Abstract
There has been a marked rise in the number of avoidable deaths in health services around the world. At the same time there has been a growing increase in antibiotic resistant so-called “superbugs.” We examine here the potential role of body temperature measurement in these adverse trends. Electronic based thermometers have replaced traditional mercury (and other liquid-in-glass type) thermometers for reasons of safety rather than superiority. Electronic thermometers are in general less robust from a measurement perspective than their predecessors. We illustrate the implications of unreliable temperature measurement on the diagnosis and management of disease, including COVID-19, through statistical calculations. Since a return to mercury thermometers is both undesirable and impractical, we call for better governance in the current practice of clinical thermometry to ensure the traceability and long-term accuracy of electronic thermometers and discuss how this could be achieved.
1. Introduction
Accurate body temperature measurement is key to detect the presence or absence of high temperature or fever (and in the case of sepsis, sometimes hypothermia) [Citation1]. It is one of the major components of the diagnosis and management of suspected infection, including the decision to prescribe antibiotics, or to step up care (e.g., via referral from primary to secondary care). If the temperature is inaccurately measured and fever is incorrectly detected, doctors may incorrectly believe that bacterial infection is present and antibiotics may be prescribed, adding to the risk of antibiotic resistance. Alternatively, if a fever is not detected, and a true bacterial infection or even sepsis is missed, then a patient may not receive timely treatment, leading to additional morbidity or even mortality. Fever can be the only early sign of sepsis, particularly in immunocompromised patients [Citation2], and hypothermia in sepsis can be an indicator of increased risk of severe illness and death [Citation1]. Although not the sole indicator of sepsis (see NICE sepsis pathways http://pathways.nice.org.uk/pathways/sepsis) it is nevertheless particularly important that accurate temperature measurement is made if sepsis is suspected. Incorrect temperature measurement in either case increases the likelihood of an adverse patient outcome, which may lead to less effective clinical interventions.
Despite modern hygiene and antibiotics, acute infection remains a considerable cause of both morbidity and mortality. Pneumonia and influenza alone were responsible for 5.4% of all deaths in England and Wales in 2018 [Citation3] and are the 4th most common cause of death in young children [Citation4], while antepartum infections still account for around 10% of neonatal deaths [Citation5].
Clinical guidelines are used to support decision making. Where these include temperature measurements, they typically use one or more thresholds to determine which patients require intervention, which might include antibiotics, stepping up of care, or further investigation. Examples of these guidelines include the NHS NEWS2Footnote1 score [Citation6], the UK COVID-19 case definition [Citation7], and the Systemic Inflammatory Response Syndrome (SIRS) criteria for defining sepsis [Citation8].
In the case of the containment and management of a pandemic infection, both the issues of missed or delayed diagnosis, and additional resource use are of particular importance. In both pandemic influenza (“swine flu”) [Citation9] and COVID-19 [Citation7], the presence of fever has been a key diagnostic marker used to identify cases. In particular in the latter case a modest fever threshold of 37.8 °C was set. It is clear that accurate body temperature measurement for triaging suspected infected individuals is key to managing and containing such widespread infectious diseases.
In response to the issue of unreliable body temperature measurement the National Body Temperature Measurement Group was formed on 29 April 2019. The authors of this paper are the members of that group. The mission statement of the group is “to ensure robust and reliable body temperature measurement throughout the NHS and wider community.”
In this paper we describe how unreliable clinical thermometry has arisen. We discuss the health implications and we outline possible solutions as to how this situation can be addressed. Some of the findings discussed in this paper have special relevance to the effectiveness of body temperature measurement for the containment of infectious diseases and so has direct application in pandemic situations where fever is a key indicator.
2. Unreliable body temperature measurement
2.1. Background
The rise in unreliable body temperature measurement has coincided with the introduction of electronic based clinical thermometers and the phasing out of mercury thermometersFootnote2.
Mercury thermometers were the ubiquitous temperature measurement device in health services throughout the world before the 1980s, and had a number of advantages, which were:
That they were established for a long time in clinical practice.
Exacting international standards and a chain of measurement traceability [Citation10] existed for producing reliable clinical mercury thermometers.
