Abstract
Protowenella is an early–middle Cambrian, isostrophically coiled, calcareous microfossil originally described from the middle Cambrian (Miaolingian) of Australia; it has been referred previously to the molluscan classes Monoplacophora, Helcionelloida or Gastropoda. A unique specimen from the Henson Gletscher Formation (Miaolingian Series, Wuliuan Stage) of North Greenland has a bilaterally symmetrical operculum preserved in place within the shell aperture. Paired cardinal processes and clavicles on the inner side of the operculum indicate that Protowenella was a hyolith morphologically close to the orthothecid Conotheca. Protowenella is transferred from Mollusca to Hyolitha, Order Orthothecida, Family Protowenellidae nov., representing a novel morphological departure from the generally slender cones of other hyoliths.
John S. Peel [[email protected]], Department of Earth Sciences (Palaeobiology), Uppsala University, Villavägen 16, SE-75236, Uppsala, Sweden.
HELCIONELLOIDS and hyoliths are among the most prominent groups of Cambrian Small Shelly Fossils, the mainly phosphatic or phosphatized microfossils extracted from carbonate sediments by dissolution in weak acids. Their remains are most commonly preserved as phosphatic encrustations or as internal moulds of the calcareous shells (Bengtson et al. Citation1990, Creveling et al. Citation2014). While members of both groups are usually bilaterally symmetrical, hyoliths are characterized by a straight to slightly curved narrow conch, the aperture of which is closed by a robust calcareous operculum (Marek & Yochelson Citation1976, Berg-Madsen & Malinsky Citation1999, Martí Mus & Bergström Citation2005, Malinky & Yochelson Citation2007, Martí Mus et al. Citation2014). Two orders of hyoliths are recognized in the Cambrian. Hyolithids are distinguished by a shelf-like extension of the apertural margin, the ligula (), which is not present in orthothecids (). Furthermore, the outer surface of the operculum is divided into clearly demarcated cardinal and conical shields in most hyolithids, while orthothecid opercula are usually flat.
Figure 1. A, Hyolithid in lateral view. B, Orthothecid in lateral view. C, Protowenella with operculum in place reconstructed as an orthothecid hyolith. D–F, Alternative reconstructions of Protowenella; D, as an exogastric monoplacophoran (Runnegar & Jell Citation1976); E, as an endogastric helcionelloid (Peel Citation1991a, b); F, as a gastropod. Inferred digestive tract shown schematically with mouth (black dot) and anus (×).

Helcionelloids are cap-shaped, often laterally compressed, and coiled through a quarter to slightly more than a full whorl (Peel Citation1991a, b, Jacquet & Brock Citation2016). There are no records of an operculum in helcionelloids. As in modern limpetoid gastropods, the presence of an operculum would be incompatible with their clamping or semi-infaunal mode of life (Vermeij Citation2016).
The general morphology of the shell of Protowenella Runnegar & Jell, Citation1976 supports its placement as a member of the helcionelloid group of molluscs (Berg-Madsen & Peel Citation1978, Missarzhevsky Citation1989, Peel Citation1991a, b), albeit with a distinctive, tightly coiled, form (). The type species, Protowenella flemingi Runnegar & Jell, Citation1976 from the Miaolingian of Australia (Runnegar & Jell Citation1976), is known principally from internal moulds characterized by a tightly coiled, rather globose, isostrophic, shell without a median sinus in the apertural margin, but with a circumbilical channel impressed into the mould surface on each umbilico-lateral surface. The bilaterally symmetrical morphology may be described as ‘bellerophontiform’ by comparison to the coiling in the late Cambrian–Triassic mollusc group resembling Bellerophon Montfort, Citation1808. This similarity prompted Runnegar & Jell (Citation1976) to interpret Protowenella as the oldest genus within a widely drawn Order Bellerophontida Ulrich in Ulrich & Scofield, Citation1897, which they transferred from its traditional place within the gastropods (Knight et al. Citation1960) to an expanded Class Monoplacophora Knight, Citation1952 (). In describing Protowenella from the Miaolingian of Bornholm, Denmark, Berg-Madsen & Peel (Citation1978) rejected this wholesale interpretation of bellerophontids as untorted monoplacophorans, returning most to the gastropods, but they considered Protowenella to be an untorted mollusc. Their opinion was based on a functional morphological analysis of the channels in the umbilico-lateral areas of Protowenella flemingi, a character not discussed by Runnegar & Jell (Citation1976) although visible in their illustrations (Runnegar & Jell Citation1976, fig. 6G, H). Peel (Citation1991a, b) placed Protowenella within Class Helcionelloida Peel, Citation1991a (), an opinion followed by Gubanov et al. (Citation2004), Wotte (Citation2021) and Claybourn et al. (Citation2019). MacKinnon (Citation1985) followed Runnegar & Jell (Citation1976) in assigning Protowenella to the Order Bellerophontida, while Brock (Citation1998) considered Protowenella to be a bellerophontid, and tentatively a gastropod (). Parkhaev (Citation2008, Citation2017) placed Protowenella as a heterobranch of the Family Khairkhaniidae Missarzhevsky, Citation1989, but within the Order Khairkhaniformes Parkhaev, Citation2001 of the gastropod Subclass Divisabranchia Minichev & Starobogatov, Citation1979. In contrast, Parkhaev in Bouchet et al. (Citation2017, p. 330) classified Khairkhaniidae as Palaeozoic molluscs (gastropods or monoplacophorans) of uncertain position, unassigned to superfamily.
The interpretation of bellerophontiform molluscs as untorted molluscs, stem group gastropods, gastropods, or a conglomeration of all three, remains contentious (Missarzhevsky Citation1989, Peel Citation1991a, b, Wahlman Citation1992, Geyer Citation1994, Harper & Rollins Citation2000, Bouchet & Rocroi Citation2005, Frýda et al. Citation2008, Frýda Citation2012, Bouchet et al. Citation2017, Ponder et al. Citation2020). Peel (Citation2016) considered the morphological group to be polyphyletic and that opinion is maintained here. However, the controversy in the literature concerning the phylogenetic position of Protowenella within molluscs loses relevance in the present context, with the description of an in-place operculum in a unique specimen of Protowenella from the Miaolingian of North Greenland (). The discovery would represent the first occurrence of an operculum in a bellerophontiform mollusc, be it torted or untorted. Remarkably, however, the morphology of the bilaterally symmetrical operculum clearly invites comparison with hyolith opercula. Protowenella is not a mollusc, but an orthothecid hyolith ().
