Abstract
Two pairs of equidimensional muscle-attachment scars are described on the dorso-lateral surfaces of internal moulds of the helcionelloid molluscs Hensoniconus gen. nov. and Vendrascospira from the Henson Gletscher Formation (Cambrian, Miaolingian Series, Wuliuan Stage) of North Greenland (Laurentia). Two patterns of muscle scars are recognized in helcionelloids. In the Hensoniconus–Vendrascospira group, two pairs of sub-equally sized scars are located on the dorso-lateral surfaces. In the Bemella–Figurina group, thin, band-like scars are distributed on the sub-apical surface and along the dorsal (supra-apical) surface. Comparison of these rare described occurrences of muscle scars in bilaterally symmetrical molluscs indicates that the simple shell form may obscure recognition of distinct lineages within Cambrian univalves that are based on anatomical features, such as musculature.
John S. Peel [[email protected]], Department of Earth Sciences (Palaeobiology), Uppsala University, Villavägen 16, SE-75236, Uppsala, Sweden.
DEVELOPMENT of a limpet-like univalved shell is a recurrent theme in gastropod evolution, with Vermeij (Citation2016) noting more than 50 independent lineages, while similar simple shell forms also occur within untorted molluscs of the classes Helcionelloida and Tergomya. This simplicity frustrates hypotheses concerning the origin of the gastropods, since the early history of patellogastropods, considered the most primitive of gastropod stocks, and of basal univalve mollusc groups, is largely unchartered in the Palaeozoic (Ponder et al. Citation2020). Limpet-like shells are widely distributed and may be locally common in the Palaeozoic, but establishing their relationships on the basis of the conical or slightly coiled shells is often problematic on account of their lack of diagnostic morphological features. However, the pattern of muscle attachment scars on the shell interior, usually preserved as replicates on internal moulds, has proven to be a valuable tool in assessing the relationships of univalves. Most successfully, a solid lineage has been established between present tryblidiid tergomyans such as Neopilina Lemche, Citation1957 (Wingstrand Citation1985, Lindberg Citation2009) and their Cambrian ancestors, including Proplina Kobayashi, Citation1933, with the distinctive series of paired muscles along the supra-apical (dorsal) surface (Stinchcomb & Angeli Citation2002). Tryblidium Lindström, 1880 and Pilina Koken & Perner, Citation1925, Silurian representatives of this lineage with excellently preserved muscle attachment scars, were described already by Lindström (1880, Citation1884), but a variety of Palaeozoic taxa has been documented by Koken & Perner (Citation1925), Horný (Citation1963, Citation2002), Peel (Citation1977), Stinchcomb (Citation1986), Wahlman (Citation1992), Yochelson & Webers (Citation2006) and others.
The dominant group of limpet-like univalves in the early and middle Cambrian was referred to the Class Helcionelloida by Peel (Citation1991a, Citation1991b), which is approximately equivalent in terms of generic composition to the Order Helcionelliformes (within the gastropod Subclass Archaeobranchia) of Parkhaev (Citation2019). Most helcionelloids are coiled through a fraction of a whorl, but forms with conical shells and one or two complete whorls are known. Numerous helcionelloid genera have been described, especially from Australia, China and Russia (Missarzhevsky Citation1989, Parkhaev & Demidenko Citation2010, Jacquet & Brock Citation2016, Parkhaev Citation2019 for references). Historically, most Laurentian material has been referred to classic taxa such as Scenella Billings, Citation1872 () and Helcionella Grabau & Shimer, Citation1909, although Landing (Citation1988), Geyer (Citation1994), Landing & Bartowski (Citation1996), Skovsted (Citation2004, 2006a, Citation2006b), Skovsted & Peel (Citation2007), Atkins & Peel (Citation2008), Peel et al. (Citation2016), Wotte & Sundberg (Citation2017) and Devaere et al. (Citation2019) have introduced later nomenclature.
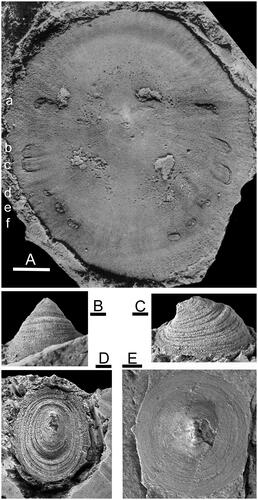
Figure 1. A, Protoconchoides? rasettii Geyer, Citation1994, USNM 123374, holotype, dorsal view of internal mould with paired muscle scars, collected by Franco Rasetti from 57 m feet above the base of the Mt. Whyte Formation, about 320 m North-east of the upper end of Ross Lake, Yoho National Park, British Columbia. Cambrian, Miaolingian Series (after Rasetti Citation1954, reproduced with permission from the Journal of Paleontology). B–D, Scenella reticulata Billings, Citation1872, USNM 18232, Cambrian Series 2, Conception Bay, Newfoundland. E, Protoconchoides amii (Matthew, Citation1902), GSC 137151, Burgess Shale, Phyllopod bed, British Columbia. Cambrian, Miaolingian Series, Drumian Stage. Scale bar: 1 mm (B–D), 2 mm (A, E).
Records of muscle scars in helcionelloids or morphologically similar shells from the lower and middle Cambrian are rare (Rasetti Citation1954, Parkhaev Citation2002, Citation2014, Vendrasco et al. Citation2010, Li et al. Citation2021; ) and have not yet contributed significantly to elucidating the relationship between these basal molluscs and subsequent crown groups. Indeed, Missarzhevsky (Citation1989) proposed the informal name Eomonoplacophora for forms without known muscle scars and distributed its genera between seven families that are now mainly grouped within Helcionelloidea. Thus, helcionelloids have been interpreted variously as gastropods, endogastric untorted molluscs and exogastric untorted molluscs, as reviewed by Runnegar (Citation1981), Peel (Citation1991a,Citationb), Parkhaev (Citation2008, Citation2017, Citation2019), Geyer et al. (Citation2019) and Li et al. (Citation2021). Interpretations of Scenella as a chondrophorine (Stanley Citation1982, Citation1986, Yochelson & Gil Cid Citation1984, Babcock & Robison Citation1988) are now discounted.
This paper describes muscle attachment scars on internal moulds of two helcionelloid taxa from the early-middle Cambrian (Miaolingian Series, Wuliuan Stage) of North Greenland, referred to Vendrascospira Peel & Kouchinsky, Citation2022 and a new genus Hensoniconus, with type species Scenella? siku Peel & Kouchinsky, Citation2022. The muscle scar pattern in these Greenland specimens is distinct from those recorded by Parkhaev (Citation2002, Citation2014), Vendrasco et al. (Citation2010) and Li et al. (Citation2021) in helcionelloids from the lower and middle Cambrian. The scar pattern is also unlike the distribution of muscle scars in the familiar (but systematically problematic) Scenella sp. undet. of Rasetti (Citation1954; ) from the Mt. Whyte Formation (Wuliuan Stage) of British Columbia. The latter material was redescribed as Protoconchoides? rasettii sp. nov. by Geyer (Citation1994) and is reviewed herein on account of discussions concerning the significance of its musculature in studies of the early evolution of univalve molluscs (Rasetti Citation1954, Runnegar & Pojeta Citation1985, Peel Citation1991b, Geyer Citation1994).The substantial differences between the discussed muscle scar patterns, within just a few of the more than 50 genera of Cambrian univalve molluscs, strongly suggest that helcionelloids do not represent a single, uniform lineage.
Materials and methods
Samples from North Greenland were collected from the Henson Gletscher Formation in the southern Lauge Koch Land–western Peary Land region. Peel & Kouchinsky (Citation2022) gave full details of their geological derivation and collection localities as part of the description of assemblages of molluscs and mollusc-like fossils. The Henson Gletscher Formation in this region is a highly fossiliferous unit within a prograding complex of shelf carbonates and siliciclastic sediments, referred to the Brønlund Fjord Group (Higgins et al. Citation1991, Ineson & Peel Citation1997, Geyer & Peel Citation2011, Peel et al. Citation2016). It is composed mainly of dark, recessive, bituminous and cherty limestones, dolostones and mudstones, with a middle member of pale fine-grained sandstones.
