Abstract
Australia hosts the richest diversity of extant cicadas in the world, but a scarcity of fossils means that little is known about their evolutionary history on the continent. Here we describe the first fossilized Cicadidae from Australia. Laopsaltria ferruginosa gen. et sp. nov., Burbungoides gulgongensis gen. et sp. nov. and Tithopsaltria titan gen. et sp. nov. were all found at McGraths Flat near Gulgong, New South Wales, a recently discovered Miocene Lagerstätte. These cicada fossils preserve remarkable detail, including setae and wing membrane surface structures. Wing size varies considerably between the three newly described species and, in T. titan sp. nov., reaches a maximum size beyond what is known from extant Australian cicadas (or any other known cicada fossil), indicating a disparate cicada fauna in Australia’s Miocene rainforests.
Max Moulds [[email protected]], Australian Museum Research Institute, 1 William Street, Sydney, New South Wales 2010, Australia. Michael Frese [[email protected]], Faculty of Science and Technology, University of Canberra, Bruce, Australian Capital Territory 2601, Australia; Australian Museum Research Institute, 1 William Street, Sydney, New South Wales 2010, Australia; Commonwealth Scientific and Industrial Research Organisation, Health and Biosecurity, Black Mountain, Australian Capital Territory 2601, Australia. M. R. McCurry [[email protected]], Australian Museum Research Institute, 1 William Street, Sydney, New South Wales 2010, Australia; Earth and Sustainability Science Research Centre, School of BEES, The University of New South Wales, Sydney, New South Wales 2052, Australia; Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, District of Columbia 20560, USA.
AUSTRALIA is home to a diverse and highly endemic cicada fauna. Two families are extant on the Australian continent: Tettigarctidae (known as hairy cicadas that communicate via substrate vibrations; Claridge et al. Citation1999); and Cicadidae (known as singing cicadas that communicate using loud airborne calls; Moulds Citation1990).
The fossil record of Tettigarctidae is scarce in the Southern Hemisphere. Only three tettigarctid fossils have been recorded from Australia, Mesodiphthera grandis Tillyard, Citation1919, Tardilly prosboloides (Tillyard, Citation1922) and Tardilly dunstani (Tillyard, Citation1922) (Lambkin Citation2019); these specimens have the distinction of being the oldest known cicada fossils, all three dating back to the Late Triassic (Norian). A further three are known from other parts of the Southern Hemisphere, Tettagalma striata Menon, Citation2005 and Architettix compacta Hamilton, Citation1990, both from the Early Cretaceous (Aptian) of Brazil, and Paratettigarcta zealandica Kaulfuss & Moulds, Citation2015 from the Miocene of New Zealand (23–16 Ma: Kaulfuss & Moulds Citation2015). Approximately 38 species in 23 genera are recorded from the Northern Hemisphere ranging in age from the latest Triassic (Rhaetian) to the Middle Eocene (Fu et al. Citation2019, Jiang et al. Citation2019, Moulds Citation2018). All are compression fossils except for two described from Burmese amber (Fu et al. Citation2019, Jiang et al. Citation2019).
The fossil record of the Cicadidae (true or singing cicadas) is even more limited, with only one specimen known from the Southern Hemisphere: Fonsecacicada mineira Martins-Neto & Mendes, Citation2002 from the upper Eocene (Priabonian) Fonseca Formation of Brazil. Without an Australian record for the Cicadidae, it has been impossible to track how the family has changed over geological time.
The hot and warm climate of the early to middle Miocene facilitated the existence of a mesic biome throughout much of the Australian continent, a stark contrast to the dry shrublands, forests and deserts of today (Byrne et al. Citation2008, Christophel Citation1989). The recent discovery of cicada fossils from the McGraths Flat Lagerstätte in central New South Wales (McCurry et al. Citation2022) dating to the Miocene (16–11 Ma, based on palynology) provides our first glimpse into what the cicada fauna was like in these ancient environments.
Here we record the first fossilized Cicadidae from Australia, three new species in three new genera, two species belonging to the subfamily Cicadinae (tentatively identified as aff. Psaltoda in McCurry et al. Citation2022, Table S4), and one to the Cicadettinae, including the largest known fossilized cicada with a wingspan estimated at 165 mm. Some specimens show a remarkably detailed wing nanostructure never before found in fossilized cicadas.
Materials and methods
Four fossil cicada specimens were collected from McGraths Flat during two separate expeditions between 2019 and 2021 (). The fossil specimens were prepared where necessary using an airscribe (ME-9100, PaleoTools, Brigham City, UT, USA). A pinned specimen of the extant species Burbunga hillieri (Distant, Citation1907), used for comparative imaging (Australian Museum registration No. K.537240), was collected near Talyealye Homestead, New South Wales (29°05′28ʺS; 144°27′59ʺE) in January, 1999. All fossil and extant specimens were photographed using a Canon EOS 7 D Mark II camera mounted on a BK Plus imaging system (Dun Inc., Charlottesville, VA, USA). Selected fossil specimens were imaged using a FEI Quanta 650 F field-emission scanning electron microscope (FESEM) (FEI Company, Hillsboro, OR, USA). The FEI microscope was operated at 15 kV, with a spot size of 2.45, an aperture setting of 5, and working distances of about 10–15 mm. Of note, the high conductivity of the rocks from McGraths Flat allowed high-resolution SEM imaging without coating. An uncoated extant B. hillieri wing was imaged using a Zeiss Crossbeam 550 FIB/FESEM (Carl Zeiss AG, Oberkochen, Baden, Württemberg, Germany) operated at 0.5 kV, ∼10-pA probe current and a working distance of 2 mm. Photos were processed using Adobe Photoshop and Illustrator. All specimens are accessioned in the palaeontology collection of the Australian Museum (AM), Sydney, Australia. Wing terminology used in the descriptions () follows that of Moulds (Citation2005, Citation2012).
Figure 1. Forewing of Laopsaltria ferruginosa sp. nov. (F.147104). A, Wing venation, part (counterpart illustrated in and ). B, Forewing venation.