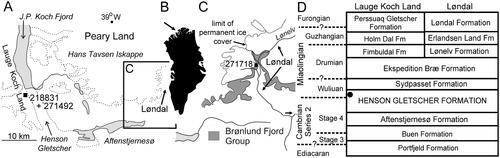
Figure 2. Locality and geological information. A, Lauge Koch Land – Løndal region of North Greenland showing collection site of GGU samples 218831 and 271492. B, Greenland, with arrow indicating location of A. C, Løndal region of Western Peary Land (see inset in A) showing collection locality for GGU sample 271718. D, Cambrian stratigraphic nomenclature of the Lauge Koch Land – Løndal region showing derivation of GGU samples from the Henson Gletscher Formation (black dot).
Hyolitha was proposed as a separate class within Mollusca by Marek & Yochelson (Citation1976) and Malinky & Yochelson (Citation2007) but regarded as a separate phylum by others (Runnegar et al. Citation1975, Runnegar Citation1980, Sysoev Citation1984, Missarzhevsky Citation1989, Val’kov Citation1990). Sun et al. (Citation2016) envisaged a relationship to sipunculids. Moysiuk et al. (Citation2017) suggested they were lophophorates, although Liu et al. (Citation2020a) considered that hyoliths were probably basal lophotrochozoans rather than lophophorates linked with brachiopods. Li et al. (Citation2020) stressed similarities in shell structure in concluding that hyoliths might be part of the total group Mollusca, but left their phylogenetic position unresolved.
Geological background and material
All described material is derived from the Henson Gletscher Formation of North Greenland (). The Henson Gletscher Formation is a component of a prograding complex of shelf sediments that accumulated in North Greenland on the present day southern margin of the transarctic Franklinian Basin (Higgins et al. Citation1991, Ineson & Peel Citation1997, Geyer & Peel Citation2011, Peel et al. Citation2016). The formation is mainly composed of dark, recessive, shaly-weathering, bituminous and cherty limestones, dolostones and mudstones, with a middle member of pale fine-grained sandstones. The Henson Gletscher Formation is 62 m thick at its type locality in Lauge Koch Land (, 82°10′N, 40°24′W, Ineson & Peel Citation1997, fig. 31, Geyer & Peel Citation2011, fig. 3, ), from which GGU samples 218831 and 271492 were collected at 56.5 m above the base. GGU sample 271718 was collected 1 m below the top of the formation in Løndal, to the east (, 82°18′N, 37°03′W, Clausen & Peel Citation2012, fig. 1), where the formation has thinned to 47 m.
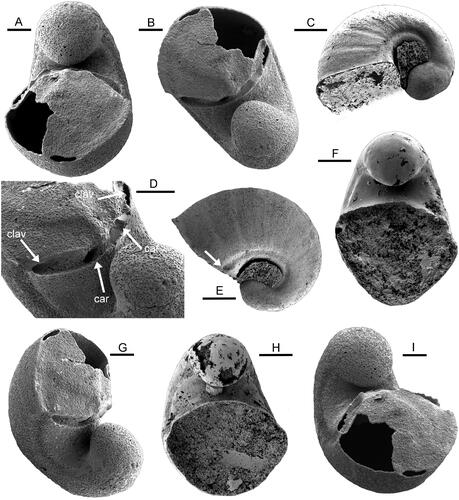
Figure 3. Protowenella flemingi Runnegar & Jell, Citation1976, internal moulds from the Henson Gletscher Formation, North Greenland, Cambrian, Miaolingian Series, Wuliuan Stage, Ptychagnostus gibbus Biozone. A, B, D, G, I, PMU 38329 from GGU sample 218831, Lauge Koch Land. A, B, apertural views showing isostrophic form and bilaterally symmetrical operculum. D, detail of umbilical area showing pits representing the cardinal processes (car) and clavicles (clav). G, I, oblique apertural views. C, E, PMU 38330 from GGU sample 271718 in oblique lateral and lateral views, with arrow in E showing termination of circumbilical channel prior to the broken aperture. F, PMU 38331 from GGU sample 271718, apertural view. H, PMU 38332, apertural view. Scale bars: 50 µm (A, B, D, G, I), 100 µm (C, E, F, H).
GGU sample 218831 was collected by Peter Frykman on 24 June 1979 from the same locality and horizon as GGU sample 271492 (J. S. Peel 25 June 1978). GGU sample 271718 was collected by J. S. Peel on 15 July 1978.
The Henson Gletscher Formation in southern Lauge Koch Land and southwestern Peary Land ranges in age from Cambrian Series 2 (Stage 4) to the Miaolingian Series (Wuliuan Stage, Ptychagnostus gibbus Biozone), although Drumian strata occur along the north coast of North Greenland (Higgins et al. Citation1991, Robison Citation1994, Blaker & Peel Citation1997, Ineson & Peel Citation1997, Geyer & Peel Citation2011, ).
Carbonate rock samples were digested in weak acetic acid and the dried, sieved residues were handpicked under a binocular microscope. Selected specimens were gold-coated prior to scanning electron microscopy, using a Zeiss Supra 35VP scanning electron microscope, and images were assembled using Adobe Photoshop CS4.
Repositories and institutional abbreviations.
GGU, a sample collected during field work by Grønlands Geologiske Undersøgelse (Geological Survey of Greenland), now part of the Geological Survey of Denmark and Greenland, Copenhagen, Denmark. PMU, palaeontological type collection of the Museum of Evolution, Uppsala University, Sweden.
Operculum of Protowenella
Almost all of the more than 100 specimens from GGU samples 218831, 271492 and 271718 are assigned to Protowenella flemingi Runnegar & Jell, Citation1976. They are preserved as compact apatite internal moulds of the type documented by Creveling et al. (Citation2014) in the Thorntonia Limestone (Cambrian, Miaolingian Series) of Australia, and widespread in Cambrian samples from North Greenland () and elsewhere. Rare specimens additionally preserve patches of a thin phosphatized encrustation that was formed on the outside of the shell (). The void between the encrustation and the internal mould thus corresponds to the dissolved calcareous shell. In contrast, the unique operculate specimen from GGU sample 218831 is preserved as a thin encrusting layer deposited on the inner surface of the shell (). Dissolution of the carbonate infilling in this specimen during sample preparation has left a hollow interior. The internal mould of the shell preserves no trace of the shallow comarginal corrugation sometimes visible on solid internal moulds () or the fine growth lines present on the external shell surface (, arrow). In all the available specimens of Protowenella, the circumbilical channels on the umbilico-lateral walls are clearly delimited ( arrow).
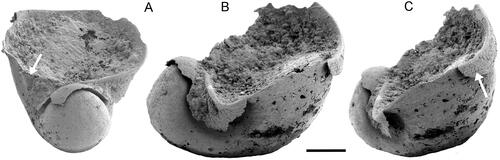
Figure 4. Protowenella flemingi Runnegar & Jell, Citation1976, PMU 38333 from GGU sample 271492, largely exfoliated internal mould, Henson Gletscher Formation, North Greenland, Cambrian, Miaolingian Series, Wuliuan Stage, Ptychagnpstus gibbus Biozone. A, oblique apertural view with channel on internal mould (arrow). B, oblique lateral view showing apertural margin lacking emargination corresponding to the circumbilical channel on the internal mould. C, oblique lateral view showing growth lines (arrow) near apertural margin. Scale bar 100 µm.