Fossil assemblages from the Henson Gletscher Formation in the southern Lauge Koch Land region range in age from Cambrian Series 2 (Stage 4) to the Miaolingian Series (Wuliuan Stage; Ptychagnostus gibbus Biozone). However, younger strata (Miaolingian, Drumian Stage) occur along the northern coast of North Greenland, to the west (Higgins et al. Citation1991, Robison Citation1994, Blaker & Peel Citation1997, Ineson & Peel Citation1997, Geyer & Peel Citation2011). Trilobites from the Henson Gletscher Formation are mainly Laurentian in character, but the assemblages also include universal agnostoids and taxa that are important for correlation with Siberia and South China (Blaker & Peel Citation1997, Geyer & Peel Citation2011, Sundberg et al. Citation2022). Other elements of the diverse fauna were described by Clausen & Peel (Citation2012), Peel et al. (Citation2016), Peel (Citation2017, Citation2019, Citation2021, Citation2022) and Peel & Kouchinsky (Citation2022).
GGU sample 271492 was collected by J.S. Peel on 25 June 1978 at 56.5 m above the base of the Henson Gletscher Formation at its type locality (thickness 62 m) in southern Lauge Koch Land (82°10′N, 40°24′W; Ineson & Peel Citation1997, Geyer & Peel Citation2011, Peel Citation2021, Citation2022, Peel & Kouchinsky Citation2022, ). GGU sample 271718 was collected on 15 July 1978 from about 1 m below the top of the formation (thickness 47 m) on the west side of Løndal, western Peary Land, North Greenland, some 40 km to the east of the type section (82°18′N, 37°03′W; Clausen & Peel Citation2012, Peel & Kouchinsky Citation2022, ).
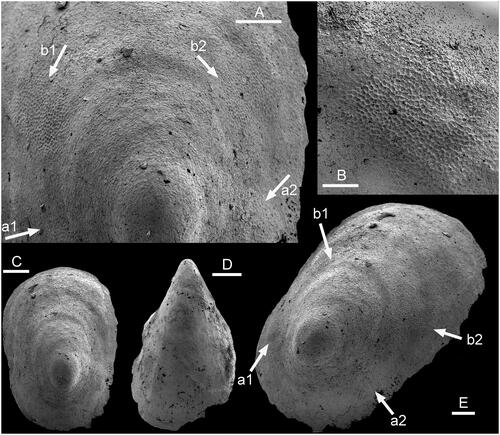
Figure 2. Muscle-attachment scars in Hensoniconus siku (Peel & Kouchinsky, Citation2022), A–C, E, PMU 39206, internal mould from GGU sample 271718, Henson Gletscher Formation (Miaolingian Series, Wuliuan Stage), Løndal, North Greenland. A, Detail of apical region of supra-apical surface showing two pairs of muscle scars (arrows a, b). B, Detail of muscle scar b2. C, Dorsal view. E, Dorso-lateral view with muscle scars (arrows a1, a2, b1, b2). D, PMU 39207, holotype, internal mould, oblique dorsal view from supra-apical margin. Scale bars: 50 µm (B), 100 µm (A, C–E).
The described Greenland specimens are phosphatized internal moulds derived from limestone samples dissolved in 10% acetic acid prior to imaging of selected specimens by scanning electron microscopy. Images were assembled in Adobe Photoshop CS4.
Institutional abbreviations
GGU, Grønlands Geologiske Undersøgelse (Geological Survey of Greenland), now the Geological Survey of Denmark and Greenland, Copenhagen, Denmark. GSC, Geological Survey of Canada, Ottawa. PMU, Palaeontological Collection, The Museum of Evolution, Uppsala University, Sweden. USNM, Natural History Museum, Smithsonian Institution, Washington D.C., USA.
Systematic palaeontology
Phylum MOLLUSCA Cuvier, Citation1797
Class HELCIONELLOIDA Peel Citation1991a
Hensoniconus gen. nov.
Diagnosis
Isostrophic, limpet-like, elongate with apex lying close to the sub-apical margin. Two pairs of sub-equal muscle-attachment scars are located symmetrically about the median dorsal plane, on the lateral areas of the internal mould.
Etymology
For Matthew Henson (1866–1955), companion of American explorer Robert Peary (1856–1920) on numerous Arctic expeditions. Matthew Henson was the first man to reach the site subsequently erroneously identified by Peary in 1909 as the North Pole.
Type species
Scenella? siku Peel & Kouchinsky, Citation2022, Cambrian (Miaolingian Series, Wuliuan Stage), Henson Gletscher Formation, Løndal, western Peary Land, North Greenland.
Remarks
A full description of the type and only known species, Hensoniconus siku (Peel & Kouchinsky, Citation2022), is given below. Parkhaev (Citation2001, Citation2002, Citation2014) referred morphologically similar, relatively smooth shells to Bemella communis Parkhaev, Citation2001 from Cambrian Series 2 in South Australia, although the holotype and other specimens of the latter species are strongly corrugated. The type species of Bemella, Bemella jacutica Missarzhevsky, Citation1969 from the Tommotian of Siberia, as figured by Missarzhevsky (Citation1969), is corrugated and more tightly coiled, although internal moulds attributed to the same species by Rozanov et al. (Citation2010, pl. 27, ) are laterally compressed and smooth. Parkhaev & Demidenko (Citation2010, pl. 59) illustrated a number of smooth internal moulds from the earliest Cambrian Meishucunian Stage of China, which they referred to Bemella simplex Yu, Citation1979, although they are not similar to the type species, Bemella jacutica. Some of these illustrated specimens are similar in shape to Hensoniconus, although others are taller and with a bluntly rounded apex, more closely resembling Vendrascospira. Bemella simplex is significantly older than the currently described Miaolingian material of Hensoniconus and Vendrascospira from North Greenland; its musculature is not known.
Geyer et al. (Citation2019) questioned the morphological distinction between Bemella and Helcionella, the type species of which was illustrated by Knight (Citation1941), but in the present context Bemella communis and Hensoniconus siku are clearly separated by the patterns of their muscle attachment scars, as discussed below. Li et al. (Citation2021) described smooth internal moulds assigned to Bemella communis from the Xinji Formation (Cambrian Series 2) of North China but the illustrated specimens are more oval in plan view than Hensoniconus siku, without the overhanging apex.
The question of the relationship between Scenella and Protoconchoides Shaw, Citation1962 was discussed in detail by Geyer (Citation1994). In the type species, Protoconchoides hermetensis (Resser, Citation1945), and Protoconchoides douli Geyer, Citation1994 from the Miaolingian of Idaho, the apex of the oval, conical, shell is located almost centrally, in contrast to its marginal position in the slightly coiled shell of Hensoniconus siku. This is also the case in Protoconchoides? rasettii Geyer, Citation1994, from the Mt. Whyte Formation of British Columbia (), originally described as Scenella sp. by Rasetti (Citation1954).
Hensoniconus siku (Peel & Kouchinsky, Citation2022)
()
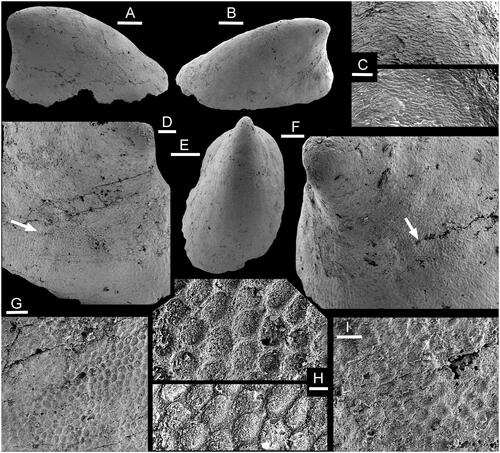
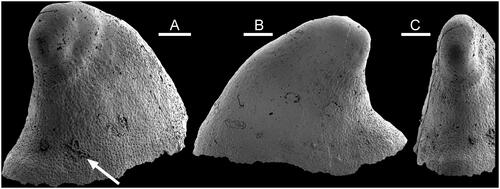
Figure 3. Muscle-attachment scars in Hensoniconus siku (Peel & Kouchinsky, Citation2022), internal mould, PMU 39207, holotype, from GGU sample 271718, Henson Gletscher Formation (Miaolingian Series, Wuliuan Stage), Løndal, North Greenland. A, Lateral view. B, Oblique lateral view. C, Detail of imbricate shell structure from apex of E, with the surface of the internal mould digitally inverted in the lower image to show the relief on the shell interior. Shell apex upwards in image. D, Oblique lateral view of sub-apical surface (in B) with muscle scar (arrow, detail in G, H). E, Oblique dorsal view. F, Oblique lateral view of sub-apical surface (in A) with muscle scar (arrow, detail in I). G, H, Details of muscle scar (arrow in D). The upper image in H is digitally inverted in the lower image to showing the relief of the muscle scar on the interior surface of the shell. I, detail of muscle scar (arrow in F). Scale bars: 5 µm (H), 10 µm (I), 20 µm (C, G), 50 µm (D, F) 200 µm (A, B, E).