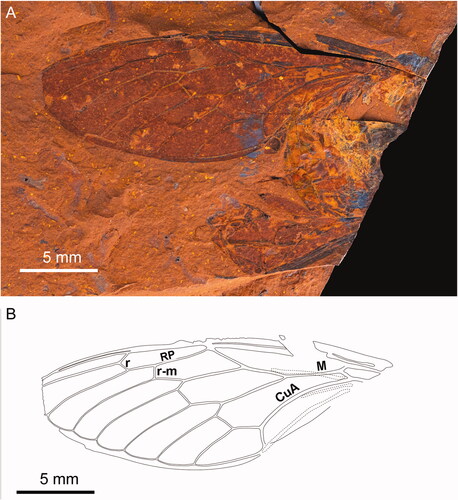
Figure 2. Forewing and hind wing of Burbungoides gulgongensis sp. nov. (F.147105). A, Part. B, Counterpart. C, Wing venation as on the fossil. D, Wing venation with forewing and hind wing separated.
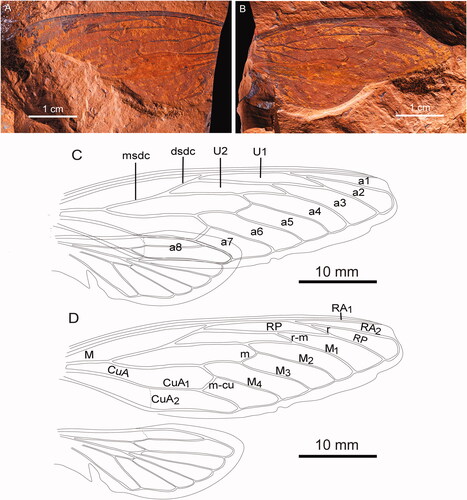
Figure 3. Forewing of Tithopsaltria titan sp. nov. (F.147103). A, Distal portion of the forewing. B, Proximal section of the forewing. C, Venation and relative positions of each of the wing sections.
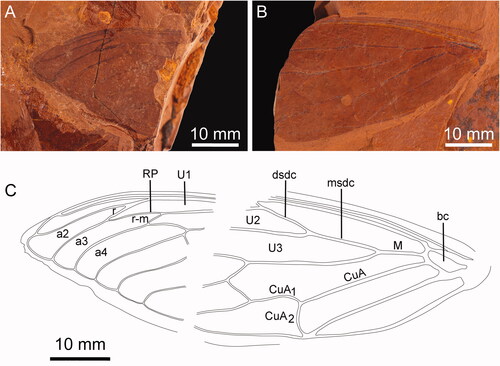
Table 1. Cicada specimens from McGraths Flat.
Systematic palaeontology
CICADOMORPHA Evans, Citation2009
CICADOIDEA Westwood, Citation1840
CICADIDAE Latreille, Citation1802
CICADETTINAE Buckton, Citation1890
CICADETTINI, Buckton, Citation1890
Laopsaltria gen. nov.
Type species
Laopsaltria ferruginosa sp. nov.
Zoobank identifier
Zoobank LSID: urn:lsid:zoobank.org:act:7FB58C24-EC2B-419E-B31A-CC7F840B771E.
Diagnosis
Forewing with eight apical cells; ulnar cell 3 angled to radial cell; veins M and CuA meeting basal cell wide apart, the departure of CuA far more proximal than M; vein RP gently curved; vein CuA strongly bowed along its length; crossveins r and r-m far apart, far more than the length of those crossveins; crossvein r similarly angled to crossvein m; apical cell 2 much expanded towards wing margin; ulnar cell 1 not markedly tapering; wing margin fully formed, narrow but not contiguous with ambient vein; costal margin narrowly ampliate to node; wing tending to be rounded in shape with a likely broadly rounded apex.
Differs from all other documented genera in the following character state combination: M and CuA widely separated at basal cell, CuA strongly bowed; RP gently curved; crossveins r and r-m far apart (far more than length of crossveins), and crossveins r and r-m misaligned making r instead similarly aligned with crossvein m.
Etymology
From the Greek genitive ‘laos’ meaning rock or stone and referring to the fossil status of the specimen. Feminine.
Laopsaltria ferruginosa sp. nov.
()
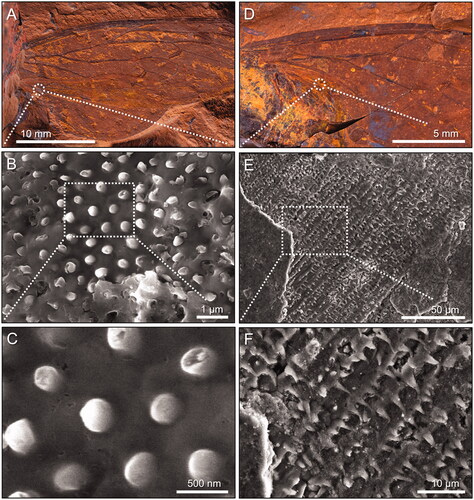
Figure 4. Setae. A–C, Forewing of Tithopsaltria titan sp. nov. (F.147103). A, Overview. B, Position and density of setae on the leading edge of the wing (ca 5 setae/mm). C, SEM image showing the base of a seta. D, Seta spine protruding from the leading edge of the forewing of Burbungoides gulgongensis sp. nov. (F.147105, counterpart). E, Seta base on the leading edge of the forewing of Laopsaltria ferruginosa sp. nov. (F.147104; counterpart). F, G, Leg of possible Tithopsaltria titan sp. nov. (F.147106; identity uncertain; although the specimen is well preserved only parts of the wing base and of a leg are present, but these closely match T. titan) with numerous preserved setal bases; the arrows in F point along the length of a seta that has part of its spine exposed.
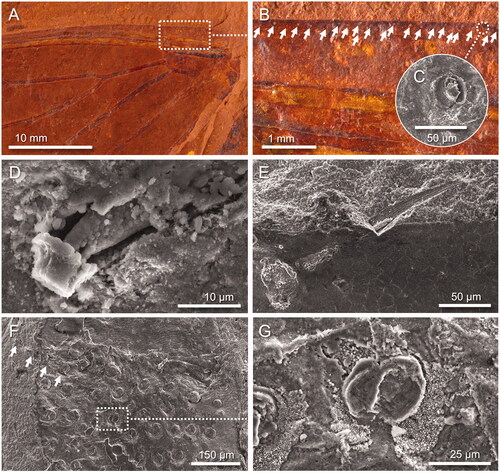
Figure 5. Wing surface nanostructures. A–C, Forewing of Laopsaltria ferruginosa sp. nov. (F.147104; counterpart). A, Overview. B, C, SEM images of nano-holes (well-preserved sections show a distinctive hexagonal pattern). D–F, Extant cicada Burbunga hillieri (Distant, Citation1907). D, Overview (whole specimen and left forewing). E, F, SEM images of the wing surface with nano-protrusions (nanopillars) that are arranged in a near-perfect hexagonal pattern. Average centre-to-centre distances between neighbouring nano-holes (197 ± 19 nm [standard deviation]; n = 20) or nanopillars (178 nm ± 21 nm; n = 23) are given for the fossil and the extant wing, respectively.