Description
Although partly broken, the preserved inner surface of the unique operculum is clearly bilaterally symmetrical about the same plane as the coiled shell () and fits closely to the shell aperture. Both the margins of the operculum and the shell aperture are coplanar, lacking invaginations. The shallowly convex surface of the internal mould, corresponding to the concave inner surface of the operculum itself, is marked by a triangular raised area, narrowing towards the umbilical area of the shell from points located about half way between the lateral extremities of the shell aperture (and the operculum) and the median dorsal line of the supra-apical surface of the shell (). The apex of this triangle continues as a short ridge to the margin of the operculum, below the shell apex ().
The adumbilical margin of the operculum is a deep channel that in its width extends between the circumbilical channels of the shell internal mould (). It represents a prominent comarginal ridge on the interior of the operculum that fits within the apertural margin of the shell (). The channel is U-shaped in cross-section, with two deep holes penetrating to the interior on each side of the median line (). These holes equate with prongs on the interior surface of the operculum.
Discussion
The role of opercula as a primary defensive element in tube-dwelling or cone-dwelling organisms is documented already in the early Cambrian where heavily calcified, bilaterally symmetrical, opercula are present in hyolithid and orthothecid Hyolitha (Marek & Yochelson Citation1976, Berg-Madsen & Malinsky Citation1999, Martí Mus & Bergström Citation2005, Peel Citation2010, Citation2021, Martí Mus et al. Citation2014, Peel & Willman Citation2018). While the identity of the associated tube is uncertain, phosphatic mobergellan opercula may be locally abundant in lower Cambrian strata (Bengtson Citation1968, Streng & Skovsted Citation2006, Skovsted & Topper Citation2018), but even rare Cambrian corallomorphs developed an operculum to cover the calice (Jell & Jell Citation1976, Peel Citation2011).
The most familiar association of opercula is with gastropods. Most gastropod groups develop an operculum at some stage in their ontogeny and fossil gastropod opercula are known from the early Ordovician to the present day (Yochelson Citation1979). While isolated heavily calcified opercula may be common at certain horizons in the Lower Palaeozoic (Lindström Citation1884, Yochelson Citation1979, Boucot & Poinar Citation2010), the general lack of calcification in most gastropod opercula precludes their preservation as fossils (Checa & Jiménez-Jiménez Citation1998, Ponder et al. Citation2020). Records of Palaeozoic gastropods with the operculum preserved in place are rare, and therefore well documented (Lindström Citation1884, Perner Citation1903, Yochelson & Linsley Citation1972, Horný & Peel Citation1995, Peel & Horný Citation1996, Rohr & Frýda Citation2001, Rohr Citation2004, Peel Citation2015). The opercula do not display bilateral symmetry, reflecting their association with anisostrophically coiled gastropod groups (Checa & Jiménez-Jiménez Citation1998). Opercula are not present in limpets or in bellerophontoidean gastropods, and have not been described from supposed Cambrian gastropods. Northrop (Citation1939) reported two possible opercula within the aperture of the holotype of the bellerophontoidean Salpingostoma inornatum Northrop, Citation1939 from the Silurian of Gaspé, but the two bodies were identified as a bivalve and a trilobite cephalon by Peel (Citation1972).
The morphology of the operculum in Protowenella demonstrates its hyolith character. Key features are the bilateral symmetry and the paired cardinal processes and clavicles represented by the deep holes into the interior (, arrowed as car and clav). While clavicles and cardinal processes are present in both hyolithids () and orthothecids (), the operculum outer surface in most hyolithids is divided into clearly demarcated cardinal and conical shields () that are not seen in Protowenella. Furthermore, a shelf-like ligula that is associated with the development on the operculum of cardinal and conical shields is not developed in Protowenella, although diagnostic of hyolithids. Additionally, a pronounced clavicular ridge (), locking the operculum into the shell aperture, is a characteristic feature of Conotheca Missarzhevsky, Citation1969 () and other orthothecid hyoliths. In Protowenella, the clavicular ridge is represented by the U-shaped channel at the adumbilical margin of the internal mould of the operculum (). The flattened marginal area peripheral to the clavicular ridge in Conotheca () is equivalent therefore to the thickness of the orthothecid conch wall and is not seen in the preserved internal mould of Protowenella.
Figure 5. Hyolith opercula, Cambrian Series 2 (Stage 4), North Greenland. A, PMU 22963, Buen Formation, internal surface of hyolithid operculum showing cardinal processes and clavicles. B, PMU 22969, Buen Formation, external surface of hyolithid Kalaallitia myliuserichseni Peel & Willman, Citation2018 showing cardinal and conical shields. C, Conotheca? sp., PMU 36944, Aftenstjernesø Formation. showing internal surface with cardinal processes and clavicles set in clavicular ridge. D, H, Conotheca laurentiensis Landing & Bartowski, Citation1996, PMU 36952, Aftenstjernesø Formation, oblique views showing flat external surface with protoconch (arrow in D), and clavicle and cardinal processes (H). E, Parkula bounites Bengtson in Bengtson et al., Citation1990, PMU 36931, Aftenstjernesø Formation, external surface. F, Conotheca? sp., PMU 36947, Aftenstjernesø Formation, showing internal surface with cardinal processes and clavicles set in clavicular ridge. G, PMU 22968, Buen Formation, internal surface of hyolithid Kalaallitia myliuserichseni. Scale bars: 100 µm (C–F, H), 1 mm (A, B, G).
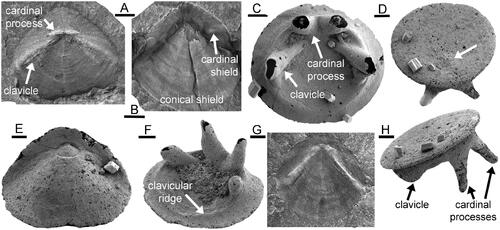
The clavicles in Conotheca may vary in degree of expression from narrow prongs () to lower, more blade-like ridges (), with the former comparing best to the holes present in the internal mould of Protowenella (). In Neogloborilus Qian & Zhang, Citation1983, as illustrated by Pan et al. (Citation2019) and Peel (Citation2021), the clavicles are raised, but differ from Protowenella in being directed almost horizontally towards the ventral margin of the operculum.
The outer surface of the operculum of Protowenella is not known but it was likely flat or shallowly convex as in Conotheca (), with the protoconch placed just above the cardinal processes (arrow in ).