2022, Scenella? siku Peel & Kouchinsky, p. 86, fig 10G, K, M–O.
Holotype
PMU 39207, internal mould from GGU sample 271718, Henson Gletscher Formation, Løndal, western Peary Land, North Greenland, Cambrian (Miaolingian Series, Wuliuan Stage).
Referred material
PMU 39205 and PMU 39206, internal moulds from GGU sample 271718.
Description
Isostrophic, low, limpet-like, in which the width of the elongate shell is about two-thirds of its length; lateral margins sub-parallel in dorsal perspective (). Apex lying close to the sub-apical margin, slightly overhanging the steeply inclined sub-apical wall (). Supra-apical surface shallowly convex in lateral profile, with the point of greatest height above the apertural plane lying at about one-fifth of the distance from the sub-apical margin to the supra-apical margin. Surface of internal mould may retain a few, shallow, widely spaced comarginal depressions. Two pairs of muscle-attachment scars are located symmetrically about the median dorsal plane, on the lateral areas of the internal mould (). External ornamentation not known.
Remarks
Hensoniconus siku (Peel & Kouchinsky, Citation2022), the only described species of the genus, was originally tentatively assigned to Scenella by Peel & Kouchinsky (Citation2022); about ten specimens are available. Described specimens of Scenella, including the type species (Knight Citation1941, pl. 2, ; ), differ from Hensoniconus siku in having the apex placed more centrally and an oval to sub-circular dorsal plan. Comarginal corrugation in Scenella reticulata Billings, Citation1872 is frequent but only weakly developed. Broad, widely spaced comarginal depressions on the internal mould of Hensoniconus siku seem to represent shell thickening at occasional growth halts. Ornamentation of the shell exterior is not known from Hensoniconus siku, whereas Scenella is ornamented with a fine reticulation of growth lines and radial ribs ().
Protoconchoides amii (Matthew, Citation1902), abundant in the Burgess Shale of British Columbia, differs from Hensoniconus siku in the sub-circular dorsal plan and more central location of the apex on the cap-shaped shell (Conway Morris & Peel Citation2013; ). Specimens illustrated by Babcock & Robison (Citation1988) indicate slight steepening of the sub-apical surface relative to the longer, very shallowly convex supra-apical surface and finely reticulate ornamentation. The sub-apical surface is also shorter than the supra-apical surface in Protoconchoides? rasettii (), which is also readily distinguished from Hensoniconus siku by its six pairs of muscle scars.
Muscle scars in Protoconchoides? rasettii
Protoconchoides? rasettii Geyer, Citation1994 was established for Scenella sp. undet. of Rasetti (Citation1954) from the Mt. Whyte Formation, Cambrian (Miaolingian Series) of British Columbia. As noted by Horný (Citation2009, p. 26), Protoconchioides of Geyer (Citation1994) appears to be an inadvertent mispelling of Protoconchoides Shaw, Citation1962 that unfortunately was perpetuated by Peel & Kouchinsky (Citation2022).
Rasetti (Citation1954) assigned to Scenella sp. undet. specimens that display six pairs of muscle scars along the supra-apical surface (). The shell is oval, almost circular in plan view, with the raised, elongate apex slightly closer to the sub-apical margin. Muscle scars are located comarginally on the lateral areas close to the apertural margin (). Rasetti (Citation1954) referred Scenella to Class Tergomya (as the isopleuran Order Monoplacophora of the then current usage by Knight Citation1952) where a series of paired muscle scars occurs along the dorsum (supra-apical surface) in the present day Neopilina and the familiar Ordovician–Silurian molluscs Tryblidium and Pilina, an opinion supported by Runnegar & Pojeta (Citation1985). Peel (Citation1991b) preferred placement of Scenella within Class Helcionelloidea, on the basis of the morphology of typical specimens (), but expressed concern about the relationship of the Mt. Whyte specimens () with muscle scars.
Yochelson & Webers (Citation2006) appeared to dismiss Scenella sp. undet. of Rasetti (Citation1954) as a mollusc, suggesting comparisons with the lobopod Microdictyon Bengtson, Matthews & Missarzhevsky, Citation1986 (as Microdiction).
Geyer (Citation1994) redescribed Protoconchoides Shaw, Citation1962, the type species of which was derived from the Gateway Formation (Miaolingian Series) of the Grand Canyon region (Resser Citation1945, Shaw Citation1962). He noted that the almost conical shell with a central apex contrasted with the curved shell of Scenella. The locally abundant Metoptoma amii Matthew, Citation1902 from the Burgess Shale, generally referred to Scenella, can be referred to Protoconchoides (). Geyer (Citation1994) transferred Scenella sp. undet. of Rasetti (Citation1954) from the Mt. Whyte material to Protoconchoides? rasetti sp. nov., although he noted that the preserved musculature was distinct from the comarginal circular depression on the internal mould interpreted as the muscle scar of the genus. However, a similar depression corresponding to a thickening of the shell interior is visible in Rasetti’s (Citation1954) specimen (USNM 123374; ), suggesting that the comarginal depression is not directly associated with musculature; it may reflect comarginal thickening associated with a pause in growth. Three pairs of scars (, a–c) are much larger than the other three (, d–f). The first pair (a) is relatively widely spaced and located at the transition of the sub-apical surface to the supra-apical surface.
Rasetti (Citation1954) compared the muscle scar pattern of Protoconchoides? rasetti (as Scenella sp. undet.) to that of the tryblidiid Tergomya, but the muscles in the latter typically form a pattern distributed on the median dorsal (supra-apical) surface behind the anterior apex (Lindström Citation1884, Peel Citation1977, Stinchcomb Citation1986). If Protoconchoides? rasetti is interpreted as a tryblidiid, the anteriormost pair of scars (a in ) differs in lying slightly anterior and downslope of the apex. However, Peel (Citation1991b) and others have pointed out that underlying muscle scar patterns may be readily modified by changes in shell form and function. Thus, the pattern in Protoconchoides? rasetti could reflect migration of a typically tryblidiid muscle scar circlet towards the apertural margin associated with a sessile limpet-like habit. Muscles forming the anterior pair of muscle scars in fossil tryblidiids, such as Pilina and Tryblidium, may be complex and enlarged, but the pattern of three pairs of large and three pairs of small muscle scars seen in Protoconchoides? rasetti is distinctive.
Dzik (Citation2010) commented that the almost centrally located shell apex and the arrangement of muscles of Rasetti’s (Citation1954) specimens are more similar to the kirengellids than to tryblidiid tergomyans, the former group considered to include the late Cambrian (Furongian Series) Kiringella Rosov, 1968 and some species assigned to Hypseloconus Berkey, Citation1898. Dzik (Citation2010) separated this kirengellid group, which is generally equivalent to the Order Hypseloconida Peel, Citation1991b, from typical tryblidiids, which he did not recognize before the Late Ordovician. However, late Cambrian specimens from Missouri with well-preserved tryblidiid muscle scars were assigned to Proplina by Stinchcomb (Citation1986) and to Pilina liaoningensis Yu & Yochelson, Citation1999 from the late Cambrian of Liaoning, China, by Yu & Yochelson (Citation1999).
Dzik (Citation2010) developed an evolutionary model based on similarities in muscle scar patterns in which hypseloconids (kirengellids) might be interpreted as brachiopods rather than molluscs. This part of the model is rejected, as regards the placement of hypseloconids, but agreement is expressed with Dzik’s (Citation2010) suggestion that the muscle scar pattern of Protoconchoides? rasettii (as Scenella sp. undet. of Rasetti Citation1954) is more closely similar to hypseloconids (kirengellids) than to tryblidiids. There is little to support its assignment to Helcionelloida and the species is referred to Class Tergomya, Order Hypseloconida, as suggested by Geyer (Citation1994).
