Abstract
The bowerbirds (Passeriformes, Ptilonorhynchidae) are a family of Australo-Papuan songbirds that are renowned for their unique bower-building behaviour. Prior to this study, the fossil record of Ptilonorhynchidae was limited to late Quaternary remains from Victoria, Australia. A new genus and species of bowerbird is described here from the late Oligocene (ca 26–23 Ma) deposits of the Riversleigh World Heritage Area, in Waanyi Country, Queensland, Australia. This fossil bowerbird is smaller than most extant species of bowerbirds, and represents the earliest known evidence of Ptilonorhynchidae, although its intrafamilial affinities remain uncertain pending additional material. A second, larger ptilonorhynchid is identified from the early Miocene (ca 16 Ma) of Riversleigh. These fossils considerably extend the temporal range of Ptilonorhynchidae, and indicate the presence of two species of bowerbirds in the Oligo-Miocene of northern Australia. The ages of these fossils are consistent with molecular estimates of the divergence time between Ptilonorhynchidae and its sister group, Climacteridae (Australo-Papuan treecreepers). The new bowerbird is the ninth species of passerine to be described from the pre-Pleistocene of Australia, and highlights the importance of the Riversleigh fossil deposits in our understanding of the early evolutionary history of passerines.
Jacqueline M.T. Nguyen [[email protected]], Australian Museum Research Institute, 1 William Street, Sydney NSW 2010 Australia; College of Science and Engineering, Flinders University, GPO Box 2100, Adelaide SA 5001 Australia; and South Australian Museum, North Terrace, Adelaide SA 5000 Australia.
BOWERBIRDS (Passeriformes, Ptilonorhynchidae) are medium-sized, chunky songbirds that are renowned for their unique bower-building behaviour for mating displays. Male bowerbirds skilfully construct bowers using sticks and other plant materials, and decorate these structures with brightly coloured natural objects such as fruits, flowers, insects, feathers, and stones, as well as human-made objects (Higgins et al. Citation2006, Rowland Citation2008). The structure and complexity of these displays are species-specific, as are the nature, positioning, and colour of the objects that adorn them (Schodde & Mason Citation1999). Within Ptilonorhynchidae, there are 28 species in eight genera, all of which are confined to Australia and New Guinea (Gill et al. Citation2022). These birds are highly frugivorous, and predominantly occur in rainforests and adjacent wet forests in Australia and in hill and montane forests in New Guinea. The grey bowerbird species (Chlamydera Gould, Citation1837a) have adapted to drier and more open habitats on both land masses (Schodde & Mason Citation1999, Frith & Frith Citation2008, Beehler & Pratt Citation2016).
Until recent decades, bowerbirds were thought to be closely related to birds-of-paradise (Paradisaeidae) because of similarities in sexual plumage dimorphism, courtship displays, polygynous behaviour, and geographical distribution (Higgins et al. Citation2006, Rowland Citation2008). Phylogenetic analyses based on molecular data indicate that Ptilonorhynchidae is the sister group of Climacteridae (Australo-Papuan treecreepers), and that this clade forms the sister group to all other oscine passerines except Menurides (lyrebirds + scrubbirds) (e.g., Barker et al. Citation2004, Selvatti et al. Citation2015, Moyle et al. Citation2016). Based on their architectural designs, bowerbirds have been categorized into four groups: (1) catbirds (Ailuroedus Cabanis & Heine, Citation1851), which do not construct bowers or courts; (2) the court-building Tooth-billed Bowerbird (Scenopoeetes dentirostris (Ramsay, Citation1876)); (3) maypole bower builders (Prionodura De Vis, Citation1883, Amblyornis Elliot, Citation1872), including the mat-building Archbold’s Bowerbird (Archboldia papuensis Rand, Citation1940); and (4) avenue bower builders (Ptilonorhynchus Kuhl, Citation1820, Chlamydera, Sericulus Swainson, Citation1825) (Frith & Frith Citation2008, Beehler & Pratt Citation2016). A recent phylogenomic study showed that catbirds form the sister group to a clade comprising the court and maypole builders (including Archboldia), and that the avenue builders constitute the sister group to all other bowerbirds (Ericson et al. Citation2020).
The fossil record of bowerbirds was previously limited to the late Quaternary of Victoria in Australia. Reported fossils include remains of the extant Satin Bowerbird Ptilonorhynchus violaceus (Vieillot, Citation1816) from Mabel Cave and Pyramids Cave in the Buchan region (Baird Citation1991, Citation1993), as well as from Amphitheatre Cave in the Glenelg River area (Baird Citation1992). This study describes a new genus and species of ptilonorhynchid, and reports a second indeterminate bowerbird from the Oligo-Miocene deposits of the Riversleigh World Heritage Area, in Waanyi Country, Queensland, Australia. These fossils constitute the earliest known evidence of Ptilonorhynchidae and expand the diversity of songbirds identified from the pre-Pleistocene of Australia.
Materials and methods
Direct comparisons were made with specimens of the following extant taxa: Ptilonorhynchidae: Ailuroedus crassirostris (Paykull, Citation1815) Green Catbird AM O.56645, AM O.64754, AM O.70188; Ailuroedus melanotis (Gray, Citation1858) Black-eared Catbird AM O.70421; Scenopoeetes dentirostris Tooth-billed Bowerbird AM O.65902, AM O.71414; Amblyornis macgregoriae De Vis, Citation1890 MacGregor’s Bowerbird NHMD 141557, NMV B19278;Amblyornis subalaris? Sharpe, Citation1884 Streaked Bowerbird? ANWC PASS-1023; Prionodura newtoniana De Vis, Citation1883 Golden Bowerbird ANWC PASS-1526, QM O.12694; Sericulus chrysocephalus (Lewin, Citation1808) Regent Bowerbird AM O.60064, AM O.65350, AM O.67926, SAMA B20548, SAMA B51160; Ptilonorhynchus violaceus Satin Bowerbird AM O.57127, AM O.64555, SAMA B19568, SAMA B21910; Chlamydera guttata Gould, Citation1862 Western Bowerbird NMV B24788; Chlamydera nuchalis (Jardine & Selby, Citation1830) Great Bowerbird AM O.65727, AM O.70457, SAMA B7008, SAMA B7009; Chlamydera maculata (Gould, Citation1837b) Spotted Bowerbird AM O.64603, AM O.67037, AM O.71574. Menuridae: Menura novaehollandiae Latham, Citation1801 Superb Lyrebird AM O.60220. Artamidae: Gymnorhina tibicen (Latham, Citation1801) Australian Magpie AM O.71169. Campephagidae: Coracina novaehollandiae (Gmelin, Citation1789) Black-faced Cuckooshrike AM O.67876. Oriolidae: Sphecotheres vieilloti Vigors & Horsfield, Citation1827 Australasian Figbird AM O.60498. Corvidae: Corvus coronoides Vigors & Horsfield, Citation1827 Australian Raven AM O.76571. Corcoracidae: Struthidea cinerea Gould, Citation1837b Apostlebird AM O.60444. Paradisaeidae: Ptiloris victoriae Gould, Citation1850a Victoria’s Riflebird AM O.68473. Skeletal specimens of the monotypic Archboldia papuensis were unavailable for comparison.