That the mercury thermometers were not subject to significant drift in use or with time in storage, so that thermometers were generally both accurate at time of construction and throughout their useful lifetime.
When acute damage happened, it was likely to be immediately visible (e.g., a bubble in the mercury column or other visible defect) and therefore a device either gave the right body temperature, or was visibly unusable.
The use of mercury thermometers was well established and understood by the medical profession.
However, with the (justified) concern about toxic effects of mercury from broken thermometers as well as the more immediate concerns of broken glass causing injury to patients and staff, such thermometers were replaced with electronic based devices which operated at a number of different anatomical sites and in a number of different ways. But this step, whilst appropriate for patient and staff safety, may well have been detrimental for reliable body temperature measurement as many of the advantages of mercury thermometers were either compromised or lost. This is unfortunate because the requirement for low uncertainty body temperature measurement remains, either for one-off temperature measurement for fever detection in infectious disease triaging or for building a reliable diagnosis score when using guidelines such as NEWS2.
2.2. Electronic temperature measurement devices
Modern electronic clinical thermometers are largely based on two technical approaches, non-contact or contact thermometry.
Non-contact thermometers sense the infra-red radiation emitted from the body measurement site and convert that measurement by an algorithm into a temperature. This is the basis of tympanic and forehead thermometers as well as thermal imaging.
Contact thermometers sense a physical quantity (usually electrical resistance) of the sensor when in contact with the body measurement site and converts that measurement into a temperature – this is the basis of oral, rectal and axillary thermometry. In fact, modern contact thermometers generally do not wait to measure body temperature at equilibrium, but algorithmically predict it,Footnote3 in order to save clinical time.
All these devices have an uncertainty when new (as does any measurement device). For purposes of clarity we are using the term uncertainty to mean a “non-negative parameter characterizing the dispersion of the quantity values being attributed to a measurand, based on the information used” which is the definition given in the International Vocabulary of Metrology [Citation11].
Over-reliance has been put on CE and other quality, or certification measures, as an indicator of performance. However, performance, even when new, cannot be guaranteed and will always have a spread in values for a given batch and type of thermometer.
For clinical thermometers, according to manufacturer’s information, stated uncertainty generally ranges from ± 0.1 °C to ± 0.3 °C. Unfortunately, not all manufacturers state whether this is one or two standard deviations and for the purposes of uniformity we assume that they mean two. Over time the device uncertainty changes, due to several factors. These could be due to unavoidable drifts in the electronics of the thermometer, or the device receiving a shock of some kind such as dropping on the floor or extended exposure to a strong heat source like sunlight or just through prolonged use without performance confirmation.
2.3. In hospital validation of thermometers
The fact that clinical thermometers drift in service is anticipated by at least some manufacturers who provide calibration sourcesFootnote4 with which to periodically check the performance of their thermometers. If such calibration sources are used on a routine and periodic basis this is certainly better than no checks. However, the calibration devices themselves need to be periodically calibrated and to ensure reliability of calibration, the calibration sources themselves should be calibrated by an accredited provider whose services are certified according to the international standard ISO17025:2017Footnote5 [Citation12]. Only such a provider gives the assurance that the calibrations they undertake are traceable to reliable national and international standards.Footnote6
3. Issues with the body temperature measurement sites
3.1. Background
The devices themselves are not the only source of uncertainty. The measurement site itself also contributes, sometimes significantly, to the final temperature uncertainty achieved, potentially doubling (or even more) the attained uncertainty of body temperature. The gold standard site for temperature measurement is the core and the optimum site is generally regarded as the temperature of pulmonary artery blood [Citation13]. However, because of the invasive nature of that measurement in routine clinical practice core body temperature is inferred from different sites around the body which are; tympanic membrane (ear), forehead (skin), oral, rectal or axilla (underarm). Each site, and indeed each thermometer modality, presents particular challenges for reliable thermometry (e.g., [Citation14,Citation15]).
Health care professionals are aware that temperature measurement differs by site, but the perception that this can be corrected by simple addition or subtraction of a constant correction factor is over-simplistic [Citation16] as a variety of issues affect measurement at each site. As a result the true uncertainty of any body temperature measurement is higher than the uncertainty given by the thermometer manufacturer because of the additional sources of uncertainty associated with the measurement.
