Abstract
Long-term neurological conditions (LTNCs) cause physical and psychological symptoms that have a significant impact on activities of daily living and quality of life. Multidisciplinary teams are effective at providing treatment for people with LTNCs; however, access to such services by people with disabilities can be difficult and as a result, good quality care is not universal. One potential solution is telehealth. This review describes the potential of telehealth to support people with LTNCs, the challenges of designing and implementing these systems, and the key recommendations for those involved in telehealth to facilitate connected services that can benefit patients, carers and healthcare professionals. These recommendations include understanding the problems posed by LTNCs and the needs of the end-user through a person-centred approach. We discuss how to work collaboratively and use shared learning, and consider how to effectively evaluate the intervention at every stage of the development process.
1. Long-term neurological conditions and care
Long-term neurological conditions (LTNCs) such as stroke, dementia, motor neuron disease (MND) and multiple sclerosis, cause physical and psychological symptoms that have significant effects on activities of daily living [Citation1,Citation2]. Physical symptoms include difficulties with speech, swallowing, breathing and muscle weakness, which may cause dexterity problems and necessitate walking aids or communication devices [Citation3,Citation4]. Psychological symptoms include anxiety, depression, apathy, fatigue and cognitive impairments [Citation3,Citation4]. People with neurological conditions have the highest levels of pain, anxiety and depression, and the lowest health-related quality of life, compared to other long-term illnesses [Citation5,Citation6]. These conditions also affect informal carers (e.g., family or friends) because they deliver a substantial amount of care, which can result in a high degree of burden and distress [Citation7].
Neurological conditions are the highest cause of long-term disability and early death, and the second leading cause of all death worldwide [Citation8]. Between 2001 and 2014, deaths in people with a neurological condition increased by 39%, whereas deaths from all causes decreased by 6% [Citation9]. This is due to such conditions being more common in people who are older; therefore, the ageing population is increasing the prevalence of neurological conditions and the need for medical and social care [Citation8]. Emergency department attendance and hospital admittance in people with LTNCs has increased by 21% and 24%, respectively [Citation10]. Alongside other aspects of neurological care, this costs the United Kingdom’s (UK) National Health Service (NHS) £4.4 billion per year [Citation10].
In the absence of cures, the majority of neurological care focuses on symptom management. The complexity of the biopsychosocial needs of patients is best met by a multidisciplinary team (MDT) approach, with regular reviews by a range of healthcare professionals with continuing support and a focus on quality of life [Citation3,Citation4,Citation11,Citation12]. The MDT approach has been associated with positive benefits, such as increased survival, quality of life, independence and likelihood of living at home [Citation13–16]. illustrates the complexity of an MDT approach for people with MND, which is similar in other LTNCs. The large number of different healthcare professionals across multiple organisations emphasises the need for effective information sharing, usually through digital communication technology, to provide the best care for patients.
Figure 1. Recommended components of an MDT for management of MND. Reproduced with permission from the authors Hobson and McDermott [Citation11].
![Figure 1. Recommended components of an MDT for management of MND. Reproduced with permission from the authors Hobson and McDermott [Citation11].](/cms/asset/66165971-3a40-4bdf-81d6-1ed7981d8122/ijmt_a_2040625_f0001_c.jpg)
2. Difficulties delivering neurological care
Despite the positive effects of an MDT approach for LTNCs, access is a common barrier [Citation17,Citation18]. Due to the large number of specialists required for a full MDT [Citation11], neurological care is often located in large, tertiary centres, although some parts of the country have limited access [Citation17]. Travel to tertiary centres can be challenging and arduous for people with disabilities, where attendance at clinical appointments can be as low as 43% [Citation15,Citation16]. Attendance is lower in people who have difficulty walking, live in rural areas, or are from a lower socioeconomic background [Citation19].
Although LTNCs as a whole are prevalent, individual conditions can be rare [Citation10]. This rarity can cause primary care facilities to have limited direct experience treating neurological conditions [Citation6,Citation17]; thus, reducing access to specialised care outside of the large, tertiary centres. There is also a lack of neurology specialists [Citation20], which compounds the issues surrounding access further and may delay people with neurological conditions receiving timely care. In some areas, even large centres can lack the required specialists [Citation21].
Multidisciplinary team appointments for people with neurological conditions are conducted at regular fixed intervals [Citation3]. However, there is limited information available to healthcare professionals regarding their patients’ health between clinical appointments. As disease progression rates vary between different people [Citation22], a patient’s health can rapidly deteriorate with time-sensitive healthcare interventions not being implemented until their next clinical appointment.
Therefore, there is a need for new approaches to be developed to provide more coordinated care and increase access to neurological healthcare provision [Citation11]. These approaches should be personalised to each individual [Citation3,Citation4], to provide greater support and information for patients [Citation18]. Digital health has been suggested as a key method to achieve these goals [Citation6]. Implementing more digital technologies into healthcare is recommended by multiple international organisations including the NHS and World Health Organisation [Citation23,Citation24], which is also supported by patients, carers and researchers [Citation6,Citation25].
