Abstract
The pre-burial history of a partial elasmosaurid plesiosaur skeleton is reconstructed from analysis of the distribution and modification of bones preserved in a calcareous concretionary mass. The specimen lacks the skull, cervical vertebrae, left limb bones and some girdle elements, but the remaining bones are interpreted to have been deposited on the sea floor from a semi-buoyant carcass and their relative positions modified by the action of scavengers. Bioerosive agents caused loss of bone, particularly on joint surfaces and vertebral centra, as the carcass lay exposed on the sea floor, perhaps for several years before burial.

TAPHONOMIC attributes of individual vertebrate fossils have been noted in many palaeontological works. They include documentation of the types of elements preserved (proportion of the skeleton represented), the degree of articulation or association, the condition of the bones (fragmentation, corrosion, scratches and other surface damage), associated fossils, and the sedimentological context of the remains. The taphonomic processes affecting terrestrial vertebrates, especially mammals, are reasonably well known (Martin Citation1999) but those relating to marine forms are less common and have focused mainly on whale, dolphin, seal and turtle carcasses (Schäfer Citation1972, Behrensmeyer Citation1991, Citation2001). Schäfer (Citation1972) noted that the lifestyle, death, disintegration, and burial of these aquatic animals are similar to that of large marine reptiles. Some taphonomic studies of ichthyosaur skeletons have been published (e.g. Martill Citation1987) but little has been written about plesiosaurs.
Here we consider the taphonomic attributes of a Late Cretaceous elasmosaurid plesiosaur from North Canterbury, South Island, New Zealand and interpret the post-mortem history of the skeleton (Canterbury Museum, Christchurch, catalogue number CM Zfr 145). The specimen can not be identified precisely without the skull or cervical vertebrae, but the animal would have been at least 6 m long (Hiller & Mannering Citation2005).
Methods
Discovery and preparation
A large calcareous concretion (measuring ca 2.6 m × 2.3 m × 1.0 m) containing plesiosaur bones was discovered on the banks of the Waipara River of North Canterbury, New Zealand () in 1982, following slumping of cliff exposures of the Conway Formation. Canterbury Museum staff and volunteers retrieved the concretion, which had broken into two large blocks and a third smaller one, and undertook initial preparation in early 1983. Further work, carried out over the next 14 years, involved splitting the two larger blocks into 15 smaller ones () and gradually exposing the bones using mechanical methods. Most individual elements were not completely removed from the matrix so as to preserve their taphonomic context. Where necessary, some small bones (phalanges, caudal vertebrae and ribs, rib fragments) were completely removed from the rock but their positions were recorded. The river banks near the source of the fossil were searched for several years but no further bones were recovered.
Fig. 1. Locality maps. A, map of New Zealand showing the position of the North Canterbury region (shaded square); B, simplified geological map of North Canterbury showing the Upper Cretaceous outcrop and the locations of the Waipara River and Haumuri Bluff.
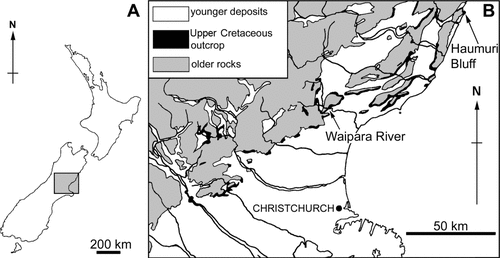
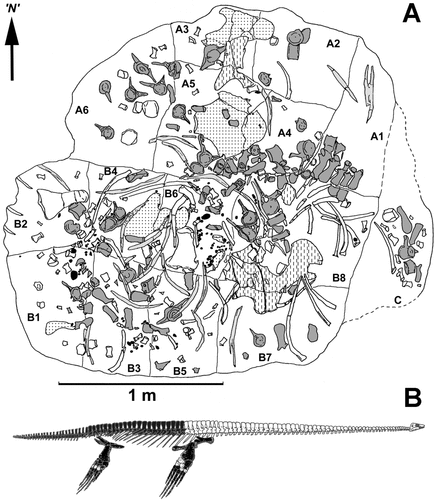
Fig. 2. A, Bone map of CM Zfr 145. A, B and C are the original blocks of rock recovered from the Waipara River field site; A1–A6 and B1–B8 denote the smaller blocks into which the two largest original blocks were split. Dark grey, vertebrae and detached neural spines; pale grey, gastralia; stipple, pelvic girdle elements; dashed, pectoral girdle elements; unshaded, limb bones and ribs. N, arbitrary north used for measuring orientations of long bones. B, Diagram showing (in black) the bones represented in CM Zfr 145.