Circumbilical channels on the internal mould are a diagnostic character of Protowenella and were interpreted as internal thickenings of the shell wall or possible shallow emarginations in the aperture by Berg-Madsen & Peel (Citation1978). The latter interpretation promoted the suggestion that these emarginations in Protowenella represented the loci of inhalant currents into the mantle cavity (Berg-Madsen & Peel Citation1978, Peel Citation1991a, b). However, almost all described specimens of Protowenella are internal moulds and precise details of the apertural margin generally are not preserved. Poorly preserved silicified material from the Kuonamka Formation (Miaolingian) described by Gubanov et al. (Citation2004) lacked any indication of the circumbilical channels on the shell exterior. The notion that the channels may form emarginations in the aperture of some specimens is not discounted, but the channel in one illustrated specimen terminates prior to the margin (, arrow), suggesting that the channel was a ridge on the shell interior rather than a fold in the shell wall. Ridges on the shell interior would form a support for the margin of the operculum, as also would simple folds in the margin that do not form emarginations.
A second specimen of Protowenella () preserves the shell apertural margin due to a thin encrustation of the external surface, but no emargination is visible. A circumbilical channel is preserved on the internal mould (, arrow) but does not produce an invagination in the apertural margin. Runnegar (Citation1985, fig. 1A–C) noted that the interior surface of some helcionelloids (not morphologically close to Protowenella) developed comarginal thickenings that formed deep channels on the internal mould without any visible indication of their presence on the external surface.
Hyolithes Eichwald, Citation1840, a hyolithid and the eponymous genus of Hyolitha, develops lateral, longitudinal, furrows on the dorsal surface close to the lateral extremities of the conch in Ordovician specimens (Malinky Citation2002, pl. 3, figs 10, 14, 22), similar structures are present in Rodentilites Marek, Malinky & Geyer, Citation1997 and several other genera (Marek et al. Citation1997). The furrows are located in an analogous position to the circumbilical channels in Protowenella, although the latter is much more tightly coiled than the longitudinally shallowly concave Hyolithes.
The small size and unusually tight curvature may suggest that Protowenella is the protoconch of a larger orthothecid hyolith. The North Greenland samples contain internal moulds (and isolated protoconchs) of several hyoliths but coiled protoconchs similar to Protowenella that are attached to mature shells have not been observed. Such associations are not known in other samples from North Greenland nor reported in the literature.
A single phosphatized specimen from the early Cambrian Meishucunian Stage of Yunnan, China, described by Feng et al. (Citation2007), was considered to combine a hyolith-type microstructure with a mollusc-like shell. The open-coiled, cyrtoconic, shell (length about 1 mm) is coiled through more than half a whorl, although the apex and aperture are broken (Feng et al. Citation2007, fig. 2). The outer surface is ornamented by numerous, closely spaced, acute transverse ribs separated by concavities. The circular aperture is planar without median or circumbilical emarginations. Feng et al. (Citation2007) described a shell structure consisting of two layers, which they considered indicative of hyolith affinity. The outer layer preserved longitudinal fibres, perpendicular to the transverse ribs, whereas the inner layer consisted of transverse elements, similar to structures described in Siberian material by Kouchinsky (Citation2000). Duan (Citation1984) assigned similar shells to four new species assigned to Paragloborilus Qian, Citation1977 (see also Qian Citation1989), a genus of orthothecid hyoliths. Illustrated specimens may be curved through about one third of a whorl near the apex (Duan Citation1984). The degree of coiling and nature of the ornamentation bears little resemblance to described helcionelloid molluscs. As indicated by the shell structure, the Chinese material is interpreted as curved orthothecid hyoliths. Protowenella is readily distinguished by its tightly coiled shell and lack of the prominent transverse ribbing.
In summary, Protowenella is interpreted as an orthothecid hyolith with an operculum closely similar to Conotheca (). However, the tightly coiled shell is unlike other described orthothecids or hyolithids, which have straight or shallowly curved shells, promoting its assignment to a new Family Protowenellidae (Order Orthothecida Marek, Citation1966).
Systematic palaeontology
Phylum uncertain
Class HYOLITHA Marek, Citation1963
Discussion.
The phylogenetic placement of Hyolitha is controversial. Hyoliths have been regarded as molluscs (Marek & Yochelson Citation1976, Malinky & Yochelson Citation2007), a separate phylum (Runnegar et al. Citation1975, Runnegar Citation1980, Sysoev Citation1984, Missarzhevsky Citation1989, Val’kov Citation1990), lophophorates (Moysiuk et al. Citation2017), basal lophotrochozoans (Liu et al. Citation2020a) or possibly members of the stem lineage within total group Mollusca (Li et al. Citation2020). While increasing the known morphological diversity, the present transfer of Protowenella to Hyolitha does not contribute materially to this discussion.
Hyoliths are traditionally grouped within two orders (Hyolithida and Orthothecida), mainly based on the morphology of the apertural margin and operculum, although recent studies have described Cambrian hyoliths that are intermediate or possibly ancestral to the two groups (Malinky & Skovsted Citation2004, Li et al. Citation2020, Liu et al. Citation2020b, Skovsted et al. Citation2020). The authorship of Hyolithida is the subject of discussion (Fischer Citation1962, Malinky Citation1990, Malinky and Yochelson Citation2007, Geyer Citation2018) but Malinky & Yochelson (Citation2007) argued for the maintenance of Sysoev (Citation1957) as its author. Peel & Yochelson (Citation1984) proposed a late Palaeozoic Order Toxeumorphorida Shimansky, Citation1962, referred to as Toxeumorphida by Malinky & Yochelson (Citation2007), but their proposal has generated little comment.
Order ORTHOTHECIDA Marek, Citation1966
Family PROTOWENELLIDAE fam. nov.
Diagnosis.
Strongly coiled, globose, isostrophic (to anisostrophic?), with broad circumbilical channels on the umbilico-lateral walls of the internal mould. Operculum interior with prong-like cardinal processes and clavicles rising from a prominent comarginal clavicular ridge.
Discussion.
In additional to Protowenella, the anisostrophic Xinjispira Yu & Rong in Yu, Citation1987 originally described from Cambrian Series 2 in Henan, China (Zhou & Xiao Citation1984, Yu Citation1987, Li et al. Citation2021), is tentatively placed here on account of the development of circumbilical channels on the internal mould described from Antarctica by Claybourn et al. (Citation2019). The channels leave no indication in the shell ornamentation (Claybourn et al. Citation2019). An operculum is not known in Xinjispira.
Yu & Rong (Citation1991) considered Xinjispira to be a macluritid gastropod. Parkhaev (Citation2001, Citation2008, Citation2019) placed Xinjispira together with Protowenella within the molluscan Family Khairkhaniidae Missarzhevsky, Citation1989. Claybourn et al. (Citation2019) tentatively placed Xinjispira in the Family Pelagiellidae Knight, Citation1956 within total group Gastropoda Cuvier, Citation1797, on account of its anisometry, while Li et al. (Citation2021) regarded it as probably a stem group gastropod of uncertain ordinal position.