3. Telehealth
There are multiple, different types of digital health and technology that can support the care of people with LTNCs. This review will focus on telehealth, which is the remote monitoring of patients in their own homes to anticipate exacerbations early and build their self-care competencies [Citation26]. This is one of five different ways of using technology to manage long-term conditions [Citation26] (see ). Examples of telehealth include remote monitoring of physical abilities (such as step counters, actigraphy and spirometry), or by collecting patient reported data. Telehealth in MND (TiM) system is one example already used in the NHS which can collect fortnightly patient/carer reported outcome measures and readings using questionnaires that identify signs of deterioration in the condition, uncontrolled symptoms or emotional distress [Citation27] (see Section 3.1 for a full description of the TiM system).
Figure 2. Five categories of healthcare technology included in TECS. Adapted from NHS Commissioning Assembly [Citation26].
![Figure 2. Five categories of healthcare technology included in TECS. Adapted from NHS Commissioning Assembly [Citation26].](/cms/asset/8a7ee417-5818-472b-b2ef-c1843748c979/ijmt_a_2040625_f0002_c.jpg)
3.1. The telehealth in MND system
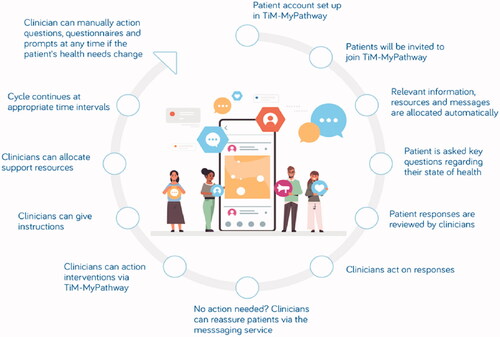
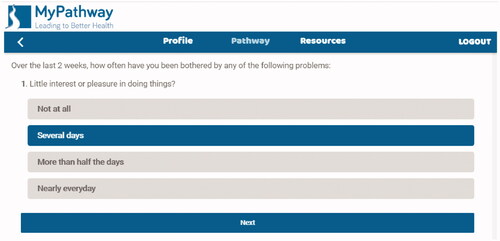
The TiM system was developed using iterative co-design with potential users of the services including patients, carers and healthcare professionals at Sheffield Teaching Hospitals NHS Foundation Trust, in collaboration with researchers at the University of Sheffield, NIHR Devices for Dignity MedTech Cooperative and telehealth experts (see ) [Citation27]. The service uses MyPathway, an NHS approved service that sends patients and carers fortnightly questionnaires collecting data about their physical and emotional health (see ). It can identify troublesome symptoms and displays core measurements graphically to detect signs of deterioration (see ). TiM also collects records of physical measures such as weight and respiratory function. Data are immediately available to their specialist nurse who can identify problems and liaise with the specialist MDT. The TiM system is being tested in a real-world implementation study as part of usual care in Sheffield (UK) and Dublin (Ireland) and research suggests this is valued by patients and carers, can facilitate communication, identify and treat problems earlier, and make more efficient use of face-to-face appointments [Citation29,Citation30]. TiM can also provide service level analysis of data to identify common issues amongst the caseload (e.g., the TiM system has identified high levels of depression because of COVID), which can drive further service improvements [Citation31,Citation32].
Figure 5. The TiM telehealth system presents data graphically to enable identification of progression in the disease. ALSFRS-R: amyotrophic lateral sclerosis – Functional Rating Scale – revised [Citation28] (a commonly used functional rating scale in MND/ALS).
![Figure 5. The TiM telehealth system presents data graphically to enable identification of progression in the disease. ALSFRS-R: amyotrophic lateral sclerosis – Functional Rating Scale – revised [Citation28] (a commonly used functional rating scale in MND/ALS).](/cms/asset/e72b8e09-695d-4b75-b958-db481579c641/ijmt_a_2040625_f0005_c.jpg)
4. Benefits of using telehealth in neurology
4.1. Specialist access and convenience
Removing the need to travel long distances to hospital has positive implications for patients and carers including reducing fatigue, travel costs [Citation33], the negative impact on employment [Citation34] and other social responsibilities. It also could have a positive environmental impact and reduce the need for hospital premises. Telehealth can facilitate triage systems, whereby only those patients whose condition has deteriorated need to attend an appointment. Not only would this reduce time spent attending unnecessary appointments, it may free up capacity for all patients who need it, including those unable to use the telehealth system [Citation25].
Telehealth can be used by MDTs for LTNCs to collect data and facilitate timely access for neurological diagnoses, treatment, support and information [Citation25,Citation27,Citation35–38]. For example, Zhan et al. [Citation36] used smartphones to monitor people with Parkinson’s disease. The data collected (e.g., finger tapping, balance and reaction time) was used to detect responses to therapies, enabling specialists to modify treatments remotely. Telehealth can enable early diagnosis and facilitate timely treatment [Citation39], which is especially important for LTNCs. For example, treatment of breathing difficulties for people with MND or assessing cognitive impairments [Citation40,Citation41].
Given the difficulties travelling to clinical appointments and the subsequent 43% attendance rate [Citation15,Citation16], providing people with LTNCs remote access to specialists could increase adherence to services. As the diseases progress and patients become very frail, telehealth systems can support people within their homes during the end of their lives; where dying at home is a frequently expressed preference [Citation42].