Muscle scars in Hensoniconus siku
In proposing Scenella? siku on the basis of internal moulds from GGU sample 271718 from Løndal, western Peary Land, Peel & Kouchinsky (Citation2022) noted that the assignment to Scenella was only tentative and referred to discussion of Scenella by Geyer (Citation1994). The Løndal material is designated herein as type species of a new genus, Hensoniconus, which differs from most described species of Scenella, including the type species illustrated by Knight (Citation1941; ), in the marginal position of the apex on the elongate conch. Additionally, Hensoniconus is defined by the presence of two pairs of muscle attachment scars located on its lateral surfaces (). Unfortunately, muscle scar patterns are not yet known in typical Scenella.
The two pairs of muscle attachment scars are located on the lateral areas of the internal mould, where they are disposed symmetrically about the median dorsal plane (). Their location, the raised polygonal pattern and the size of individual cells are consistent with Vendrascospira frykmani (). However, the coarser botryoidal texture (referring to the pattern of convex surfaces) preserved on the internal mould of the apex of Vendrascospira frykmani () is not seen in Hensoniconus siku, although a similar microstructure of finely imbricate lamellae is present in the apical area of both (–L).
Figure 4. Muscle-attachment scars in Vendrascospira. Internal moulds from GGU sample 271492, Henson Gletscher Formation (Miaolingian Series, Wuliuan Stage), southern Lauge Koch Land, North Greenland. A, B, E, Vendrascospira cf. frykmani Peel & Kouchinsky, Citation2022, PMU (24744). A, Detail of left muscle scar, as illustrated in B. B, Dorsal view locating pair of lateral muscle scars (arrows), enlarged in E. C, D, F–K, Vendrascospira frykmani Peel & Kouchinsky, Citation2022, PMU 39208. C, Lateral view with muscle scars a1 and b1 (arrows). D, Dorsal view with three preserved muscle scars (arrows a, b), a fourth scar (a2, upper right) is obscure. F, Supra-apical surface showing pair of muscle scars (b1, b2, arrows), with detail in I. G, Oblique lateral view with two muscle scars, enlarged in C, on left side as illustrated in F, I. H, Muscle scars (a1, b1) as illustrated in C, F, I. I, Muscle scars on apical region of supra-apical surface. J, Detail of muscle scar a1 as illustrated in G. K, finely imbricate shell structure on internal mould, detail of I. L, Digital replica of K showing the stepwise imbrication on the shell interior, opposite in relief to that on the internal mould. Scale bars: 20 µm (A, J–L), 50 µm (H, I), 100 µm (C, E), 200 µm (B, D, F, G).
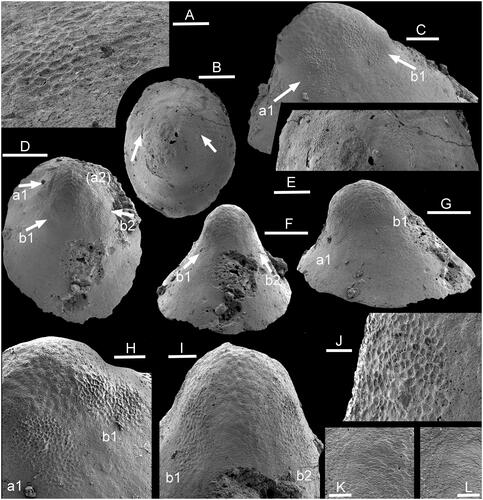
On the internal mould, each scar consists of a polygonal pattern of well-defined ridges forming the margin of concave depressions that are about 5 µm in diameter (–I). The ridges thus correspond to grooves in the internal surface of the shell (). The scars pass into the surrounding surface of the internal mould without sharp delimitation of their margins. They are equidimensional, but slightly irregular in form owing to their gradual passage into the surrounding shell surface; the margins of scars a1 and a2 (arrowed in ) are less clearly delimited than those in scars b1 and b2. Individual scars are isolated and lack connection with adjacent scars, either laterally or across the sub-apical and supra-apical surfaces. The flanks of the internal mould, adapertural of the muscle scars, display a weak, comarginal, wrinkled pattern on the supra-apical surface (), but this becomes radial on the sub-apical surface near the aperture ().
Muscle scars in Vendrascospira frykmani
Vendrascospira frykmani was established by Peel & Kouchinsky (Citation2022) on the basis of a single internal mould from GGU sample 271492 (PMU 39208; –L) from southern Lauge Koch Land, North Greenland. However, an additional specimen tentatively assigned to the species, but unfortunately destroyed after scanning, is illustrated here (). Vendrascospira frykmani is distinguished from the co-occurring Vendrascospira troelseni, the type species of Vendrascospira (), by its less laterally compressed form and oval aperture in plan view (Peel & Kouchinsky Citation2022).
Figure 5. Vendrascospira troelseni Peel & Kouchinsky, Citation2022, PMU 39211 from GGU sample 271492, holotype, internal mould. A, Oblique lateral view showing smooth early growth stage, wrinkled intermediate stage and finely pitted adapertural area; arrow indicates cast of a euendolith. B, Lateral view, with a crescentic patch with wrinkled texture below the apex. C, Sub-apical surface. Scale bars: 100 µm.
Two pairs of sub-equal muscle scars are preserved on the holotype internal mould of Vendrascospira frykmani near the bluntly rounded apex () just adapical of the point where the shell increases its rate of expansion (). The scars are placed symmetrically on each side of the median plane of symmetry, and each scar is irregularly oval in shape, with its long axis lying along a radial line. As in Hensoniconus siku, each scar consists of a polygonal pattern of well-defined ridges surrounding concave depressions that are about 5 µm in diameter (). The scars pass quickly into the surrounding surface of the internal mould, but their margins are not clearly defined. There is no indication that scars are connected laterally or across the sub-apical and supra-apical surfaces.
The flanks of the internal mould are smooth, but the apical area is covered by a reversed polygonal pattern of grooves around shallowly convex elevations in a botryoidal texture that is morphologically opposite in relief to that of the muscle attachment scars (). Relief of this surface is more subdued than that of the muscle scars, and individual cells are two or three times larger in diameter (). The muscle attachment surfaces overlie this botryoidal pattern on the internal mould and therefore are impressed through it on the shell interior. With passage from the apex down the supra-apical surface, the botryoidal pattern develops a weak imbricated structure, with individual elements overlain by succeeding elements as the margin is approached (). In detail, this pattern reflects the finely imbricate pattern of growth lamellae moulded from the shell interior onto the internal mould (). It is also observed in Hensoniconus siku ().
Muscle scars forming pair a are well preserved in Vendracospira cf. frykmani () but those of pair b are obscure.
Discussion
Preservation and growth
The height of the muscle scars of Hensoniconus siku and Vendrascospira frykmani above the apertural plane, in lateral perspective, is not certainly known, since the internal moulds do not preserve any indication of the apertural margin of the original shell. This is also the case with most other helcionelloid specimens from GGU samples 271492 and 271718 described by Peel & Kouchinsky (Citation2022), suggesting that phosphatization was restricted to the earlier parts of the shell. In contrast, specimens of the laterally compressed helcionelloid Mellopegma schizoceras Vendrasco, Kouchinsky, Porter & Fernandez, Citation2011a from GGU sample 271492 often preserve the apertural margin (Peel & Kouchinsky Citation2022) but lack recognized muscle scars.
Due to the logarithmic expansion of the coiled shell, early growth stages of isometric shells are commonly more tightly coiled than later stages (Thompson Citation1942, Raup Citation1961, Peel Citation1974, Geyer et al. Citation2019). Thus, the supra-apical surface of the coiled shell in lateral view becomes progressively more shallowly convex with growth (), a feature also affected by both the initial rate of expansion of the shell cone and by allometry during growth. Thus, morphological comparison of specimens with broken apertures, or incomplete internal moulds, must take particular account of ontogenetic variation, not least that between juvenile and more mature growth stages (Jacquet & Brock Citation2016, Jackson & Claybourn Citation2018). Of particular relevance is awareness of the potential morphological and taxonomic significance of the size difference between micromorphic specimens recovered by acid digestion of carbonates (Small Shelly Fossils) and macromorphic individuals (Jacquet & Brock Citation2016, Geyer et al. Citation2019). Specimens of Hensoniconus siku and Vendrascospira () are a size order smaller than illustrated ‘crack-out’ specimens ().