![Figure 5. Wing surface nanostructures. A–C, Forewing of Laopsaltria ferruginosa sp. nov. (F.147104; counterpart). A, Overview. B, C, SEM images of nano-holes (well-preserved sections show a distinctive hexagonal pattern). D–F, Extant cicada Burbunga hillieri (Distant, Citation1907). D, Overview (whole specimen and left forewing). E, F, SEM images of the wing surface with nano-protrusions (nanopillars) that are arranged in a near-perfect hexagonal pattern. Average centre-to-centre distances between neighbouring nano-holes (197 ± 19 nm [standard deviation]; n = 20) or nanopillars (178 nm ± 21 nm; n = 23) are given for the fossil and the extant wing, respectively.](/cms/asset/354bae4f-d335-47f6-9eb0-0313f2ecf166/talc_a_2112287_f0005_c.jpg)
Diagnosis
Differs from the species in the closely similar genus Terepsalta Moulds, Citation2012 in having the three inner sections of the discal cell all of similar length (the proximal section in Terepsalta is very short); from Adelia Moulds, Citation2012 in having apical cell 3 very long (short in Adelia) and a costal margin gradually reducing (abruptly narrowed at the node in Adelia); and from Diemeniana Distant, Citation1905c in having crossveins r and r-m far apart (close together in Diemeniana) and the distal section of the discal cell as long as medial section (shorter in Diemeniana).
Holotype
AM F.147104, an almost complete forewing in ventral view together with a small part of hind wing, remnants of the other forewing, ventral thorax and an eye; part and counterpart each in two pieces ().
Etymology
From the Latin ‘ferruginosus’ meaning rust-coloured or rusty, pertaining to the reddish brown colour of the fossil caused by iron minerals.
Zoobank identifier
Zoobank LSID: urn:lsid:zoobank.org:act:C9E560C6-2E2E-4154-95AB-5F117567C128.
Type locality, unit and age
McGraths Flat, ∼25 km northeast of Gulgong in central New South Wales, Australia. Unspecified middle Miocene unit within the Canthiumidites bellus Zone (16–11 Ma: McCurry et al. Citation2022).
Description
Small cicada, forewing length 23 mm; the three inner sections of the discal cell all of similar length; apical cells 2–4 longer than ulnar cells 1 and 2; without infuscations. The ventral body (and the crumpled other forewing) are too decomposed to show diagnostic features.
Remarks
Laopsaltria ferruginosa appears closest to the extant genera Terepsalta, Adelia and Diemeniana. The forewing division of CuA1 by crossvein m-cu so that the proximal section is shortest for the most part separates the Cicadettinae from the Cicadinae, the only two subfamilies of Cicadidae found in Australasia (Moulds Citation2005), although there are some exceptions to this character. Among extant Australian Cicadettinae, Laopsaltria is similar in several significant ways to Terepsalta infans Walker, Citation1850, Adelia borealis Goding & Froggatt, Citation1904, and some species of Diemeniana; all located in two close early-branching clades in the species-rich tribe Cicadettini (Marshall et al. Citation2016). In particular, Laopsaltria, Terepsalta, Adelia and Diemeniana all have veins M and CuA meeting the basal cell far apart, vein RP bowed at its proximal end and what is likely a broadly rounded wing apex.
It is difficult to determine how closely related Laopsaltria is to Terepsalta, Adelia and Diemeniana as Laopsaltria does differ in other attributes as discussed above under Distinguishing features. It is possible that Laopsaltria is ancestral to the sister clades in Marshall et al. (Citation2016) that incorporate both Terepsalta, Adelia and Diemeniana, and possibly ancestral to a large radiation of Cicadettini that is sister to Terepsalta + Adelia + Diemeniana and that has not only radiated extensively in Australia but is also found in fewer species through Asia, Africa, Europe and North America.
Two other extant Australian genera have attributes somewhat similar to the aforementioned genera, Kobonga Distant, 1906 and Marteena Moulds, 1986, each having M and CuA separated at the basal cell but very close together or in some Kobonga fused as one. These fall within the clade branching above Terepsalta+Adelia + Diemeniana and can be considered more highly derived (later branching) than Laopsaltria because of the closer proximity of M and CuA (Moulds Citation2005, p. 412).
CICADIDAE Latreille, Citation1802
CICADINAE Latreille, Citation1802
BURBUNGINI Moulds, Citation2005
Burbungoides gen. nov.
Type species
Burbungoides gulgongensis sp. nov.
Zoobank identifier
Zoobank LSID: urn:lsid:zoobank.org:act:C2474100-9336-413A-B18A-CD2248F61750.
Diagnosis
Forewing with eight apical cells; ulnar cell 3 angled to radial cell; veins M and CuA meeting basal cell wide apart, their departure with little divergence for much of their length making the medial cell unusually narrow at its proximal end; vein CuA1 divided by crossvein at about mid length; costal margin abruptly narrowing at node; vein CuA bowed along its length; vein M3 + 4 curved inwards on medial cell; crossveins r and r-m far apart, far more than the length of those crossveins, and similarly angled; crossvein r similarly angled to crossvein m; crossvein m broadly S-shaped; ulnar cell 1 narrow and not markedly tapering; apical cells tending shorter than ulnar cells; wing margin fully formed; costal margin not ampliate; wing tending slender in shape. Hind wing with six apical cells; apical cells about as long as costal, radial and medial cells.
Differs from Burbunga Distant, Citation1905b in possessing very long hind wing apical cells (about as long as costal, radial and medial cells) and the costal margin abruptly narrowing at the node. Distinguished from all other documented genera in the following character state combination: forewing veins M and CuA meeting basal cell wide apart (and the departure of CuA probably more proximal than M); the medial cell very narrow at its proximal end; vein CuA1 divided by crossvein at about mid length; vein CuA bowed; the costa abruptly narrowed at node; and the hind wing apical cells long, about as long as costal, radial and medial cells.
Etymology
A combination of the generic name Burbunga and the suffix ‘-oides’, implying likeness and referring to the similarity of this new genus to Burbunga. Masculine.
Burbungoides gulgongensis sp. nov.