4.2. Facilitating coordinated care
MDT care is thought to have a positive impact on management because of the multiple specialists involved () and locating all specialists together mean decisions are made quickly and specialist knowledge shared [Citation11]. However, many LTNCs are rare, meaning that specialist MDTs may be geographically dispersed [Citation21]. Telehealth can facilitate more coordinated neurological care by enabling multiple healthcare professionals to simultaneously review patient data, regardless of whether they are all physically located at the same place or not (see Section 3.1) [Citation27].
4.3. Facilitating self-management
Self-management is an important aspect of care for people with LTNCs [Citation4,Citation39]. Self-management has been associated with multiple benefits across different chronic conditions, including objective outcomes (e.g., blood sugar levels, blood pressure and asthma attacks), subjective outcomes (e.g., quality of life and activities of daily living) and decreased healthcare utilisation [Citation43–45].
Telehealth can deliver remote support for self-management, removing the necessity for patients and carers to physically attend programmes [Citation26]. The “Big CACTUS” is one example that implemented a self-managed computerised speech and language therapy intervention for people with speech problems after stroke; resulting in significant improvements in word-finding exercises [Citation46]. Other self-management programmes delivered via telehealth have had high adherence and provide improvements in balance, emotional state and mobility [Citation47,Citation48].
4.4. Facilitating research
Although some LTNCs have effective treatments, many have few or none [Citation3,Citation4]. This is partially because of the limited understanding of the biological causes of these diseases; however, clinical trials in LTNCs also face significant barriers [Citation49]. Traditional randomised controlled trials need large numbers of participants to demonstrate effectiveness of therapies. For rare diseases such as MND (with a UK population of 5000 and a life expectancy of only two to four years [Citation50]), it is difficult to recruit and retain these numbers in a reasonable time frame. Frail and disabled patients also face barriers attending trial visits, resulting in a disparity in characteristics between research participants and the wider neurological clinical population [Citation51,Citation52].
Telehealth can overcome barriers to clinical research by enabling the remote collection of data, which can reduce participant burden and facilitate faster research [Citation49,Citation53,Citation54]. The more times a study collects data, the less participants they need to recruit to demonstrate the impact of the intervention. For example, 1208 participants are required for a six-month trial collecting activity data once per month; however, only 214 participants are required for the same trial if activity data are collected daily [Citation49]. Face-to-face daily measurements are infeasible but could be obtained using telehealth to enable this 82% reduction in sample size.
Telehealth also enables researchers to interrogate the large amount of remotely collected data to identify trends and provide recommendations for clinical care, with multiple authors describing successes in this regard [Citation54,Citation55]. For example, over 4000 patient reported outcome measures relating to all aspects of MND have been collected within just one year of a UK MND service implementing the TiM system [Citation27,Citation32]. As the telehealth system collects these outcome measures automatically, data collection costs and delays are far smaller.
If telehealth systems are being used to facilitate clinical research, measures should be continually validated to show their accuracy and reliability [Citation56]. Healthcare authorities that determine whether novel interventions are safe (e.g., the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK and the Food and Drug Administration (FDA) in the USA) are unlikely to accept research data collected through un-validated digital measures. Studies following this guidance have repeatedly found no differences between original measures and their digital counterparts [Citation57–61]. There are also some circumstances where remote measurements are preferable. For example, people with Parkinson’s disease over-perform in walking tests in clinical settings [Citation62]; thus, telehealth could be a method to achieve more valid results.
5. Challenges to telehealth in neurology and potential solutions
5.1. Changes in medical services and staff behaviours
The implementation of telehealth into LTNCs requires staff to change their daily practices, which can be perceived as a threat to traditional models of care [Citation33]. Few studies have evaluated neurological healthcare professionals’ experiences of using telehealth [Citation63]. In studies that did, staff were more likely to report barriers to the use of technology, such as increase in workload, loss of control, changes in patient–nurse relationships and changes in work processes [Citation64]. This is similar to other chronic conditions [Citation63,Citation65,Citation66]. The focus on perceived negatives of telehealth can act as a barrier to healthcare professionals offering services to patients, despite the patients themselves being willing to engage with digital services [Citation65].
The rapid shift to remote working during the COVID-19 pandemic is a great example of the challenges and benefits of changing the model in which care is delivered. Remote working was initially welcomed as an efficient and “COVID-secure” way to deliver care and interact with the MDT but staff are increasingly recognising the considerable ongoing effort required to adapt traditional services [Citation67]. Services are likely to move towards a more hybrid model with remote consultations being supported by telehealth, where information can be collected by patients, carers and staff at different times and locations, but are available to support MDT working. Developing intuitive systems and providing continued training enables healthcare professionals to overcome barriers to changing practices [Citation64]. Promoting the benefits of telehealth for patients and using staff digital champions to support colleagues can increase digital literacy [Citation68] and staff engagement [Citation63,Citation64].