The fossils described here are registered in the Queensland Museum palaeontology collection (QM F); precise details of fossil localities have been lodged with the QM. Fossil specimens were prepared and initially registered in the temporary collections (AR) of the Vertebrate Palaeontology Laboratory at the University of New South Wales in Sydney, Australia. Taxonomic nomenclature follows Gill et al. (Citation2022), osteological terminology follows Baumel & Witmer (Citation1993), and myological terminology follows Vanden Berge & Zweers (Citation1993), except where noted. Anatomical abbreviations used in the text: lig., ligamentum/ligamenti; M., musculus/musculi; proc., processus; tub., tuberculum. Measurements were taken using a Leica M80 stereo microscope with an eyepiece reticule calibrated to a reference scale accurate to 0.1 mm. This published work and the introduced nomenclatural acts have been registered with ZooBank with the following LSID: urn:lsid:zoobank.org:pub:3B4E92E9-3E2D-480E-B894-16CBF65031FD.
Institutional abbreviations: AM, Australian Museum, Sydney, Australia; ANWC, Australian National Wildlife Collection, CSIRO, Canberra, Australia; NHMD, Natural History Museum of Denmark Zoological Museum, Copenhagen, Denmark; NMV, Museums Victoria, Melbourne, Australia; QM, Queensland Museum, Brisbane, Australia; SAMA, South Australian Museum, Adelaide, Australia.
Systematic palaeontology
Order PASSERIFORMES Linnaeus, Citation1758
Suborder PASSERI Linnaeus, Citation1758
Infraorder CLIMACTERIDES Cracraft, Citation2014
Family PTILONORHYNCHIDAE Gray, Citation1841
Sericuloides gen. nov.
(urn:lsid:zoobank.org:act:75FEF204-6BC4-4E77-9813-1A0DA313C708)
Type species
Sericuloides marynguyenae, sp. nov.
Etymology
The generic name derives from Sericulus (from Greek sērikos, silken) and -oidēs (Greek, resembling), and refers to similarities to species of the genus Sericulus.
Diagnosis
As for type and only species.
Sericuloides marynguyenae sp. nov.
(
(urn:lsid:zoobank.org:act:14A358BC-D6CF-400E-A0A0-0934A90823D6)
Fig. 1. Sericuloides marynguyenae gen. et sp. nov. from the late Oligocene White Hunter Site, Riversleigh, Australia, compared with the extant Sericulus chrysocephalus. A, B, Sericuloides marynguyenae, holotype right carpometacarpus (QM F57971). C, D, Sericulus chrysocephalus, right carpometacarpus. E, G–J, Sericuloides marynguyenae, paratype left tarsometatarsus (QM F57972). F, K–N, Sericulus chrysocephalus, left tarsometatarsus. A, C, G, K, dorsal view, B, D, ventral view, E, F, proximal view, H, L, plantar view, I, M, medial view, J, K, lateral view. Scale bar = 2 mm. Abbreviations: b, bulge; cpl, crista plantaris lateralis; fcc, fovea carpalis caudalis; fdl, canal for M. flexor digitorum longus tendon; fhl, canal for M. flexor hallucis longus tendon; flv, fovea lig. ventralis; fp2, canal for M. flexor perforatus digiti II; fp3, fp4, canal for Mm. flexores perforati digiti III et IV tendons; fpp2, canal for M. flexor perforans et perforatus digiti II tendon; fpp3, canal for M. flexor perforans et perforatus digiti III tendon; mfdm, depression for attachment of M. flexor digiti minoris; ms, medial shaft surface; pc, proc. cranialis; si, spatium intermetacarpale; tmf, tub. m. fibularis; tmtc, tuberositas m. tibialis cranialis.
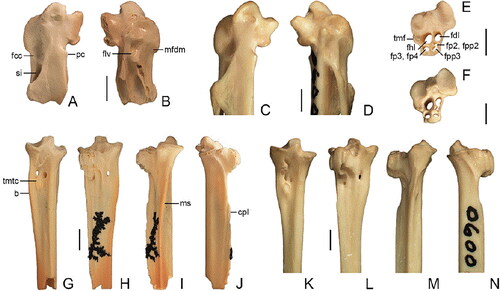
Diagnosis
The fossils are referred to Ptilonorhynchidae because they exhibit the following combination of features. Carpometacarpus: the trochlea carpalis ventralis is (a) oblong, (b) abruptly joins the os metacarpale minus, and (c) slightly protrudes caudally from the latter; the fovea lig. ventralis (sensu Livezey & Zusi Citation2006) is (d) large, deep, and (e) located distocaudally of the craniocaudal midpoint of the proc. pisiformis; (f) the fovea carpalis caudalis is large and deep; (g) in dorsal view, the proximal part of the spatium intermetacarpale located proximally of the proc. intermetacarpalis is widely open. Tarsometatarsus: (h) the plantar extent of the crista plantaris lateralis is less than that of the hypotarsus; (i) the medial shaft depth is very shallow; (j) the fossa infracotylaris dorsalis is deep; the tuberositas m. tibialis cranialis is (k) approximately centred in the sulcus extensorius, and (l) is located distally of the arcus extensorius by a distance equal to about 1–1.5 times the length of the medial foramen vasculare proximale.
The large size of the fossils excludes them from families characterized by small to very small species, such as Acanthisittidae, Maluridae, Pardalotidae and Acanthizidae. It can be challenging to distinguish passerine families because they are osteologically very similar, but the fossils can be excluded from other families characterized by medium- to large-sized species based on the following character states. Differs from Menuridae in character states a, b, d, f, g, i, j, l, and in having two distinct foramina vascularia proximalia in the tarsometatarsus (Menuridae are characterized by having two foramina within a subsidiary fossa). Differs from Cinclosomatidae in features b, e, h, i, l, and in having a proc. cranialis (sensu Manegold Citation2008) situated about proximodistally level with the fovea carpalis caudalis (cinclosomatids are characterized by a distally located proc. cranialis; Nguyen et al. Citation2018). Differs from Artamidae in features b, d, i, k, in having a relatively shorter trochlea carpalis ventralis, in lacking an incompletely ossified retinaculum extensorium tarsometatarsi, and in having a plantarly open hypotarsal canal for the Mm. flexores perforati digiti III et IV (fp3, fp4) tendons (the latter two features are synapomorphic for cracticines; Nguyen et al. Citation2013). Differs from Campephagidae in character states b, d, g, i, j, k, l. Differs from Oriolidae in features a, b, c, e, f, j, k, l. Differs from Corvidae in features b, d, e, g, i, k. Differs from Corcoracidae in features b, c, e, j, k, and in having a shallower crista plantaris lateralis. Differs from Paradisaeidae in features d, e, g, j, k, l, and in having a deeper cranial notch between the proc. extensorius and proc. alularis.