()
Diagnosis
Distinguished from most extant Australian species in having a very narrow proximal half to the medial cell. There are infuscations overlaying the proximal end of RA2, crossveins r and r-m and the length of RP between them, proximal part of M1, crossveins m and m-cu, and at distal extremities of M1–M4 and CuA1 (extremities of RA1 and RP not visible), a pattern otherwise found among extant Australian genera with veins M and CuA widely separated at the basal cell only in some Macrotristria Stål, Citation1870, Henicopsaltria Stål, Citation1866 and Burbunga. Differs from Macrotristria and Henicopsaltria in having CuA bowed; differs from Burbunga as detailed under Burbungoides above.
Holotype
AM F.147105, an almost complete forewing missing base, and hind wing partly obscured; part and counterpart ().
Etymology
Named after Gulgong, the town close to the type locality of this species.
Zoobank identifier
Zoobank LSID: urn:lsid:zoobank.org:act:2D14C692-3ACA-4701-9D6B-28ED5E9A1222.
Type locality, unit and age
McGraths Flat, ∼25 km northeast of Gulgong in central New South Wales, Australia. Unspecified middle Miocene unit within the Canthiumidites bellus Zone (16–11 Ma: McCurry et al. Citation2022).
Description
Forewing length 40 mm; distal section of the discal cell very much shorter than either medial or basal sections, the medial section especially long; apical cells 2–4 shorter than ulnar cells 1 and 2; infuscation overlaying proximal end of RA2, crossveins r and r-m and the length of RP between them, proximal part of M1, crossveins m and m-cu, and at extremities of M1–M4 and CuA1 (extremities of RA1 and RP not visible).
Remarks
Burbungoides gulgongensis is close to the extant genus Burbunga. The forewing division of CuA1 by crossvein m-cu so that the proximal section is longest is indicative of the subfamily Cicadinae (Moulds Citation2005), otherwise found only in the Tibicininae of Eurasia and North and South America but absent from Australasia and Africa. Features suggestive of tribal placement are the separation of forewing veins M and CuA at the basal cell and more significantly their departure with only minimal divergence making the proximal end of the medial cell much narrower than is usually found in cicadas, a feature accentuated by the inward curvature of M3 + 4 at its proximal end. Further, crossvein m is broadly S-shaped, and the apical crossveins r and r-m are far apart, much more so than the length of the crossveins themselves and these crossveins are similarly angled.
Infuscations on the forewing are a striking feature. These overlay crossveins r, r-m and m plus the venation adjoining them, in addition to crossvein m-cu, and the distal extremities of veins M1–M4 and CuA1. Infuscations distributed like this are found in relatively few extant Australian species, indeed among New Guinea and South-East Asian cicadas, although close variants are common. Within the Australian fauna, an identical or near identical pattern is found in Burbunga, Henicopsaltria, Arenopsaltria Ashton, Citation1921, Macrotristria, Illyria Moulds, Citation1985, Psaltoda Stål, Citation1861, Tamasa Distant, Citation1905a and Diceropyga subapicalis Walker, Citation1868. While wing infuscations patterns of this nature are not diagnostic for genera, their presence and pattern do appear to reflect some degree of relationship.
The venation attributes mentioned above, in conjunction with the wing infuscation pattern discussed, in particular the narrow proximal end to the medial cell, are only found among the extant Australian species Burbunga gilmorei Distant, 1882 (), Burbunga queenslandica Moulds, Citation1994, Burbunga inornata Distant, Citation1905b and Burbunga parva Moulds, Citation1994. While this implies a close relationship of B. gulgongensis with the genus Burbunga, and consequently the Burbungini, the fossil differs from all Burbunga except Burbunga hillieri (which differs in its gradually tapered costal margin) in having much longer hind wing apical cells. Consequently, we place the fossil in the Burbungini but allocate it to the new genus Burbungoides.
ARENOPSALTRIINI Moulds, Citation2018 (in Marshall et al. Citation2018)
Tithopsaltria gen. nov.
Type species
Tithopsaltria titan sp. nov.
Zoobank identifier
Zoobank LSID: urn:lsid:zoobank.org:act:D5F9551B-DCD8-4620-BB1B-8186DCC13584.
Diagnosis
Forewing with 8 apical cells; ulnar cell 3 angled to radial cell; veins M and CuA meeting basal cell wide apart, with the departure of CuA distinctly closer to the cell base than M; vein CuA1 divided by crossvein so that proximal portion shortest and considerably bowed; median veins M1–M4 with their distal ends forming apical cells increasingly curved; vein M3 + 4 barely curved inwards on medial cell; crossveins r and r-m far apart, far more than the length of those crossveins, and both similarly angled; crossvein r markedly different to angle of crossvein m; crossvein m straight; ulnar cell 1 narrow and not markedly tapering; wing margin wide, fully formed; costal margin ampliate for basal quarter or so; wing of average proportions with anal angle tending to be angulate; the costal margin evenly curved, except a little more so in subapical region.
Distinguished from all other documented genera in the following character state combination: forewing veins M and CuA meeting basal cell wide apart with the departure of CuA far more proximal than M; vein CuA1 divided by crossvein so that proximal portion a little shorter than distal portion and considerably bowed; crossveins r and r-m widely separated (distance between them about twice the length of a crossvein); and in having the distal and medial sections of the discal cell both very long and much longer than the proximal one.
Etymology
Derived from the Latin ‘Tithonus’ (Greek ‘Tithonos’), the mythical consort of Aurora, symbolic of decrepit old age and changed into a cicada, and from the Latin ‘psaltria’, a female harpist and a traditional ending for cicada generic names. Feminine.
Tithopsaltria titan sp. nov.
()
Diagnosis
Distinguished from all extant Australian species with forewing veins M and CuA separated at the basal cell, in having the distal and medial section of the discal cell (the base of ulnar cells 2 and 3) both similar in length and each much longer than the basal section (the stem of vein M). Only some Arenopsaltria, Cyclochila Amyot & Serville, Citation1843 and Psaltoda fumipennis Ashton (Citation1912) have a similar venation but differ noticeably in having the proximal section of CuA1 straight instead of curved, and Cyclochila and Psaltoda clearly do not have vein CuA departing the basal cell far more proximal than M.
Holotype
AM F.147103, complete forewing; part and counterpart, both in two pieces ().
Etymology
Greek ‘Titan’, referring to the family of giants in Greek mythology that ruled the earth until overthrown by the Olympian gods. The name symbolizes the large size of this species, the largest known fossilized cicada.
Zoobank identifier
Zoobank LSID: urn:lsid:zoobank.org:act:062EADC8-B96C-402F-B0A9-AB16ABFBC144.