Once fully exposed, evidence of decomposition was found on the dorsal vertebrae in block A1 (). Transverse processes on the left-hand side were well preserved but those on the right-hand side were missing. We concluded that the left-hand side of the vertebrae had been preserved by early burial within the sediment (Martill Citation1987, Allison et al. Citation1991), whilst the right-hand side was probably exposed to destructive agents on the sea floor. Hence, preparation of the concretion began from the stratigraphically lower surface.
Methods of analysis
A map () recording the distribution and orientation of individual bones (less some distal caudal vertebrae and phalanges) was compiled together with an inventory of preserved and missing elements (). Prior to collection, several parts of the concretion had been displaced from their original positions so could not be orientated with respect to true north. Therefore, orientations of long bones were measured from an arbitrary ‘north’ (, ) and the heavier end noted where applicable.
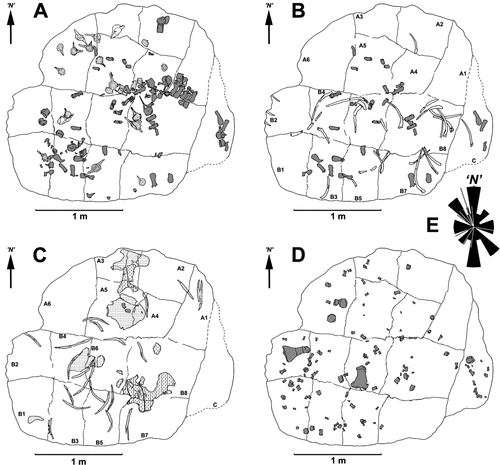
Fig. 3. Diagrams showing the distribution of groups of bones. A, dorsal vertebrae and detached neural spines (dark grey), caudal vertebrae (pale grey) and sacral vertebrae (unshaded). B, detached neural spines and caudal ribs (dark grey) and ribs (unshaded). C, pectoral (dashed) and pelvic (stipple) girdle bones and gastralia (pale grey). D, limb bones. E, rose diagram derived from orientations of gastralia and propodials (n = 25) and lines showing orientation of ribs (n = 11). N, arbitrary north used for measuring orientations of long bones.
Modification of bone surfaces was assessed visually and elements were compared with the equivalent elasmosaurid remains held at Canterbury Museum. Pre-burial damage was carefully distinguished from preparation damage, the latter marks being consistently fresh, lacking signs of degradation along the margins, and free of sediment infill.
Observations
Geological setting
The bones were encased in a large, oblate, calcareous concretion from the middle part of the Conway Formation (Upper Cretaceous) which is predominantly a grey, poorly indurated, massive, jarositic, slightly glauconitic fine silty sandstone (Browne & Field Citation1985). Pervasive bioturbation has almost entirely obliterated primary sedimentary structures; only a few major bedding surfaces are preserved. The sediment hosting the concretion is poorly sorted fine sandstone (framework grains 0.1–0.2 mm) dominated by quartz with lesser feldspar and labile rock fragments all set in a matrix of clay minerals with minor glauconite and finely disseminated pyrite. Within the concretion, the clay matrix has been replaced by calcite cement. The concretions at Waipara River occur in thick beds of dark brown–grey, organic-rich sediment that alternate with light grey beds largely devoid of concretions. Intensive bioturbation of all beds incorporates indeterminate vertical, horizontal and inclined burrows consistent with an ichnofabric index of 4 or 5 for shelf environments (sensu Droser & Bottjer Citation1986).