3.2. Tympanic (ear) thermometry
Tympanic membrane thermometers are widely used in health services and by the general public. In use the measurement head of the thermometer is covered by a single-use plastic sheath which is disposed of after use. The thermometers operate by sensing the infra-red (thermal) radiation emitted by the tympanic membrane (or more usually the thermal radiation emitted by the lower ear canal and tympanic membrane). This is generally thought to be a good measurement site because blood flows from the hypothalamus to the tympanic membrane. However, the site has several issues which render it non-ideal for measuring body temperature. These are:
Ear canal geometry. The ear canal is not straight, but curved, generally obscuring at least part of the tympanic membrane. While appropriate “ear-tugging” can help to partially straighten the ear canal, and is recommended practice, even if one follows this process one is still not certain that the tympanic membrane is brought into unobscured view. This obscuration would lead to the thermometer reading a lower temperature because the sensed infra-red radiation is from both the lower ear canal and the tympanic membrane and not the tympanic membrane alone.
That wax in the ear can partially or fully block the view of the tympanic membrane, also giving a false low reading.
That fluid in the ear will generally fully block access to the tympanic membrane and since water based fluids are opaque to infra-red what is measured is the temperature of the fluid not the body.
These issues have led to variable performance of tympanic membrane thermometers in practice (e.g., [Citation13,Citation17]) nevertheless such thermometers are certainly the best understood and characterised of the clinical infra-red based thermometers.
3.3. Infra-red skin thermometry
Attempts have been made to infer body temperature from measuring the temperature of the skin. Some of these approaches relied on the use of chemical based patch thermometers. However, such thermometers were found not-fit-for purpose and are not recommended by the NHS for body temperature measurement [Citation18].
In principle the infra-red radiation emitted by skin can be used to measure its temperature. This is because the emissivity of skin is high (reported to be around 0.98 [see for e.g., [Citation19]]). However, in general skin temperature does not relate directly to body temperature. This is because:
Skin temperature is particularly different from core body temperature, varies significantly across the body and is influenced by environmental conditions.
The infra-red radiation sensed by a skin thermometer is a combination of that emitted by the skin and that reflected from the environmentFootnote7 which could lead to an excess high or low body temperature reading depending on the specific thermal environment of the patient.
Two body sites have been the particular focus of attention regarding body temperature measurement; the forehead and the inner canthi of the eye, the latter usually determined by thermal imaging.
3.3.1. Forehead thermometry
The forehead is proposed to be a good measurement site because the temporal artery feeds core blood to the temple. Thermometers have been developed specifically for measuring the temperature of the site of the temporal arteryFootnote8 (e.g., [Citation20,Citation21]). However the measurement site is prone to the two issues outlined previously, and a further issue, that of locating the actual site of the artery, is not feasible due to the variability of its location on the temple. In addition the relatively large target size of the thermometers themselves means that the thermometer targets a larger area than is actually taken up by the temporal artery site. More generally studies to date have found measurements at this site, either by temporal artery or more general forehead thermometers, to be an unreliable indicator of body temperature [Citation20,Citation22,Citation23]Footnote9. There is also evidence that at least some of the devices themselves have poor measurement characteristics [Citation24]. Such findings led to the MHRA issuing a press release [Citation25] warning not to use such devices for fever detection in the context of the COVID-19 pandemic.
3.3.2. Inner canthi temperature by thermal imaging
In principle thermal imaging using the inner canthi is an attractive option for measuring body temperature. However, it is prone to the same issues as outlined previously. In addition, most thermal imagers have a typical uncertainty of ±2 °C or 2% whichever is higher, which render them, without appropriate calibration, as unreliable for body/skin temperature measurement.
With appropriate design, validation/calibration processes, using a temperature-controlled environment and allowing the subject time to thermally equilibrate (recommended time 10-15 min [Citation26]), it is possible to reduce the uncertainty of skin temperature measurement with thermal imagers.