Type locality, unit and age
McGraths Flat, ∼25 km northeast of Gulgong in central New South Wales, Australia. Unspecified middle Miocene unit within the Canthiumidites bellus Zone (16–11 Ma: McCurry et al. Citation2022).
Description
Forewing length 76 mm, the largest known fossil cicada, surpassed in size only by extant species in the genus Megapomponia Boulard, Citation2005; distal and medial section of the discal cell both very long and each longer than the basal section; apical cells 2–4 similar in length to ulnar cells 1 and 2; without infuscations (the darkening overlaying crossvein r and distal half of RP between r and r-m is treated as a doubtful infuscation and is probably an artefact as its distribution is abnormal and it abnormally darkens the vein).
Remarks
Tithopsaltria titan most closely resembles species of Psaltoda and Macrotristria, Anapsaltoda Ashton, Citation1921, Henicopsaltria, Thopha, Arunta and Jassopsaltria Ashton, Citation1914 (all subfamily Cicadinae). These all share with T. titan a similar forewing shape, a broad outer margin, veins M and CuA widely separated at the basal cell, strong distal curvature of the median veins forming the apical cells, a partially ampliate costa, crossveins r and r-m widely spaced and directed at a similar angle, and crossveins m and m-cu angled similarly to each other but very differently to crossveins r and r-m. While many species in these genera also have CuA1 curved, only some species of Psaltoda have the proximal section of CuA1 strongly bowed as in T. titan, in particular Psaltoda magnifica Moulds, Citation1984 and Psaltoda flavescens Distant, 1892. Among the aforementioned genera, including Psaltoda, all have forewing veins M and CuA departing the basal cell similarly distant from its base, the one exception being Henicopsaltria (tribe Arenopsaltriini) that has CuA departing closer to the base of the basal cell than M as does T. titan, in particular Henicopsaltria eydouxii (Guérin-Méneville, Citation1838). This suggests that the nearest extant genera to T. titan are Psaltoda and Henicopsaltria, or more specifically, the species previously mentioned. However, these differ slightly from T. titan by one character, in having vein CuA1 divided by crossvein m-cu so that the proximal section is longest, not a little shorter as in T. titan. This has implications at subfamily level, its division with the proximal section shortest usually implies that a species belongs to the subfamily Cicadettinae (Moulds Citation2005), not to the Cicadinae as suggested by the close similarity of T. titan with Psaltoda and Henicopsaltria. This, however, is not a ‘hard and fast rule’ and there are exceptions. Among the 15 extant species of Psaltoda, there are three species (Psaltoda fumipennis, Psaltoda insularis Ashton, Citation1914, and Psaltoda adonis Ashton, Citation1914) where the proximal section of CuA1 is shorter instead of longer, and there are others where the division is about mid-length (see Moulds Citation1990, pls 9–11). It is reasonable to assume that Tithopsaltria is another exception in this regard, its similarities to Psaltoda, Henicopsaltria and their allies far outweighing similarity to any species in the Cicadettinae. While there are some genera in Cicadettinae, even among the Australian fauna such as Aleeta Moulds, Citation2003, Tryella Moulds, Citation2003 and Diemeniana, these show only a superficial resemblance to Tithopsaltria, because they differ markedly in wing shape, have a much narrower clavus, a much narrower wing margin, as well as a closer separation of forewing veins M and CuA, all common attributes in Cicadettinae.
While T. titan shows clear affinities with extant species of Psaltoda and Henicopsaltria, these two genera belong to different tribes: Psaltoda to the Psaltodini and Henicopsaltria to the Arenopsaltriini. The attribute associating T. titan with Psaltoda is the strong curvature of the proximal section of CuA1, a variable feature within the genus and not of high phylogenetic value. On the other hand, Henicopsaltria has in common with T. titan vein CuA departing the basal cell closer to the cell base than vein M. This is plesiomorphic compared to the more distal departure of CuA (Moulds Citation2005, p. 412). As this is more likely to indicate phylogenetic relationships within the Cicadinae, we place Tithopsaltria in the tribe Arenopsaltriini, a tribe containing just two extant genera, Henicopsaltria and Arenopsaltria. Interestingly, Arenopsaltria has vein CuA departing the basal cell even more basal than in Henicopsaltria, very similar to that of T. titan.
Preservation of setae, nanostructures and other anatomical details
Fossils from McGraths Flat locality usually occur with part and counterpart components, often split through the middle, which, in the case of insect wings, means that the inside of veins or the internal cuticle membrane is exposed. For example, the holotype of Tithopsaltria titan (AM F.147103) possesses a single line of setae on the leading edge of the forewing (), of which only the bases can be seen since the spines are usually hidden underneath the wing (). However, a single seta on the leading wing edge in the holotype of Laopsaltria ferruginosa (AM F.107104) shows a dislodged internal base and the first 10 µm of its spine (), while a seta on the leading wing edge in the holotype of Burbungoides gulgongensis (AM F.107105) preserves an almost complete spine (). The spine shown in has also split in half exposing details of its internal structure, with suggestions of longitudinal groves. The fine ornamental patterns seen in the dark lower half of the spine in , and in parts of the spine illustrated in , may represent nanoscale structures of the cuticle. Furthermore, the spine in preserve a close array of setal bases, suggesting that the leg of at least one unidentified species (AM F.147106) carried a covering of very small setae, a feature not unusual on extant cicada legs.
In some cases, the McGraths Flat fossil cicadas do not split through the centre, and structures near the surface can be observed. SEM imaging of AM F.107104 revealed evidence of a regular nanostructure on the wing surface (). The observed holes have the near-perfect hexagonal pattern and dimensions of a ‘nanopillar array’, a surface nanostructure on the wings of extant cicadas. For example, the wing surface topology of the extant Australian species Burbunga hillieri is characterized by small, cone-shaped protrusions (nanopillars) that are (mostly) arranged in a hexagonal pattern with an average centre-to-centre distance of 178 nm between two pillars (). The arrangement and dimensions of these nanopillars are very similar to the arrangement and dimensions of the nano-holes in L. ferruginosa, which likely possessed a wing topology similar to modern cicadas. The actual nanopillars on AM F.107104, however, have not survived, but were preserved as a detailed mould that formed when the iron mineral goethite precipitated around the insect. Interestingly, similar looking high-fidelity casts have been generated from extant cicada wings using poly(methyl metha-crylate) (see Zhang et al. Citation2006), gold (see Xie et al. Citation2008) nickel (see Xie et al. Citation2017) or titanium dioxide (see Zada et al. Citation2017), which further supports our hypothesis that L. ferruginosa had a modern wing surface topology.