The Conway Formation in the Waipara River area has been dated as Haumurian–Teurian (Late Cretaceous–early Paleocene) based on dinoflagellate biostratigraphy (Roncaglia et al. Citation1999, Crampton et al. Citation2000). Sediment samples from CM Zfr 145 yielded a dinocyst assemblage attributable to the upper Alterbidinium acutulum Zone or lower Manumiella druggii Zone (early late Maastrichtian; Wilson et al. Citation2005). Hence, this plesiosaur is slightly younger than the well-known remains in the Maungataniwha Sandstone Member of the Tahora Formation, North Island, New Zealand (Vajda & Raine Citation2010).
Associated fossils
The Conway Formation contains few macrofossils other than reptile bones. Well-preserved calcareous shells are conspicuously absent apart from a few very poorly preserved molluscs and foraminifera. Accordingly, only teleost bones, teeth and scales, linguloid brachiopods and plant remains (leaves and branches) were recovered with CM Zfr 145. Rich dinocyst assemblages are also present, and the concretion contains numerous trace fossils. These fossils were distributed randomly throughout the concretion rather than being concentrated on a single bedding plane. The bivalves and brachiopods are infaunal types and were probably responsible for some of the bioturbation. Elsewhere, the formation has also yielded some shark teeth and vertebrae.
Skeletal elements present
The plesiosaur skeleton was almost completely disarticulated when buried. Recovered bones were scattered over an area of about 5 m2 but non-repetition of elements indicates they derive from a single individual (; Hiller & Mannering Citation2005). They comprise 17 dorsal, three sacral and 30 caudal vertebrae (, ), the right scapula, right and partial left coracoid, the right pubis, both left and right ilia and both ischia. Only the right fore- and hindlimbs are present and include the humerus, radius, ulna, femur, tibia, fibula and metapodials; 77 isolated phalanges were also recovered from the bone aggregation.
The skull, cervical vertebrae, left scapula and both sets of clavicles and interclavicles, left pubis, and the left fore- and hind paddles are absent from the concretion. The clavicles and interclavicles were probably cartilaginous, hence much less likely to be preserved than bone. Phalanges from the right paddles are certainly also missing; the 77 accounted for are far less than the 140 or more that might be expected in two limbs of a Late Cretaceous elasmosaur (Welles & Bump Citation1949).
A total of 347 quartzose gastroliths, 11–58 mm in diameter, are scattered amongst the bones (Hiller & Mannering Citation2005). Gastroliths occur in each block but are much more common in block B6 (), perhaps indicating the original position of the visceral mass.
Bone distribution
Five of the dorsal vertebrae in near articulation were used as a tentative guide to the orientation of the torso. Based on the position of their anterior and posterior zygapophyses, we placed the missing cranial end of the carcass in an ‘easterly’ position (, ), with the tail extending towards the ‘west.’ The remaining vertebrae superficially appear to be scattered throughout the concretion but the array of succeeding dorsals () may not have moved far from their original position, although the sacrals and caudals are irregularly jumbled. Apart from two clusters, ribs, caudal ribs and detached neural spines () are widely scattered. The gastralia, originally embedded within muscle tissue between the pectoral and pelvic girdles, are dispersed in a weak arc (). The pectoral and pelvic girdle elements are also disarticulated () with the right ischium and pubis lying on top of the scapula, and the left ischium separated by a considerable distance. The right humerus and right femur () have also been moved away from the central body area. The smaller paddle bones (including the epipodials, metapodials and phalanges) and distal caudal vertebrae are dispersed both horizontally and vertically within the bed (, D).
Skeletal elements were also categorized according to their hydrodynamic properties to evaluate water movement and direction of flow. The small paddle bones and distal caudal vertebrae have the highest potential for transport, since they are small, light and capable of being moved by currents of moderate strength in any direction. Transported elongate bones most commonly orientate parallel and perpendicular to flow, but the array of ribs, propodials and gastralia in this specimen have no consistent orientation (), suggesting they were not subject to strong unidirectional flow. However, they may have obstructed the movement of small bones by weak flows as evidenced on block C (), where some phalanges have come to rest against a rib.