The status of other coiled Small Shelly Fossils, such as Khairkhania Missarzhevsky, Citation1980 and Ardrossania Runnegar in Bengtson et al. Citation1990 invites investigation, although their similarity to Protowenella is limited to their bilateral symmetry. While hyoliths are diverse and widely distributed through Lower Palaeozoic strata, no obvious descendants of Protowenella have been described from post-Miaolingian strata.
Protowenella Runnegar & Jell, Citation1976
Type species.
Protowenella flemingi Runnegar & Jell, Citation1976 from the Currant Bush Limestone (Gowers Formation), Queensland, Australia, Cambrian, Miaolingian Series.
Emended diagnosis.
Strongly coiled, globose, isostrophic, with broad circumbilical channels on the umbilico-lateral walls of the internal mould. Whorl profile elliptical to circular, aperture simple, its margins coplanar. Operculum bilaterally symmetrical, seemingly with flat external surface, with prong-like cardinal processes and clavicles rising from a prominent comarginal clavicular ridge on the interior at the adumbilical margin. Shell ornamentation of comarginal growth lines and shallow corrugations, the latter sometimes visible on the internal mould.
Discussion.
Berg-Madsen & Peel (Citation1978) emended the original diagnosis of Runnegar & Jell (Citation1976) to include the circumbilical channels on the internal mould; this is further emended here to include characters of the operculum. Circumbilical channels are present in Protowenella lancaraensis Wotte, Citation2021 from the Miaolingian of Spain, which may prove to be a junior synonym of Protowenella flemingi. Protowenella cobbensis MacKinnon, Citation1985 from the Miaolingian of New Zealand lacks the circumbilical channels on the internal mould and has a more pointed early growth stage in apertural view (MacKinnon Citation1985, fig. 9A–G); it is tentatively excluded from the genus. Specimens of Protowenella plena Missarzhevsky in Missarzhevsky & Mambetov, Citation1981 illustrated by Missarzhevsky & Mambetov (Citation1981) and Missarzhevsky (Citation1989) from the early Cambrian of Maly Karatau are too poorly preserved for confident assignment. Material from Cambrian Series 2 in China, assigned to Protowenella primaria Zhou & Xiao, Citation1984 and Protowenella huainanensis Zhou & Xiao, Citation1984, was placed in synonymy of Protowenella flemingi by Li et al. (Citation2021).
Li et al. (Citation2021, fig. 25) described the early ontogeny of the type species Protowenella flemingi based on internal moulds from the Xinji Formation (Cambrian Series 2, Stages 3–4) of North China. Vendrasco et al. (Citation2010) described shell structure with bundles of fibres parallel to growth lines in Australian material of the type species. A fine transverse fibrous texture is present on the surface of some internal moulds from Greenland (). The surface of the phosphate coating moulding the shell interior in the operculate specimen shows a fine pitting on the shell surface, but this merges into a pattern of low ridges on the preserved internal surface of the operculum ().
Xinjispira possesses the globose form and broad circumbilical channels on the internal mould characteristic of Protowenella (Claybourn et al. Citation2019) but differs in its clear anisostrophic coiling, referred to as hyperstrophic in the comparison to Maclurites Lesueur, Citation1818 made by Yu & Rong (Citation1991). Oriented in apertural view (as in ), the aperture is displaced to the right in specimens of Xinjispira simplex (Zhou & Xiao Citation1984), the type species of Xinjispira (Li et al. Citation2021) from the Xinji Formation China. However, in specimens from the Shackleton Limestone (Cambrian Series 2) of Antarctica, the aperture is displaced to the left (Claybourn et al. Citation2019).
Profound variation in coiling, including from sinistral to dextral morphologies, is well known in early Cambrian helcionelloid molluscs and often can be attributed to variation within species or genera (Landing Citation1988, Gubanov & Peel Citation2000, Jacquet et al. Citation2017). Parkhaev (Citation2001, Citation2008, Citation2019) included several sinistral, isostrophic and dextral forms, including Protowenella and Xinjispira, within the molluscan Family Khairkhaniidae Missarzhevsky, Citation1989, although Claybourn et al. (Citation2019) questioned the grouping together of the differently coiled genera. If Xinjispira (Cambrian Series 2) is a protowenellid hyolith, the variation in anisostrophic coiling may have stabilized into the isostrophic norm for Protowenella by the Miaolingian.
Acknowledgements
All material was collected during the North Greenland Project (1978–80) of the Geological Survey of Greenland. Christian B. Skovsted is thanked for comments concerning an earlier version of the manuscript. Remarks from two anonymous reviewers and journal editors are gratefully acknowledged.
References
- Bengtson, S., 1968. The problematic genus Mobergella from the Lower Cambrian of the Baltic area. Lethaia 1, 325–351.
- Bengtson, S., Conway Morris, S., Cooper, B.J., Jell, P.A. & Runnegar, B.N., 1990. Early Cambrian fossils from South Australia. Memoirs of the Australasian Association of Palaeontologists 9, 1–364.
- Berg-Madsen, V. & Malinsky, J.M., 1999. A revision of Holm’s late Mid and Late Cambrian hyoliths of Sweden. Palaeontology 42, 841–885.
- Berg-Madsen, V. & Peel, J.S., 1978. Middle Cambrian monoplacophorans from Bornholm and Australia, and the systematic position of the bellerophontiform molluscs. Lethaia 11, 113–125.
- Blaker, M.R. & Peel, J.S., 1997. Lower Cambrian trilobites from North Greenland. Meddelelser om Grønland, Geoscience 35, 1–145.
- Bouchet, P. & Rocroi, J.-P., 2005. Classification and nomenclator of gastropod families. Malacologia 47, 1–397.
- Bouchet, P., Rocroi, J.-P., Hausdorf, B., Kaim, A., Kano, Y., Nützel, A., Parkhaev, P., Schrödl, M. & Strong, E.E., 2017. Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 61, 1–526.
- Boucot, A.J. & Poinar, G.O., Jr, 2010. Fossil Behavior Compendium. CRC Press, Boca Raton, FL, 391. pp.
- Brock, G.A., 1998. Middle Cambrian molluscs from the southern New England fold belt, New South Wales, Australia. Geobios 31, 571–586.
- Checa, A.G. & Jiménez-Jiménez, A.P., 1998. Constructional morphology, origin, and evolution of the gastropod operculum. Paleobiology 24, 109–132.
- Clausen, S. & Peel, J.S., 2012. Middle Cambrian echinoderm remains from the Henson Gletcher Formation of North Greenland. GFF 134, 173–200.
- Claybourn, T.M., Jacquet, S.M., Skovsted, C.B., Topper, T.P., Holmer, L.E. & Brock, G.A., 2019. Mollusks from the upper Shackleton Limestone (Cambrian Series 2), Central Transantarctic Mountains, East Antarctica. Journal of Paleontology 93, 437–459.