5.2. Patient factors
Symptoms of LTNCs pose potential barriers for telehealth use, for example, difficulties with arm weakness and dexterity, memory, attention or fatigue [Citation3,Citation4,Citation39]. A service designed with end-users in mind can overcome many barriers [Citation69]. For example, even the most disabled people with MND could continue to use telehealth [Citation29,Citation61,Citation70]. When low finger dexterity and unfamiliarity with the computer tablet caused challenges in one study, simple solutions such as offering face-to-face support, providing a touch screen and stylus, and enabling the patient to use their own equipment removed the barriers [Citation29]. Despite age being assumed an inherent barrier of all technology, for people with LTNCs age has not been found to affect uptake and adherence [Citation71,Citation72].
Socioeconomic background and health literacy are other patient barriers that reduce engagement with general healthcare and thus require consideration [Citation19,Citation73]. Requiring access to technology and internet connections can increase inequalities and, for those unable to use these facilities, services must provide adequate alternatives [Citation74]. Whilst many LTNCs do not disproportionately affect those with lower socioeconomic status, some do. Risk factors associated with poverty, including obesity and smoking [Citation75,Citation76], are major causes of stroke and dementia [Citation77], whilst patients with any LTNC often experience loss of employment and financial hardship as a result of their disabilities [Citation78].
Other patient factors that may affect telehealth adherence relate to acceptability, usability and motivation to engage in digital health interventions. Telehealth systems that are highly complex, require daily engagement, do not provide feedback, or are only used to create health service cost-savings have poor user adherence [Citation53,Citation79,Citation80]. Whereas, telehealth services that facilitate choice, control and feedback – or an alternative method to “give something back” to the patient – have high uptake and adherence [Citation29,Citation54,Citation61,Citation70,Citation81]. Providing education and training alongside telehealth services also improves user motivation and acceptability [Citation82]. People with LTNCs are likely to have different perceptions regarding different telehealth services and acceptability can also be different between patients and carers [Citation83]. When developing telehealth services, it is therefore important to adopt person-centred approaches (see Section 5.2.1) of co-design to collect and respond to end-user preferences [Citation69].
5.2.1. Person-centred approach
A person-centred approach describes a systematic process of involving the end-users at every stage of intervention development, and understanding and accommodating their perspectives [Citation69]. The approach has a keen focus on co-design principles with all the relevant stakeholders, such as patients, carers, healthcare professionals and technology developers [Citation69] (see Section 3.1). The inclusion of healthcare professionals is especially important to respond to the lack of representation in the current telehealth literature [Citation63]. Introducing technology developers to end-users also enables them to fully understand key aspects of the condition and patients’ intervention needs. However, there needs to be understanding between the different stakeholders and how each approaches the development and evaluation of technology systems [Citation84].
Person-centred design involves quantitative and qualitative data collection methods from the planning stage to the implementation and trialling stage. Including end-users within the early design stages can facilitate the creation of guiding principles, which are the key objectives and key features of the intervention. These aspects can then be iteratively tested, with key features that do not achieve their desired effects being replaced with other components alongside further testing. Features that do not respond to objectives or guiding principles can be avoided, which reduces project scope creep. Within the implementation and trialling stage, the guiding principles and objectives form the basis of clinic trial research questions. Through the iterative collection of stakeholder perspectives, intervention components that increase usability and acceptability can be incorporated, such as facilitating choice, control and feedback as described above.
Although person-centred design can be beneficial for any long-term condition [Citation69], it can be particularly valuable in understanding the unique barriers in LTNCs. Co-production techniques have successfully been used within dementia, multiple sclerosis, MND and stroke [Citation27,Citation38,Citation47,Citation85], and can be combined with other methods to include individuals from under-served groups [Citation86]. The positive of developing telehealth for such complex and changeable conditions is that if the system is successful for people with neurological conditions, it will likely work in other long-term conditions too.
5.3. Clinical governance and costs
Prior to implementation, financial and technical governance arrangements are required to be in place, along with arrangements for oversight and accountability. Governance arrangements must consider data processing agreements, privacy statements and the levels of service that the healthcare organisation expects from the telehealth provider.
Companies wishing to work within the NHS must meet the National Data Guardian’s 10 data security standards and can use the online self-assessment Data Security and Protection Toolkit to measure their performance against these standards. Healthcare providers have a duty to ensure these requirements are met and having these governance processes in place can reduce the time taken to implement a new service.
Fortunately, there are now a wealth of providers already established that meet these requirements and NHSX provides a list of suppliers that meet these basic requirements. Services need to look for good companies who will not only meet these standards but should demonstrate an understanding of the needs and nuances of health services and their target users and should be able to adopt a person-centred approach to development. A successful telehealth service should also integrate with existing technology to ensure seamless transition of data and avoid the frustrations felt by users when multiple different platforms are needed [Citation49].
Although requiring an initial investment, multiple studies have reported that telehealth systems can be cost-effective for LTNCs if they reduce hospitalisation costs [Citation71,Citation87]. However, it seems these cost savings are largely explained by small differences in the number of intensive care admissions [Citation63,Citation88]. Telehealth interventions in LTNCs tend not to be targeted at reducing intensive care admissions and so cost-savings would need to be found from other healthcare resource use, which is challenging to measure when patients have multiple interactions with many different clinical services.