Large flat pectoral and pelvic elements, especially when lying flat against the sediment, would presumably have resisted movement by currents and may also have acted as barriers to small bone transport. Irregularly shaped bones (e.g. vertebrae with attached neural spines and/or transverse processes) would also have resisted drag in the sediment and formed obstacles to the movement of smaller bones with higher hydrodynamic potential. This may have occurred where small bones have been dropped within the articulated dorsal vertebrae.
The skeleton map () shows that bones with the highest hydrodynamic potential are scattered amongst those with the least. Hence, it appears that little or no current sorting has occurred. If the current directions of ‘barrier-lags’ can be inferred by smaller bones amassing on the up-current side of larger bones, there seems to be no preferred direction. The orientations of long bones (ribs, gastralia, propodials) plotted on a rose diagram () suggest weak current alignment of gastralia and propodials, but the relatively few measurements (n = 25) are considered insufficient evidence to infer flow alignment of these bones. The orientations of ribs (, n = 12) also appear random.
Bone modification
Although skeletal elements appear well preserved, several types of bone modification were identified on the elasmosaur. Loss of bone is evident, including partial destruction of the five associated dorsal vertebrae, the capitula of both humerus and femur, and other cartilage-covered joints. Also, a range of scratches, gouges and indentations were identified (see below) on otherwise well-preserved bone surfaces. The bones are not significantly compacted or distorted; all preserve their original dimensions. Calcite veins cutting both the concretion and the bones are clearly younger diagenetic features.
Loss of bone
There are two categories of bone loss. The first was generated by mechanical gouging or breakage of well-defined areas of bone, mostly on the edges of large bones. A good example includes a prominent gouge (42 mm long, 23 mm wide and 20 mm deep) on the anterior edge of the distal end of the humerus opening towards the proximal end (). A similar gouge at the distal end of the femur opens distally, with a small fragment of displaced bone adhering to the external surface (). The right coracoid is missing a large, almost rectangular area of bone from its posterior ramus (). This feature is unique in the specimen and represents the largest single item of bone loss, affecting the thinnest bone.
Fig. 4. A, Dorsal view of the humerus showing the loss of bone from the distal end of the anterior edge. B, Distal corner of the femur showing fragment (arrowed) gouged from the bone. Scale bars = 10 mm.
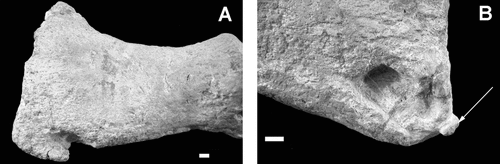
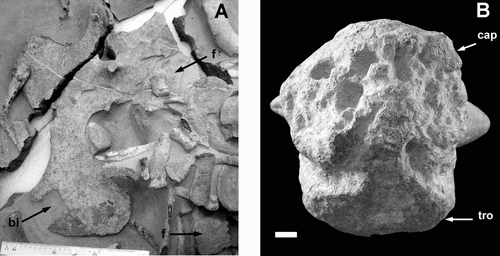
Fig. 5. A, Ventral view of the fused coracoids showing broken fragments of the left ramus (f) and the piece of bone lost from the right ramus (bl). Ruler = 300 mm. B, Proximal end of the femur showing bone loss from the capitulum (cap) and the development of deep pits; the trochanter (tro) is well preserved. Stratigraphic up towards the top of the photograph. Scale bar = 10 mm.
The second type of bone loss was probably not mechanical and affected more extensive, less well defined areas particularly on the capitulum of the femur or the centra of the articulated dorsal vertebrae. Modification appears to have been progressive, beginning with the development of subcircular pits, up to 16 mm deep, perpendicular to the bone surface with flat or concave floors (). Degradation of the intervening bony walls led to coalescence of adjacent pits into irregular depressions or linear troughs, ultimately facilitating the complete destruction of the bone as evident in the articulated dorsal centra.
Brittle fracture
A single example of this category is evident: the left ramus of the coracoid has fractured into at least three pieces ().