The new taxon is smaller in size than most extant species of ptilonorhynchids, but is comparable in size to the Golden Bowerbird (Prionodura newtoniana). It differs from other ptilonorhynchid genera examined in the following features. Carpometacarpus: (1) The proc. cranialis is smaller in size, and (2) situated further distally from the distal edge of the proc. alularis. (3) The trochlea carpalis dorsalis is proximodistally shorter. (4) The fossa infratrochlearis is shallower. (5) On the ventral side of the trochlea carpalis ventralis, the depression for attachment of M. flexor digiti minoris is shallower and appears near planar with the ventral surface of the trochlea carpalis. (6) The proximal margin of the depression for attachment of M. flexor digiti minoris is situated distocaudally of the proc. pisiformis, and about proximodistally level with the proximal margin of the fovea lig. ventralis. (7) The proximal margin of the depression for attachment of M. flexor digiti minoris is indistinctly circumscribed. (8) The fovea carpalis cranialis is shallower. (9) The notch situated cranially of the fovea lig. ventralis is deeper. (10) The fovea carpalis caudalis is shallower. (11) In dorsal view, the ridge that separates the fovea carpalis caudalis and the proximal opening of the spatium intermetacarpale is less pronounced. (12) The proximodistal distance between the fovea carpalis caudalis and the proximal part of the spatium intermetacarpale is shorter. Tarsometatarsus: The cotyla medialis is (13) slightly more proximad than its lateral counterpart, and (14) protrudes further dorsally. (15) The fossa infracotylaris dorsalis is deeper. The foramina vascularia proximalia are (16) proportionately larger, and (17) more widely set apart. (18) The tuberositas m. tibialis cranialis is proportionately shorter. (19) The sulcus extensorius is deeper. (20) In dorsal view, the medial edge of the shaft bulges out medially. (21) The medial portion of the shaft is shallower and forms a sharper crest. (22) In lateral view, the plantar extent of the crista plantaris lateralis is greater than that of the tub. m. fibularis.
The fossil taxon differs from species of Ailuroedus in character states 3, 4, 8, 9, 11, 13, 14, 17–21; differs from Scenopoeetes in features 1–9, 11, 15, 16, 19–22; differs from Amblyornis in features 1, 3–5, 8, 9, 15, 17; differs from Prionodura in features 3–9, 13–16, 19, 21; differs from Sericulus in features 3, 8, 10, 11, 15–19, 21; differs from Ptilonorhynchus in features 2, 3, 5–8, 13, 20; and differs from Chlamydera in features 2, 3, 12, 15, 20.
Etymology
The specific name is named in honour of the author’s mother, Mary Thị Minh Châu Nguyễn.
Holotype
QM F57971 (AR19718), proximal end of right carpometacarpus (). Measurements (mm): preserved length 8.1, proximal width >4.6, proximal length ca 3.8; length of os metacarpale alulare 2.8.
Referred material
Paratype: QM F57972 (AR19715), proximal end of left tarsometatarsus (–J). Measurements (mm): preserved length 15.7, proximal width 4.2, proximal depth (cotyla lateralis to crista lateralis hypotarsi) ca 4.1.
Type locality, unit and age
White Hunter Site, D-Site Plateau, Riversleigh World Heritage Area, in Waanyi Country, Queensland, Australia. White Hunter Site is allocated to Faunal Zone A in Riversleigh biochronology, and is interpreted to be late Oligocene in age (ca 26–23 Ma), based on mammal biocorrelation, biostratigraphy, and multivariate analyses (e.g., Archer et al. Citation1989, Travouillon et al. Citation2006, Arena et al. Citation2016). Radiometric U-Pb dating of Faunal Zone A sites, including White Hunter Site, has been attempted but has so far been unsuccessful (Woodhead et al. Citation2016).
Description
The holotype carpometacarpus QM F57971 has breakage to the distal edge of the proc. alularis and the ventral side of the ossa metacarpalia majus et minus, as well as minor abrasion to the trochlea carpalis and proc. pisifomis. The paratype tarsometatarsus QM F57972 has breakage to the arcus extensorius and abrasion to the hypotarsus, crista plantaris lateralis, and edge of the cotyla lateralis. There are some black mineral dendrites on the plantar shaft surface of the fossil tarsometatarsus. In addition to the features that characterize Ptilonorhynchidae and those used to diagnose the new taxon, Sericuloides marynguyenae exhibits the following features.
Carpometacarpus
The distal edge of the proc. alularis is broken but, based on what is preserved, it is about proximodistally level with the midpoint of the fovea lig. ventralis. In the species of Amblyornis, Sericulus, and Ptilonorhynchus studied, the distal edge of this process does not reach as far distally with respect to the midpoint of the fovea lig. ventralis. The trochlea carpalis dorsalis is rounded in proximal profile and bears a deep fossa on the dorsal surface. The distal edge of the trochlea carpalis ventralis is about level with the proximal portion of the proc. cranialis, and is slightly more angular than observed in Scenopoeetes dentirostris and Chlamydera guttata. The tip of the proc. cranialis is broken but, based on what is preserved, the process appears to be smaller and less protuberant than in most of the extant ptilonorhynchids studied, but similar in size to that observed in Ailuroedus melanotis and larger than in Prionodura newtoniana. The proximal edge of the proc. pisiformis is abraded but it appears to protrude slightly less ventrally than in the species of Scenopoeetes, Ailuroedus, Amblyornis, Sericulus, and Ptilonorhynchus studied. Cranially of the proc. pisiformis is a shallow fossa. The fovea lig. ventralis is oval-shaped, slightly narrower than in species of Scenopoeetes, Sericulus, and Ptilonorhynchus and in Ailuroedus melanotis, and much deeper than in Scenopoeetes. The fovea carpalis caudalis is wide and cranially recessed. The distal margin of the fovea carpalis caudalis is about proximodistally level with the distal portion of the proc. cranialis. The caudal edge of the proc. intermetacarpalis is slightly abraded, but it appears to be level with that of the os metacarpale minus. Viewed dorsally, the proximal edge of the proc. intermetacarpalis is gently concave and cranially bounds the fovea carpalis caudalis, as in most of the ptilonorhynchid specimens examined. This process only partially bounds the distal portion of the fovea carpalis caudalis in some specimens of Scenopoeetes, Ailuroedus melanotis, Ai. crassirostris, Amblyornis subalaris?, Chlamydera nuchalis, and C. maculata. The sulcus tendinosus is wide and deep. In dorsal view, the proximal opening of the spatium intermetacarpale proximally of the proc. intermetacarpalis is slightly smaller than in species of Amblyornis studied, but slightly larger than in Prionodura newtoniana.