Thermal imagers can then, in principle, be used to detect people with fevers, there is even a standard [Citation27] that aims to do this reliably at airports, though we are not aware of any airports that actually follow the standard. That document recommends that the subject’s temperature be determined from viewing the inner canthus of the eye with a thermal imager. If the thermal imager is appropriately calibrated, preferably through use of a co-located or in image reference source [Citation28,Citation29] it may be possible that a good estimate of body temperature can be obtained [Citation30]. However, by way of caution, as far as the authors are aware, the correlation between inner canthi and body temperature has not yet been demonstrated with all aspects of the measurement traceable to reliable temperature standards.
3.4. Contact (oral, rectal and axilla) thermometryFootnote10
Electronic oral thermometers are available, usually with the sensing part of the thermometer covered with a single-use plastic sheath. The thermometer sensor itself, as with any contact thermometer, only measures its own temperature so it is very important that the thermometer is in good contact with the site being measured. This is one reason why axilla (armpit) temperature measurements are generally quite poor because of the poor contact of the site with the thermometer. Whilst not prone to the sources of uncertainty that beset infra-red based thermometers, such thermometers are relatively slowFootnote11 and require more intrusion than the other types. As with any electronic based device, they do require periodic recalibration to ensure that the temperatures they measure reliable.
4. Clinical practice of thermometry
For the purposes of clarity, we distinguish monitoring, involving repeated measurements on the same individual, from diagnosis and screening based on a measurement at a single point in time.
For monitoring (e.g., to identify a trend, or an abrupt change, in temperature) some forms of uncertainty may be acceptable: a thermometer may have an offset of 0.5 °C below actual patient temperature yet would be able to identify a trend (providing its response was linear with temperature). However, for diagnosis and screening (e.g., triage of patients arriving at an emergency department) it is not enough to identify a trend, and the true temperature in relation to diagnostic thresholds must be measured.
The most complex, but not uncommon, situation is that a patient journey goes from admission, then to treatment and finally to discharge. This involves measurement of temperature by different staff in multiple departments and could involve using different measurement devices and at different measurement sites. Due to the different mode of operation of electronic clinical thermometer types this can create a false picture of any underlying trend. Ideally once a particular thermometer and site has been used this should be repeated for subsequent measurements [Citation31] but this is not routinely done nor are the consequences well understood of not doing so. Comparability of all the thermometers used in that patient journey is essential for the later measurements to be interpreted correctly in reference to earlier measurements taken on admission.
Single point screening has different challenges. Here one is trying to determine whether a patient is above a certain decision criteria threshold. In this case the calibration (i.e., temperature performance) and effective use of the thermometer are critical to obtaining a reliable one-off determination of body temperature.
5. The implications of unreliable body temperature measurement
5.1. Background
We now consider the implication of introducing a thermometer into a clinical environment that has an uncertainty of ±0.3 °C (two standard deviation interval) which is a typical uncertainty of a new thermometer, and is in accordance with international standards specifying clinical thermometer performance (e.g., [Citation32]), and then extend this to the sorts of uncertainties that might be seen in a typical clinical environment, where devices may not be new, and may have been exposed to mechanical or thermal shocks. These will be examined in the context of the National Early Warning Score 2 (NEWS2) and also the COVID-19 UK NHS threshold of 37.8 °C [Citation7]. The implications of these results are discussed in the context of avoidable deaths, antibiotic resistance and slowing the spread of infectious diseases. We assume that this uncertainty is normally distributed and has an expansion factor of two.
5.2. Implications of uncertainty in a clinical thermometer
We consider two additional scenarios: firstly, that of a thermometer which has been in use for a period of time and whose uncertainty has increased to ± 0.5 °C; and secondly, a thermometer (with increased uncertainty) that has been subjected to a thermal or mechanical shock, resulting in a loss of calibration, such that all measurements are offset by 0.2 °C, in addition to increased uncertainty. We do not consider additional sources of uncertainty which arise from the measurement itself.
demonstrates the range of temperatures that would be measured from these three thermometers, when measuring 1000 different patients. The green bars represent measurements that correspond to the patients’ true temperature, while the grey bars represent measurements that differ from the patients’ true temperature.