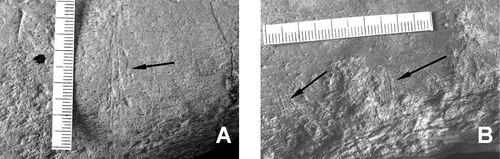
Surficial scratches
Scratches are of two main types. The first are up to 4.2 mm long, thin, teardrop-shaped indentations occurring mostly as subparallel pairs (). Entry (gradual deepening and widening) and exit (an abrupt perpendicular wall) points can be identified in each indentation. paired scratches occur either in isolation or, more commonly, in groups ().
The second type encompasses subtle fine scratches averaging 8 mm long, occurring mostly in parallel sets of 2–5 (). Two subcategories are recognized: faint relatively long, straight and parallel to subparallel (<5° divergence) ‘scratches’ that are spaced up to 3.7 mm apart (), and faint ‘serrations’ that occur in groups, are shorter than ‘scratches,’ and in all instances are exactly parallel ().
Interpretation
Sedimentology
Warren & Speden (Citation1977) interpreted the Conway Formation at Haumuri Bluff, ca 95 km northeast of the Waipara River site (), as having been deposited in an offshore barred submarine depression marked by gentle bottom currents with sedimentation mostly from suspension. The jarosite likely derives from authigenic bacterial reduction of seawater sulfates associated with a supply of organic matter in a dysaerobic environment. This view was reiterated by Browne & Field (Citation1985) for the formation in the North Canterbury area. The degree of bioturbation indicates a soft sea floor, slow sedimentation rates and an absence of strong wave or current activity, most likely below wave base. Warren & Speden (Citation1977) envisaged a setting similar to the modern Santa Barbara Basin off southern California with water depths of several hundred metres.
Bone distribution
The plesiosaur skeleton (CM Zfr 145) is almost completely disarticulated and disassociated, indicating that processes have acted on the carcass to redistribute the bones. The missing head, neck, left pectoral, pelvic and limb elements were apparently removed before burial, and probably prior to the body reaching the sea floor. The head and neck may have been removed by a predator at the time of death or by a large scavenger shortly afterwards, or they detached from the rest of the body during the early stages of a ‘bloat-and-float’ decompositional phase, as observed in cetacean carcasses (Schäfer Citation1972). The head and neck likely detached from the torso at the shoulders and may have been transported a considerable distance from the rest of the carcass. Loss of limb and girdle bones from the left side of the torso must also have happened during a buoyant stage given the left-side down orientation in the sediment. Missing right-paddle phalanges may have been removed by currents, but the smaller caudal vertebrae (vulnerable to the same processes) are accounted for, so more likely, they were moved by scavengers. Redistribution of larger bones almost certainly occurred after the carcass came to rest. If hydrostatic pressure and low temperatures inhibited gas production and flotation, the carcass would have sunk to the sea floor and remained there during decomposition. Subsequent scattering of bones and twisting of the axial skeleton would have been facilitated by shrinkage and retraction of ligaments and tendons. Alternatively, if gas evacuation and rainout took place from a buoyant torso, this must have occurred close to the sea floor (i.e. low in the water column) as indicated by the distribution of visceral elements such as gastralia and the numerous gastroliths, which would have been more widely dispersed if the gut had burst high in the water column (decomposition of soft tissues begins close to the intestinal tract and progresses more rapidly than elsewhere in the body; Jans et al. Citation2004).
The smaller paddle elements have completely disarticulated and dispersed. Although some small bones appear to have collected against larger ones, if water transport was responsible for this, there was no consistent flow direction. Depending on when individual small paddle bones detached, they may have simply settled between larger ones, although even very weak currents may have moved such bones. This may partly explain their degree of dissociation (). Bioturbation caused downward displacement of some small bones. Certainly, no strong current action appears to have transported or re-oriented the major bones (). Long bones reached their final orientation or position through weak water movement or, more likely, via decomposition and gravity-assisted and/or biological redistribution. As the last elements to disarticulate, the vertebrae would have remained associated the longest and undergone the least reworking.