Tarsometatarsus
The cotylae lateralis et medialis flare out lateromedially. In proximal view, the cotyla lateralis is about equal in dorsal extent to the eminentia intercotylaris. The eminentia intercotylaris is low, trapezoid in proximal profile, and bears a deep fossa on the dorsolateral surface. The tub. m. fibularis is large, but is not as protuberant as in Prionodura newtoniana. Although there is some minor damage to the proximoplantar section of the hypotarsus, the six-canal pattern that is characteristic of Eupasseres is preserved in the fossil. The hypotarsal canal for the M. flexor hallucis longus (fhl) tendon is larger than that for the M. flexor digitorum longus (fdl) tendon. The canal for the fp3, 4 tendons is situated plantad of the canal for the fhl tendon. In some specimens of Ailuroedus crassirostris, Sericulus chrysocephalus, and Ptilonorhynchus violaceus this canal is located plantolaterally of the canal for the fhl tendon. The hypotarsal canals for the M. flexor perforatus digiti II (fp2) and M. flexor perforans et perforatus digiti II (fpp2) tendons are dorsally closed and separated from the canal for the fdl tendon, but they merge with the canal for the M. flexor perforans et perforatus digiti III (fpp3) tendon. In the extant ptilonorhynchids studied, the canals for the fp2 and fpp2 tendons were either confluent with the canal for the fpp3 tendon (as in the fossil), merged with the canal for the fdl tendon, or were separately enclosed. The sulcus within the arcus extensorius is proximolaterally directed to the midpoint of the eminentia intercotylaris. The foramina vascularia proximalia are distally adjacent to the arcus extensorius. The tuberositas m. tibialis cranialis is very low, oval-shaped, and is centred in the sulcus extensorius. In some specimens of Ailuroedus melanotis, Scenopoeetes, Prionodura, Sericulus, and Chlamydera maculata, this tuberosity is located slightly medially. At about the proximodistal level of the tuberositas m. tibialis, the medial side of the shaft bulges out medially, more so than observed in specimens of Amblyornis, Prionodura, and Sericulus. The impressio lig. collateralis medialis is abraded. Viewed laterally, the notch between the hypotarsus and the proximal edge of the crista plantaris lateralis is long; its proximodistal length is about equal to that of the hypotarsus. The ridges that bound the sulcus flexorius are very low.
Ptilonorhynchidae genus and species indeterminate
()
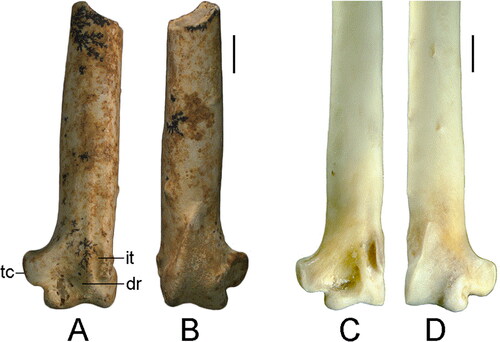
Fig. 2. An indeterminate ptilonorhynchid from the early Miocene RSO Site, Riversleigh, Australia, compared with the extant Scenopoeetes dentirostris. A, B, Indeterminate ptilonorhynchid, distal end of left ulna (QM F57970). C, D, Scenopoeetes dentirostris, right ulna (mirrored). A, C, cranial view, B, D, caudal view. Scale bar = 2 mm. Abbreviations: dr, depressio radialis; it, incisura tendinosa; tc, tub. carpale.
Referred material
QM F57970 (AR19857), distal end of left ulna (). Measurements (mm): preserved length 16.4, distal width 5.1, depth of condylus dorsalis 3.8.
Locality, unit and age
Ross Scott-Orr (RSO) Site, D-Site Plateau, Riversleigh World Heritage Area, in Waanyi Country, Queensland, Australia. This site is assigned to the youngest interval (B3) of Faunal Zone B in Riversleigh biochronology, and is interpreted to be early Miocene in age (e.g., Archer et al. Citation1989, Travouillon et al. Citation2006, Arena et al. 2016). Speleothem material associated with fossils at RSO Site have been U-Pb radiometrically dated to 16.55 ± 0.31 Ma (Woodhead et al. Citation2016).
Remarks
The fossil ulna is well preserved, with only abrasion to the depressio radialis area and the bony ridges of the incisura tendinosa. There are a few black mineral dendrites on the shaft and distal end of the ulna. The fossil is referred to Ptilonorhynchidae because it exhibits the following combination of features: the tub. carpale is (a) craniocaudally deep, (b) square in shape, and (c) projects far cranioventrally; (d) the incisura tendinosa is wide; (e) overall large size; (f) the condylus dorsalis is long relative to the length of the condylus ventralis. It can be excluded from families characterized by medium- to large-sized species based on the following features. Differs from Menuridae in features a, b, and in having less prominent papillae remiges caudales, a deeper depressio radialis, and equal distal extent of the condyles. Differs from Cinclosomatidae in feature a, f, and in having a proportionately smaller tub. carpale. Differs from Artamidae in character states a, b, and in having a shallower sulcus intercondylaris. Differs from Campephagidae in character states a, b, c, e. Differs from Oriolidae in feature a, f, and a slightly deeper sulcus intercondylaris. Differs from Corvidae in features b, c, f. Differs from Corcoracidae in features a, b, f. Differs from Paradisaeidae in features a, b, d.