Figure 1. Expected probability distribution functions of temperature measurement on 1000 patients measured with three different thermometers. (a) a new thermometer with uncertainty of ±0.3 °C. (b) an old thermometer with uncertainty of ±0.5 °C. (c) a damaged thermometer with the same standard deviation as (b) in addition to a consistent offset of 0.2 °C. Accurate readings (matching the true patient temperature) are highlighted by a vertical line.
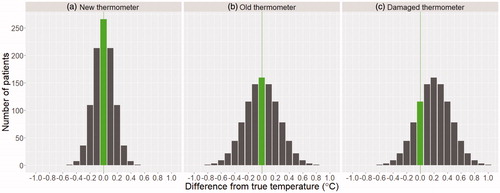
These graphs are helpful in considering the effect of inaccurate thermometer measurements where action thresholds are employed, such as in NEWS2, where action thresholds are set at ≥38.1 °C, ≥39.1 °C, ≤36 °C, and ≤35 °C. This would also apply to the temperature definition for COVID-19, which uses a threshold of ≥37.8 °C. If a patient’s true temperature is at or near an action threshold, even small uncertainties in measurement can result in changes to patient care. For e.g., a patient with a true temperature of 37.6 °C would trigger the NEWS2 threshold at ≥38.1 °C if the thermometer used was in error by at least 0.5 °C. For a new thermometer this would occur in 1 in 1000 cases (0.1% of the time) and would increase to 35 in 1000 (3.5%) for the old thermometer, and 157 in 1000 (15.7%) for the damaged thermometer.
For the same patient to trigger the COVID-19 case definition threshold would only require an error of at least 0.2 °C. This would occur in 154 in 1000 cases (15.4% of the time) for the new thermometer, 273 in 1000 cases (27.3% of the time) for the old thermometer, and 579 in 1000 cases (57.9% of the time) for the damaged thermometer.
So thermometer uncertainty has the potential to result in overdiagnosis, and hence overtreatment, either directly when used as a sole indicator for treatment or more commonly where temperature is used as part of a cumulative diagnosis scoring scheme (e.g., NEWS2). Patients who do not in fact have a high temperature may be prescribed unnecessary antibiotics, fuelling antibiotic resistance, or may be given additional investigations such as imaging and blood tests which are unnecessary and increase costs to the health service.
The opposite problem is also possible. Due to the thermometer uncertainty a patient’s temperature may register as more normal than it really is (for e.g., a febrile patient’s temperature may be below the action threshold for fever.) This also has considerable adverse implications, potentially resulting in missed or delayed diagnosis. With sepsis in particular, delays in recognition and treatment initiation may result in increased morbidity and mortality.
5.3. Thermometer uncertainty and the implications for the detection of fever
In the COVID-19 pandemic the UK government uses 37.8 °C as the threshold for identifying fever. Poor body temperature measurement will lead to a significant number of false positives (i.e., identifying people with a fever when they haven’t) and vice versa, false negatives (i.e., ruling out a fever even when fever is present). This could lead to members of the public unnecessarily quarantining themselves, and so disrupting their lives, on the mistaken belief they had an elevated temperature. But more seriously it could lead to infected people going about their normal business when in reality they were carrying a fever and so potentially carrying (and hence by their behaviour spreading) the disease. Improving body temperature measurement is essential so as to reduce both false positives and false negatives to minimise both life disruption and also spread of disease.
6. Essential next steps to solve the body temperature measurement conundrum
We believe there are three key steps that need to be taken to significantly improve the practice of clinical thermometry. These are; ensure that the thermometers used are fit for purpose, ensure that the thermometers have an on-going traceable calibration, and, that users are fully trained in their use.
6.1. Ensure that thermometers in use are fit-for-purpose
The health community is faced with a confusing variety of variably performing body temperature measurement devices. Simple things that would improve the situation immediately are; only use body temperature measurement devices that are correctly certified, and which are manufactured in accordance with medical device regulations as medical devices (that is to [Citation33]). In addition, checking the specification of the thermometer and ensuring that the manufacturers uncertainty statement is credible and appropriate for the body temperature measurement required is also important.
The next step would be to undertake objective device validation studies with the aim of identifying which thermometers perform as required. These would then be licenced for use within the NHS and other health care environments. Both the approval of type and validation studies should be performed by an organisation independent of the manufacturer to avoid, for e.g., biased reporting of results. Ideally this organisation should be the sole authority to approve thermometers for use in the NHS and wider healthcare.