Bone modification
Large gouges are interpreted to have been caused by the teeth of vertebrate predators or scavengers. Their size is consistent with tooth punctures from a mosasaur and teeth of Prognathodon waiparaensis, recovered from the Conway Formation at Waipara River (Welles & Gregg Citation1971), fit neatly into the gouges on the humerus and femur. More difficult to explain is the rectangular piece of bone lost from the right coracoid (). It does not appear to be associated with bite marks, but probably resulted from compressional fracturing during burial produced by the force of overlying sediment, or more likely, a small bone (possibly removed during preparation) impacting on the extremely thin posterior ramus ().
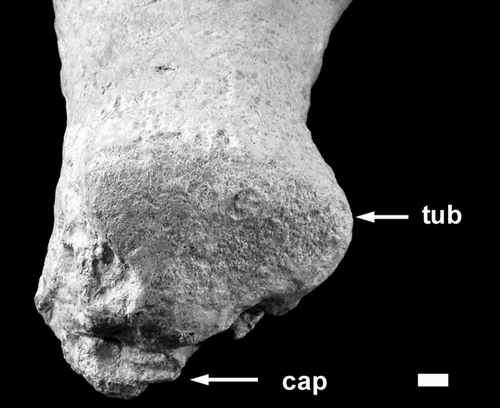
Loss of bone leaving deep pitting () on the centra and transverse processes of dorsal vertebrae, from the capitula of humerus and femur and from articulation surfaces of girdle bones seems to have resulted from an entirely different process. The position and variable size and depth of these depressions preclude derivation by biting. The rarity and degraded quality of fossil microfauna and the absence of calcareous shells in the surrounding sediment (Warren & Speden Citation1977, Hollis & Strong Citation2003) suggest chemical dissolution, but this did not affect phosphatic skeletons. We attribute this damage to bioerosion effected by some bone-excavating/consuming organism, potentially including echinoids, molluscs and worms (Glover et al. Citation2008) although none of these is preserved among the associated fossils. The smallest pits are interpreted as the points of initiation of bone destruction, possibly focused on blood vessel foramina. Similarly, loss of bone from the dorsal vertebrae may have originated at the nutritive foramina on the ventral surface of the centra. Bioerosion would have occurred while the bones lay exposed on the sea floor prior to burial. The femoral trochanter and the humeral tuberosity do not show significant damage and may have been protected by burial in the sediment (, ).
Fig. 8. Proximal end of the humerus showing the well-preserved tuberosity (tub). The capitulum (cap) has been badly degraded like that of the femur. Stratigraphic up towards the bottom of the photograph. Scale bar = 10 mm.
Type 1 scratches are common on the fossil, reflecting a frequent process. They do not result from fragmentation or dissolution, and their subparallel expression is inconsistent with mechanical abrasion. These scratches likely represent impressions made by the apex of a tooth from a large shark, similar to or smaller than Cretoxyrhina mantelli (see Shimada & Hooks Citation2004). The animal responsible was smaller than the one that produced the gouges and likely interacted with the carcass later in the mobile-scavenger stage of Smith & Baco (Citation2003), although some overlap in activities is expected. The paired scratches record the actions of upper and lower jaws; the shorter scratches made by lower jaw teeth, which are used to anchor the bone, and the longer scratches by upper jaw teeth that are responsible for the removal of flesh (Charles Maxwell pers com.). Chipping has probably occurred where a tooth has dug under the surface and lifted a portion of periosteum intact. A predator/scavenger's objective is not to score the bone, but to remove flesh, so the number of impressions made is probably not representative of the intensity of the activity on the carcass. Scratches in this category are of similar size, possibly indicating production by one species, similar-sized individuals, or both.
Type 2 scratches are less common and much smaller than type 1. These subtle, fine features can not have been produced through fragmentation or chemical dissolution. Although abrasive origin is possible, such damage (both indistinct ‘scratches’ and faint ‘serrations’) has been previously attributed to serrated-toothed sharks such as Squalicorax (Everhart Citation2004, Schwimmer et al. Citation1997). These sharks are well-known scavengers of bloat-and-float carcasses. Distribution of the scratches across multiple surfaces of many bones suggests that the bones were repositioned to expose fresh tissue; this process may have further contributed to the disarticulation and re-orientation of the skeletal elements. Alignment of ribs and gastralia may preserve not current flow but traces of ‘grab-drag’ behaviour, where sharks and other fish have dragged the end of a long-bone to the edge of the mass.