High-fidelity preservation is not restricted to setae or nanostructures on the wing surface of the McGraths Flat cicada fossils (see ). Potential internal structures of the cuticle are observable but are difficult to interpret because the taphonomy is not completely understood (McCurry et al. Citation2022).
Discussion
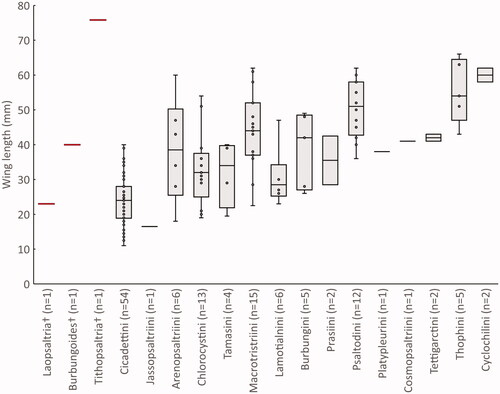
A scarcity of fossil cicadas from the Australian continent has led to ambiguity about how the fauna has changed over time. The cicada specimens described here include an unexpected degree of morphological difference to the modern fauna. Tithopsaltria titan, with a forewing length of 76 mm, is the largest known fossilized cicada and is by far the largest known Australian cicada (). It is only slightly rivalled in size by extant species in the genus Megapomponia Boulard, Citation2005 that today inhabit Southeast Asia (Lee & Sanborn Citation2010). The largest extant Australian species, Thopha saccata Fabricius, Citation1803, has a forewing length of up to 67 mm (Moulds Citation2008). The association of Tithopsaltria to Psaltoda and Henicopsaltria, two genera of large species and closely allied to Thopha Amyot & Serville, Citation1843, suggests that T. titan represents an independent evolution of extremely large cicadas in Australia.
Figure 7. Wing length of fossil (red) and extant (black) Australian cicadas. Wing size data are estimates for each species sourced from Moulds (Citation1990). Boxplots represent the size distribution of wing length within the tribe. Tribal classification follows Marshall et al. (Citation2018).
Wing surface nanostructures have been documented for a range of extant cicada species, including several Australian species (e.g., Kelleher et al. 2016, Pogodin et al. Citation2013, Ivanova et al. Citation2012, Sun et al. Citation2009, Sun et al. Citation2012, Xie et al. Citation2017). The topology of these nanostructures varies from species to species, with differences in structure homogeneity and in the shape, size and spacing of the nanopillars. Furthermore, Sun et al. (Citation2012) noted that ageing and storage conditions affected the outermost ‘wax’ layer(s) of the epicuticle in extant species, and thus changes the shape and height of the pillars. To compare the wing nanostructure of extant specimens of different ages, and extant specimens with our fossil cicadas (in which a mould of the nanopillar array rather than the actual pillars is preserved), we measured the average centre-to-centre distance between two pillars (sometimes called the ‘pitch’) rather than pillar diameter and spacing. Our analysis suggests that the wing surface nanostructure of Laopsaltria ferruginosa is similar to that of an extant Australian species in the same tribe, Burbunga hillieri (). However, more research is warranted to determine whether nanostructure details are indeed valuable taxonomic characters, as the intra-species variation of cicada wing nanostructures has never been thoroughly investigated, and a comprehensive investigation of extant species that fully reflect the diversity of the family has not yet been performed.
A lack of well-preserved fossil material has, until now, not allowed tracing the evolution of these wing surface nanostructure in cicadas (as well as in other insects). Thus, our description of a Miocene cicada wing surface nanostructure is an important first step to close this knowledge gap. Given that fossil cicadas are not particularly rare at McGraths Flat (so far, in only 3 years of excavation, seven specimens have been discovered that represent at least three species), it is tempting to speculate that additional fossil nanostructures will be reported in time.
The surface nanostructure on cicada wings possesses a range of properties including antireflection, superhydrophobicity, self-cleaning and control of microbial adhesion/growth. The versatility of these structures has sparked considerable interest outside the field of entomology. For example, the ability of cicada nanostructures to destroy bacteria through direct physical contact rather than chemical interactions (Ivanova et al. Citation2012, Jenkins et al. Citation2020) makes such nanostructures an attractive template for the engineering of surfaces that kill bacterial contaminants and inhibit biofilm formation. Kelleher et al. (Citation2016) established a functional assay that can quantify and compare bactericidal properties of wing nanostructures from different cicada species. So far, however, only the nanostructures of extant cicada wings have been studied. Our discovery of the extraordinary fidelity of preservation at McGraths Flat shows that key parameters of wing nanostructures can be measured in fossil cicadas. This not only helps to elucidate the evolutionary origins of wing surface nanostructures, but also opens up the potential for examining the function of these structures in these extinct species.
Zoobank registration
http://zoobank.org/urn:lsid:zoobank.org:pub:7BB4E6CC-88A7-4E31-BBC4-6CD293FB8D0C.
Acknowledgements
We thank Nigel McGrath, and Lyn and Geoff Gale for their generosity and help in accessing the site. David Marshall is thanked for helpful discussion and comments on an early draft of the manuscript; we thank Rolf Oberprieler for kindly providing advice on Latin grammar and Frank Brink for expert advice and help with the imaging of extant wing specimens. Furthermore, we acknowledge the scientific and technical assistance of Microscopy Australia, especially from the Centre for Advanced Microscopy, Australian National University (jointly funded by the Australian National University and the Australian Federal Government), and we thank the Australian National Insect Collection, Commonwealth Scientific Industrial Research Organisation for access to imaging equipment. Derek Smith kindly accessioned our extant cicada voucher. We are grateful to the Etheridge family descendants who funded this work in addition to internal grants to M.R.M. from AM, and to M.F. from the University of Canberra.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Amyot, C.J.B. & Serville, J.G.A., 1843. Histoire Naturelle des Insects. Hémiptères. Librairie Encyclopedique de Roret. Fain & Thunot, Paris. Text, lxxvi, 675 pp. Atlas, 12 pls.
- Ashton, J.H., 1912. Some new Australian Cicadidae. Proceedings of the Royal Society of Victoria, New Series, 24, 221–229, pls XLIX–LI.
- Ashton, J.H., 1914. Catalogue of the Cicadidae in the South Australian Museum with descriptions of several new species. Transactions and Proceedings of the Royal Society of South Australia 38, 345–358, pl. 17.