As some LTNCs can be rare [Citation18], per-patient costs can be higher for these conditions due to a lack of economies of scale. Telehealth developers and funders therefore need to be aware of cost-effectiveness issues in rare conditions and reduce costs where possible. Sharing costs and expertise across different neurology centres and/or different conditions is one solution. This also avoids time and cost wasted with inexperienced services trying to develop telehealth systems from scratch.
5.4. Research evidence for telehealth
Traditionally, new treatments are evaluated using randomised controlled trials that aim to demonstrate whether the treatment is effective within a strictly controlled environment to determine whether the costs of the treatment are worth the potential benefits and cost-savings. Clinical trials in LTNCs are already challenging because of the barriers described earlier; furthermore, randomised controlled trials may not be appropriate when evaluating telehealth systems [e.g., Citation30,Citation89] due to their complexity [Citation90]. A telehealth service incorporates a number of different features and behaviours, and depends on individual expertise and skills to deliver the treatment [Citation90]. Complexity may also be added when there is a permitted element of flexibility within the intervention; for example, allowing patients to enter data into telehealth systems at different intensities. Complex interventions are more difficult to evaluate compared to trials of medication, because of the need to capture wide-ranging data that can answer questions relating to: what works?; for whom?; how?; and why? The rapid development of technology compounds these difficulties further, which can often outpace the slow, traditional randomised controlled trial.
Evaluation of complex interventions should involve four phases: development or identifying the intervention; feasibility; evaluation; and implementation [Citation90]. Progress should be conducted iteratively, refining the intervention and repeating phases if necessary [Citation90]. Intervention developers need to understand the level of evidence that is required to demonstrate the value of their telehealth system [Citation91,Citation92]. Through the National Institute for Health and Care Excellence’s (NICE) evidence standard framework, most telehealth systems will require medium to high levels of supportive evidence, depending on the level of risk and the intended outcome of the product [Citation91]. Technologies that are simple, low risk and/or cheap (such as a patient diary) require less supportive evidence compared to interventions intended to deliver treatment. Research on telehealth for people with LTNCs should consider solutions for low sample sizes, the need for more cost-effectiveness analyses [Citation63], and develop a method to systematically define telehealth systems to facilitate the aggregation of findings. Healthcare funders need to consider these methodological issues when evaluating the evidence of telehealth interventions for LTNCs and not solely rely on randomised controlled trials.
6. Key recommendations
This review has described the benefits, challenges and solutions involved in developing telehealth systems. From these, we propose the following key recommendations.
6.1. Use a person-based approach
This review has highlighted the importance of understanding the needs of those who will use the telehealth system and the services in which they will be embedded into. The key challenges within LTNCs include overcoming the disabilities and complexity caused by the condition and the MDT approach that healthcare services employ to support people. However, each service operates differently and people with LTNCs and carers may have different perspectives. To understand stakeholder perspectives and the specific needs of each individual service, a person-based approach should be used. This approach facilitates the systematic collection of data from different stakeholders to enable the creation of a telehealth system that best fits the needs of the end-users.
6.2. Work collaboratively and use shared learning
Developing telehealth systems and the necessary clinical governance can be expensive and time consuming to complete. Developers need to recognise that a successful person-based approach can also take time to implement rigorously. Therefore, working collaboratively with MDTs is a useful method to share the workload, broaden the potential usefulness of the product. Further time and money can be saved though by adapting existing systems and using shared learning, rather than trying to start from the beginning. Support for this can be gained through several organisations, such as NHSX, which aims to share best practice and establish methods to ensure collaboration. As part of these aims, NHSX has released a list of accredited technology suppliers that people wishing to develop telehealth services can use to avoid reinventing an already available system and therefore save time and money.
6.3. Consider intervention impact and effectiveness at every stage of the development process based upon guidelines
Telehealth interventions need to demonstrate that the system functions, is usable, and is effective before healthcare authorities will recommend that services implement them. The best demonstration of an intervention’s impact and effectiveness is conducted throughout the development process, ensuring that key decisions are based upon evidence. At each stage, the developers need to understand what data to collect and analyse and the level of evidence that is required to demonstrate the service’s positive and negative impacts. It is important to follow the available guidelines and recommendations to ensure evidence is collected to an appropriate standard and provide healthcare authorities the ability to recommend the telehealth service.
6.4. Recognise the potential value of telehealth to the care of LTNCs
LTNCs place a large burden on individuals, healthcare services and society as a whole. There is huge scope to improve services and address many of the barriers we have identified. Patients are already comfortable using technology such as email, telephone and video to access healthcare services and the COVID-19 pandemic has produced a rapid increase in the use of digital technology to facilitate remote medical care [Citation93]. There is now increasing acceptance of these new ways of providing medical care and an expectation that this will continue long after the pandemic. Therefore, there is currently a great opportunity to engage with patients, carers and healthcare professionals to expand the use of telehealth systems, which can have a direct and profound impact on the lives of people living with LTNCs.
Acknowledgements
CJM is an NIHR Research Professor.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Additional information
Funding
References
- Burridge J, Alt Murphy M, Buurke J, et al. A systematic review of international clinical guidelines for rehabilitation of people with neurological conditions: what recommendations are made for upper limb assessment? Front Neurol. 2019;10:567.