While the described muscle scars in Vendrascospira frykmani and Hensoniconus siku are clearly visible, it should not be assumed that all muscle attachment areas have been preserved in the small available sample. Personal examination of large samples of Carboniferous isometric bellerophontoidean gastropods has demonstrated substantial variation in the preservation of muscle scars on internal moulds, both within the same specimen and in specimens from the same sample.
Surface textures in Cambrian molluscs
Vendrasco et al. (Citation2010, Citation2011a, Citation2011b, Citation2016) noted the wide distribution of polygonal and other textures on phosphatic internal moulds of Cambrian molluscs, following pioneering studies by Runnegar (Citation1985), and presented a series of arguments that the polygonal structures were imprints of prismatic shell microstructure. This countered the earlier interpretation by Ushatinskaya and Parkhaev (Citation2005) that such structures in brachiopods and Cambrian molluscs represented imprints of cells of the outer mantle epithelium. Winrow & Sutton (Citation2012) summarized extensive earlier literature in describing polygonal mosaics in brachiopods that were formed as moulds of epithelial cells, rather than shell microstructure, in describing acrotretoid brachiopods from the Cambrian of southern Great Britain. As such, they questioned some of the arguments advanced by Vendrasco et al. (Citation2010), at least in brachiopods. Vendrasco et al. (Citation2010, p. 125) noted that polygon size in their Cambrian mollusc material varied from 10 to 50 µm, noting that ‘mantle epithelial cells … are typically much smaller.’ In most of the Cambrian brachiopod material described by Winrow & Sutton (Citation2012), epithelial cells varied between about 10 µm and 16 µm in diameter, although they reported instances of cell diameters of 30 µm (Ushatinskaya & Parkhaev Citation2005 and others).
Studies by Li et al. (Citation2019, Citation2023) indicated that polygonal textures in Cambrian molluscan internal moulds probably have multiple origins. Li et al. (Citation2019) confirmed the existence of polygonal cellular imprints on the adductor muscle attachment zone of recent bivalve shells. These muscle attachment zones often displayed distinct boundaries with respect to the rest of the shell surface, making them easily distinguishable. Li et al. (Citation2023) provided evidence that polygonal texture is formed by the prismatic organic matrix rather than as imprints of mineral prisms. They demonstrated that the preserved organism matrix can form both concave and convex polygonal textures on internal moulds.
The polygonal pattern in Vendrascospira frykmani and Hensoniconus siku is comparable in dimensions and form to the polygonal structure in the muscle scars of extant craniid brachiopods described by Williams & Wright (Citation1970) and Parkhaev (Citation2014). Dong et al. (Citation2022) noted that prismatic prisms of aragonite forming the myostracal layer are widespread in modern molluscan classes, and this origin is readily applied to the Henson Gletscher Formation material interpreted as muscle scars. However, the internal moulds of these Greenland specimens also preserve other textures not associated with the muscle attachment areas. The holotype internal mould of Vendrascospira troelseni preserves three distinct surface textures (). The apical region is mainly smooth, although a weak, dispersed tuberculation is present at the apex, and some wrinkling is observed on the sub-apical surface at the comarginal ruga that may represent the apertural margin of the juvenile (). The adapertural margins of the specimen show a pattern of sharply defined circular pits, similar to those illustrated by Vendrasco et al. (Citation2010, Citation2011a) in Mellopegma georginensis Runnegar & Jell, Citation1976 from the Miaolingian Series of Australia. Intermediate areas of the lateral flanks show a wrinkled structure with a radial orientation that passes gradually into the adapertural, pitted area.
The apical region of Vendrascospira frykmani displays a polygonal texture (‘botryoidal pattern’) that is opposite in relief to that of the muscle scars and with individual cells that are two or three times larger (). Similar structures were illustrated by Li et al. (Citation2019) in specimens of Anabarella australis Runnegar in Bengtson et al., Citation1990 from the Xinji Formation of China. The botryoidal pattern fades abapically, not extending beyond the muscle scars, and is more subdued in relief. The polygons of the muscle scars appear to overlie this botryoidal texture on the internal mould and were therefore more deeply embedded into the inner surface of the shell. In detail, the botryoidal textured surface is seen not to be prismatic but composed of finely imbricated lamellae () that are stacked such that each inclined lamella on the supra-apical surface is overlain by a succession of lamellae sloping away from the apex, towards the apertural margin (). This inclination is opposite to that seen on the apical area of Hensoniconus siku on the shell interior, where individual lamellae slope towards the apex. The lower part of each image of Hensoniconus siku () has been digitally inverted to illustrate the structure on the interior of the shell compared with that on the internal mould. Vendrasco et al. (Citation2010, Citation2011a, Citation2011b) and Li et al. (Citation2023) have discussed similar lamellar structures in a number of Cambrian univalve and bivalve molluscs. While the relationship between this imbricate lamellar shell microstructure and the botryoidal texture of the inner surface in Vendrascospira frykmani is unresolved, their close juxtaposition suggests that the botryoidal texture is an epithelial texture imposed on the surface of the underlying lamellar shell.
Comparisons
In contrast to the two pairs of muscle scars preserved in Vendrascospira frykmani and Hensoniconus siku, the muscle attachment area in Anhuiconus microtuberus Zhou & Xiao, Citation1984, as illustrated by Parkhaev (Citation2002) from Cambrian Series 2 in Australia, consists of a single scar on the umbilico-lateral (sub-apical) surface of a shell that is tightly coiled through about one whorl, with a strongly overhanging apex (). The scar passes continuously from one lateral surface to the other, under the apex. Additional scars have not been recognized on the dorsal area.
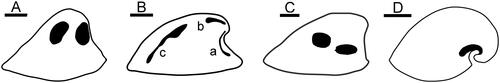
Figure 6. A, Vendrascospira frykmani Peel & Kouchinsky, Citation2022 in lateral view showing muscle-attachment scars on internal mould. B, Bemella communis Parkhaev, Citation2001 showing muscle scars on internal mould (after Parkhaev Citation2014); muscle scars on sub-apical surface (a) and supra-apical surface (b, c). Li et al. (Citation2021) considered b and c to represent the same muscle scar. C, Hensoniconus siku (Peel & Kouchinsky, Citation2022) showing muscle-attachment scars on internal mould. D, Anhuiconus microtuberus Zhou & Xiao, Citation1984 showing muscle scar below apex (after Parkhaev Citation2002). Scale bars: 100 µm (D), 200 µm (A–C).
In their position on the umbilical (sub-apical) shoulders, close to the axis of coiling, the lateral terminations of the muscle scar in Anhuiconus microtuberus are reminiscent of the muscle-attachment area of some patelliform gastropods (Ponder et al. Citation2020). Similar scars have also been described in Palaeozoic bellerophontoidean gastropods and some pseudo-isometric pleurotomarioideans, and may extend from the dorso-lateral areas towards the median line of the umbilical (sub-apical) surface (Peel Citation1972, Citation1982, Citation1986, Citation1993, Citation2001, Citation2004). The similarity likely reflects the shell form of the respective molluscs, since Anhuiconus microtuberus is more strongly coiled than most helcionelloids. A muscle scar passing beneath the apex is also known in the Palaeozoic patelliform gastropods Archinacella Ulrich & Scofield, Citation1897 and Archinacellina Horný, Citation1961, but the terminations of the scar ascend to the dorsal surface before extending almost to the supra-apical margin, in contrast to Anhuiconus microtuberus (Peel & Horný, Citation1999; Peel in press).
Smooth internal moulds of species of Bemella communis Parkhaev, Citation2001 from Cambrian Series 2 in Australia described by Parkhaev (Citation2002, Citation2014) display three pairs of muscle scars preserved symmetrically as narrow radial bands located beneath the apex (), dorsally near the apex () and dorsally near the supra-apical margin (). Specimens of Bemella incomparabilis Parkhaev, Citation2001 described by Parkhaev (Citation2002, Citation2014) also from Cambrian Series 2 in Australia show a pair of small ribbon-like scars on the dorsum near the apex, but scars are not described from other parts of the strongly corrugated shell. The ribbon-like scars contrast with the two pairs of almost equidimensional scars in Hensoniconus siku and Vendrascospira frykmani.