- Creveling, J.R., Knoll, A.H. & Johnston, A.H., 2014. Taphonomy of Cambrian phosphatic small shelly fossils. Palaios 29, 295–308.
- Cuvier, G., 1797. Tableau Élementaire de l’historie Naturelle des Animaux. Baudouin, Paris, 710 pp.
- Duan, C., 1984. Small shelly fossils from the lower Cambrian Xihaoping Formation in the Shennongjia district, Hubei Province ––hyoliths and fossil skeletons of unknown affinities. Bulletin of the Tianjin Institute, Geological and Mineral Resources 7, 144–186. (in Chinese with English summary).
- Eichwald, K.E. von, 1840. Ûber das silurische Schichtensystem in Esthland. Zeitschrift für Natur- und Heilkunde der Königlichen Medicinisch-chirurgischen Akademie St. Petersburg 1–2, 1–210.
- Feng, W., Mu, X. & Kouchinsky, A.V., 2007. Hyolith-type microstructure in a mollusc-like fossil from the Early Cambrian of Yunnan. Lethaia 34, 303–308.
- Fischer, D., 1962. Calyptoptomatids. In Treatise on Invertebrate Palaeontology, Part W Miscellanea: Conodonts, Conoidal Shells of Uncertain Affinities, Worms, Trace Fossils and Problematica. Moore, R.C. Geological Society of America, New York and University of Kansas Press, Lawrence, 116–130, 289 pp.
- Frýda, J., 2012. Phylogeny of Palaeozoic gastropods inferred from their ontogeny. In Earth and Life: Global Biodiversity, Extinction Intervals and Biogeographic Pertubations Through Time. Talent, J., ed. Springer Legacy Series, Berlin, 395–435.
- Frýda, J., Nützel, A. & Wagner, P.J., 2008. Paleozoic Gastropoda. In Phylogeny and Evolution of the Mollusca. Ponder, W.F. & Lindberg, D.R. eds. University of California Press, Berkeley, CA, 239–270, 469 pp.
- Geyer, G., 1994. Middle Cambrian mollusks from Idaho and early conchiferan evolution. New York State Museum Bulletin 481, 69–86.
- Geyer, G., 2018. A new enigmatic hyolith from the Cambrian of west Gondwana and its bearing on the systematics of hyoliths. Papers in Palaeontology 4, 85–100.
- Geyer, G. & Peel, J.S., 2011. The Henson Gletscher Formation, North Greenland, and its bearing on the global Cambrian Series 2–Series 3 boundary. Bulletin of Geosciences 86, 465–534.
- Gubanov, A.P., Kouchinsky, A.V., Peel, J.S. & Bengtson, S., 2004. Middle Cambrian molluscs of ‘Australian’ aspect from northern Siberia. Alcheringa 28, 1–20.
- Gubanov, A.P. & Peel, J.S., 2000. Cambrian monoplacophoran molluscs (Class Helcionelloida). American Malacological Bulletin 15, 139–145.
- Harper, J.A. & Rollins, H.B., 2000. The bellerophont controversy revisited. American Malacological Bulletin 15, 147–156.
- Higgins, A.K., Ineson, J.R., Peel, J.S., Surlyk, F. & Sønderholm, M., 1991. Lower Palaeozoic Franklinian Basin of North Greenland. Bulletin Grønlands Geologiske Undersøgelse 160, 71–139.
- Horný, R.J. & Peel, J.S., 1995. A new Silurian gastropod from Bohemia with the operculum in situ. Journal of the Czech Geological Society 40, 79–88.
- Ineson, J.R. & Peel, J.S., 1997. Cambrian shelf stratigraphy of North Greenland. Geology of Greenland Survey Bulletin 173, 1–120.
- Jacquet, S.M. & Brock, G.A., 2016. Lower Cambrian helcionelloid macromolluscs from South Australia. Gondwana Research 36, 333–358.
- Jacquet, S.M., Brougham, T., Skovsted, C.B., Jago, J.B., Laurie, J.R., Betts, M.J., Topper, T.P. & Brock, G.A., 2017. Watsonella crosbyi from the lower Cambrian (Terreneuvian, Stage 2) Normanville Group in South Australia. Geological Magazine 154, 1088–1104.
- Jell, P.A. & Jell, J.S., 1976. Early Middle Cambrian corals from western New South Wales. Alcheringa 1, 181–195.
- Knight, J.B., 1952. Primitive fossil gastropods and their bearing on gastropod classification. Smithsonian. Miscellaneous Collections 117, 56 pp.
- Knight, J.B., 1956. New families of Gastropoda. Journal of the Washington Academy of Sciences 46, 41–42.
- Knight, J.B., Cox, L.R., Keen, A.M., Smith, A.G., Batten, R.L., Yochelson, E.L., Ludbrook, N.H., Robertson, R., Yonge, C.M. & Moore, R.C., 1960. Treatise on Invertebrate Paleo/ztology. Part 1, Mollusca 1. Geological Society of America and University of Kansas, Boulder and Lawrence, 1–351 pp.
- Kouchinsky, A.V., 2000. Skeletal microstructures of hyoliths from the Early Cambrian of Siberia. Alcheringa 24, 65–81.
- Landing, E., 1988. Lower Cambrian stratigraphy of Eastern Massachusetts: Stratigraphy and small shelly fossils. Journal of Paleontology 62, 661–695.
- Landing, E. & Bartowski, K.E., 1996. Oldest shelly fossils from the Taconic allochthon and late early Cambrian sea-levels in eastern Laurentia. Journal of Paleontology 70, 741–761.
- Lesueur, C.A., 1818. Observations on a new genus of fossil shells. Journal of the Academy of Natural Sciences of Philadelphia 1, 310–313.
- Li, L., Skovsted, C.B., Yun, H., Betts, M.J. & Zhang, X., 2020. New insight into the soft anatomy and shell microstructures of early Cambrian orthothecids (Hyolitha). Proceedings of the Royal Society B 287, 20201467. https://doi.org/10.1098/rspb.2020.1467
- Li, L., Zhang, X., Skovsted, C.B., Yun, H., Pan, B. & Li, G., 2021. Revisiting the molluscan fauna from the Cambrian (series 2, stages 3–4) Xinji Formation of North China. Papers in Palaeontology 7, 521–564.
- Lindström, G., 1884. On the Silurian Gastropoda and Pteropoda of Gotland. Kungliga Svenska Vetenskaps Akademiens Handlingar 19, 250 pp.
- Liu, F., Skovsted, C.B., Topper, T.P. & Zhang, Z., 2020b. Revision of Triplicatella (Orthothecida, Hyolitha) with preserved digestive tracts from the early Cambrian Chengjiang Lagerstätte, South China. Historical Biology 33, 1857–1871.
- Liu, F., Skovsted, C.B., Topper, T.P., Zhang, Z. & Shu, D., 2020a. Are hyoliths Palaeozoic lophophorates? National Science Review 7, 453–469.