- Mitchell LA, Hirdes J, Poss JW, et al. Informal caregivers of clients with neurological conditions: profiles, patterns and risk factors for distress from a home care prevalence study. BMC Health Serv Res. 2015;15(1):350.
- National Institute for Health and Care Excellence. Motor neurone disease: assessment and management. NICE Guideline [NG42]; London, 2019.
- National Institute for Health and Care Excellence. Multiple sclerosis in adults: management. Clinical guideline [CG186]; London, 2019.
- NHS England. GP patient survey 2015; London, 2015.
- Thames Valley Strategic Clinical Network. Transforming community neurology. What commissioners need to know; Oxford, 2016.
- Etters L, Goodall D, Harrison BE. Caregiver burden among dementia patient caregivers: a review of the literature. J Am Acad Nurse Pract. 2008;20(8):423–428.
- GBD Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):459–480.
- Public Health England. Deaths associated with neurological conditions in England; London, 2018.
- The Neurological Alliance. Neuro numbers 2019. A report by The Neurological Alliance; London, 2019.
- Hobson E, McDermott CJ. Supportive and symptomatic management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2016;12(9):526–538.
- National Institute for Health and Care Excellence. Dementia: assessment, management and support for people living with dementia and their carers. NICE Guideline [NG97]; London, 2018.
- Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2013;2013(9):CD000197.
- Clarke DJ, Forster A. Improving post-stroke recovery: the role of the multidisciplinary health care team. J Multidiscip Healthc. 2015;8:433–442.
- Hogden A, Foley G, Henderson RD, et al. Amyotrophic lateral sclerosis: improving care with a multidisciplinary approach. J Multidiscip Healthc. 2017;10:205–215.
- Rooney J, Byrne S, Heverin M, et al. A multidisciplinary clinic approach improves survival in ALS: a comparative study of ALS in Ireland and Northern Ireland. J Neurol Neurosurg Psychiatry. 2015;86(5):496–501.
- The Neurological Alliance. Issues affecting neurology services. Neurological Alliance briefing April 2016; London, 2016.
- The Neurological Alliance. The invisible patients. Revealing the state of neurology services; London, 2015.
- Minden SL, Hoaglin DC, Hadden L, et al. Access to and utilization of neurologists by people with multiple sclerosis. Neurology. 2008;70(13 Pt 2):1141–1149.
- Burton A. How do we fix the shortage of neurologists? Lancet Neurol. 2018;17(6):502–503.
- Patel UK, Malik P, DeMasi M, et al. Multidisciplinary approach and outcomes of tele-neurology: a review. Cureus. 2019;11(4):e4410.
- Turner MR, Parton MJ, Shaw CE, et al. Prolonged survival in motor neuron disease: a descriptive study of the King's database 1990–2002. J Neurol Neurosurg Psychiatry. 2003;74(7):995–997.
- NHS England. Five year forward view; London, 2018.
- World Health Organisation. Global strategy on digital health 2020–2025; Geneva, 2021.
- Bloem BR, Henderson EJ, Dorsey ER, et al. Integrated and patient-centred management of Parkinson's disease: a network model for reshaping chronic neurological care. Lancet Neurol. 2020;19(7):623–634.
- NHS Commissioning Assembly. Technology enabled care services: resource for commissioners; London, 2015.
- Hobson E, Baird WO, Partridge R, et al. The TiM system: developing a novel telehealth service to improve access to specialist care in motor neurone disease using user-centered design. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19(5–6):351–361.
- Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1–2):13–21.
- Hobson E, Baird W, Bradburn M, et al. Process evaluation and exploration of telehealth in motor neuron disease in a UK specialist centre. BMJ Open. 2019;9(10):e028526.
- Hobson E, Baird WO, Bradburn M, et al. Using telehealth in motor neuron disease to increase access to specialist multidisciplinary care: a UK-based pilot and feasibility study. BMJ Open. 2019;9(10):e028525.
- Harold M, Knox L, Booth J, et al. Exploring the inclusivity of telehealth for people living with motor neuron disease, and the validity of a telehealth version of the ALSFRS-R. In: MNDA, editor. 32nd International Symposium on ALS/MND; Virtual; 2021.
- Sutherland J, Knox L, Mayberry E, et al. Exploring the natural history data captured using telehealth in routine clinical practice. In: MNDA, editor. 32nd International Symposium on ALS/MND; Virtual; 2021.
- Dorsey ER, Venkataraman V, Grana MJ, et al. Randomized controlled clinical trial of "virtual house calls" for Parkinson disease”. JAMA Neurol. 2013;70(5):565–570.
- Jaglal SB, Guilcher SJT, Bereket T, et al. Development of a chronic care model for neurological conditions (CCM-NC). BMC Health Serv Res. 2014;14(1):409.
- Paganoni S, Simmons Z. Telemedicine to innovate amyotrophic lateral sclerosis multidisciplinary care: the time has come. Muscle Nerve. 2019;59(1):3–5.
- Zhan A, Mohan S, Tarolli C, et al. Using smartphones and machine learning to quantify Parkinson disease severity: the mobile Parkinson disease score. JAMA Neurol. 2018;75(7):876–880.