The specimen represents a ptilonorhynchid that is larger in size than Sericuloides marynguyenae. The papillae remigales caudales are low tubercles. The condyli dorsalis et ventralis are approximately equal in distal extent. The condylus dorsalis is oval-shaped, and does not protrude far dorsally from the shaft. This condyle is slightly longer proximodistally than in Prionodura. The sulcus intercondylaris and depressio radialis are shallow. The tub. carpale bears a very shallow depression on its proximal edge. This tuberosity is slightly shorter proximodistally than in species of Ailuroedus, Sericulus, and Ptilonorhynchus, and slightly smaller and less angular in shape than in Scenopoeetes. It is slightly wider than in Sericulus, but narrower than in Chlamydera. The distal edge of the tub. carpale is roughly perpendicular to the long axis of the shaft, but in Chlamydera it is more acute. In Amblyornis subalaris? the distal edge of this tuberosity is more rounded than in the fossil. The fragmentary nature of the fossil ulna and its morphological similarity across species of bowerbirds studied allows its attribution to family, but precludes its confident referral to a lower taxonomic level. However, the fossil ulna indicates the presence of a bowerbird comparable in size to the extant Black-eared Catbird (Ailuroedus melanotis) in the early Miocene of Riversleigh.
Discussion
Sericuloides marynguyenae from the late Oligocene of Riversleigh represents the earliest known evidence of Ptilonorhynchidae, and predates the previous fossil record for the bowerbird family by nearly 23 million years. This fossil bowerbird shares some similarities with members of the clade comprising Sericulus, Ptilonorhynchus, and Chlamydera, such as a small proc. cranialis, a shallow fossa infratrochlearis, a deep notch located cranially of the fovea lig. ventralis on the carpometacarpus, a cotyla medialis that protrudes further dorsally, and a crista plantaris lateralis that extends plantarly beyond the tub. m. fibularis on the tarsometatarsus. However, in a passerine time-tree inferred by Oliveros et al. (Citation2019), this clade is inferred to have diverged from its sister group ca 22–8 Ma, which would place the stem branch of the avenue bower builders slightly later than the estimated age of the new taxon. This raises the possibility that these morphological similarities may be plesiomorphies. Based on the material available, the relationships of Sericuloides marynguyenae to other ptilonorhynchids remain uncertain, and will only be improved with the recovery and study of additional material. A larger indeterminate species of bowerbird that is comparable in size to the extant Black-eared Catbird (Ailuroedus melanotis) is also reported here from the early Miocene (ca 16 Ma) of Riversleigh. Together, these fossils represent the first pre-Pleistocene records for bowerbirds and considerably extend the temporal range of this family.
The Palaeogene record for passerines is mostly known from Europe. Fossils of several stem-group passerines have been described from early Eocene deposits in Europe and North America (summarized by Mayr Citation2022). Early Oligocene fossils referred to Passeriformes have been found in Europe (e.g., Bocheński et al. Citation2011, Citation2013, Citation2018), including the earliest records of suboscines (Tyranni) (Mayr & Manegold Citation2004, Citation2006a, Citation2006b, Riamon et al. Citation2020, Bocheński et al. Citation2021). The oldest known oscine (Passeri) fossils in the Northern Hemisphere come from the late Oligocene of France and Germany (Mourer-Chauviré et al. Citation1989, Manegold Citation2008). Outside of Europe, the Palaeogene record for passerines is very limited. It includes two fragmentary bones from the early Eocene Tingamarra Local Fauna in Australia, which are the only pre-Oligocene fossils attributed to Passeriformes (Boles Citation1995a, Citation1997), and fossils of the logrunner Orthonyx kaldowinyeri Boles, Citation1993 from the late Oligocene of Riversleigh (Nguyen et al. Citation2014). Sericuloides marynguyenae constitutes the third Palaeogene passerine reported from outside of Europe. The new bowerbird taxon and Orthonyx kaldowinyeri are also significant because they comprise the oldest known fossils that can be referred to extant oscine subclades and to extant passerine families.
Phylogenetic analyses of genetic and genomic data have shown that Ptilonorhynchidae and Climacteridae are reciprocally monophyletic sister groups (e.g., Prum et al. Citation2015, Selvatti et al. Citation2015, Moyle et al. Citation2016, Oliveros et al. Citation2019, Ericson et al. Citation2020). Molecular dating studies have estimated the divergence between Ptilonorhynchidae and Climacteridae to have occurred some time during the Eocene to early Oligocene. Phylogenetic analyses of mitochondrial and nuclear data by Selvatti et al. (Citation2015) placed the split between the bowerbird and treecreeper families at ca 39–28 Ma. A phylogenomic analysis of birds by Prum et al. (Citation2015) yielded a mean estimate of ca 28 Ma for the age of the Ptilonorhynchidae–Climacteridae clade. Using genomic data, a time-calibrated phylogeny of songbirds (oscine passerines) inferred by Moyle et al. (Citation2016) provided an estimate of ca 31–23 Ma for the divergence between these two families, whereas a time-calibrated phylogeny of passerines by Oliveros et al. (Citation2019) estimated this split to have occurred ca 37–26 Ma. The age of Sericuloides marynguyenae is consistent with these molecular date estimates and provides a minimum bound of late Oligocene (ca 26–23 Ma) for the divergence between Ptilonorhynchidae and Climacteridae, which is older than the earliest record of Climacteridae from the early Miocene (ca 23–16 Ma) of Riversleigh (Nguyen Citation2016). The indeterminate ptilonorhynchid ulna from the early Miocene RSO Site at Riversleigh, which has been U-Pb radiometrically dated to 16.86–16.24 Ma (Woodhead et al. Citation2016), also does not conflict with these molecular estimates.
Like many other birds, bowerbirds have specific ecological requirements and are sensitive to environmental changes. The availability of food (mostly fruit) and materials for constructing nests and bowers, as well as suitable nest and display sites, are key to their survival (Rowland Citation2008). Bowerbirds are mostly found in wet forests and rainforests, though they can show some flexibility in their habitat preferences. These birds can also inhabit rainforest edges, ecotones between rainforest and surrounding habitats, and wet sclerophyll forests and woodlands adjacent to rainforests. Species such as the Satin Bowerbird Ptilonorhynchus violaceus, Regent Bowerbird Sericulus chrysocephalus, and Spotted Bowerbird Chlamydera maculata often move into modified habitats such as suburban parks, gardens, orchards, and similar open areas if water and food sources are available (Higgins et al. Citation2006, Frith & Frith Citation2008, Rowland Citation2008). Species of Chlamydera (grey bowerbirds) have adapted to drier, more open habitats such as open sclerophyll forests and woodlands, including riparian associations, forest edges, and patches of scrub in savannah and grassland (Higgins et al. Citation2006, Beehler & Pratt Citation2016). The Fawn-breasted Bowerbird C. cerviniventris Gould, Citation1850b and Yellow-breasted Bowerbird C. lauterbachi Reichenow, Citation1897 display intermediate habitat preferences between the grey bowerbirds and other taxa; these species occur at the edges and in mosaics of rainforest-woodland and rainforest-grassland, respectively (Frith & Frith Citation2008).