There should be a general aim to reduce the varieties of body temperature measurement devices within the health service environment. This would improve the uniformity of measurement practice, intercomparability of results and the measurement of longer-term temperature trends.
In the approval process, the results might show that research and innovation is required into a new type of thermometer that does not suffer from the problems outlined in the previous sections.
6.2. Ensure clinical thermometers remain fit for purpose during use by traceable calibration
The outputs of electronic devices can drift or change over time. This can effectively be solved by having within each healthcare organisation a means of traceably calibrating the clinical thermometers. This means that there would have to be processes established which mandated both the calibration of the thermometers on a periodic basis and that the temperature calibration equipment used within the clinical setting was also periodically calibrated traceable back to national standards. Best practice would be to establish ISO17025:2017 calibration capability within the healthcare laboratories responsible for thermometer calibration. This would provide a level of confidence in such measurements which currently does not exist.
6.3. Ensure all users are trained in the performance and use of clinical thermometers
It is important to ensure that all users who perform body temperature measurement are appropriately trained in the use of the thermometers that are used in their particular environment. There is already some guidance available (e.g., [Citation34]) but it is often not well known or uniformly followed [Citation15]. In addition, courses on the use of clinical thermometers are available (e.g., [Citation35]) and these could be used to teach the essentials of body temperature measurement.
Training should also be available to patients who are expected to make a life-saving self-referral based on self-measured temperature. We refer in particular to immunocompromised patients in haematology and oncology, asked to monitor their temperature at home for signs of potentially fatal sepsis. In these cases, it is important to stress to the patient that a baseline temperature should be obtained whilst they are well, with the thermometer they are using. Then any temperature increase above that baseline would be indicative of elevated temperature, irrespective of the actual reading of the thermometer itself.
7. Summary
The change from well known, well understood “under-the-tongue” mercury thermometers to electronic temperature measurement devices used at a range of different sites has raised several issues that have yet to be properly addressed in the NHS and health services elsewhere. The fact that such thermometers do not operate in the same way, nor have the same performance as the previously used mercury thermometers has not been fully appreciated. The requirement for periodic performance checking (calibration) to traceable and accredited temperature standards has not been widely implemented, leading to potentially unreliable thermometers being in routine clinical use.
8. Conclusions
We have outlined the key issues of contemporary clinical thermometry. It is clear that the wide variety of clinical thermometers of variable performance has led to a significant reduction in the quality of body temperature measurement in health services throughout the world.
This situation can be significantly improved if:
It is ensured that clinical thermometers in use are fit-for-purpose. This step will require validation and approval.
It is ensured that clinical thermometers remain fit for purpose during use by regular traceable calibration.
it is ensured that all users are trained in the performance and use of clinical thermometers and that guidelines are followed.
We strongly believe that it is essential to act as soon as possible so that reliable body temperature measurement is reinstated in the healthcare sector. By doing so:
There will be an improvement in patient outcomes by reducing avoidable deaths.
The useful life of precious antibiotics will be potentially extended.
The thresholding for correct identification of febrile individuals will be greatly improved, thus greatly helping reduce/slow the spread of infection in pandemic situations where elevated temperature is a key indicator of infection.
Acknowledgements
We would like to acknowledge Professor Dame Sue Hill for drawing our attention to the importance of undertaking this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Notes
1 The NEWS2 score (https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2) is widely used in secondary and acute care settings across the UK. It is an additive score based on 7 parameters: heart rate, respiration rate, body temperature, oxygen saturation, need for supplemental oxygen, systolic blood pressure, and conscious level. A parameter in the normal range scores 0, with deviations from normal scoring between 1 and 3 points. Various total score thresholds from 5 upwards are used to initiate escalating levels of response. A score of 3 on any single parameter also triggers an urgent response, irrespective of scores on any other parameter.
The thresholds for body temperature in the NEWS2 score are as follows:
•Less than or equal to 35.0 °C (scores 3 points).
•Less than or equal to 36.0 °C (scores 1 point).
•Greater than or equal to 38.1 °C (scores 1 point).