- Sola-Valls N, Blanco Y, Sepúlveda M, et al. Telemedicine for monitoring MS activity and progression. Curr Treat Options Neurol. 2015;17(11):47.
- Talbot CV, Briggs P. The use of digital technologies by people with mild to moderate dementia during the COVID-19 pandemic: a positive technology perspective. Dementia. 2021.
- National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. NICE Guideline [NG128]; London, 2019.
- Stavroulakis T, Walsh T, Shaw PJ, et al. Gastrostomy use in motor neurone disease (MND): a review, meta-analysis and survey of current practice. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(2):96–104.
- O'Malley RPD, Mirheidari B, Harkness K, et al. Fully automated cognitive screening tool based on assessment of speech and language. J Neurol Neurosurg Psychiatry. 2021;92(1):12–15.
- Flemming K, Turner V, Bolsher S, et al. The experiences of, and need for, palliative care for people with motor neurone disease and their informal caregivers: a qualitative systematic review. Palliat Med. 2020;34(6):708–730.
- Hodkinson A, Bower P, Grigoroglou C, et al. Self-management interventions to reduce healthcare use and improve quality of life among patients with asthma: systematic review and network meta-analysis. BMJ. 2020;370:m2521.
- Jones F, Riazi A. Self-efficacy and self-management after stroke: a systematic review. Disabil Rehabil. 2011;33(10):797–810.
- Warsi A, Wang PS, LaValley MP, et al. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164(15):1641–1649.
- Palmer R, Dimairo M, Cooper C, et al. Self-managed, computerised speech and language therapy for patients with chronic aphasia post-stroke compared with usual care or attention control (big CACTUS): a multicentre, single-blinded, randomised controlled trial. Lancet Neurol. 2019;18(9):821–833.
- Huijbregts MPJ, McEwen S, Taylor D. Exploring the feasibility and efficacy of a telehealth stroke self-management programme: a pilot study. Physiother Can. 2009;61(4):210–220.
- Hwang N-K, Park J-S, Chang M-Y. Telehealth interventions to support self-management in stroke survivors: a systematic review. Healthcare. 2021;9(4):472.
- van Eijk RPA, Beelen A, Kruitwagen ET, et al. A road map for remote digital health technology for motor neuron disease. J Med Internet Res. 2021;23(9):e28766.
- Hoppitt T, Pall H, Calvert M, et al. A systematic review of the incidence and prevalence of long-term neurological conditions in the UK. Neuroepidemiology. 2011;36(1):19–28.
- Chiò A, Canosa A, Gallo S, et al. ALS clinical trials: do enrolled patients accurately represent the ALS population? Neurology. 2011;77(15):1432–1437.
- van Eijk RPA, Westeneng HJ, Nikolakopoulos S, et al. Refining eligibility criteria for amyotrophic lateral sclerosis clinical trials. Neurology. 2019;92(5):e451–e460.
- Rutkove SB, Narayanaswami P, Berisha V, et al. Improved ALS clinical trials through frequent at-home self-assessment: a proof of concept study. Ann Clin Transl Neurol. 2020;7(7):1148–1157.
- Rutkove SB, Qi K, Shelton K, et al. ALS longitudinal studies with frequent data collection at home: study design and baseline data. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(1–2):61–67.
- Block VAJ, Pitsch E, Tahir P, et al. Remote physical activity monitoring in neurological disease: a systematic review. PLOS One. 2016;11(4):e0154335.
- Espay AJ, Hausdorff JM, Sánchez-Ferro Á, et al. A roadmap for implementation of patient-centered digital outcome measures in Parkinson's disease obtained using mobile health technologies. Mov Disord. 2019;34(5):657–663.
- Harper KA, Butler EC, Hacker ML, et al. A comparative evaluation of telehealth and direct assessment when screening for spasticity in residents of two long-term care facilities. Clin Rehabil. 2021;35(4):589–594.
- Hoffmann T, Russell T, Cooke H. Remote measurement via the internet of upper limb range of motion in people who have had a stroke. J Telemed Telecare. 2007;13(8):401–405.
- van Oirschot P, Heerings M, Wendrich K, et al. Symbol digit modalities test variant in a smartphone app for persons with multiple sclerosis: validation study. JMIR Mhealth Uhealth. 2020;8(10):e18160.
- Verduzco-Gutierrez M, Romanoski NL, Capizzi AN, et al. Spasticity outpatient evaluation via telemedicine: a practical framework. Am J Phys Med Rehabil. 2020;99(12):1086–1091.
- van Eijk RPA, Bakers JN, Bunte TM, et al. Accelerometry for remote monitoring of physical activity in amyotrophic lateral sclerosis: a longitudinal cohort study. J Neurol. 2019;266(10):2387–2395.
- Lord S, Godfrey A, Galna B, et al. Ambulatory activity in incident Parkinson’s: more than meets the eye? J Neurol. 2013;260(12):2964–2972.
- Helleman J, Kruitwagen ET, van den Berg LH, et al. The current use of telehealth in ALS care and the barriers to and facilitators of implementation: a systematic review. Amyotroph Lateral Scler Frontotemporal Degener. 2019;21(3–4):1–16.