Currently the Great Bowerbird Chlamydera nuchalis and the Spotted Bowerbird are the only ptilonorhynchid species found in the Riversleigh region (Atlas of Living Australia Citation2022). The early Miocene (Faunal Zone B) palaeoenvironment at Riversleigh is interpreted to have been closed wet forest based on the abundance and diversity of rainforest-characteristic mammalian taxa (Black et al. Citation2012 and references therein). However, palaeoecological studies of the mammalian fauna of late Oligocene (Faunal Zone A) Riversleigh sites infer a structurally heterogeneous palaeoenvironment with forest elements (Myers Citation2002, Bassarova Citation2005, see also Nguyen et al. Citation2014). If we assume that the ecological requirements of the fossil bowerbirds are similar to those of their present-day relatives, the presence of these birds in the late Oligocene and early Miocene of Riversleigh does not conflict with these palaeohabitat reconstructions. Chlamydera cerviniventris and C. lauterbachi, which occur in mosaic habitats, could be considered present-day analogues of the new fossil bowerbird species. A phylogenomic study by Ericson et al. (Citation2020) proposed that bower-building behaviour was not an ancestral condition, but developed in parallel in two groups: the maypole bower builders (Amblyornis, Archboldia, Prionodura), and the avenue bower builders (Chlamydera, Ptilonorhynchus, Sericulus). The authors suggested that the evolution of bower-building and male display behaviour was facilitated by stable environmental conditions comprising tropical and subtropical forests with abundant food supply and low predator pressure, which is not too different from the palaeoenvironmental interpretation of Oligo-Miocene Riversleigh.
Sericuloides marynguyenae is the ninth species of passerine to be described and named from Riversleigh and from the pre-Pleistocene of Australia overall. It is also only the second passerine to be identified from the Oligocene of Australia (Worthy & Nguyen Citation2020). Fossils representing Climacteridae, the sister group of Ptilonorhynchidae, have been discovered from the early Miocene deposits at Riversleigh and have been attributed to the extant genera Cormobates and Climacteris (Nguyen Citation2016). Other oldest representatives of the ‘basal oscine’ radiation have also been found at Riversleigh, including a lyrebird Menura tyawanoides Boles, Citation1995b and a bristlebird Dasyornis walterbolesi Nguyen, Citation2019 from early Miocene deposits, and a logrunner Orthonyx kaldowinyeri, which was found at sites that span the late Oligocene to early late Miocene (Boles Citation1993, Nguyen et al. Citation2014). The earliest known fossils of honeyeaters derive from middle Miocene sites at Riversleigh (Boles Citation2005) and early Pleistocene representatives of Maluridae, Acanthizidae, and Pomatostomidae have been identified from this fossil heritage area (Nguyen et al. Citation2016). Given that representatives of the closest present-day relatives of Ptilonorhynchidae have been found in the Oligo-Miocene deposits of Riversleigh, it is not surprising to find members of the bowerbird lineage that date from this time period. The new bowerbird fossils described in this study add to the diversity of songbirds known from the pre-Pleistocene of Australia, and highlight the global importance of the Riversleigh deposits in our understanding of the early evolutionary history of this avian radiation.
Acknowledgements
For access to comparative specimens, I thank the following staff and institutions: Leah Tsang (AM), Maya Penck (SAMA), Leo Joseph (ANWC), Peter Hosner (NHMD), Karen Roberts (NMV), and Heather Janetzki (QM). Thanks to Mike Archer and Sue Hand (UNSW) for access to fossil specimens, Anna Gillespie, Troy Myers, and Karen Black (UNSW) for fossil preparation, and Karen Black for assistance with images. The Riversleigh project is supported by an Australian Research Council Discovery Project grant to M. Archer and S. Hand (DP170101420), UNSW Sydney, Queensland Museum, Environment Australia, Queensland Parks and Wildlife Service, Outback at Isa, Mount Isa City Council, Riversleigh Society Inc., Phil Creaser and the CREATE Fund, the Rackham family, K. and M. Pettit, A. and D. Jeanes, the Waanyi people of northwestern Queensland, and the field assistance of staff, students, and volunteers. I am grateful to Walter Boles and Sue Hand for helpful comments that improved an earlier draft of this manuscript. Thanks also to the editors Benjamin Kear and Robin Beck and anonymous reviewers for their constructive feedback.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Archer, M., Godthelp, H., Hand, S.J. & Megirian, D., 1989. Fossil mammals of Riversleigh, Northwestern Queensland: preliminary overview of biostratigraphy, correlation and environmental change. Australian Zoologist 25, 29–66.
- Arena, D.A., Travouillon, K.J., Beck, R.M.D., Black, K.H., Gillespie, A.K., Myers, T.J., Archer, M. & Hand, S.J., 2016. Mammalian lineages and the biostratigraphy and biochronology of Cenozoic faunas from the Riversleigh World Heritage Area, Australia. Lethaia 49, 43–60.
- Atlas of Living Australia 2022. Available at http://www.ala.org.au (accessed 31 October 2022).
- Baird, R.F., 1991. Avian fossils from the Quaternary of Australia. In Vertebrate Palaeontology of Australasia, Vickers-Rich, P., Monaghan, J.M., Baird, R.F. & Rich, T.H., eds., Pioneer Design Studio in Cooperation with Monash University Publications Committee, Melbourne, 809–870.
- Baird, R.F., 1992. Fossil avian assemblage of pitfall origin form Holocene sediments in Amphitheatre Cave (G-2), south-western Victoria, Australia. Records of the Australian Museum 44, 21–44.
- Baird, R.F., 1993. Pleistocene avian fossils from Pyramids Cave (M-89), eastern Victoria, Australia. Alcheringa 17, 383–404.
- Barker, F.K., Cibois, A., Schikler, P., Feinstein, J. & Cracraft, J., 2004. Phylogeny and diversification of the largest avian radiation. Proceedings of the National Academy of Sciences of the United States of America 101, 11040–11045.
- Bassarova, M., 2005. Taphonomy and Palaeoecology of Oligo-Miocene Riversleigh Fossil Sites. PhD Thesis, University of New South Wales, Sydney, Australia, 233 pp. (unpublished)
- Baumel, J.J. & Witmer, L.M., 1993. Osteologia. In Handbook of Avian Anatomy: Nomina Anatomica Avium (second edition). Baumel, J.J., King, A.S., Breazile, J.E., Evans, H.E. & Vanden Berge, J.C., eds., Publications of the Nuttall Ornithological Club 23, 45–132., Nuttall Ornithological Club, Cambridge.