•Greater than or equal to 39.1 °C (scores 2 points).
2 Hereafter the phrase “mercury thermometers” is to be taken to encompass all forms of liquid-in-glass based clinical thermometers.
3 Note that the predictive algorithm itself is a source of uncertainty in body temperature measurement.
4 That is a source or sources of known temperature.
5 ISO/IEC 17025:2017 “General requirements for the competence of testing and calibration laboratories”.
6 This requirement was recognised long ago in industry. This led to the introduction of third party accreditation of calibration services by the United Kingdom Accreditation Service (UKAS). UKAS accreditation ensures that any calibration provider has the correct processes, trained staff and appropriate reference standards, traceable to the national standard, required to perform reliable, trustworthy calibrations. For any critical measurement in an industrial process generally that measurement will be traceable back to national standards via an accredited calibration laboratory, or to the national standard itself. All calibration providers who are accredited by UKAS will issue certificates which has the UKAS logo. The accreditation process guarantees the reliability of the services provided.
7 This is only a few percent (∼2%) however if there is a cold or hot object nearby the reflected contribution can still cause significant error to the measured temperature.
8 Standard ASTM E1965-98 (2016) “Standard specification of infrared thermometers for intermittent determination of patient temperature”: “addresses assessing a subject’s body internal temperature through measurement of thermal emission from the ear canal and performance requirements for noncontact temperature measurements of skin” This standard is explicit that skin thermometers are intended for determining the skin temperature of a patient, they are not intended for measuring (core) body temperature.
9 More studies are required, particularly of forehead thermometers that adhere closely to ISO 80601-2-56:2017, to determine whether in appropriate measurement conditions efficacy of body temperature measurement can be achieved by such an approach.
10 The discussion here is mainly in the context of oral thermometers but the same issues apply to rectal ones.
11 Which is why predictive algorithms are often used to predict what the final attained temperature for the thermometer would be given the rate of change whilst in contact with the measurement site.
References
- NICE. National Institute for Health and Care Excellence, Sepsis: recognition, diagnosis and early management. NICE guideline. 2016; [cited 2016]. Available from: www.nice.org.uk/guidance/ng51.
- NICE. National Institute for Health and Care Excellence, Neutropenic sepsis: prevention and management in people with cancer. NICE guideline. 2012; [cited September 2012]. Available from: www.nice.org.uk/guidance/cg151.
- ONS1. Office of National Statistics. Mortality statistics – underlying cause, sex and age. ONS. 2018; [cited 2020 Apr 17]. Available from: https://www.nomisweb.co.uk/query/construct/summary.asp?mode=construct&version=0&dataset=161.
- ONS2. Office of National Statistics. Deaths registered in England and Wales (Dataset). ONS 2019; [cited 2020 Jan 11]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsregisteredinenglandandwalesseriesdrreferencetables.
- ONS 2020. Office of National Statistics. Child and infant mortality in England and Wales: 2018. ONS. 2020; [cited 2020 Feb 11]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/childhoodinfantandperinatalmortalityinenglandandwales/2018/pdf.
- RCP. Royal College of Physicians. National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Updated report of a working party London: RCP 2017; [cited 2020 Jan 11]. Available from: https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2.
- PHE. Public Health England. COVID-19: investigation and initial clinical management of possible cases Guidance: PHE, 6 April 2020; [cited 2020 Apr 21]. Available from:https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases/investigation-and-initial-clinical-management-of-possible-cases-of-wuhan-novel-coronavirus-wn-cov-infectionon.
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- Bicanic T, Solomon AW, Karunaharan N, et al. Positive predictive value of the UK clinical case definition for H1N1/09 (‘swine’) influenza. J Infect. 2010;60(5):405–407.
- BS 691:1979. “Specification for clinical maximum thermometers (mercury-in-glass)” Published: December 1979 Replaced By: BS 691:1987
- VIM. “The International Vocabulary of Measurement” 2008 ed. (minor corrections 2012); [cited 2020 Jan 14]. Available from: https://www.bipm.org/utils/common/documents/jcgm/JCGM_200_2012.pdf.