- Mackinnon, D.I., 1985. New Zealand late Middle Cambrian molluscs and the origin of Rostroconchia and Bivalvia. Alcheringa 9, 65–81.
- Malinky, J.M., 1990. Hyolitha from Northeast Canada: reappraisal of the hyolith orders Camerothecida and Diplothecida. Journal of Paleontology 64, 587–595.
- Malinky, J.M., 2002. A revision of early to mid-Ordovician hyoliths from Sweden. Palaeontology 45, 511–555.
- Malinky, J.M. & Skovsted, C.B., 2004. Hyoliths and small shelly fossils from the lower Cambrian of north-east Greenland. Acta Palaeontologica Polonica 49, 551–578.
- Malinky, J.M. & Yochelson, E.L., 2007. On the systematic position of the Hyolitha (Kingdom Animalia). Memoirs of the Association of. Australasian Palaeontologists 34, 521–536.
- Marek, L., 1963. New knowledge on the morphology of Hyolithes. Sborník geologickych věd, řada Paleontologie 1, 53–72.
- Marek, L., 1966. New hyolithid genera from the Ordovician of Bohemia. Časopis národního muzea 135, 89–92.
- Marek, L., Malinky, J.M. & Geyer, G., 1997. Middle Cambrian fossils from Tizi N’Tichka, the High Atlas, Morocco, Part 2. Hyolitha. Journal of Paleontology 71, 638–656.
- Marek, L. & Yochelson, E.L., 1976. Aspects of the biology of Hyolitha (Mollusca). Lethaia 9, 65–82.
- Martí Mus, M. & Bergström, J., 2005. The morphology of hyolithids and its functional implications. Palaeontology 48, 1139–1167.
- Martí Mus, M., Jeppsson, L. & Malinky, J.M., 2014. A complete reconstruction of the hyolithid skeleton. Journal of Paleontology 88, 160–170.
- Minichev, Y.S. & Starobogatov, Y.I., 1979. Subclass Gastropoda and their phylogenetic relationships. Zoological Journal 58, 293–305.
- Missarzhevsky, V.V., 1969. Descriptions of hyoliths, gastropods, hyolithelminths, camenids, and forms of an obscure systematic position. The Tommotian Stage and the problem of the lower boundary of the Cambrian. Trudy Ordena Trudovogo Krasnogo Znameni Geologicheskij Institut. Akademiya Nauk SSSR 206, 105–175. (in Russian).
- Missarzhevsky, V.V., 1980. Early Cambrian Mongolian Hyolitha and Gastropoda. Paleontological Journal 15, 18–25.
- Missarzhevsky, V.V., 1989. Oldest skeletal fossils and stratigraphy of Precambrian and Cambrian boundary beds. Trudy Geologicheskogo Instituta, Akademiya Nauk SSSR 443, 237 pp. (in Russian).
- Missarzhevsky, V.V. & Mambetov, A.M., 1981. Stratigraphy and fauna Cambrian and Precambrian boundary beds of Maly Karatau (in Russian). Trudy Geologicheskogo Instituta, Akademiya Nauk SSSR 326, 92 pp. (in Russian).
- Montfort, P.D. de, 1808–1810. Conchyliologie systématique, et classification méthodique des coquille, offrant leurs figures, leur arrangement générique, leurs descriptions caractéristiques, leurs noms, ainsi que leur synonymie en plusieurs langues. F. Schoell, Paris. 409 pp.
- Moysiuk, J., Smith, M.R. & Caron, J.B., 2017. Hyoliths are Palaeozoic Lophophorates. Nature 541, 394–397.
- Northrop, S.A., 1939. Paleontology and stratigraphy of the Silurian rocks of the Port Daniel–Black Cape region, Gaspé. Geological Society of America Special Paper 21, 302 pp.
- Pan, B., Skovsted, C.B., Sun, H. & Li, G., 2019. Biostratigraphical and palaeogeographical implications of early Cambrian hyoliths from the North China Platform. Alcheringa 43, 351–380.
- Parkhaev, P.Y., 2001. Molluscs and siphonoconchs. In The Cambrian Biostratigraphy of the Sainsbury Basin, South Australia. Transactions of the Palaeontological Institute 282. Alexander, E.M., Jago, J.B., Rozanov, A.Yu. & Zhuravlev, A.Yu., eds., Russian Academy of Sciences, Moscow, 133–210.
- Parkhaev, P.Y., 2008. The early Cambrian radiation of Mollusca. In Phylogeny and Evolution of the Mollusca. Ponder, W.F. & Lindberg, D.R., eds. University of California Press, Berkeley, 33–69.
- Parkhaev, P.Y., 2017. Origin and the Early Evolution of the Phylum Mollusca. Paleontological Journal 51, 663–686.
- Parkhaev, P.Y., 2019. Cambrian molluscs of Australia: overview of taxonomy, biostratigraphy and paleobiogeography. Stratigraphy and Geological Correlation 27, 181–206.
- Peel, J.S., 1972. Observations on some Lower Palaeozoic tremanotiform Bellerophontacea (Gastropoda) from North America. Palaeontology 15, 412–422.
- Peel, J.S., 1991a. Functional morphology of the Class Helcionelloida nov., and the early evolution of the Mollusca, 157–177. In The Early Evolution of Metazoa and the Significance of Problematic Taxa. Simonetta, A. & Conway Morris, S. eds. Cambridge University Press, Cambridge.
- Peel, J.S., 1991b. The Classes Tergomya and Helcionelloidea, and early molluscan evolution. Bulletin Grønlands Geologiske Undersøgelse 161, 1–65.
- Peel, J.S., 2010. Articulated hyoliths and other fossils from the Sirius Passet Lagerstätte (early Cambrian), North Greenland. Bulletin of Geosciences 85, 385–394.
- Peel, J.S., 2011. The coral Cothonion from the lower Cambrian of North Greenland. Alcheringa 35, 405–411.
- Peel, J.S., 2015. Operculum regeneration and failed predation in the Silurian gastropod Oriostoma. Palaeontology 58, 229–237.
- Peel, J.S., 2016. Gastropods from the Carboniferous (Namurian) of Congleton Edge, Cheshire, UK. Papers in Palaeontology 2, 399–438.
- Peel, J.S., 2021. An outer shelf shelly fauna from Cambrian Series 2 (Stage 4) of North Greenland (Laurentia). Journal of Paleontology 95, Memoir 83, 1–41.
- Peel, J.S. & Horný, R.J., 1996. Sinistral hyperstrophic coiling in a Devonian gastropod from Bohemia with an in situ operculum. Palaeontology 39, 709–718.
- Peel, J.S., Streng, M., Geyer, G., Kouchinsky, A. & Skovsted, C.B., 2016. Ovatoryctocara granulata assemblage (Cambrian Series 2–Series 3 boundary) of Løndal, North Greenland. Australasian Palaeontological Memoirs 49, 241–282.