- Beehler, B.M. & Pratt, T.K., 2016. Birds of New Guinea: Distribution, Taxonomy, and Systematics. Princeton University Press, Princeton, 672 pp.
- Black, K.H., Archer, M., Hand, S.J. & Godthelp, H., 2012. The rise of Australian marsupials: a synopsis of biostratigraphic, phylogenetic, palaeoecologic and palaeobiogeographic understanding. In Earth and Life: Global Biodiversity, Extinction Intervals and Biogeographic Perturbations through Time. Talent, J.A., ed., Springer, London, 983–1078.
- Bocheński, Z.M., Tomek, T., Bujoczek, M. & Salwa, G., 2021. A new passeriform (Aves: Passeriformes) from the early Oligocene of Poland sheds light on the beginnings of Suboscines. Journal of Ornithology 162, 593–604.
- Bocheński, Z.M., Tomek, T., Wertz, K. & Swidnicka, E., 2013. The third nearly complete passerine bird from the early Oligocene of Europe. Journal of Ornithology 154, 923–931.
- Bocheński, Z.M., Tomek, T., Bujoczek, M. & Wertz, K., 2011. A new passerine bird from the early Oligocene of Poland. Journal of Ornithology 152, 1045–1053.
- Bocheński, Z.M., Tomek, T., Wertz, K., Happ, J., Bujoczek, M. & Swidnicka, E., 2018. Articulated avian remains from the early Oligocene of Poland adds to our understanding of passerine evolution. Palaeontologia Electronica 21.2, 1–12.
- Boles, W.E., 1993. A logrunner Orthonyx (Passeriformes: Orthonychidae) from the Miocene of Riversleigh, north-western Queensland. Emu 93, 44–49.
- Boles, W.E., 1995a. The world’s oldest songbird. Nature 374, 21–22.
- Boles, W.E., 1995b. A preliminary analysis of the Passeriformes from Riversleigh, Northwestern Queensland, Australia, with the description of a new species of lyrebird. Courier Forschungsinstitut Senckenberg 181, 163–170.
- Boles, W.E., 1997. Fossil songbirds (Passeriformes) from the early Eocene of Australia. Emu 97, 43–50.
- Boles, W.E., 2005. Fossil honeyeaters (Meliphagidae) from the late Tertiary of Riversleigh, North-western Queensland. Emu 105, 21–26.
- Cabanis, J. & Heine, F., 1851. Museum Heineanum. Verzeichniss der ornithologischen Sammlung des Oberamtmann Ferdinand Heine, auf Gut St. Burchard vor Halberstadt. Volume 1. R. Frantz, Halberstadt, 233. pp.
- Cracraft, J., 2014. Avian higher level relationships and classification: Passeriformes. In The Howard and Moore Complete Checklist of the Birds of the World (Fourth Edition). Volume 2: Passerines, Dickinson, E.C. & Christidis, L., eds, Aves Press, Eastbourne, xvii–xlvi.
- De Vis, C.W., 1883. Description of two new birds of Queensland. Proceedings of the Linnean Society of New South Wales 7, 561–563.
- De Vis, C.W., 1890. Appendix G. Report on birds from British New Guinea. Annual Report on British New Guinea from 4th September, 1888, to 30th June, 1889, 57–62.
- Elliot, D.G., 1872. Descriptions of two genera of Paradiseidae, with remarks on some of the species. IBIS 2, 111–114.
- Ericson, P.G.P., Irestedt, M., Nylander, J.A., Christidis, L., Joseph, L. & Qu, Y., 2020. Parallel evolution of bower-building behavior in two groups of bowerbirds suggested by phylogenomics. Systematic Biology 69, 820–829.
- Frith, C.B. & Frith, D.W., 2008. Bowerbirds: Nature, Art, and History. Frith & Frith, Malanda, 304 pp.
- Gill, F., Donsker, D. & Rasmussen, P. (eds). 2022. International Ornithological Committee World Bird List. Version 12.2. https://www.worldbirdnames.org/
- Gmelin, J.F., 1789. Caroli a Linné. Systema naturae per regna tria naturae: secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I, pars II. Georg. Emanuel. Beer, Lipsiae, 501–1032.
- Gould, J., 1837a. The Birds of Australia. Volume 1. J. Gould, London, 278 pp.
- Gould, J., 1837b. A Synopsis of the Birds of Australia, and Adjacent Islands. J. Gould, London, 8 pp.
- Gould, J., 1850a. On new species of Mammalia and birds from Australia. Proceedings of the Zoological Society of London 1849, 109–112.
- Gould, J., 1850b. On new species of birds from Australia. Proceedings of the Zoological Society of London 1850, 200–201.
- Gould, J., 1862. On a new species of Chlamydera, or Bower-Bird. Proceedings of the Zoological Society of London 1862, 161–162.
- Gray, G.R., 1841. A List of the Genera of Birds, with Their Synonyms and an Indication of the Typical Species of Each Genus. R. & J. Taylor, London, xii + 115. pp.
- Gray, G.R., 1858. A list of the birds, with descriptions of new species, obtained by Mr Alfred R. Wallace in the Aru and Ké Islands. Proceedings of the Zoological Society of London 1858, 169–198.
- Higgins, P.J., Peter, J.M. & Cowling, S.J. (eds.) 2006. Handbook of Australian, New Zealand and Antarctic Birds. Volume 7: Boatbill to Starlings. Oxford University Press, Melbourne, 1055 pp.
- Jardine, W. & Selby, P.J., 1830. Illustrations of Ornithology. Volume 2. Daniel Lizars, Edinburgh, 114 pp.
- Kuhl, H., 1820. Beiträge zur Zoologie und vergleichenden Anatomie. Abth. 1. Verlag Der Hermannschen Buchhandlung, Frankfurt-am-Main, 151 pp.
- Latham, J., 1801. Supplementum Indicis Ornithologici, sive Systematis Ornithologiae. G. Leigh, J. & S. Sotheby, London, 74 pp.
- Lewin, J., 1808. Birds of New Holland with their Natural History. White & Bagster, London, 22 pp.
- Linnaeus, C., 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Volume 1. Tenth edition, revised. Laurentii Salvii, Holmiae, 533 pp.
- Livezey, B.C. & Zusi, R.L., 2006. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy: I. – Methods and characters. Bulletin of the Carnegie Museum of Natural History 37, 1–544.
- Manegold, A., 2008. Passerine diversity in the late Oligocene of Germany: earliest evidence for the sympatric coexistence of Suboscines and Oscines. IBIS 150, 377–387.
- Mayr, G., 2022. Paleogene Fossil Birds, Second Edition. Springer Nature Switzerland AG, Cham, 239 pp.