- Ashton, J.H., 1921. A revision of the Australian Cicadidae. Part 1. Proceedings of the Royal Society of Victoria (n.s.) 33, 87–107.
- Boulard, M., 2005. Création du genre Megapomponia et description de Mp. clamorigravis n. sp. (Rhynchota, Cicadoidea, Cicadidae). Ecole Pratique des Hautes Etudes, Travaux du Laboratoire Biologie et Evolution des Insectes Hemipteroidea 15, 93–110.
- Buckton, G.B., 1890. Monograph of the British Cicadidae or Tettigidae. Vol. 1. Macmillan and Co., London, 133 pp.
- Byrne, M., Yeates, D.K., Joseph, L., Kearney, M., Bowler, J., Williams, M.A.J., Cooper, S., Donnellan, S.C., Keogh, J.S., Leys, R., Melville, J., Murphy, D.J., Porch, N. & Wyrwoll, K.-H., 2008. Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417.
- Christophel, D.C., 1989. Evolution of the Australian flora through the Tertiary. In Woody Plants—Evolution and Distribution Since the Tertiary. Ehrendorfer, F., ed., Springer, Wien, 63–78.
- Claridge, M.F., Morgan, J.C. & Moulds, M.S., 1999. Substrate-transmitted acoustic signals of the primitive cicada, Tettigarcta crinita Distant (Hemiptera Cicadoidea, Tettigarctidae). Journal of Natural History 33, 1831–1834.
- Distant, W.L., 1905a. Rhynchotal notes.–XXXI. Annals and Magazine of Natural History 15, 379–387.
- Distant, W.L., 1905b. Rhynchotal notes.–XXXIII. Annals and Magazine of Natural History 16, 22–35.
- Distant, W.L., 1905c. Rhynchotal notes.–XXXIV. Annals and Magazine of Natural History 16, 203–216.
- Distant, W.L., 1907. Rhynchotal notes.–XLIII. Annals and Magazine of Natural History 20, 411–423.
- Evans, J.W., 2009. A natural classification of leaf-hoppers (Jassoidea, Homoptera). Part 1. External morphology and systematic position. Transactions of the Royal Entomological Society of London 96, 47–60.
- Fabricius, J.C., 1803. Systema Rhyngotorum secundum ordines, genera, species, adiectis synonyms, locis, observationibus, descriptionibus. Akademische Druck- u. Verlagsanstalt, Graz, Austria, pp. i–x, 1–314, index 1–21. [Cicadas viii, 33–44. Second edition, 1822. Facsimilie reprint, 1971.]
- Fu, Y., Cai, C. & Huang, D., 2019. First hairy cicadas in mid-Cretaceous amber from northern Myanmar (Hemiptera: Cicadoidea: Tettigarctidae). Cretaceous Research 93, 285–291.
- Goding, F.W. & Froggat, W.W., 1904. Monograph of the Australian Cicadidae. Proceedings of the Linnean Society of New South Wales, 29(3), 561–670, pls XVIII + XIX.
- Guérin-Méneville, F.E., 1838. Insectes. In: L.I. Duperrey, Voyage autour du monde, exécuté par ordre du Roi, sur la corvette de sa Majesté, La Coquille, pendant les années 1822, 1823, 1824 et 1825, sous le ministère et conformément aux instructions de S.E.M. le Marquis de Clermont-Tonnerre, Ministre de la Marine; et publié sous les auspices de son Excellence Mgr le Cte de Chabrol, Ministre de la Marine et des Colonies. A. Bertrand, Paris. Text; Zoologie 2(2), Division 1, pp. 57–302.
- Hamilton, K.G.A., 1990. Homoptera. In Insects from the Santana Formation, Lower Cretaceous, of Brazil. Grimaldi, D.A., ed., Bulletin of the American Museum of Natural History 195, 82–122.
- Ivanova, E.P., Hasan, J., Webb, H.K., Truong, V.K., Watson, G.S., Watson, J.A., Baulin, V.A., Pogodin, S., Wang, J.Y., Tobin, M.J., Löbbe, C. & Crawford, R.J., 2012. Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small (Weinheim an der Bergstrasse, Germany) 8, 2489–2494.
- Jenkins, J., Mantell, J., Neal, C., Gholinia, A., Verkade, P., Nobbs, A.H. & SU, B., 2020. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nature Communications 11, 1–14.
- Jiang, H., Chen, J., Jarzembowski, E. & Wang, B., 2019. An enigmatic fossil hairy cicada (Hemiptera, Tettigarctidae) from mid-Cretaceous Burmese amber. Cretaceous Research 96, 14–18.
- Kaulfuss, U. & Moulds, M.S., 2015. A new genus and species of Tetti-garctid cicada from the early Miocene of New Zealand: Paratettigarcta zealandica (Hemiptera, Auchenorrhyncha, Tettigarctidae). ZooKeys 484, 83–94.
- Kelleher, S.M., Habimana, O., Lawler, J., O’Reilly, B., Daniels, S., Casey, E. & Cowley, A., 2016. Cicada wing surface topography: an investigation into the bactericidal properties of nanostructural features. ACS Applied Materials & Interfaces 8, 14966–14974. Epub 2015.
- Lambkin, K.J., 2019. Mesodiphthera Tillyard, 1919, from the Late Triassic of Queensland, the oldest cicada (Hemiptera: Cicadomorpha: Cicadoidea: Tettigarctidae). Zootaxa 4567, zootaxa.4567.2.8.
- Latreille, P.A., 1802. Histoire naturelle, générale et particulière des Crustacés et des Insectes. Ouvrage faisant suite aux oeuvres de Laclerc de Buffon et partie du cours complet d’Histoire naturelle rédigé p. C.S. Sonnini. 3. Familles naturelles et genres. Dufart, Paris, xii + 467 pp.
- Lee, Y.J. & Sanborn, A.F., 2010. Three new species of the genus Megapomponia (Hemiptera: Cicadidae) from Indochina, with a key to the species of Megapomponia. Journal of Asia-Pacific Entomology 13, 31–39.
- Marshall, D.C., Hill, K.B.R., Moulds, M., Vanderpool, D., Cooley, J.R., Mohagan, A.B. & Simon, C., 2016. Inflation of molecular clock rates and dates: molecular phylogenetics, biogeography, and diversification of a global cicada radiation from Australasia (Hemiptera: Cicadidae: Cicadettini). Systematic Biology 65, 16–34. [First issued online 22 October, 2015, pp. 1–19.]