- Machin G, Rusby R, McEvoy HC, et al. Traceability and calibration in temperature measurement: a clinical necessity. IPEM Abstracts, The Institute of Physics and Engineering in Medicine, p.83, Patersons, UK 2004.
- Fulbrook P. Core body temperature measurement: a comparison of axilla, tympanic membrane and pulmonary artery blood temperature. Intensive Crit Care Nurs. 1997;13(5):266–272.
- Asadian S, Khatony A, Moradi GR, et al. Accuracy and precision of four common peripheral temperature measurement methods in intensive care patients. Med Devices. 2016;9:301–308.
- Davie A, Amoore J. Best practice in the measurement of body temperature. Nurs Stand. 2010;24 (42):42–49.
- Sund-Levander M, Grodzinsky E, Loyd D, et al. Errors in body temperature assessment related to individual variation, measuring technique and equipment. Int J Nurs Pract. 2004;10(5):216–223.
- McCarthy P, Heusch A, Kenkre J, et al. Infrared ear thermometers versus rectal thermometers. Letter to the Lancet. 2002;360:1882.
- NICE; [cited 2013 May]. Available from: https://www.nice.org.uk/donotdo/forehead-chemical-thermometers-are-unreliable-and-should-not-be-used-by-healthcare-professionals.
- Ammer K, Ring F. The thermal human body: a practical guide to thermal imaging. Singapore: Jenny Stanford Publishing Pte Ltd., Section 4.3.1;2019.
- Aw J. The non-contact handheld cutaneous infra-red thermometer for fever detection during the COVID-19 global emergency. J Hospital Infection. 2020;104(4):P451.
- Hebbar K, Fortenberry JD, Rogers K, et al. Comparison of temporal artery thermometer to standard temperature measurements in pediatric intensive care unit patients. Pediatric Crit Care Med. 2005;6:557–561.
- Bolton S, Latimer E, Clark D. Temporal artery and non-contact infra-red thermometers: is there sufficient evidence to support their use in secondary care? Globa lCE. 2020;2(2):8–16.
- Crawford M. Use of temporal artery or forehead thermometers is misleading. Br Med J. 2015;351:6125.
- Fletcher T, Whittam A, Simpson R, et al. Comparison of non-contact infrared skin thermometers. J Med Eng Technol. 2018;42(2):65–71.
- MHRA news release. [cited 2020 July 3]. Available from: https://www.gov.uk/government/news/dont-rely-on-temperature-screening-products-for-detection-of-coronavirus-covid-19-says-mhra.
- Ring EFJ, Ammer K. The technique of infra red imaging in medicine. Thermol Int. 2000;10:7–14.
- ISO/TR 13154:2017. Medical electrical equipment— Deployment, implementation and operational guidelines for identifying febrile humans using a screening thermograph. 2017.
- Simpson R, Machin G, McEvoy HC, et al. Traceability and calibration in temperature measurement: a clinical necessity. J Med Eng Technol. 2006;30(4):212–217.
- Simpson R, McEvoy HC, Machin G, et al. In field-of-view thermal image calibration system for medical thermography applications. Int J Thermophys. 2008;29(3):1123–1130.
- Ring EFJ, Jung A, Kalicki B, et al. “Ch. 5, New standards for fever screening with thermal imaging systems. In: A casebook in clinical medicine. Published © IOP Publishing Ltd; 2015.
- McCallum L, Higgins D. Measuring body temperature. Nurs Times. 2012;108(45):20–22.
- ISO 80601-2-56:2017(en). Medical electrical equipment —Part 2–56: Particular requirements for basic safety and essential performance of clinical thermometers for body temperature measurement. 2017.
- ISO 13485:2016. Medical devices—Quality management systems—Requirements for regulatory purposes. 2016.
- NICE 2013b. NICE guideline: “Management of feverish illness in children”; [cited 2013]. Available from: https://www.nice.org.uk/guidance/ng143/evidence/may-2013-full-guideline-pdf-6960663038 and https://www.nhs.uk/common-health-questions/accidents-first-aid-and-treatments/how-do-i-take-someones-temperature/.
- McEvoy HC. Medical thermometry training course for nursing staff using infrared ear thermometers in a clinical environment. 2008, NPL Training.