Conclusions
The early taphonomic history of the Waipara River elasmosaur skeleton is consistent with that reported for modern whale falls (Smith & Baco Citation2003). An initial mobile scavenger stage began with settling of the carcass on the sea floor and removal of flesh by sharks, bony fish and probably crustaceans, inflicting tooth marks on, and repositioning, the bones. The succeeding enrichment/opportunist stage involved smaller organisms (invertebrates and bacteria) infesting and eroding the bones to exploit the enclosed organic matter. It is unlikely that the bones of CM Zfr 145 reached the sulfophilic stage, since Braby et al. (Citation2007) suggested that a comparable 10 m long whale may not have had bones large enough to fuel a sulfophilic community. Burial of the skeleton finally took place before a terminal reef stage developed since none of the bone surfaces hosts encrusting organisms.
Smith & Baco (Citation2003) suggested that the mobile scavenger stage for large vertebrate carcasses can last between four months and two years. However, Braby et al. (Citation2007) reported that whale carcasses in shallower water can be completely stripped of flesh within three months. Fujiwara et al. (Citation2007) noted that the succession of ecological communities developed much more rapidly in shallow water whale falls, a scenario that is probably comparable to that involving CM Zfr 145, which was buried in a shallow near-shore marine palaeoenvironment.
Acknowledgements
We are deeply indebted to Al Mannering, not only for his role in the discovery of the specimen, but also for the sterling job he did in preparing it to the point where we could carry out this study and for the many fruitful discussions on aspects of its taphonomy. We are grateful to Pat Druckenmiller and the editors for their many helpful and constructive suggestions. The project was supported by a Foundation for Research Science and Technology fellowship to KMB and for this we are very grateful.
References
- Allison, P. A., Smith, C. R., Kukert, H., Deming, J. W., and Bennett, B. A., 1991. Deep-water taphonomy of vertebrate carcasses: A whale skeleton in the bathyal Santa Catalina Basin, Paleobiology 17 (1991), pp. 78–89.
- Behrensmeyer, A. K., 1991. "Terrestrial vertebrate accumulations". In: Allison, P. A., and Briggs, D. E.G., eds. Taphonomy: Releasing the Data Locked in the Fossil Record. New York: Plenum Press; 1991. pp. 291–327.
- Behrensmeyer, A. K., 2001. "Terrestrial vertebrates". In: Briggs, D. E.G., and Crowther, P. R., eds. Palaeobiology II. Oxford: Blackwell Science; 2001. pp. 318–321.
- Braby, C. E., Rouse, G. W., Johnson, S. B., Jones, W. J., and Vrijenhoek, R. C., 2007. Bathymetric and temporal variation among Osedax boneworms and associated megafauna on whale-falls in Monterey Bay, California, Deep-Sea Research I 54 (2007), pp. 1773–1791.
- Browne, G. H., and Field, B. D., 1985. The lithostratigraphy of Late Cretaceous to Early Pleistocene rocks of northern Canterbury, New Zealand, New Zealand Geological Survey Record 6 (1985), pp. 1–63.
- Crampton, J. S., Mumme, T. C., Raine, J. I., Roncaglia, L., Schiøler, P., Strong, C. P., Turner, G., and Wilson, G. J., 2000. Revision of the Piripauan and Haumurian local stages and correlation of the Santonian–Maastrichtian (Late Cretaceous) in New Zealand, New Zealand Journal of Geology and Geophysics 43 (2000), pp. 309–333.
- Droser, M. L., and Bottjer, D. J., 1986. A semiquantitative field classification of ichnofabric, Journal of Sedimentary Petrology 56 (1986), pp. 558–559.
- Everhart, M. J., 2004. Late Cretaceous interaction between predators and prey. Evidence of feeding by two species of shark on a mosasaur, PalArch, Vertebrate Palaeontology Series 1 (2004), pp. 1–7.