- Mayr, G. & Manegold, A., 2004. The oldest European fossil songbird from the early Oligocene of Germany. Die Naturwissenschaften 91, 173–177.
- Mayr, G. & Manegold, A., 2006a. New specimens of the earliest European passeriform bird. Acta Palaeontologica Polonica 51, 315–323.
- Mayr, G. & Manegold, A., 2006b. A small suboscine-like passeriform bird from the Early Oligocene of France. The Condor 108, 717–720.
- Mourer-Chauviré, C., Hugueney, M. & Jonet, P., 1989. Découverte de Passeriformes dans l’Oligocène supérieur de France. Comptes Rendus de l’Académie des Sciences de Paris, Série II 309, 843–849.
- Moyle, R.G., Oliveros, C.H., Andersen, M.J., Hosner, P.A., Benz, B.W., Manthey, J.D., Travers, S.L., Brown, R.M. & Faircloth, B.C., 2016. Tectonic collision and uplift of Wallacea triggered the global songbird radiation. Nature Communications 7, 12709.
- Myers, T.J.M., 2002. Palaeoecology of Oligo-Miocene Local Faunas from Riversleigh. PhD Thesis, University of New South Wales, Sydney, Australia, 243 pp. (unpublished)
- Nguyen, J.M.T., 2016. Australo-Papuan treecreepers (Passeriformes: Climacteridae) and a new species of sittella (Neosittidae: Daphoenositta) from the Miocene of Australia. Palaeontologia Electronica 19, 1A.
- Nguyen, J.M.T., 2019. A new species of bristlebird (Passeriformes, Dasyornithidae) from the early Miocene of Australia. Journal of Vertebrate Palaeontology 39, e1575838.
- Nguyen, J.M.T., Archer, M. & Hand, S.J., 2018. Quail-thrush birds from the Miocene of northern Australia. Acta Palaeontologica Polonica 63, 493–502.
- Nguyen, J.M.T., Boles, W.E., Worthy, T.H., Hand, S.J. & Archer, M., 2014. New specimens of the logrunner Orthonyx kaldowinyeri (Passeriformes: Orthonychidae) from the Oligo-Miocene of Australia. Alcheringa 38, 245–255.
- Nguyen, J.M.T., Hand, S.J. & Archer, M., 2016. The late Cenozoic passerine avifauna from Rackham’s Roost Site, Riversleigh, Australia. Records of the Australian Museum 68, 201–230.
- Nguyen, J.M.T., Worthy, T.H., Boles, W.E., Hand, S.J. & Archer, M., 2013. A new cracticid (Passeriformes: Cracticidae) from the early Miocene of Australia. Emu 113, 374–382.
- Oliveros, C.H., Field, D.J., Ksepka, D.T., Barker, F.K., Aleixo, A., Andersen, M.J., Alström, P., Benz, B.W., Braun, E.L., Braun, M.J. & Bravo, G.A., 2019. Earth history and the passerine superradiation. Proceedings of the National Academy of Sciences of the United States of America 116, 7916–7925.
- Paykull, G., 1815. Lanius crassirostris, avis antea ignotae. Nova Acta Regiae Societatis Scientiarum Upsaliensis 7, 282–285.
- Prum, R.O., Berv, J.S., Dornburg, A., Field, D.J., Townsend, J.P., Lemmon, E.M. & Lemmon, A.R., 2015. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526, 569–573.
- Ramsay, E.P., 1876. List of birds met with in north-eastern Queensland, chiefly at Rockingham Bay. Proceedings of the Linnean Society of London 1875, 578–603.
- Rand, A.L., 1940. New birds from the 1938-1939 expedition. American Museum Novitates 1072, 1–14.
- Reichenow, A., 1897. Neue Vogelarten von Kaiser-Wilhelms-Land. Ornithologische Monatsberichte 5, 24–26.
- Riamon, S., Tourment, N. & Louchart, A., 2020. The earliest Tyrannida (Aves, Passeriformes), from the Oligocene of France. Scientific Reports 10, 9776.
- Rowland, P., 2008. Bowerbirds. CSIRO Publishing, Collingwood, 136 pp.
- Schodde, R. & Mason, I.J., 1999. The Directory of Australian Birds: Passerines. CSIRO Publishing, Collingwood, 851 pp.
- Selvatti, A.P., Gonzaga, L.P. & de Moraes Russo, C.A., 2015. A Paleogene origin for crown passerines and the diversification of the Oscines in the New World. Molecular Phylogenetics and Evolution 88, 1–15.
- Sharpe, R.B., 1884. Contributions to the Ornithology of New Guinea. Part IX. On further collections made by Mr. A. Goldie in the Astrolabe Mountains. Journal of the Linnean Society of London, Zoology 17, 405–408.
- Swainson, W., 1825. On the characters and natural affinities of several new birds from Australasia; including some observations on the Columbidae. Zoological Journal London 1, 463–484.
- Travouillon, K.J., Archer, M., Hand, S.J. & Godthelp, H., 2006. Multivariate analyses of Cenozoic mammalian faunas from Riversleigh, north-western Queensland. Alcheringa 30, 323–349.
- Vanden Berge, J.C. & Zweers, G.A., 1993. Myologia. In Handbook of Avian Anatomy: Nomina Anatomica Avium (Second edition). Baumel, J.J., King, A.S., Breazile, J.E., Evans, H.E. & Vanden Berge, J.C., eds., Publications of the Nuttall Ornithological Club 23. Nuttall Ornithological Club, Cambridge, 189–247.
- Vieillot, L.P., 1816. Nouveau Dictionnaire d'Histoire Naturelle, appliquée aux arts, à l'Agriculture, à l'Écomomie rurale et domestique, à la Médecine, etc. Par une société de naturalistes et d'agriculteurs. Volume 6. Chez Deterville, Paris, 585 pp.
- Vigors, N.A. & Horsfield, T., 1827. A description of the Australian birds in the collection of the Linnean Society; with an attempt at arranging them according to their natural affinities. Transactions of the Linnean Society of London 15, 170–331.
- Woodhead, J., Hand, S.J., Archer, M., Graham, I., Sniderman, K., Arena, D.A., Black, K.H., Godthelp, H., Creaser, P. & Price, E., 2016. Developing a radiometrically-dated chronologic sequence for Neogene biotic change in Australia, from the Riversleigh World Heritage Area of Queensland. Gondwana Research 29, 153–167.
- Worthy, T.H. & Nguyen, J.M.T., 2020. An annotated checklist of the fossil birds of Australia. Transactions of the Royal Society of South Australia 144, 66–108.
