroImage 19 (4): 1835–1842. doi: 10.1016/S1053-8119(03)00230-1
- Schneider, D., V. P. Slaughter, S. I. Becker, and P. E. Dux. 2014. “Implicit False-Belief Processing in the Human Brain.” NeuroImage 101: 268–275. doi:10.1016/j.neuroimage.2014.07.014.
- Schurz, M., and J. Perner. 2015. “An Evaluation of Neurocognitive Models of Theory of Mind.” Frontiers in Psychology 6: 1610. doi:10.3389/fpsyg.2015.01610.
- Schurz, M., J. Radua, M. Aichhorn, F. Richlan, and J. Perner. 2014. “Fractionating Theory of Mind: A Meta-Analysis of Functional Brain Imaging Studies.” Neuroscience and Biobehavioral Reviews 42C: 9–34. doi:10.1016/j.neubiorev.2014.01.009.
- Shamay-Tsoory, S. G., A. Abu-Akel, S. Palgi, R. Sulieman, M. Fischer-Shofty, Y. Levkovitz, and J. Decety. 2013. “Giving Peace a Chance: Oxytocin Increases Empathy to Pain in the Context of the Israeli-Palestinian Conflict.” Psychoneuroendocrinology 38 (12): 3139–3144. doi:10.1016/j.psyneuen.2013.09.015.
- Singer, T., and O. M. Klimecki. 2014. “Empathy and Compassion.” Current Biology 24 (18): R875–R878. doi:10.1016/j.cub.2014.06.054.
- Singer, T., B. Seymour, J. O’Doherty, H. Kaube, R. J. Dolan, and C. D. Frith. 2004. “Empathy for Pain Involves the Affective But Not Sensory Components of Pain.” Science 303 (5661): 1157–1162. doi:10.1126/science.1093535.
- Trautwein, F. M., T. Singer, and P. Kanske. 2016. “Stimulus-Driven Reorienting Impairs Executive Control of Attention: Evidence for a Common Bottleneck in Anterior Insula.” Cerebral Cortex 26 (11): 4136–4147. doi: 10.1093/cercor/bhw225
- Tusche, A., A. Bockler, P. Kanske, F. M. Trautwein, and T. Singer. 2016. “Decoding the Charitable Brain: Empathy, Perspective Taking, and Attention Shifts Differentially Predict Altruistic Giving.” Journal of Neuroscience 36 (17): 4719–4732. doi:10.1523/JNEUROSCI.3392-15.2016.
- Valk, S. L., B. C. Bernhardt, A. Bockler, P. Kanske, and T. Singer. 2016. “Substrates of Metacognition on Perception and Metacognition on Higher-Order Cognition Relate to Different Subsystems of the Mentalizing Network.” Human Brain Mapping 37 (10): 3388–3399. doi:10.1002/hbm.23247.
- Valk, S. L., B. C. Bernhardt, A. Bockler, F. M. Trautwein, P. Kanske, and T. Singer. 2017. “Socio-Cognitive Phenotypes Differentially Modulate Large-Scale Structural Covariance Networks.” Cerebral Cortex 27 (2): 1358–1368.
- van Leeuwen, E. J. C., J. Call, and D. B. M. Haun. 2014. “Human Children Rely More on Social Information Than Chimpanzees Do.” Biology Letters 10 (11). doi:10.1098/rsbl.2014.0487.
- Wessa, M., J. Heissler, S. Schonfelder, and P. Kanske. 2013. “Goal-Directed Behavior Under Emotional Distraction is Preserved by Enhanced Task-Specific Activation. Social Cognitive and Affective Neuroscience 8 (3): 305–312. doi:10.1093/scan/nsr098.
- Wimmer, H., and J. Perner. 1983. “Beliefs About Beliefs: Representation and Constraining Function of Wrong Beliefs in Young Children’s Understanding of Deception.” Cognition 13 (1): 103–128. doi:10.1016/0010-0277(83)90004-5.
- Winter, K., S. Spengler, F. Bermpohl, T. Singer, and P. Kanske. 2017. “Social Cognition in Aggressive Offenders: Impaired Empathy, But Intact Theory of Mind.” Scientific Reports 7 (1): 670. doi:10.1038/s41598-017-00745-0.
- Wolf, I., I. Dziobek, and H. R. Heekeren. 2010. “Neural Correlates of Social Cognition in Naturalistic Settings: A Model-Free Analysis Approach.” NeuroImage 49 (1): 894–904. doi: 10.1016/j.neuroimage.2009.08.060
- Zaki, J., J. Weber, N. Bolger, and K. Ochsner. 2009. “The Neural Bases of Empathic Accuracy.” Proceedings of the National Academy of Sciences of the USA 106 (27): 11382–11387. doi:10.1073/pnas.0902666106.