- Fujiwara, Y., Kawato, M., Yamamoto, T., Yamanaka, T., Sato-Okoshi, W., Noda, C., Tsuchida, S., Komai, T., Cubelio, S. S., Sasaki, T., Jacobsen, K., Kubokawa, K., Fujikura, K., Maruyama, T., Furushima, Y., Okoshi, K., Miyake, H., Miyazaki, M., Nogi, Y., Yatabe, A., and Okutani, T., 2007. Three-year investigations into sperm whale-fall ecosystems in Japan, Marine Ecology 28 (2007), pp. 219–232.
- Glover, A. G., Kemp, K. M., Smith, C. R., and Dahlgren, T. G., 2008. On the role of bone-eating worms in the degradation of marine vertebrate remains, Proceedings of the Royal Society B 275 (2008), pp. 1959–1961.
- Hiller, N., and Mannering, A. A., 2005. An unusual new elasmosaurid plesiosaur (Sauropterygia) from the Upper Haumurian (Maastrichtian) of the South Island, New Zealand, Memoirs of the Queensland Museum 51 (2005), pp. 27–37.
- Hollis, C., and Strong, C., 2003. Biostratigraphic review of the Cretaceous/Tertiary boundary transition, mid-Waipara River section, North Canterbury, New Zealand, New Zealand Journal of Geology and Geophysics 46 (2003), pp. 243–253.
- Jans, M. M.E., Nielsen-Marsh, C. M., Smith, C. I., Collins, M. J., and Kars, K., 2004. Characterisation of microbial attack on archaeological bone, Journal of Archaeological Science 31 (2004), pp. 87–95.
- Martill, D. M., 1987. A taphonomic and diagenetic case study of a partially articulated ichthyosaur, Palaeontology 30 (1987), pp. 543–555.
- Martin, R. E., 1999. Taphonomy: a process approach. Cambridge: Cambridge University Press; 1999. p. 508.
- Roncaglia, L., Field, B. D., Raine, J. I., Schiøler, P., and Wilson, G. J., 1999. Dinoflagellate biostratigraphy of Piripauan–Haumurian (Upper Cretaceous) sections from northeast South Island, New Zealand, Cretaceous Research 20 (1999), pp. 271–314.
- Schäfer, W., 1972. Ecology and Palaeoecology of Marine Environments. Edinburgh: Oliver & Boyd; 1972. p. 568.
- Schwimmer, D. R., Stewart, J. D., and Williams, G. D., 1997. Scavenging by sharks of the genus Squalicorax in the Late Cretaceous of North America, Palaios 12 (1997), pp. 71–83.
- Shimada, K., and Hooks, I., 2004. Shark-bitten protostegid turtles from the Upper Cretaceous Mooreville Chalk, Alabama, Journal of Paleontology 78 (2004), pp. 205–210.
- Smith, C. R., and Baco, A. R., 2003. Ecology of whale falls at the deep-sea floor, Oceanography and Marine Biology Annual Review 41 (2003), pp. 311–354.
- Vajda, V., and Raine, J. I., 2010. A palynological investigation of plesiosaur-bearing rocks from the Upper Cretaceous Tahora Formation, Mangahouanga, New Zealand, Alcheringa (2010).
- Warren, G., and Speden, I., 1977. The Piripauan and Haumurian stratotypes (Mata Series, Upper Cretaceous) and correlative sequences in the Haumuri Bluff district, South Marlborough, New Zealand Geological Survey Bulletin 92 (1977), pp. 1–60.
- Welles, S. P., and Bump, J. D., 1949. Alzadasaurus pembertoni, a new elasmosaur from the Upper Cretaceous of South Dakota, Journal of Paleontology 23 (1949), pp. 521–535.
- Welles, S. P., and Gregg, D. R., 1971. Late Cretaceous marine reptiles of New Zealand, Records of the Canterbury Museum 9 (1971), pp. 5–111.
- Wilson, G. J., Schiøler, P., Hiller, N., and Jones, C. M., 2005. Age and provenance of Cretaceous marine reptiles from the South Island and Chatham Islands, New Zealand, New Zealand Journal of Geology and Geophysics 48 (2005), pp. 377–387.