- Koivunen M, Saranto K. Nursing professionals' experiences of the facilitators and barriers to the use of telehealth applications: a systematic review of qualitative studies. Scand J Caring Sci. 2018;32(1):24–44.
- Keenan J, Rahman RJ, Hudson J. Exploring the acceptance of telehealth within palliative care: a self-determination theory perspective. Health Technol. 2021.
- Knox L, Gemine R, Dunning M, et al. Reflexive thematic analysis exploring stakeholder experiences of virtual pulmonary rehabilitation (VIPAR). BMJ Open Respir Res. 2021;8(1):e000800.
- Shaw S, Wherton J, Vijayaraghavan S, et al. Health services and delivery research. Advantages and limitations of virtual online consultations in a NHS acute trust: the VOCAL mixed-methods study. Southampton (UK): NIHR Journals Library; 2018.
- NHS Health Education England. Improving digital literacy; London, 2017.
- Yardley L, Morrison L, Bradbury K, et al. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res. 2015;17(1):e30.
- Helleman J, Van Eenennaam R, Kruitwagen ET, et al. Telehealth as part of specialized ALS care: feasibility and user experiences with "ALS home-monitoring and coaching". Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(3–4):183–192.
- Pinto A, Almeida JP, Pinto S, et al. Home telemonitoring of non-invasive ventilation decreases healthcare utilisation in a prospective controlled trial of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2010;81(11):1238–1242.
- Haase R, Schultheiss T, Kempcke R, et al. Modern communication technology skills of patients with multiple sclerosis. Mult Scler. 2013;19(9):1240–1241.
- Levy H, Janke A. Health literacy and access to care. J Health Commun. 2016;21(Suppl. 1):43–50.
- Honeyman M, Maguire D, Evans H, et al. Digital technology and health inequalities: a scoping review; Cardiff, 2020.
- Blakely T, Hales S, Kieft C, et al. The global distribution of risk factors by poverty level. Bull World Health Organ. 2005;83(2):118–126.
- Ezzati M, Lopez AD, Rodgers A, et al. Comparative quantification of health risks. Global and regional burden of disease attributable to selected major risk factors. Geneva: World Health Organization; 2004. p. 1987–1997.
- Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120(3):472–495.
- Hategeka C, Traboulsee AL, McMullen K, et al. Association of unemployment and informal care with stigma in multiple sclerosis: evidence from the survey on living with neurological conditions in Canada. Int J MS Care. 2019;21(5):214–225.
- Rixon L, Hirani S, Cartwright M, et al. What influences withdrawal because of rejection of telehealth – the whole systems demonstrator evaluation. J Assist Technol. 2013;7(4):219–227.
- Garcia-Gancedo L, Kelly ML, Lavrov A, et al. Objectively monitoring amyotrophic lateral sclerosis patient symptoms during clinical trials with sensors: observational study. JMIR Mhealth Uhealth. 2019;7(12):e13433.
- Simblett SK, Evans J, Greer B, et al. Engaging across dimensions of diversity: a cross-national perspective on mHealth tools for managing relapsing remitting and progressive multiple sclerosis. Mult Scler Relat Disord. 2019;32:123–132.
- Maeder A, Poultney N, Morgan G, et al. Patient compliance in home-based self-care telehealth projects. J Telemed Telecare. 2015;21(8):439–442.
- Kruse CS, Fohn J, Umunnakwe G, et al. Evaluating the facilitators, barriers, and medical outcomes commensurate with the use of assistive technology to support people with dementia: a systematic review literature. Healthcare. 2020;8(3):278.
- Blandford A, Gibbs J, Newhouse N, et al. Seven lessons for interdisciplinary research on interactive digital health interventions. Digit Health. 2018;4:2055207618770325.
- Griffin N, Kehoe M. A questionnaire study to explore the views of people with multiple sclerosis of using smartphone technology for health care purposes. Disabil Rehabil. 2018;40(12):1434–1442.
- Witham MD, Anderson E, Carroll C, et al. Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process. Trials. 2020;21(1):694.
- de Almeida JPL, Pinto AC, Pinto S, et al. Economic cost of home-telemonitoring care for BiPAP-assisted ALS individuals. Amyotroph Lateral Scler. 2012;13(6):533–537.
- Hobson E, Baird WO, Cooper CL, et al. Using technology to improve access to specialist care in amyotrophic lateral sclerosis: a systematic review. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(5–6):313–324.
- Kwasnicka D, Keller J, Perski O, et al. White paper: open digital health – accelerating transparent and scalable health promotion and treatment. Health Psychol Rev.; 2022; Accepted for publication.
- Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of medical research council guidance. BMJ. 2021;374:n2061.
- National Institute for Health and Care Excellence. Evidence standards framework for digital health technologies; London, 2018.
- World Health Organization. Monitoring and evaluating digital health interventions: a practical guide to conducting research and assessment; Geneva, 2016.
- Hobson E, Fazal S, Shaw PJ, et al. "Anything that makes life's journey better." Exploring the use of digital technology by people living with motor neurone disease. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(5–6):378–387.