- Peel, J.S. & Willman, S., 2018. The Buen Formation (Cambrian Series 2) biota of North Greenland. Papers in Palaeontology 4, 381–432.
- Peel, J.S. & Yochelson, E.L., 1984. Permian Toxeumorphorida from Greenland: an appraisal of the molluscan class Xenoconchia. Lethaia 17, 211–221.
- Perner, J., 1903. Systême Silurien du centre de la Bohême par Joachim Barrande. Vol. IV Gastéropodes 1, Texte (Patellidae et Bellerophontidae) et Planches 1 à 89. Fr. Rivnác, Prague, 164 pp.
- Ponder, W.F., Lindberg, D.R. & Ponder, J.M., 2020. Biology and Evolution of the Mollusca, Volume 2. CRC Press, Boca Raton, FL, 870 pp.
- Qian, Y., 1977. Hyolitha and some problematica from the Lower Cambrian Meishucun Stage in Central and SW China. Acta Palaeontologica Sinica 16, 255–278. (in Chinese with English summary).
- Qian, Y., 1989. Early Cambrian small shelly fossils of China with special reference to the Precambrian–Cambrian boundary. In Stratigraphy and Palaeontology of Systemic Boundaries in China, Precambrian–Cambrian Boundary 2, 342 pp. Nanjing University Publishing House, Nanjing.
- Qian, Y. & Zhang, S.-B., 1983. Small shelly fossils from the Xihaoping Member of the Tongying Formation in Fangxian County of Hubei Province and their stratigraphical significance. Acta Palaeontologica Sinica 22, 82–94. (in Chinese).
- Robison, R.A., 1994. Agnostoid trilobites from the Henson Gletscher and Kap Stanton formations (Middle Cambrian), North Greenland. Bulletin Grønlands Geologiske Undersøgelse 169, 25–77.
- Rohr, D.M. & FrÝda, J., 2001. A new Ordovician gastropod and operculum from the Czech Republic. Journal of Paleontology 75, 461–462.
- Rohr, D.M., Fix, M.F. & Darrough, G.U.Y., 2004. Life association of shell and operculum of Ceratopea Ulrich, 1911 (Ordovician, Gastropoda). Journal of Paleontology 78, 218–220.
- Runnegar, B., 1980. Hyolitha: status of the phylum. Lethaia 13, 21–25.
- Runnegar, B., 1985. Shell microstructure of Cambrian molluscs replicated by phosphate. Alcheringa 9, 245–257.
- Runnegar, B. & Jell, P.A., 1976. Australian Middle Cambrian molluscs and their bearing on early molluscan evolution. Alcheringa 1, 109–138.
- Runnegar, B., Pojeta, J.J.R., Morris, N.J., Taylor, J.D., Taylor, M.E. & McCLUNG, G., 1975. Biology of the Hyolitha. Lethaia 8, 181–191.
- Shimansky, V.N., 1962. Ob odnoi maloizvestnoi gruppe molluscov. Byullyetin Moskoskogov Obshchestva Ispytatelei Prirody. Odtelenie geologii 37, 164–165. 2,
- Skovsted, C.B., Martí Mus, M., Zhang, Z., Pan, B., Li, L., Liu, F., Li, G. & Zhang, Z., 2020. On the origin of hyolith helens. Palaeogeography, Palaeoclimatology, Palaeoecology 555, 109848.
- Skovsted, C.B. & Topper, T.P., 2018. Mobergellans from the early Cambrian of Greenland and Labrador: new morphological details and implications for the functional morphology of mobergellans. Journal of Paleontology 92, 71–79.
- Streng, M. & Skovsted, C.B., 2006. A new mobergellan (small shelly fossils) from the early Middle Cambrian of Morocco and its significance. Paläontologische Zeitschrift 80, 209–220.
- Sun, H., Babcock, L.E., Peng, J. & Zhao, Y., 2016. Three dimensionally preserved digestive systems of two Cambrian hyolithides (Hyolitha). Bulletin of Geosciences 91, 51–56.
- Sysoev, V.A., 1957. K morfologii, sistematike i sistematicheskomu polozheniyu khiolitiov. Doklady Akademii Nauk SSSR 116, 304–307.
- Sysoev, V.A., 1984. Morfologiya i sistematicheskoye polozheniye khiolitov. Paleontologicheskiy Zhurnal 1984, 3–14.
- Ulrich, E.O. & Scofield, W.H., 1897. Silurian Gastropoda of Minnesota. Final Report Minnesota Geological Survey 3, 813–1081.
- Val’Kov, A.K., 1990. Taksonomiya vysshikh kategorii khiolitov. Trudy Institut Geologii i Geofiziki Sibiskoi otdelenie Akademya Nauk SSSR 783, 34–50.
- Vendrasco, M.J., Porter, S.M., Kouchinsky, A., Li, G. & Fernandez, C.Z., 2010. New data on molluscs and their shell microstructures from the Middle Cambrian Gowers Formation, Australia. Palaeontology 53, 97–135.
- Vermeij, G.J., 2016. The limpet form in gastropods: evolution, distribution, and implications for the comparative study of history. Biological Journal of the Linnean Society 120, 22–37.
- Wahlman, G.P., 1992. Middle and Upper Ordovician symmetrical univalved mollusks (Monoplacophora and Bellerophontina) of the Cincinnati Arch Region. US Geological Survey Professional Paper 1066–O, 213 pp.
- Wotte, T., 2021. New Middle Cambrian molluscs from the Láncara Formation of the Cantabrian Mountains (northwestern Spain). Spanish Journal of Palaeontology 21, 145–158.
- Yochelson, E.L., 1979. Gastropod opercula as objects for paleobiogeographic study. In Historical Biogeography, Plate Tectonics, and the Changing Environment. Gray, J. & Boucot, A.J. eds. Oregon State University Press, Corvallis, Oregon, 37–43.
- Yochelson, E.L. & Linsley, R.M., 1972. Opercula of two gastropods from the Lilydale Limestone (Early Devonian) of Victoria, Australia. Memoirs of the National Museum of Victoria 33, 1–13.
- Yu, W., 1987. Yangtze micromolluscan fauna in Yangtze Region of China with notes on Precambrian-Cambrian boundary. Stratigraphy and Palaeontology of Systemic Boundary in China 1, 19–344.
- Yu, W. & Rong, R., 1991. Lower Cambrian gastropods from Fangcheng County, Henan. Acta Micropalaeontologica Sinica 8, 339–345.
- Zhou, B. & Xiao, L., 1984. Early Cambrian monoplacophorans and gastropods from Huainan and Huoqiu Counties, Anhui Province. Proceedings of Stratigraphy and Paleontology 13, 125–140. (in Chinese with English abstract).