Li et al. (Citation2021) described muscle scars on internal moulds from the Xinji Formation (Cambrian Series 2, Stages 3–4) of North China referred to Figurina figurina Parkhaev, Citation2001, originally described from Cambrian Series 2 in South Australia (Parkhaev Citation2001). Two pairs of scars were described, similar in form and position to those in Bemella communis, although Parkhaev (Citation2002, Citation2014) had considered that the pair of elongate dorsal scars was broken into two pairs in the latter (, scars a and b). As with Bemella communis, the narrow band-like scars are readily distinguished from the more equidimensional scars of Hensoniconus siku and Vendrascospira frykmani.
Vendrasco et al. (Citation2010) described a pair of small, elongate muscle scars located on the supra-apical surface adjacent to the apex in the tall, slender, helcionelloid Yochelcionella snorkorum Vendrasco, Porter, Kouchinsky, Li & Fernandez, Citation2010 from the Gowers Formation (Cambrian, Miaolingian Series) in Australia. The scars occur on the early growth stage of Yochelcionella Runnegar & Pojeta, Citation1974 prior to the development of the prominent comarginal rugae and were interpreted as the imprint of prismatic myostracum (Vendrasco et al. Citation2010). As with Anhuiconus, it is not known if additional muscle scars were present elsewhere on the shell. In terms of their shape and location, the pair of elongate muscle scars in Yochelcionella snorkorum is reminiscent of the pair of muscle scars located on the supra-apical surface, near the apex, in Bemella (Parkhaev Citation2014) and Figurina (Li et al. Citation2021). Muscle scars in Vendrascospira frykmani and Hensoniconus siku differ in their greater relative size and equidimensional shape.
Conclusions
Only a few examples of muscle scars, reviewed herein, are known in helcionelloids, despite their role as the most diverse and common molluscs in the early–middle Cambrian. However, two patterns of muscle scars can be recognized at this time, both of which are readily delimited from that seen in the hypseloconid Protoconchoides? rasettii. In the Bemella–Figurina group, the thin, band-like scars are distributed on the sub-apical surface and along the dorsal (supra-apical) surface. In the Hensoniconus–Vendrascospira group, two pairs of more equally dimensioned muscle scars are located on the dorso-lateral areas. Variation in shell morphology within helcionelloids likely promoted the development of other muscle scar patterns, such as the pair of adapical scars on the supra-apical surface in Yochelcionella snorkorum and the sub-apical scar of Anhuiconus microtuberus, in response to their mode of life. However, the simple limpet-like morphology of most helcionelloids may obscure the recognition of several lineages based on internal anatomy, such as muscle patterns.
Muscle scar patterns in the Bemella–Figurina and Hensoniconus–Vendrascospira groups offer little information as to their relationship with molluscan crown groups. In lateral perspective (), the muscle scar pattern in Hensoniconus and Vendrascospira shows a superficial similarity to the location of adductor muscle scars in bivalves such as the Cambrian Pojetaia Jell, 1980, but this is clearly spurious when their function is considered.
Acknowledgements
Samples were collected during the North Greenland Project (1978–1980) of GGU. I am grateful for thoughtful reviews from Sarah Jacquet and an anonymous reviewer. Luoyang Li kindly provided access to literature.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data archiving statement
This published work and the nomenclatural acts it contains have been registered in ZooBank: http://zoobank.org/urn:lsid:zoobank.org:pub:00A2A60F-4940-4C14-9250-0FC5B6F0A50E.
References
- Atkins, C.J. & Peel, J.S., 2008. Yochelcionella (Mollusca, Helcionelloida) from the lower Cambrian of North America. Bulletin of Geosciences 83, 23–38.
- Babcock, L.E. & Robison, R.A., 1988. Taxonomy and paleobiology of some Middle Cambrian Scenella (Cnidaria) and hyolithids (Mollusca) from western North America. University of Kansas Paleontological Contributions, Paper 121, 1–22.
- Bengtson, S., Conway Morris, S., Cooper, B.J., Jell, P.A. & Runnegar, B.N., 1990. Early Cambrian fossils from South Australia. Association of Australasian Palaeontologists Memoir 9, 1–364.
- Bengtson, S., Matthews, S.C. & Missarzhevsky, V.V., 1986. The Cambrian netlike fossil Microdictyon. In Problematic Fossil Taxa. hoffman, a. & nitecki, M., eds, Oxford Monographs on Geology and Geophysics 5, Oxford University Press, New York, 97–115.
- Berkey, C.P., 1898. Geology of the St. Croix Dalles, III: Paleontology. The American Geologist. 21, 270–294.
- Billings, E., 1872. On some fossils from the Primordial rocks of Newfoundland. The Canadian Naturalist 6, 465–479.
- Blaker, M.R. & Peel, J.S., 1997. Lower Cambrian trilobites from North Greenland. Meddelelser om Grønland, Geoscience 35, 1–145.
- Clausen, S. & Peel, J.S., 2012. Middle Cambrian echinoderm remains from the Henson Gletcher Formation of North Greenland. GFF 134, 173–200.
- Conway Morris, S. & Peel, J.S., 2013. A new helcionelloid mollusk from the Middle Cambrian Burgess Shale, Canada. Journal of Paleontology 87, 1067–1070.
- Cuvier, G., 1797. Tableau Élementaire de l’historie Naturelle des Animaux. Baudouin, Paris, 710 p.
- Devaere, L., Clausen, S., Sosa-Leon, J.P., Palafox-Reyes, J.J., Buitrón-Sánchez, B.E. & Vachard, D., 2019. Early Cambrian small shelly fossils from northwest Mexico: Biostratigraphic implications for Laurentia. Palaeontologia Electronica 22, 1–60.
- Dong, W., Huang, J., Liu, C., Wang, H., Zhang, G., Xie, L. & Zhang, R., 2022. Characterization of the myostracum layers in molluscs reveals a conservative shell structure. Frontiers in Marine Science 9, 862929.
- Dzik, J., 2010. Brachiopod identity of the alleged monoplacophoran ancestors of cephalopods. Malacologia 52, 97–113.
- Geyer, G., 1994. Middle Cambrian mollusks from Idaho and early conchiferan evolution. New York State Museum Bulletin 481, 69–86.
- Geyer, G. & Peel, J.S., 2011. The Henson Gletscher Formation, North Greenland, and its bearing on the global Cambrian Series 2–Series 3 boundary. Bulletin of Geosciences 86, 465–534.
- Geyer, G., Valent, M. & Meier, S., 2019. Helcionelloids, stenothecoids and hyoliths from the Tannenknock Formation (traditional lower middle Stage 4/Wuliuan boundary interval) of the Franconian Forest, Germany. PalZ 93, 207–253.
- Grabau, A.W. & Shimer, H.W., 1909. North American Index Fossils, Invertebrates, Volume 1. A.G. Seiler & Company, New York, 853 p.
- Higgins, A.K., Ineson, J.R., Peel, J.S., Surlyk, F. & Sønderholm, M., 1991. Lower Palaeozoic Franklinian Basin of North Greenland. Bulletin Grønlands Geologiske Undersøgelse 160, 71–139.
- Horný, R.J., 1961. New genera of Bohemian Monoplacophora and patellid Gastropoda. Věstnik Ustředního ústavu geologického 36, 299–302.
- Horný, R.J., 1963. Lower Paleozoic Monoplacophora and patellid Gastropoda (Mollusca) of Bohemia. Sborník Ustředního ústavu geologického, oddíl paleontologický 28, 7–83.
- Horný, R.J., 2002. Ordovician Tergomya and isostrophic Gastropoda (Mollusca) of Bohemia: Types and referred specimens in the collections of the National Museum, Prague, Czech Republic. Acta Musei Nationalis Pragae, Series B, Historia Naturalis 57, 69–102.
- Horný, R.J., 2009. Patelliconus HORNÝ, 1961 and Mytoconula gen. n. (Mollusca, Tergomya) from the Ordovician of Perunica. Acta Musei Nationalis Pragae, Series B, Historia Naturalis 65, 25–36.
- Ineson, J.R. & Peel, J.S., 1997. Cambrian shelf stratigraphy of North Greenland. Geology of Greenland Survey Bulletin 173, 1–120.
- Jackson, I.S.C. & Claybourn, T.M., 2018. Morphometric analysis of inter- and intraspecific variation in the Cambrian helcionelloid mollusc Mackinnonia. Palaeontology 61, 761–773.
- Jacquet, S.M. & Brock, G.A., 2016. Lower Cambrian helcionelloid macromolluscs from South Australia. Gondwana Research 36, 333–358.