- Marshall, D.C., Moulds, M., Hill, K.B.R., Price, B.W., Wade, E.J., Owen, C.L., Goemans, G., Marathe, K., Sarkar, V., Cooley, J.R., Sanborn, A.F., Kunte, K., Villet, M.H. & Simon, C., 2018. A molecular phylogeny of the cicadas (Hemiptera: Cicadidae) with a review of tribe and subfamily classification. Zootaxa 4424, 1–64.
- Martins-Neto, R.G. & Mendes, M., 2002. The Fonseca Formation paleoentomofauna (Fonseca Basin, Oligocene of Minas Gerais State, Brazil) with description of new taxa. Acta Geologica Leopoldensia 25, 27–33.
- McCurry, M.R., Cantrill, D.J., Smith, P.M., Beattie, R., Dettmann, M., Baranov, V., Magee, C., Nguyen, J.M.T., Forster, M.A., Hinde, J., Pogson, R., Wang, H., Marjo, C.E., Vasconcelos, P. & Frese, M., 2022. A Lagerstätte from Australia provides insight into the nature of Miocene mesic ecosystems. Science Advances 8, eabm1406.
- Menon, F., 2005. New record of Tettigarctidae (Insecta, Hemiptera, Cicadoidea) from the Lower Cretaceous of Brazil. Zootaxa 1087, 53–58.
- Moulds, M.S., 1984. Psaltoda magnifica sp.n. and notes on the distribution of other Psaltoda species (Homoptera: Cicadidae). General and Applied Entomology 16, 27–32.
- Moulds, M.S., 1985. Illyria, a new genus for Australian cicadas currently placed in Cicada L. (=Tettigia Amyot) (Homoptera: Cicadidae). General and Applied Entomology 17, 25–35.
- Moulds, M.S., 1990. Australian cicadas. New South Wales University Press, Kensington. 217 pp., 24 pls.
- Moulds, M.S., 1994. The identity of Burbunga gilmorei (Distant) and B. inornata Distant (Hemiptera: Cicadidae) with descriptions of two allied new species. Australian Journal of Entomology 33, 97–103.
- Moulds, M.S., 2003. An appraisal of the cicadas of the genus Abricta Stål and allied genera (Hemiptera: Auchenorrhyncha: Cicadidae). Records of the Australian Museum 55, 245–304.
- Moulds, M.S., 2005. An appraisal of the higher classification of cicadas (Hemiptera: Cicadoidea) with special reference to the Australian fauna. Records of the Australian Museum 57, 375–446.
- Moulds, M.S., 2008. Thopha hutchinsoni, a new cicada (Cicadoidea: Cicadidae) from Western Australia, with notes on the distribution and colour polymorphism of Thopha sessiliba Distant. Australian Entomologist 35, 129–140.
- Moulds, M.S., 2012. A review of the genera of Australian cicadas (Hemiptera: Cicadoidea). Zootaxa 3287, 1–262.
- Moulds, M.S., 2018. Cicada fossils (Cicadoidea: Tettigarctidae and Cicadidae) with a review of the named fossilised Cicadidae. Zootaxa 4438, 443–470.
- Pogodin, S., Hasan, J., Baulin, V.A., Webb, H.K., Truong, V.K., Nguyen, T.H.P., Boshkovikj, V., Fluke, C.J., Watson, G.S., Watson, J.A., Crawford, R.J. & Ivanova, E.P., 2013. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophysical Journal 104, 835–840.
- Stål, C., 1861. Genera nonnulla nova Cicadinorum. Annales de la Société Entomologique de France 1, 613–622.
- Stål, C., 1866. Hemiptera Africana. Hemiptera Homoptera Latr 4, 276 p, pl. Officina Norstedtiana, Holmiae.
- Stål, C., 1870. Hemiptera insularum Philippinarum. Bidrag till Philippionska öarnes Hemipter-fauna. Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar, Stockholm 27, 607–776. pls VII–IX.
- Sun, M., Liang, A., Watson, G.S., Watson, J.A., Zheng, Y., Ju, J. & Jiang, L., 2012. Influence of cuticle nanostructuring on the wetting behaviour/states on cicada wings. PLoS One 7, e35056.
- Sun, M., Watson, G.S., Zheng, Y., Watson, J.A. & Liang, A., 2009. Wetting properties on nanostructured surfaces of cicada wings. The Journal of Experimental Biology 212, 3148–3155.
- Tillyard, R.J., 1919. Mesozoic insects of Queensland. No.7. Hemiptera Homoptera; with a note on the phylogeny of the Suborder. Proceedings of the Linnean Society of New South Wales 44, 857–896.
- Tillyard, R.J., 1922. Mesozoic insects of Queensland. No.9. Orthoptera, and additions to the Protorthoptera, Odonata, Hemiptera and Planipennia. Proceedings of the Linnean Society of New South Wales 47, 447–470.
- Walker, F., 1850. List of the specimens of homopterous insects in the collection of the British Museum. Part 1. British Museum, London, 1–260.
- Walker, F., 1868. Catalogue of the homopterous insects collected in the Indian Archipelago by Mr. A.R. Wallace, with descriptions of new species. Journal of the Linnean Society of London, Zoology 10, 82–193, pl. 3.
- Westwood, J.O., 1840. Order Homoptera Macleay. In An Introduction to the Modern Classification of Insects; Founded on the Natural Habits and Corresponding Organisation of the Different Families. Vol. 2. Longman, Orme, Brown, Green and Longmans, London, 414–450.
- Xie, G., Zhang, G., Lin, F., Zhang, J., Liu, Z. & Mu, S., 2008. The fabrication of subwavelength anti-reflective nanostructures using a bio-template. Nanotechnology 19, 095605.
- Xie, H., Huang, H.-X. & Peng, Y.-J., 2017. Rapid fabrication of bio-inspired nanostructure with hydrophobicity and antireflectivity on polystyrene surface replicating from cicada wings. Nanoscale 9, 11951–11958.
- Zada, I., Zhang, W., Zheng, W., Zhu, Y., Zhang, Z., Zhang, J., Imtiaz, M., Abbas, W. & Zhang, D., 2017. The highly efficient photocatalytic and light harvesting property of Ag-TiO2 with negative nano-holes structure inspired from cicada wings. Scientific Reports 7, 17277.
- Zhang, G., Zhang, J., Xie, G., Liu, Z. & Shao, H., 2006. Cicada wings: a stamp from nature for nanoimprint lithography. Small (Weinheim an der Bergstrasse, Germany) 2, 1440–1443.