- Knight, J.B., 1941. Paleozoic gastropod genotypes. Geological Society of America Special Paper 32, 510 p.
- Knight, J.B., 1952. Primitive fossil gastropods and their bearing on gastropod classification. Smithsonian Miscellaneous Collections 117, 56 p.
- Kobayashi, T., 1933. Faunal study of the Wanwanian (basal Ordovician) series with special notes on the Ribeiridae and the Ellesmereoceroids. Journal of the Faculty of Science Imperial University of Tokyo Section II Geology, Mineralogy, Geography, Seismology 3, 249–328.
- Koken, E. & Perner, J., 1925. Die Gastropoden des baltischen Untersilurs. Mémoires de l’Académie des Sciences de Russie, Série. 8. Classe Physico-mathématique 37, 1–326.
- Landing, E., 1988. Lower Cambrian stratigraphy of Eastern Massachusetts: Stratigraphy and small shelly fossils. Journal of Paleontology 62, 661–695.
- Landing, E. & Bartowski, K.E., 1996. Oldest shelly fossils from the Taconic allochthon and late early Cambrian sea-levels in eastern Laurentia. Journal of Paleontology 70, 741–761.
- Lemche, H., 1957. A new living deep-sea mollusk of the Cambro-Devonian class Monoplacophora. Nature 179, 413–416.
- Li, L., Betts, M.J., Yun, H., Pan, B., Topper, T.P., Li, G., Zhang, X. & Skovsted, C.B., 2023. Fibrous or prismatic? A comparison of the lamello-fibrillar nacre in early Cambrian and modern lophotrochozoans. Biology 12, 113.
- Lindberg, D.R., 2009. Monoplacophorans and the origin and relationships of mollusks. Evolution: Education and Outreach 2, 191–203.
- Lindstrom, G., 1880. Fragmenta Silurica e dono Caroli Henrici Wegelin. Samson & Wallin, Stockholm, 60 p.
- Lindström, G., 1884. On the Silurian Gastropoda and Pteropoda of Gotland. Kongliga svenska Vetenskaps-Akademiens Handlingar 19, 250 p.
- Li, L., Topper, T.P., Betts, M.J., Dorjnamjaa, D., Altanshagai, G., Enkhbaatar, B., Li, G. & Skovsted, C.B., 2023. Calcitic shells in the aragonite sea of the earliest Cambrian. Geology 51, 8–12.
- Li, L., Zhang, X., Skovsted, C.B., Yun, H., Li, G. & Pan, B., 2019. Shell microstructures of the helcionelloid mollusc Anabarella australis from the lower Cambrian (Series 2) Xinji Formation of North China. Journal of Systematic Palaeontology 17, 1699–1709.
- Li, L., Zhang, X., Skovsted, C.B., Yun, H., Pan, B. & Li, G., 2021. Revisiting the molluscan fauna from the Cambrian (Series 2, stages 3–4) Xinji Formation of North China. Papers in Palaeontology 7, 521–564.
- Matthew, G.F., 1902. Notes on Cambrian Faunas: Cambrian Brachiopoda and Mollusca of Mt. Stephen, B.C. with the description of a new species of Metoptoma. Transactions of the Royal Society of Canada 4, 107–112.
- Missarzhevsky, V.V., 1969. Descriptions of hyoliths, gastropods, hyolithelminths, camenids, and forms of an obscure systematic position. The Tommotian Stage and the problem of the lower boundary of the Cambrian. Trudy Ordena Trudovogo Krasnogo Znameni Geologicheskij Institut, Akademiya Nauk SSSR 206, 105–175. (in Russian)
- Missarzhevsky, V.V., 1989. Oldest skeletal fossils and stratigraphy of Precambrian and Cambrian boundary beds. Trudy Geologicheskogo Instituta, Akademiya Nauk SSSR 443, 237 p. (in Russian)
- Parkhaev, P.Y., 2001. Molluscs and siphonoconchs. In The Cambrian Biostratigraphy of the Sainsbury Basin, South Australia. Alexander, E.M., Jago, J.B., Rozanov, A. Yu. & Zhuravlev, A.Yu., eds. Russian Academy of Sciences, Transactions of the Palaeontological Institute, Moscow, vol. 282, 133–210.
- Parkhaev, P.Y., 2002. Muscle scars of the Cambrian univalved mollusks and their significance for systematics. Paleontological Journal 36, 453–459.
- Parkhaev, P.Y., 2008. The early Cambrian radiation of Mollusca. In Phylogeny and Evolution of the Mollusca. Ponder, W.F. & Lindberg, D.R., eds., University of California Press, Berkeley, 33–69.
- Parkhaev, P.Y., 2014. Structure of shell muscles in the Cambrian gastropod genus Bemella (Gastropoda: Archaeobranchia: Helcionellidae). Paleontological Journal 48, 17–25.
- Parkhaev, P.Y., 2017. Origin and the early evolution of the Phylum Mollusca. Paleontological Journal 51, 663–686.
- Parkhaev, P.Y., 2019. Cambrian molluscs of Australia: overview of taxonomy, biostratigraphy and paleobiogeography. Stratigraphy and Geological Correlation 27, 181–206.
- Parkhaev, P.Y. & Demidenko, Y.E., 2010. Zooproblematica and Mollusca from the Lower Cambrian Meishucun Section (Yunnan, China) and taxonomy and systematics of the Cambrian Small Shelly Fossils of China. Paleontological Journal 44, 883–1161.
- Peel, J.S., 1972. Observations on some Lower Palaeozoic tremanotiform Bellerophontacea (Gastropoda) from North America. Palaeontology 15, 412–422.
- Peel, J.S., 1974. Systematics, ontogeny and functional morphology of Silurian trilobed bellerophontacean gastropods. Bulletin of the Geological Society of Denmark 23, 231–264.
- Peel, J.S., 1977. Relationship and internal structure of a new Pilina (Monoplacophora) from the late Ordovician of Oklahoma. Journal of Paleontology 51, 116–122.
- Peel, J.S., 1982. Muscle scars in Bellerophon recticostatus (Mollusca) from the Carboniferous of Ireland. Journal of Paleontology 56, 1307–1310.
- Peel, J.S., 1986. Muscle scars in Porcellia (Gastropoda; Pleurotomariacea) from the Carboniferous of England. Bulletin of the Geological Society of Denmark 35, 53–58.
- Peel, J.S., 1991a. Functional morphology of the Class Helcionelloida nov., and the early evolution of the Mollusca. In The Early Evolution of Metazoa and the Significance of Problematic Taxa. Simonetta, A. & Conway Morris, S., eds, Cambridge University Press, Cambridge, 157–177.
- Peel, J.S., 1991b. The Classes Tergomya and Helcionelloidea, and early molluscan evolution. Bulletin Grønlands Geologiske Undersøgelse 161, 1–116.
- Peel, J.S., 1993. Muscle scars and mode of life of Carinaropsis (Bellerophontoidea, Gastropoda) from the Ordovician of Tennessee. Journal of Paleontology 67, 528–534.
- Peel, J.S., 2001. Musculature and asymmetry in a Carboniferous pseudo-bellerophontoidean gastropod (Mollusca). Palaeontology 44, 157–166.
- Peel, J.S., 2004. Asymmetry and musculature in some Carboniferous bellerophontiform gastropods (Mollusca). GFF 126, 213–220.
- Peel, J.S., 2017. First records from Laurentia of some middle Cambrian (Series 3) sponge spicules. Alcheringa 41, 306–314.
- Peel, J.S., 2019. Sponge spicule assemblages from the Cambrian (Series 2–3) of North Greenland (Laurentia): systematics and biogeography. GFF 141, 133–161.
- Peel, J.S., 2021. In-place operculum demonstrates that the Middle Cambrian Protowenella is a hyolith and not a mollusc. Alcheringa 45, 385–394.
- Peel, J.S., 2022. The oldesty tongue worm: a stem-group pentastomid arthropod from the early middle Cambrian (Wuliuan Stage) of North Greenland (Laurentia). GFF 144, 97–105.
- Peel, J.S., in press. Musculature of an Ordovician (Darriwilian) Patelliform Gastropod from Estonia. GFF.
- Peel, J.S. & Horný, R.J., 1999. Muscle scars and systematic position of the Ordovician limpets Archinacella and Barrandicella gen. nov. (Mollusca). Journal of the Czech Geological Society 44, 97–115.
- Peel, J.S. & Kouchinsky, A. 2022. Middle Cambrian (Miaolingian Series, Wuliuan Stage) molluscs and mollusc-like microfossils from North Greenland (Laurentia). Bulletin of the Geological Society of Denmark 70, 69–104.
- Peel, J.S., Streng, M., Geyer, G., Kouchinsky, A. & Skovsted, C.B., 2016. Ovatoryctocara granulata assemblage (Cambrian Series 2–Series 3 boundary) of Løndal, North Greenland. Australasian Palaeontological Memoirs 49, 241–282.
- Ponder, W.F., Lindberg, D.R. & Ponder, J.M., 2020. Biology and Evolution of the Mollusca, Volume 2. CRC Press, Boca Raton, FL, 870 p.
- Rasetti, F., 1954. Internal shell structures in the Middle Cambrian gastropod Scenella and the problematic genus Stenothecoides. Journal of Paleontology 28, 59–66.
- Raup, D.M., 1961. The geometry of coiling in gastropods. Proceedings of the National Academy of Sciences of the United States of America 47, 602–609.
- Resser, C.E., 1945. Cambrian history of the Grand Canyon Region. Part II. Cambrian fossils of the Grand Canyon. Publications of the Carnegie Institution 563, 171–220.
- Robison, R.A., 1994. Agnostoid trilobites from the Henson Gletscher and Kap Stanton formations (Middle Cambrian), North Greenland. Bulletin Grønlands Geologiske Undersøgelse 169, 25–77.
- Rozanov, A.Y., Parkhaev, P.Y., Demidenko, Y.E., Karlova, G.A., Korovnikov, I.V., Shabanov, Y.Y., Ivantsov, A.Y., Luchinina, V.A., Malakhovskaya, Y.E., Melnikova, L.M., Naimark, E.B., Ponomarenko, A.G., Skorlotova, N.A., Sundukov, V.M., Tokarev, D.A., Ushatinskaya, G.T. & Kipriyznova, L.D., 2010. Fossils from the Lower Cambrian stage stratotypes. Russian Academy of Sciences, Transactions of the Palaeontological Institute, Moscow, 228. pp. [in Russian]
- Runnegar, B., 1981. Muscle scars, shell form and torsion in Cambrian and Ordovician univalved molluscs. Lethaia 14, 311–322.
- Runnegar, B., 1985. Shell microstructure of Cambrian molluscs replicated by phosphate. Alcheringa 9, 245–257.
- Runnegar, B. & Jell, P.A., 1976. Australian Middle Cambrian molluscs and their bearing on early molluscan evolution. Alcheringa 1, 109–138.
- Runnegar, B. & Pojeta, J., 1974. Molluscan phylogeny: the paleontological viewpoint. Science (New York, N.Y.) 186, 311–317.
- Runnegar, B. & Pojeta, J., 1985. Origin and diversification of the Mollusca. In The Mollusca, Vol. 10: Evolution. Trueman, E.R. & Clarke, M. R. eds, Academic Press, New York, 1–57.
- Shaw, A.B., 1962. Paleontology of northwestern Vermont. IX. Fauna of the Monkton Quartzite. Journal of Paleontology 36, 322–345.
- Skovsted, C.B., 2004. Mollusc fauna of the early Cambrian Bastion Formation of North-East Greenland. Bulletin of the Geological Society of Denmark 51, 11–37.
- Skovsted, C.B., 2006. Small shelly fauna from the upper lower Cambrian Bastion and Ella Island formations, North-East Greenland. Journal of Paleontology 80, 1087–1112.
- Skovsted, C.B. & Peel, J.S., 2007. Small shelly fossils from the argillaceous facies of the lower Cambrian Forteau Formation of Western Newfoundland. Acta Palaeontologica Polonica 52, 729–748.
- Stanley, G.D., Jr 1982. Paleozoic chondrophores (medusoid hydrozoans) and their implications for problematic mollusc-like fossils. Third North American Paleontological Convention, Proceedings 2, 501–504.
- Stanley, G.D., Jr 1986. Chondrophorine hydrozoans as problematic fossils. In Problematic Fossil Taxa. Hoffman, A. & Nitecki. M., eds, Oxford Monographs on Geology and Geophysics No. 5, Oxford University Press, New York, 68–86.
- Stinchcomb, B.L., 1986. New monoplacophora (Mollusca) from Late Cambrian and Early Ordovician of Missouri. Journal of Paleontology 60, 606–626.
- Stinchcomb, B.L. & Angeli, N.A., 2002. New Cambrian and Lower Ordovician monoplacophorans from the Ozark uplift, Missouri. Journal of Paleontology 76, 965–974.
- Sundberg, F.A., Webster, M. & Geyer, G., 2022. Biostratigraphical significance of a new trilobite fauna from the Harkless Formation (upper Stage 4, Series 2, Cambrian), Nevada, USA. Lethaia 55, 1–12.
- Thompson, D.W., 1942. On Growth and Form. Cambridge University Press, Cambridge, 793 p.
- Ulrich, E.O. & Scofield, W.H., 1897. The Lower Silurian Gastropoda of Minnesota. Final Report Minnesota Geological Survey 3, 813–1081.
- Ushatinskaya, G.T. & Parkhaev, P.Y., 2005. Preservation of imprints and casts of cells of the outer mantle epithelium in the shells of Cambrian brachiopods, molluscs and problematics. Paleontological Journal 39, 251–263.
- Vendrasco, M.J., Checa, A.G. & Kouchinsky, A.V., 2011b. Shell microstructure of the early clam Pojetaia and the independent origin of nacre within the Mollusca. Palaeontology 54, 825–850.
- Vendrasco, M.J., Kouchinsky, A.V., Porter, S.M. & Fernandez, C.Z., 2011a. Phylogeny and escalation in Mellopegma and other Cambrian molluscs. Palaeontologia Electronica 14, 1–44.
- Vendrasco, M.J., Porter, S.M., Kouchinsky, A., Li, G. & Fernandez, C.Z., 2010. New data on molluscs and their shell microstructures from the Middle Cambrian Gowers Formation, Australia. Palaeontology 53, 97–135.
- Vendrasco, M.J., Rodríguez-Navarro, A.B., Checa, A.G., Devaere, L. & Porter, S.M., 2016. To infer the early evolution of mollusc shell microstructures. Key Engineering Materials 672, 113–133.
- Vermeij, G.J., 2016. The limpet form in gastropods: evolution, distribution, and implications for the comparative study of history. Biological Journal of the Linnean Society 120, 22–37.
- Wahlman, G.P., 1992. Middle and Upper Ordovician symmetrical univalved mollusks (Monoplacophora and Bellerophontina) of the Cincinnati Arch Region. US Geological Survey Professional Paper 1066–O, 213 p.
- Williams, A. & Wright, A.D., 1970. Shell structure of the Craniacea and other calcareous inarticulate brachiopods. Special Papers in Palaeontology 7, 15–51.
- Wingstrand, K., 1985. On the anatomy and relationships of recent Monoplacophora. Galathea Report 16, 7–94.
- Winrow, P. & Sutton, M.D., 2012. Epithelial cell moulds in acrotretoid brachiopods. Historical Biology 24, 557–565.
- Wotte, T. & Sundberg, F.A., 2017. Small shelly fossils from the Montezuman–Delamaran of the Great Basin in Nevada and California. Journal of Paleontology 91, 883–901.
- Yochelson, E.L. & Gil Cid, D., 1984. Reevaluation of the systematic position of Scenella. Lethaia 17, 331–340.
- Yochelson, E.L. & Webers, G.F., 2006. A restudy of the Late Cambrian molluscan fauna of Berkey (1898) from Taylor Falls, Minnesota. Minnesota Geological Survey Report of Investigations 64, 1–66.
- Yu, W., 1979. Earliest Cambrian monoplacophorans and gastropods from Western Hubei with their biostratigraphical significance. Acta Palaeontologica Sinica 18, 233–270. [in Chinese].
- Yu, W. & Yochelson, E.L., 1999. Some late Cambrian molluscs from Liaoning Province, China. Records of the Western Australian Museum 19, 379–389.
- Zhou, B. & Xiao, L., 1984. Early Cambrian monoplacophorans and gastropods from Hainan and Huoqiu Counties, Anhui Province. Proceedings of Stratigraphy and Paleontology 13, 125–140. [in Chinese with English abstract].
